Abstract
Congenital cytomegalovirus (CMV) affects ∼1 of 150 births and is a leading cause of hearing loss and intellectual disability. It has been suggested that transmission may occur via contaminated surfaces. CMV AD169 in filtered human saliva, applied to environmental surfaces, was recovered at various time points. Samples were evaluated by culture and real-time polymerase chain reaction. CMV was found viable on metal and wood to 1 hour, glass and plastic to 3 hours, and rubber, cloth, and cracker to 6 hours. CMV was cultured from 83 of 90 wet and 5 of 40 dry surfaces. CMV was more likely to be isolated from wet, highly absorbent surfaces at earlier time points.
Congenital cytomegalovirus (CMV) infection, which occurs as a result of infection during pregnancy, affects ∼1 of 150 live births and is a leading cause of hearing loss and intellectual disability in the United States [1–3]. In a typical cohort of 4 million live births, this translates to ∼30 000 babies born with a congenital CMV infection [1]. Of those born with CMV, ∼6000 (20%) will suffer permanent sequelae (eg, sensorineural hearing loss and intellectual disability), and ∼150 (0.5%) will die of complications of the infection.
CMV is an enveloped virus that establishes lifelong latency following primary infection and periodically reactivates, usually without symptoms. Reinfection with additional strains is known to occur [4]. Transmission occurs via direct contact with bodily fluids of an infected individual. In adults, asymptomatic shedding of virus in saliva and urine may persist for weeks to months after initial infection. Children, especially those congenitally infected or <3 years old, may actively shed CMV in saliva and urine for months to years [5–8].
Exposure to bodily fluids from young children poses substantial risk for CMV exposure among women of reproductive age. Consequently, recommendations set forth by various experts for reducing CMV exposure have centered on behavioral precautions, such as routinely washing hands after exposure to bodily fluids, refraining from sharing food or drink with young children, and avoiding contact with saliva when kissing young children [2, 9–12]. However, little is known about the duration of CMV viability on environmental surfaces and the extent to which bodily fluids deposited on surfaces may be sources of exposure.
Methods
Seven types of common surfaces were studied: rubber, glass, plastic, metal, sanded wood, cloth, and wheat crackers. Surfaces were categorized as either highly absorbent or poorly absorbent surfaces. Highly absorbent surfaces included cloth (100% cotton), whole wheat crackers, and sanded pine plywood. Standard window glass, steel sheet metal, plastic (Plexiglas), and rubber matting were categorized as poorly absorbent surfaces. Ten defined areas (∼2 cm2) were marked on each material for the application and recovery of virus. For poorly absorbent surfaces it was necessary to demarcate an area (using a grease pencil) on the surface to keep the virus solution within the test area.
CMV strain AD169 from a single large stock (5.96 × 109 genome equivalents/mL) was titered on human lung fibroblasts using a plaque assay and was suspended in filtered human saliva diluted 1:1 in phosphate-buffered saline (PBS), pH 7.4, at a concentration of 103 virons/mL. For each surface, 200 μL of viral-saliva solution (∼200 viable virions) were applied to each of 10 marked areas and recovered using polyester fiber-tipped swabs (plastic shaft; BD Falcon) at time points ranging from 1 minute to 6 hours after application. When liquid was present, the remaining liquid was measured using a Pipet-Aid (Drummond Scientific Company, Broomall, PA) pipette controller and the volume adjusted to 200 μL with PBS to maximize consistency in recovery. An additional 100 μL of PBS was used to rewet the surfaces and collected onto a swab. The swab and fluids were placed into the barrel of a 3-cm3 syringe, inserted into an open sterile 15-cm3 centrifuge tube, and centrifuged at 200g for 10 minutes to maximally recover the sample. For cloth and cracker, the entire 2-cm2 area moistened by the sample was excised, placed into a syringe barrel to which 100 μL of PBS was added, and centrifuged as for other specimens. Recovered liquid was measured and adjusted to 200 μL with PBS.
At each time point, observations regarding visible wetness of the material (wet or dry) were recorded. One hundred microliters of each recovered sample was immediately transferred to T25 sterile culture flasks (Corning) containing a confluent monolayer of primary human lung fibroblasts in complete minimal essential medium supplemented with 5% fetal calf serum and antibiotics (Gibco). Cultures were observed for ≥2 weeks for signs of viral growth. The remaining sample was frozen for subsequent quantitative CMV polymerase chain reaction (PCR) analysis. Viral growth in cultures was scored from 0 to 4+ based on the extent of cytopathic effect. A quantitative CMV real-time PCR assay was performed on all samples to confirm the presence of CMV. DNA copies per reaction were used as a control for sampling consistency.
Results
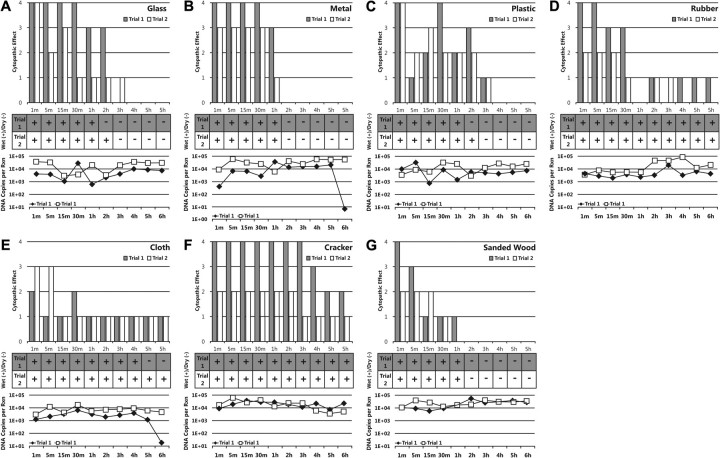
For each surface, the quantity of infectious virions declined with time, as measured by level of cytopathic effect produced in culture. In general, duration of CMV viability was shorter on poorly absorbent surfaces compared with highly absorbent surfaces (Figure 1A–D). Among poorly absorbent surfaces, CMV viability persisted longest on rubber, retaining viability for up to 6 hours. Samples applied to glass retained infectivity up to 3 hours. CMV applied to metal was rendered nonviable within 2 hours. Although viability was somewhat variable for CMV applied to plastic, viral infectivity persisted up to 3 hours. No viable virus was recovered at 1 hour for either trial of rubber, but given viability at multiple flanking time points on both sides of the timeline, this was interpreted as an experimental anomaly.
Figure 1.
Cytopathic effect, dehydration, and DNA copies after inoculation of common environmental surfaces with cytomegalovirus (CMV). Cytopathic effect seen through 2-week observation of viral culture on human lung fibroblast cells and scored from 0 to 4+. Plus sign indicate wet appearance; minus signs, dry appearance. Results of quantitative real-time CMV polymerase chain reaction assay are shown as DNA copies per reaction.
CMV survived longer on most of the highly absorbent surfaces used in the study than on poorly absorbent surfaces (Figure 1E–G). Viral infectivity was retained on cloth up to 6 hours. CMV applied to wheat cracker remained infectious for 6 hours in both trials, and, unlike on all other surfaces, in 1 trial retained 4+ cytopathic effect 3 hours after application. In contrast to the other highly absorbent surfaces, viral viability on sanded wood declined from a score of 4+ at 1 minute to a score of 0 at 2 hours.
The cracker surface remained visibly wet throughout the 6-hour study period. Among the other surfaces, the reduction of viability generally correlated with subjective visual observation of the surface becoming dry, although glass, cloth, and plastic retained viable virus for up to 1–2 hours after the surface appeared completely dry. This was observed in samples taken at the 5-hour sampling on cloth, 2-and 3-hour samplings on plastic, and 2-and 3-hour samplings on glass (Figure 1A, 1C, and 1E). No viable virus was recovered in experiments evaluating CMV survival at time points from 18 to 24 hours after application (data not shown).
As a measure of reliability and consistency of CMV sample recovery, harvested samples were separately assayed by quantitative real-time CMV PCR to estimate genome copy number. Results are displayed in the line graphs under each chart in Figure 1. These assays suggest that CMV sample recovery was highly consistent for all 10 sampling areas on all 7 surfaces. In only 1 of the trials, CMV DNA load dropped substantially at the 6-hour time point for metal (6.32 copies) and cloth (23.5 copies).
Discussion
In this systematic evaluation of CMV survival on surfaces, we found that CMV viability can sufficiently persist to enable fomite transmission. In fact, viable CMV can persist on surfaces as long as they remain wet—in some cases at least up to 6 hours. There are no additional data to conclude whether virus remains viable on surfaces that remain wet for more than 6 hours. However, the amounts of viable virus on wet surfaces decreased over a 6-hour period, suggesting that virus on wet surfaces may be less likely to survive over longer periods of time (eg, overnight). Additionally, we found that apparently dry surfaces can harbor viable virus in the 1–2-hour time period between when they are visibly dry and when, presumably, the microenvironment becomes completely dry. However, visibly wet surfaces were much more likely to harbor viable virus and to harbor higher amounts of viable virus than dry surfaces.
In most cases it appears that CMV viability is more closely related to wetness than to the particular surface where it is applied. Indeed, we found a correlation between CMV viability and the capacity of each particular material to retain moisture: with the exception of processed wood, viral viability persisted much longer in highly absorbent surfaces that would be expected to effectively retain moisture. The procedure for virus recovery from cloth and cracker, which were both centrifuged, may be most similar to a situation where cloth is placed in the mouth or a cracker is eaten. Because these highly absorbent surfaces can retain moisture internally, they may pose a higher transmission risk when they are mouthed or ingested than when only their surface is touched. CMV viability was eliminated more rapidly in wood samples than in other highly absorbent surfaces studied, suggesting a process of active neutralization. This may be attributable to substances naturally present in wood (eg, organic alcohols and aromatic liquids) or to chemicals used to treat lumber during production.
This study is the first controlled systematic research aimed at evaluating CMV viability in human fluids on surfaces over time. Few previous studies have provided evidence specifically related to opportunities for viral transmission from common surfaces. A study conducted in 1982 in a childcare center identified toddlers shedding CMV in their saliva. CMV was cultured from all 4 of the plastic toys that were swabbed immediately after removal from the mouth of a shedding child [13]. In an additional study in 1986, toys mouthed by CMV-excreting children were swabbed after removal from the child’s mouth. CMV was detected on 5 of 7 toys immediately, 4 of 7 toys after 10 minutes, and 2 of 7 toys after 30 minutes [14]. Unfortunately, it is difficult to compare results between past studies and the current study, because toy surfaces were not physically described in the former. Finally, a study from 1985 investigated CMV survival on surfaces such as Plexiglas and bedding in the immediate neonatal intensive care unit environment of congenitally infected infants [15]. That study found that virus was more likely to be recovered from surfaces that came into direct contact with the infants and that higher viral titers were associated with longer persistence on surfaces.
The strengths of the current study include the evaluation of CMV survival using titered preparations of viable virus suspended in a natural fluid (saliva) and the use of objective experimental controls (CMV real-time PCR) to monitor the consistency of sample recovery from surfaces. One possible limitation was the use of laboratory strain AD169. CMV strains adapted to growth in human lung fibroblast cell culture can rapidly lose some properties found in wild-type viruses, particularly with respect to infection of other cell types (eg, epithelial cells). However, it seems unlikely that the physical properties of the virus that contribute to survival time on inanimate surfaces would be different in laboratory-adapted strains. Laboratory strains and wild-type viruses both express the receptors needed for entry into fibroblasts, and the viral envelope is acquired from the host cell and is thus identical in both types of strains. When desiccation occurs, membrane integrity is lost, and viability along with it. Because the macromolecular composition of the viral envelope is identical for laboratory strains and wild-type viruses, comparable susceptibility to desiccation is probable. Another important limitation is that recovery of specimen was not designed to reflect real-world transmission. Instead, it was designed for comparability of different surfaces and to maximize the possibility of recovering viable virus. For these reasons, we rewet dry surfaces and centrifuged the cloth and cracker. Compared with the likelihood of recovering viable virus through these methods, we would expect that a person who touches dry surfaces or touches the cloth or cracker would be less likely to transfer viable virus to their fingers. Additionally, cultures were followed for only 2 weeks. Although it is true that some foci may have appeared after 3 or 4 weeks of observation, the intent of the study was to compare viable virus on multiple surfaces and their relative survival times.
As shown, CMV in saliva can survive long enough on surfaces to pose a transmission risk. Young children commonly shed CMV for extended periods, contaminating toys, food, and other objects in the environment. To reduce exposure to CMV, women who are pregnant should cleanse their hands after touching objects that may have been in contact with children’s saliva (eg, toys, countertops), especially if the surface appears or feels wet. Additionally, surfaces that have come in contact with children’s saliva should be cleansed regularly, especially when noticeably wet. Finally, women should take precautions to avoid sharing food or drink with a young child and avoid contact with saliva when kissing a young child.
Notes
Disclaimer.
The views included in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Financial support.
This research was supported in part by appointments (J.D.S., D.F.P., E.D., S.L.B.) at the Centers for Disease Control and Prevention (CDC), National Center for Immunization and Respiratory Disease, Division of Viral Disease, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the CDC. Additional funding for D.F.P. was provided by (Coordenação de Aperfeiçoamento de Pessoal de Nível) through an agreement between the Brazilian Ministry of Education and Universidade Federal de Sao Paulo, Sao Paulo, Brazil.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355–63. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 2.Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health. 2005 doi: 10.1186/1471-2458-5-70. 5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253–76. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 4.Boppana SB, Fowler KB, Pass RF, et al. Congenital cytomegalovirus infection: association between virus burden in infancy and hearing loss. J Pediatr. 2005;146:817–23. doi: 10.1016/j.jpeds.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 5.Leads from the MMWR. Prevalence of cytomegalovirus excretion from children in five day-care centers. JAMA. 1985;253:1236. 1239–40. [PubMed] [Google Scholar]
- 6.Jones LA, Duke PM, Yeager AS. Cytomegalovirus infections—infant development versus day care centers. Clin Res. 1984;32:A109. [Google Scholar]
- 7.Murph JR, Bale JF. The natural-history of acquired cytomegalovirus infection among children in group day care. Am J Dis Child. 1988;142:843–6. doi: 10.1001/archpedi.1988.02150080049020. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal LS, Fowler KB, Boppana SB, et al. Cytomegalovirus shedding and delayed sensorineural hearing loss results from longitudinal follow-up of children with congenital infection. Pediatr Infect Dis J. 2009;28:515–20. doi: 10.1097/INF.0b013e318198c724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove, IL: American Academy of Pediatrics; 2009. American Academy of Pediatrics. Cytomegalovirus infection; pp. 275–80. [Google Scholar]
- 10.Adler SP, Finney JW, Manganello AM, Best AM. Prevention of child-to-mother transmission of cytomegalovirus by changing behaviors: a randomized controlled trial. Pediatr Infect Dis J. 1996;15:240–6. doi: 10.1097/00006454-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Picone O, Vauloup-Fellous C, Cordier AG, et al. A 2-year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG. 2009;116:818–23. doi: 10.1111/j.1471-0528.2009.02139.x. [DOI] [PubMed] [Google Scholar]
- 12.Demmler-Harrison GJ. Congenital cytomegalovirus: public health action towards awareness, prevention, and treatment. J Clin Virol. 2009;46:S1–S5. doi: 10.1016/j.jcv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Pass RF, August AM, Dworsky M, Reynolds DW. Cytomegalovirus infection in day-care center. New Engl J Med. 1982;307:477–9. doi: 10.1056/NEJM198208193070804. [DOI] [PubMed] [Google Scholar]
- 14.Hutto C, Little EA, Ricks R, Lee JD, Pass RF. Isolation of cytomegalovirus from toys and hands in a day care center. J Infect Dis. 1986;154:527–30. doi: 10.1093/infdis/154.3.527. [DOI] [PubMed] [Google Scholar]
- 15.Faix RG. Survival of cytomegalovirus on environmental surfaces. J Pediatr. 1985;106:649–52. doi: 10.1016/s0022-3476(85)80096-2. [DOI] [PubMed] [Google Scholar]