Abstract
Prosocial decisions can be difficult because they often involve personal sacrifices that do not generate any direct, immediate benefits to the self. The current study used functional magnetic resonance imaging (fMRI) to understand how individuals decide to provide support to others. Twenty-five participants were scanned as they completed a task in which they made costly decisions to contribute money to their family and noncostly decisions to accept personal monetary rewards. Decisions to contribute to the family recruited brain regions involved in self-control and mentalizing, especially for individuals with stronger family obligation preferences. Psychophysiological interaction (PPI) analyses revealed that individuals with stronger family obligation preferences showed greater functional coupling between regions involved in self-control and mentalizing with the ventral striatum, a region involved in reward processing. These findings suggest that prosocial behavior may require both social cognition and deliberate effort, and the application of these processes may result in greater positive reinforcement during prosocial behavior.
Many prosocial behaviors benefit another individual but do not generate a direct, immediate personal benefit. In fact, such behaviors often entail personal sacrifices such as one's time, money, and personal needs. Therefore, prosocial decisions can be difficult to make because an individual must weigh the relative value of helping others against zero personal gain or potentially a personal loss. Nonetheless, people frequently engage in prosocial behaviors despite the personal sacrifices. The current study seeks to understand the processes underlying individuals' decisions to provide costly support to others when it involves overriding their own self-interests.
Traditional economic theory proposed that prosocial decisions were guided by individuals' desire to maximize their own self-interests, such as the promotion of personal positive outcomes and the prevention of personal negative outcomes (Fehr and Camerer 2007; Fehr and Schmidt 2006; Higgins 1997). A large body of work now suggests that prosocial behavior may be guided by the social preference to help others, and that such preferences are inherently rewarding (Fehr and Camerer 2007). For example, individuals gain a sense of happiness when they help others, including their own kin, their close friends, and charities (Telzer and Fuligni 2009a; Dunn et al. 2008). Furthermore, neuroeconomic research has consistently found that the striatum and ventral midbrain, regions of the brain that are sensitive to reward, are activated when helping others, suggesting that prosocial decisions may be partly guided by the rewarding nature of the activity. (Harbaugh et al. 2007; Izuma et al. 2010; Moll et al. 2006; Telzer et al. 2010).
Despite these advances, it still remains unclear what processes precipitate individuals' decisions to put the needs of others before their own. Given that prosocial behaviors often involve personal sacrifices, these decisions likely involve multiple processes. However, previous work has primarily focused on the rewarding nature of these behaviors and has been largely silent about other processes involved during the decision making process. In order to make prosocial decisions, individuals likely rely on the ability to (1) inhibit their own immediate personal desires and (2) take the perspective of others in order to understand their needs and values, processes known as self-control and mentalizing, respectively.
Prosocial Decisions and Self-control
When making prosocial decisions, it is often necessary to exhibit self-control to resolve conflict between prosocial motives and self-interests in order to put the needs of another before one's own immediate needs (Fehr and Camerer 2007). Self-control is the ability to regulate, manipulate, or control one's impulsive thoughts, feelings, and behaviors. Neuroimaging research has consistently found the bilateral ventrolateral prefrontal cortex (VLPFC) and bilateral dorsolateral prefrontal cortex (DLPFC) to be involved in self-control. The VLPFC is generally involved in response inhibition and cognitive flexibility and may help to inhibit one's own immediate reaction during the consideration of another's needs (Cohen and Lieberman 2010; O'Doherty et al. 2003; Samson et al. 2005; Vogeley et al. 2001). The DLPFC is generally involved in effortful, high level cognitive control and plays a role in selecting between competing possibilities, inhibiting self interested tendencies, and overriding selfish impulses (Frith 2000). Self-control processes have been shown to activate both the right and left VLPFC and DLPFC (Lieberman 2010), suggesting that there is often bilateral engagement of these regions during self-control. Thus, the VLPFC and DLPFC may help individuals to resolve conflict between selfish and prosocial motives in order to make decisions that benefit another, even when they come at a personal cost.
Prosocial decisions and mentalizing
Prosocial decisions may also be guided by the ability to shift attention from oneself to the needs, goals, and values of others, a process known as mentalizing. Neuroimaging research has consistently found a network of brain regions involved inmentalizing. The most commonly implicated regions include the dorsomedial prefrontal cortex (DMPFC), temporal parietal junction (TPJ) and posterior superior temporal sulcus (pSTS) (Lieberman 2010). These regions play a key role in theory of mind (i.e., understanding the minds of others), perspective taking, and shifting attention to focus on the needs and values of others (Frith and Frith 2003; Hare et al. 2010; Ruby and Decety 2003, Mitchell 2008; Saxe and Kanwisher 2003). Activation of these regions during mentalizing is largely bilateral (Lieberman 2010). In addition, the medial prefrontal cortex (MPFC) may be particularly relevant to mentalizing about specific others who are viewed as similar or close to oneself (Mitchell et al. 2005, 2006). Together, these regions may play a role in prosocial behaviors by helping an individual to shift attention away from one's own self interests to the needs and values of others, particularly when thinking about close family and friends.
Prosocial decisions and the family
In the current study, we examined the neural networks involved in prosocial decisions to help family members. Previous research has largely examined prosocial behavior towards strangers or charities. However, most helping behavior occurs between individuals who know one another well (Aronson et al. 2007).We focus on helping the family because it is a salient and frequent type of prosocial behavior, often occurring on a daily basis. For instance, 98% of adolescents from diverse cultural and economic backgrounds report helping their family on a weekly basis (Telzer and Fuligni 2009a).
Despite their frequency, decisions to help the family can be difficult, especially when they require personal sacrifices. Individuals must make important decisions that involve putting the family's needs before their own and delaying personal impulses and desires in order to help the family. For example, in our behavioral work, we found that those who assist their family more report greater demands at home, spend less time with their friends, and often show declines in their academic performance (Telzer and Fuligni, 2009a, Telzer and Fuligni 2009b; Fuligni and Pedersen 2002). Despite these personal sacrifices, when individuals help their family they report a greater sense of happiness and feelings of role fulfillment (Telzer and Fuligni 2009a) and show activation in a network of brain regions sensitive to reward, including the ventral and dorsal striatum and ventral tegmental area (Telzer et al. 2010). Together, these findings suggest that decisions to help the family are demanding and costly but also rewarding.
Family obligation values and preferences
In young adulthood, individuals often gain a deeper understanding of their social and communal obligations, and they consider ways in which they can help others, including their family (Arnett 1998; Fuligni and Pedersen 2002). As a result, individuals' sense of responsibility to assist their family and take into account the needs and wishes of their family when making decisions increases during the transition to young adulthood, often reaching levels higher than those in the adolescent years (Fuligni et al. 1999; Fuligni and Pedersen 2002). A stronger sense of family obligation during young adulthood may represent individuals' striving for a balance between independence and family solidarity and connection (Fuligni and Pedersen 2002).
Social psychologists and economists have stressed the important role that values and preferences play in determining when and how individuals choose to help others (Caprara and Steca 2007; Fehr and Fishbacher 2002). Prosocial preferences, such as family obligation, shape an individual's capacity to put others' needs before one's own (Caprara and Steca 2007; Fehr and Fishbacher 2002). In particular, individuals who more strongly value helping others and putting others' needs before their own are more likely to take another's perspective, assign priority to another's welfare, and regulate their own behavior and emotions in order to meet another's needs (Caprara and Steca 2007). Thus, individuals who more strongly endorse family obligation preferences may think about their family's needs and values and exert self-control so as to make decisions that optimize the overall benefits to their family, a process involving both self-control and mentalizing. In the current study, we measure participants' values and preferences to engage in prosocial behaviors towards their family and how these family obligation preferences relate to the recruitment of brain regions involved in self-control and mentalizing.
Linking mentalizing and self-control processing with reward activation during prosocial decisions
In our previous work, we found that activation of regions associated in reward (e.g., ventral and dorsal striatum and ventral midbrain) were modulated by cultural differences and prior family experiences when making costly contributions to the family (Telzer et al. 2010). Specifically, Latino individuals exhibited greater recruitment of reward-related regions when choosing to help their family compared to White individuals, and those who had experienced more fulfillment from assisting their family 2 years prior to the scan exhibited greater reward activation when helping their family, regardless of their cultural background. This prior work demonstrated the important role that culture and experience play in the rewarding nature of family assistance decisions. Here, we build upon this prior work by focusing on the specific role that mentalizing and self-control processes play during prosocial decisions to help the family and whether the application of these processes result in greater positive reinforcement, leading to a reward inducing, prosocial decision.
To test this possibility, we conducted psychophysiological interaction (PPI) analyses in order to examine if there is functional coupling between regions involved in self-control and mentalizing with the ventral striatum, a brain region that has consistently been linked with the reward associated with helping and supporting others (Harbaugh et al. 2007; Izuma et al. 2010; Moll et al. 2006; Telzer et al. 2010). In our previous work, we found significant variability in the extent to which individuals recruited the ventral striatum when making costly contributions to their family (Telzer et al. 2010), suggesting that ventral striatal recruitment may be modulated by other neural processes during prosocial decisions. Functional coupling between neural regions involved in self-control and mentalizing with the ventral striatum could help us understand the neural precursors that promote a reward inducing decision.
The current study
In the current study, we used fMRI to help elucidate the neural mechanisms that support prosocial behavior. We tested four key questions: (1) Do decisions to help the family recruit brain regions involved in mentalizing and self-control? (2) Do individuals who more strongly endorse family obligation preferences recruit these neural regions to a greater extent than individuals who do not value family obligation? (3) Is there functional coupling between brain regions involved in self-control and mentalizing with the ventral striatum? (4) Is this functional coupling greater for individuals who more strongly endorse family obligation?
Methods
Participants
Participants included 25 individuals from Latin American (N = 14) and European (N = 11) backgrounds (Mage = 20.2 years; 13 females), the same sample as that described in Telzer et al. (2010). Twenty-one participants were attending a 2- or 4-year college, and 12 participants were living at their parents' home at the time of the scan. Participants reported no MRI contraindications (i.e., metal in their bodies, claustrophobia, pregnancy) and spoke and read English fluently. All participants provided written consent in accordance with UCLA's Institutional Review Board.
Procedures
Participants came to the UCLA Brain Mapping Center where they completed a family assistance task during an fMRI scan (see below). Upon completion of the scan, participants completed several questionnaires including a measure indicating their family obligation preferences and were paid in cash according to their earnings on the task.
Family obligation preferences
In order to measure participants' preferences for helping their family, participants used a 5-point scale to respond to 25 questions describing their attitudes regarding (1) current assistance to the family, (2) respect for the family, and (3) future support to the family (Fuligni et al. 1999). Current assistance measured participants' expectations for how often they should assist with household tasks and spend time with the family, such as “help take care of your brothers and sisters,” “eat meals with your family,” and “spend time with your family on weekends.” Respect for the family measured participants' beliefs about the importance of respecting and following the wishes, desires, and expectations of other family members, such as “make sacrifices for your family,” “respect your older brothers and sisters,” and “show great respect for your parents.” Future support to the family measured participants' beliefs about the importance of providing support and being near their families in the future, such as “help your parents financially in the future,” “help take care of your brothers and sisters in the future,” and “have your parents live with you when you get older.” All 25 items were averaged to create one index, Family Obligation Preferences, which ranged from 1 to 5,with higher numbers indicating greater family obligation preferences. This index was used as a regressor in the fMRI analyses. Mean Family Obligation Preference in the current study was 3.22 (SD = .67), ranging from 1.63 to 4.02.
fMRI Paradigm
During the fMRI scan, participants completed the Family Assistance Task adapted from Moll and colleagues' neuroimaging task on charitable donations, (Moll et al. 2006) and used in our previous work (Telzer et al. 2010). Participants were told that they could earn as little as $0 and as much as $100 each for themselves and their family on each of the two functional runs of the task, and one run would be randomly chosen for which they would be paid in cash at the end of the scan and their families' earning would be mailed. Participants were instructed that costly decisions would result in the loss of money from their personal endowment earned during the task. In order that participants thought of their own and their families' endowments separately, they were told that they could not spend their earnings on their family and their family could not spend their earnings on the participant.
On each trial of the task, participants were presented with a payment offer that could affect their own and their family's endowments. Using a handheld buttonbox, participants were instructed to press one button to reject the entire offer and a second button to accept the entire offer. The task included four types of payments: (1) Noncostly-Reward trials, which entailed a monetary gain for the participant at no expense to their family's endowment (e.g., YOU + $2.00 FAM − $0.00); (2) Noncostly-Donation trials, which entailed a monetary gain for the family at no expense to the participant's endowment (e.g., YOU − $0.00 FAM + $2.00); (3) Costly-Reward trials, which entailed a monetary gain for the participant and a monetary loss for their family (e.g., YOU + $2.00 FAM − $1.00); and (4) Costly-Donation trials, which entailed a monetary gain for the family and a monetary loss for the participant (e.g., YOU − $1.00, FAM + $2.00). The values of these payments ranged from −$3.00 to +$8.00 to reduce participant fatigue and heuristic responding (Andreoni and Miller 2002; Harbaugh et al. 2007). The costly trials varied in terms of the ratio of the amount of gain to the amount of loss in order to vary the difficulty of the decisions and obtain a wider range of individual differences in responses. The gain, however, was always greater than the loss.
Participants completed 64 unique payment trials, each presented once per run, totaling 128 trials. Pilot testing suggested that the noncostly trials were accepted nearly 100% of the time whereas there was substantial variability in the acceptance rates for the costly trials. Therefore, the Noncostly-Reward and Noncostly-Donation payments were each shown 24 times, and the Costly-Donation and Costly-Reward payments were each shown 40 times to ensure a high enough acceptance rate to perform statistical analyses. Run order was counterbalanced across participants, and the payment types were pseudo-randomly ordered to optimize design efficiency (Wager and Nichols 2003). Each payment offer was presented for 3.5 s, followed by a fixation for an inter-trial period that was jittered, lasting 1.5 s on average. If participants did not make a decision during the payment offer, they were told it would count as a rejection. Participants were not shown the running total of their own or their family's endowments.
In the current study, we focus on two payment types: Costly-Donations and Noncostly-Rewards. Costly-Donation trials involve a conflict between participants' personal endowment and their desire to contribute to their family. Decisions to accept Costly-Donations involve a self-sacrifice that most closely approximates prosocial, altruistic decisions. In contrast, Noncostly-Reward trials only involve a personal gain that does not affect the family's endowment. These decisions are hedonistically rewarding and are consistently linked with activation in reward-relevant brain regions, such as the ventral striatum (Moll et al. 2006). Given that Costly-Donation trials tend to be more difficult and involve conflict and self-sacrifice, we examined whether making costly donations to one's family engaged neural regions associated with self-control and mentalizing and how this contrasted with gaining a personal reward.
fMRI data acquisition
Images were collected using a Siemens Trio 3-Tesla MRI scanner. For each participant, an initial 2D spin-echo image (TR = 4000 ms, TE = 40ms, matrix size 256 × 256, 4-mm thick, 1-mm gap) in the sagittal plane was acquired in order to enable prescription of slices obtained in structural and functional scans. A high-resolution structural T2*weighted echo-planar imaging volume (TR = 4000ms, TE = 54 ms, matrix size 128 × 128, FOV = 200mm, 36 slices, 1.56 mm in-plane resolution, 3 mm thick) was acquired coplanar with the functional scans for functional image registration during fMRI analysis preprocessing. The family assistance task was presented on a computer screen through MR-compatible goggles. The task was completed during two functional scans lasting 5 min, 33 s each (echo planar T2*weighted gradient-echo, TR = 3000 ms, TE = 25 ms, flip angle = 90°, MATRIX SIZE 64 × 64, FOV = 200 mm; 3 mm voxel, skip 1 mm).
fMRI data analysis
Neuroimaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM5;Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Preprocessing for each participant's images included slice-timing to adjust for temporal differences in slice acquisition within each volume, spatial realignment to correct for head motion(no participant exceeded 2 mm),normalization into a standard stereotactic space as defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping, and spatial smoothing using an 8 mm Gaussian kernel, full width at half maximum, to increase the signal-to-noise ratio.
The task was modeled as an event-related design. Using a two level procedure, we conducted a random effects fMRI data analysis. First, linear contrasts comparing Costly-Donations to Noncostly-Rewards were calculated for each participant. Events were modeled with a 3.5 s duration beginning with the appearance of the payment screen. Next, the individual subject contrasts were submitted to random-effects, group-level analyses. We conducted whole-brain regression analyses to examine how family obligation preferences related to neural activity when making financial contributions to one's family. Family obligation preferences were entered as a regressor in the contrast of Costly-Donation versus Noncostly-Reward trials.
Next, we conducted psychophysiological interaction (PPI; Friston et al. 1997) analyses to examine how a sense of family obligation relates to functional coupling between regions involved in self-control and mentalizing and the ventral striatum. For each participant, we extracted the deconvolved time-course from the ventral striatum, which was a structurally defined ROI used in our previous study (Telzer et al. 2010). We then calculated the product of this deconvolved activation time-course and the vector of the psychological variable of interest (1 for Costly-Donation; −1 for Noncostly-Reward) to create the psychophysiological interaction term. Individual level PPIs were computed for each subject, with three regressors: the interaction term, the physiological variable (ventral striatum time course), and the psychological variable (Costly-Donation versus Noncostly-Reward). Two contrasts were specified for each individual-level PPI (100 and −100) reflecting activations that were either positively or negatively related to the PPI interaction term, respectively. The individual-level PPIs were then entered into a random-effects group-level regression analysis, in which family obligation preferences were entered as a regressor, contrasting connectivity patterns between Costly-Donation versus Noncostly-Reward trials.
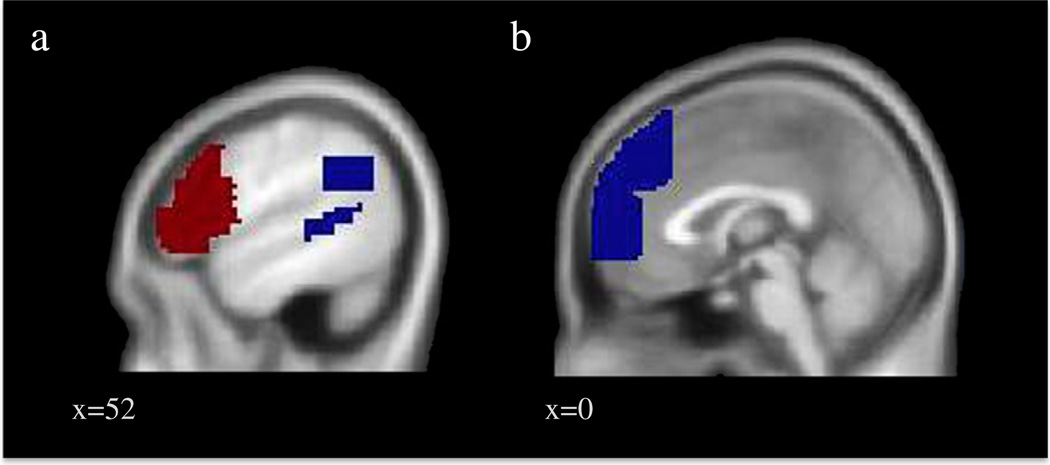
All neuroimaging analyses used a false discovery rate (FDR) correction of p < .05 to correct for multiple comparisons. A priori ROIs were created for the Self-Control and Mentalizing areas separately using the Wake Forest University Pickatlas Tool (Maldjian et al. 2003) and the Automated Anatomical Labeling atlas (AAL; Tzourio-Mazoyer et al. 2002). Using ROIs adds specificity to our analyses relative to a whole-brain search and helps to minimize the Type I error rate. The Self-Control ROI included the bilateral DLPFC and VLPFC, depicted in red in Fig. 1a. The DLPFC was defined using a combination of AAL's superior and middle frontal gyri, pars opercularis, and pars triangularis, all superior to and including the axial plane at MNI z = 2, and lateral to MNI x = 20 and x = −20 in the sagittal plane. The VLPFC was defined using a combination of AAL's superior, middle, and inferior frontal gyri, between MNI z = 0 and −10 in the axial plane, and lateral to MNI x = 20 and x = −20 in the sagittal plane. The Mentalizing ROI included the bilateral TPJ and pSTS, which are depicted in blue in Fig. 1a, as well as the MPFC and DMPFC, depicted in blue in Fig. 1b. The TPJ was defined using the union of Brodman's areas 22, 39, and 40, and restricting the ROI to include the sagittal plane lateral to x = 38 and −38, between and including the coronal plane at MNI y = −40 and −68, and between and including the axial plane at MNI z = 22 and 38. The pSTS was defined using Broadman's area 22 and restricting the ROI to include the sagittal plane lateral to and including MNI at x = 42 to −42, between and including the coronal plane at MNI y = −38 to −58, and between and including the axial plane at MNI z = 16 to −2. The DMPFC was defined using the union of Brodman's areas 8 and 9, between and including the sagittal plane at MNI x = 12 and −12, superior to and including the axial plane at MNI z = 24, and anterior to and including the coronal plane at MNI y = 30. Finally, the MPFC was defined using Brodman's area 10, between and including the sagittal plane at MNI x = 12 and −12, between and including the axial plane at MNI z = −10 and 24, and anterior to and including the coronal plane at MNI y = 30. We submitted both the Self-Control and Mentalizing ROIs to a Monte Carlo simulation implemented using AlphaSim in the software package AFNI (Ward 2000). Results of AlphaSim indicated a voxel-wise threshold of p < .005 combined with a minimum cluster size of 22 in the Mentalizing regions, 25 in the Self-Control regions, and 43 in non a priori regions, corresponding to p < .05, False Discovery Rate (FDR) corrected within each search space. Because the variables of interest often vary by ethnicity, we controlled for ethnicity by entering dummy-coded ethnicity as a covariate in all analyses. Coordinates are reported in Montreal Neurological Institute (MNI) format. For visual presentation, clusters were surface rendered using the SPM SurfRend toolbox (Kahn 2008) and overlaid on an inflated brain using NeuroLens (Hoge and Lissot 2004).
Fig. 1.
The self-control and mentalizing ROIs. (a) Self-control regions are depicted in red and include the bilateral DLPFC and VLPFC. The Mentalizing regions are depicted in blue and include the bilateral TPJ and pSTS on the lateral surface and (b) the MPFC and DMPFC on the medial surface.
Results
Prosocial decisions to the family
As reported previously (Telzer et al. 2010), participants who contributed financially to their family in their everyday life were also more likely to accept Costly-Donations during the Family Assistance Task than were participants who did not contribute to their family, t(23) = 2.98, p < .01, suggesting that the task is ecologically valid. Participants accepted more Noncostly-Reward (Macceptance = 99.2%) than Costly-Donation (Macceptance = 66.1%) trials, t(24) = 7.26, p < .001, and earned significantly more money for themselves ($77.51) than for their family ($48.29), t(24)=5.74, p < .001, suggesting that participants were sensitive to the different conditions and were concerned about their own endowments. In addition, participants took longer to make decisions to accept or reject Costly-Donations (Mrt = 1.67 s) than Noncostly-Rewards (Mrt = 1.00s) t(24) = 12.12, p < .001, suggesting that costly decisions may require more effort. Finally, to examine whether family obligation preferences were associated with participants' behavioral responses, we conducted regression analyses controlling for ethnicity. Family obligation was not associated with participants' acceptance rates, total earnings, or mean response times to the different trial types (βs = −.31–.34, ps = ns).
Neural regions engaged during prosocial decisions to the family
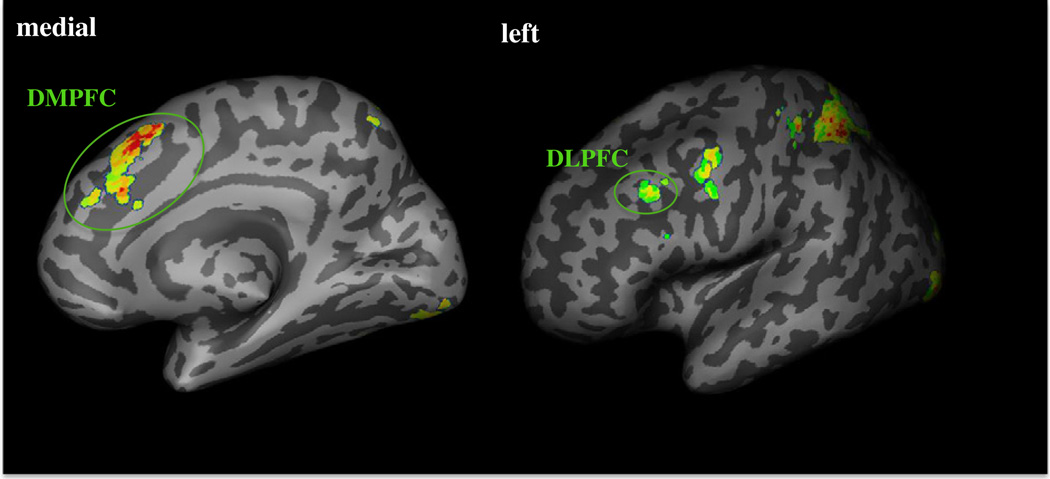
Our first set of analyses examined whether prosocial decisions to provide support to the family were related to the recruitment of brain regions involved in self-control and mentalizing. Whole brain analyses revealed that decisions to accept Costly-Donation trials relative to Noncostly-Reward trials engaged brain regions involved in mentalizing and self-control (Table 1). Specifically, participants displayed greater activity in the left DLPFC, a region linked with self-control, as well as greater activity in the DMPFC, a region linked with mentalizing (Fig. 2).
Table 1.
Neural regions engaged during decisions to accept Costly-Donation relative to Noncostly-Reward trials.
| Anatomical Region | BA | x | y | z | t | k | |
|---|---|---|---|---|---|---|---|
| DMPFC | 8 | R | 3 | 27 | 48 | 6.35 | 428 |
| Cuneus† | 18 | L | −24 | −90 | 3 | 6.02 | 585 |
| Cuneus† | 18 | R | 39 | −90 | −6 | 5.50 | 449 |
| IPL† | 40 | L | −36 | −45 | 48 | 5.41 | 482 |
| IPL† | 40 | R | 33 | −48 | 51 | 5.39 | 430 |
| DLPFC | 9 | L | −48 | 30 | 33 | 4.62 | 171 |
Note. BA refers to putative Brodmann's Area; L and R refer to left and right hemispheres; x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. The following abbreviations are used for the names of specific regions: dorsomedial prefrontal cortex (DMPFC), dorsolateral prefrontal cortex (DLPFC), inferior parietal lobule (IPL). All a priori regions are listed at p < .005, corrected for multiple comparisons within the regions of interest.
Regions marked with a cross (†) are those that were not the primary focus of this investigation but were significant at p < .005, corrected for multiple comparisons within the whole brain.
Fig. 2.
Neural regions activated during Costly-Donation relative to Noncostly-Reward trials. The left image displays the DMPFC activation and the right image displays the DLPFC activation. See Table 1 for other significant regions activated during Costly Donation versus Noncostly Reward trials.
Neural regions engaged during prosocial decisions to the family that correlated positively with family obligation preferences
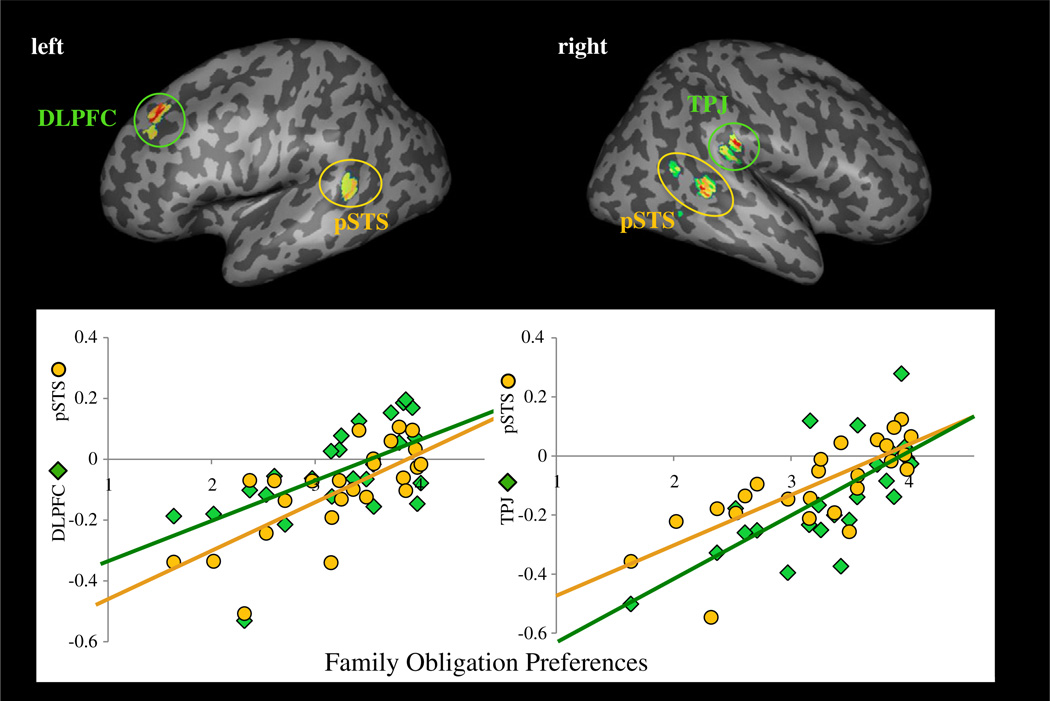
Next, we examined whether the recruitment of brain regions involved in self-control and mentalizing would be greater for individuals who placed higher value on supporting, respecting, and spending time with their family. We correlated family obligation preferences with neural activity for Costly-Donation versus Noncostly-Reward trials. Whole-brain regression analyses revealed that participants who placed greater value on supporting, respecting, and spending time with their family showed greater activation in regions involved in self-control and mentalizing (Table 2). Specifically, participants displayed greater activity in the left DLPFC, a region linked with self-control, as well as greater activity in the right TPJ, and bilateral pSTS, regions linked with mentalizing (Fig. 3).
Table 2.
Neural regions associated with family obligation preferences during decisions to accept Costly-Donation versus Noncostly-Reward trials.
| Anatomical Region | BA | x | y | z | t | r | k | |
|---|---|---|---|---|---|---|---|---|
| TPJ | 40 | R | 54 | −36 | 27 | 5.49 | .75 | 48 |
| pSTS | 22 | R | 48 | −42 | 6 | 5.32 | .74 | 68 |
| Cingulate Cortex† | 32 | R | 3 | 30 | 21 | 4.37 | .67 | 45 |
| DLPFC | 9 | L | −24 | 36 | 36 | 4.19 | .65 | 54 |
| pSTS | 22 | L | −42 | −48 | 18 | 3.84 | .62 | 38 |
Note. BA refers to putative Brodmann's Area; L and R refer to left and right hemispheres; x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); r refers to the correlation coefficient representing the strength of the association between family obligation preferences and activity in the specified region; k refers to the number of voxels in each significant cluster. The following abbreviations are used for the names of specific regions: temporal parietal junction (TPJ), posterior superior temporal sulcus (pSTS), dorsolateral prefrontal cortex (DLPFC). All a priori regions are listed at p < .005, corrected for multiple comparisons within the regions of interest.
Regions marked with a cross (†) are those that were not the primary focus of this investigation but were significant at p < .005, corrected for multiple comparisons within the whole brain.
Fig. 3.
Neural regions involved in self-control and mentalizing that correlate positively with family obligation values during the contrast of Costly-Donation versus Noncostly-Reward trials. The scatterplots provide a visual depiction of the relationship between family obligation and the differences in activity during Costly Donation versus Noncostly Reward trials in the left DLPFC (r = .65, p < .005), right TPJ (r = .75 p < .005), right pSTS (r = .74, p < .005), and left pSTS (r = .62, p < .005). Other significant regions are listed in Table 2. Note. Values on the y-axis represent parameter estimates of signal intensity from the contrast of Costly Donation–Noncostly Reward trials. Parameter estimates of signal intensity for each region were extracted for each individual from the entire, group-level cluster of activation.
Neural connectivity with the ventral striatum during prosocial decisions to the family
Next, we examined whether neural regions sensitive to self-control and mentalizing may be a precursor to the rewarding nature of giving by carrying out psychophysiological interaction analyses. Whole brain PPI analyses revealed that no brain regions were coupled with the ventral striatum during Costly-Donation relative to Noncostly-Reward decisions.
Neural connectivity with the ventral striatum during prosocial decisions to the family that correlated positively with family obligation preferences
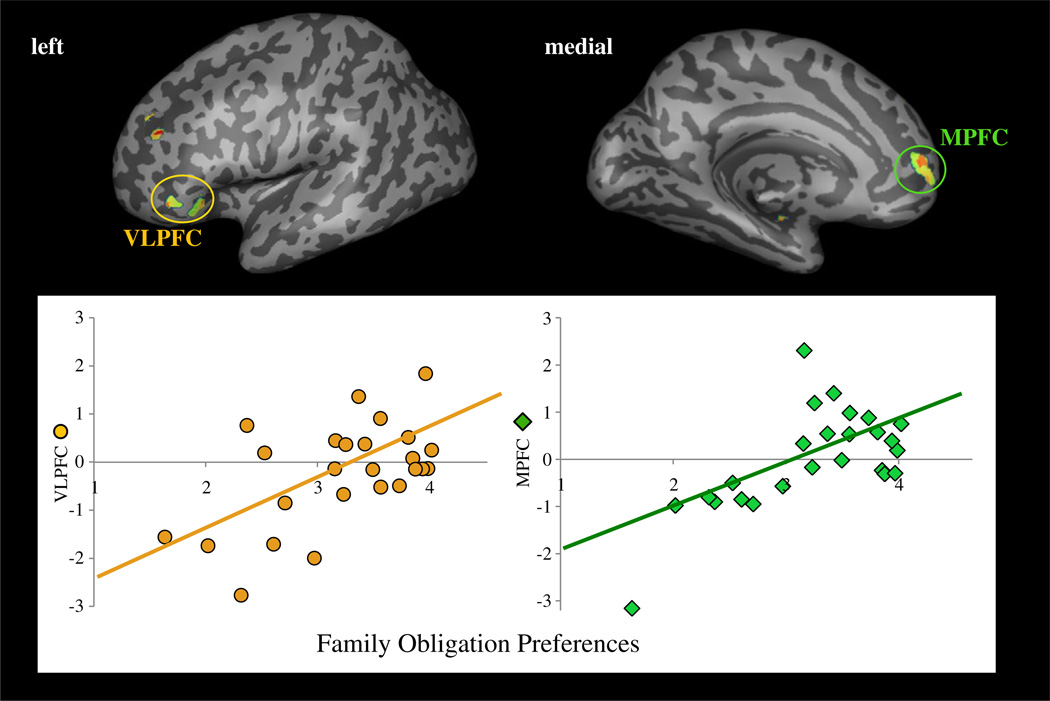
To test whether there were individual differences in the extent to which participants displayed functional coupling between neural regions associated with self-control and mentalizing with the ventral striatum, we conducted whole-brain regression analyses correlating family obligation preferences with individuals' PPI coefficients for Costly-Donation versus Noncostly-Reward trials. We expected that ventral striatum-self-control and ventral striatum-mentalizing interactions would be stronger for individuals who placed greater value on supporting, respecting, and spending time with their family. Participants who reported greater family obligation preferences showed greater functional coupling between the ventral striatum and the left VLPFC, a region linked with self-control, as well as the MPFC and DMPFC, regions linked with mentalizing (Fig. 4; Table 3).
Fig. 4.
Functional coupling between neural regions involved in self-control and mentalizing and the ventral striatum that correlate positively with family obligation preferences during the contrast of Costly Donation versus Noncostly Reward trials. The scatterplots provide a visual depiction of neural regions that showed functional coupling with the ventral striatum that correlated positively with family obligation values during Costly Donation versus Noncostly Reward trials including the left VLPFC (r = .60, p < .005) and MPFC (r = .71, p < .005). Other significant regions are listed in Table 3. Note. Values on the y-axis represent parameter estimates of functional connectivity between the ventral striatum and the brain region displayed in the figure from the PPI analysis examining Costly Donation–Noncostly Reward. Parameter estimates of signal intensity were extracted for each individual from the entire, whole-brain cluster of activation for each significant brain region.
Table 3.
Neural regions associated with the ventral striatum during decisions to accept Costly-Donation relative to Noncostly-Reward trials that correlated positively with participants' family obligation preferences.
| Anatomical Region | BA | x | y | z | t | r | k | |
|---|---|---|---|---|---|---|---|---|
| DMPFC | 8 | L | −9 | 54 | 48 | 4.26 | .66 | 22 |
| VLPFC | 10 | L | −42 | 36 | −3 | 3.71 | .60 | 28 |
| MPFC | 10 | R | 3 | 54 | 15 | 4.97 | .71 | 57 |
Note. BA refers to putative Brodmann's Area; L and R refer to left and right hemispheres; x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); r refers to the correlation coefficient representing the strength of the association between family obligation preferences and functioning coupling between the ventral striatum and the specified region; k refers to the number of voxels in each significant cluster. The following abbreviations are used for the names of specific regions: dorsomedial prefrontal cortex (DMPFC), ventrolateral prefrontal cortex (VLPFC), medial prefrontal cortex (MPFC). All a priori regions are listed at p < .005, corrected for multiple comparisons with the regions on interest. No regions outside the a priori regions were significant.
Discussion
In the current study, we examined the underlying neural mechanisms that may promote prosocial decisions. Prosocial decisions are often governed by competing rewards between one's own self-interests and the desire to help another. Our study suggests that individuals with prosocial preferences utilize neural processes involved in self-control and mentalizing when making prosocial decisions, and these neural activations may be a precursor to the rewarding nature of giving to others.
Although prosocial behaviors may be inherently rewarding, the decision to help is typically not an automatic process. Individuals must weigh the relative value of helping others with their own self interests and resolve conflict between the two in order to put the needs of another before their own. Behavioral results from our study show that costly decisions take significantly longer to make than personal monetary reward decisions, and costly decisions are related to activation of neural regions involved in self-control. These findings suggest that such prosocial behaviors are not automatic and may involve a degree of personal inhibition in order to resolve conflict between self interests and social motives to ultimately make decisions that help another. In addition to self-control, individuals recruited brain regions involved in mentalizing when making decisions to help their family, suggesting that these decisions also involve shifting attention from one's own self interests to the needs and values of the family.
Researchers have proposed that social preferences shape and govern prosocial behavior (Fehr and Fishbacher 2002; Fehr and Schmidt 2006; Caprara and Steca 2007). The extent to which individuals care about the well being of others has important consequences for their prosocial behavior. In the current study, we measured individuals' family obligation preferences and found that those who placed greater value on the support and respect of their recruited brain regions involved in self-control and mentalizing to a greater extent when making prosocial decisions. These regions were distinct from those found in the main effect of costly-donations versus noncostly-rewards. Thus, individuals with stronger family obligation preferences may be doing something qualitatively different, such as maintaining social rules in memory when they make these decisions. Individuals with lower family obligation preferences showed neural activation around and below zero, suggesting that they were not recruiting brain regions involved in self-control and mentalizing when making prosocial decisions. In fact, activation below 0 suggests that individuals with the lowest family obligation preferences were showing greater neural activation to Noncostly-Reward decisions than Costly-Donations.
Furthermore, individuals who valued helping their family more showed greater functional coupling between the ventral striatum and regions involved in self-control and mentalizing, suggesting, in part, that these neural regions may promote a reward inducing decision for individuals who value helping. Although we propose that the recruitment of brain regions involved in self-control and mentalizing facilitate prosocial behaviors and ultimately enable individuals to gain a sense of reward from helping their family, it is also possible that individuals who value family obligation feel more rewarded from helping and thus recruit more self-control and mentalizing. Finally, we did not find a main effect of ventral striatum activation to Costly-Donation compared to Noncostly-Reward decisions. This is consistent with our previous work, in which we found variability in the extent to which individuals recruited the ventral striatum when making costly contributions to their family (Telzer et al. 2010), suggesting that ventral striatal recruitment may be modulated by other neural processes during prosocial decisions. Indeed, in the current study, we found ventral striatum activation only in conjunction with neural regions involved in self-control and mentalizing for individuals who value family obligation preferences, suggesting that the extent of ventral striatal activation to prosocial decisions depends upon individual differences and is not a uniform, main effect.
We believe that the capacity to recruit self-control and mentalizing during prosocial decisions depends on individuals' values and prosocial preferences (Caprara and Steca 2007; Fehr and Fishbacher 2002), but it is also possible that individuals who recruit these neural processes to a greater extent develop more prosocial preferences over time. Longitudinal research should test this latter possibility. If it is true that prosocial preferences guide neural processing, future research could test whether making prosocial behavior motivationally significant for any individual leads to increased self-control and mentalizing activations. This could have important implications for increasing prosocial behavior in the general population.
Economic research has attempted to understand why and how prosocial behaviors occur. Most previous work has focused on prosocial behaviors towards strangers and charities. Yet, a majority of prosocial behavior occurs on a daily basis between individuals who know each other well, such as one's family. Our study extends the large body of social neuroecomomic research by exploring helping behavior towards the family. We cannot empirically differentiate whether our findings are family-specific or whether we are tapping prosocial behavior toward others more generally. We do not know from our data whether individuals would also use self-control and mentalizing processes when making decisions to help unknown others. If individuals don't know another's needs, goals, or values, they may be less likely to mentalize when making decisions to help them. Furthermore, there may be less at stake if individuals make a decision to help a stranger instead of a close other and thus the conflict between the self and other may be minimized, so they may be less likely to use self-control. Future research should examine whether charitable donations engage regions involved in self-control and mentalizing and whether these regions are related to reward activation.
In summary, prosocial decisions can be complex and difficult to make. Such decisions often involve personal sacrifices that do not incur any direct immediate benefits to the self. Thus, individuals must weigh the relative value of helping others. Our findings suggest that multiple neural processes are involved in these decisions, including mentalizing and self-control. The capacity to utilize these neural processes is shaped, in part, by individual differences in other-regarding preferences, which together may help individuals to make prosocial decisions and ultimately gain a sense of reward.
Footnotes
We would like to thank Will Moore and the University of Oregon Developmental Social Neuroscience Laboratory for constructing some of the ROIs used in the analyses. We also appreciate the support provided by the UCLA Brain Mapping Center. Support for this study was provided to A. J. Fuligni by grants from the Russell Sage Foundation and the Cousins Center for Psychoneuroimmunology, Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles. Preparation of this manuscript was supported in part by a National Research Service Award Graduate Fellowship to E.H. Telzer.
References
- Andreoni J, Miller JH. Giving according to GARP: an experimental test of the consistency of preferences for altruism. Econometrica. 2002;70:737–753. [Google Scholar]
- Arnett JJ. Learning to stand alone: the contemporary American transition to adulthood in cultural and historical context. Hum. Dev. 1998;41:295–315. [Google Scholar]
- Aronson E, Wilson TD, Akert RM. Social Psychology. 6th Edition. Prentice, Hal: New Jersey; 2007. [Google Scholar]
- Caprara GV, Steca P. Prosocial agency: the contribution of values and self-efficacy Beliefs to prosocial behavior across ages. J. Soc. Clin. Psychol. 2007;26:220–241. [Google Scholar]
- Cohen JR, Lieberman MD. The common neural basis of exerting self control in multiple domains. In: Trope Y, Hassin R, Ochsner KN, editors. Self Control in Society, Mind, and Brain. New York, NY: Oxford University Press; 2010. [Google Scholar]
- Dunn EW, Aknin LB, Norton MI. Spending money on others promotes happiness. Science. 2008;319:1687–1688. doi: 10.1126/science.1150952. [DOI] [PubMed] [Google Scholar]
- Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends in Cognitive Sciences. 2007;11:419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Fehr E, Fishbacher U. Why social preferences matter — the impact of non-selfish motives on competition, cooperation, and incentives. Econ. J. 2002;112:C1–C33. [Google Scholar]
- Fehr E, Schmidt KM. The economics of fairness, reciprocity, and altruism — experimental evidence and new theories. In: Kolm S, Ythier JM, editors. Handbook of the Economics of Giving, Altruism, and Reciprocity. Amsterdam: Elsevier; 2006. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Frith CD. The role of dorsolateral prefrontal cortex in the selection of action as revealed by functional imaging. In: Monsell Driver J., editor. Control of Cognitive Processes. Cambrdige, MA: MIT Press; 2000. [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing, Philosophical Transactions of the Royal Society, London, B. Biological Sciences. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni AJ, Pedersen S. Family obligation and the transition to young adulthood. Dev. Psychol. 2002;38:856–868. doi: 10.1037//0012-1649.38.5.856. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Tseng V, Lam M. Attitudes toward family obligations among American adolescents from Asian, Latin American, and European backgrounds. Child Development. 1999;70:1030–1044. [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Knoepfle DT, O-Doherty JP, Rangel A. Value computations in the ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J. Neurosci. 2010;30:583–590. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ET. Beyond pleasure and pain. Am. Psychol. 1997;52:1280–1300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Lissot A. NeuroLens: an integrated visualization and analysis platform for functional and structural neuroimaging. Proceedings of the International Society for Magnetic Resonance in Imaging. 2004;11:1096. [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. Journal of Cognitive Neuroscience. 2010;22:621–631. doi: 10.1162/jocn.2009.21228. [DOI] [PubMed] [Google Scholar]
- Kahn I. SPM SurfRend (Version 1.0.2) 2008 http://spmsurfrend.sourceforge.net.
- Lieberman MD. Social cognitive neuroscience. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of Social Psychology. 5th ed. New York, NY: McGraw-Hill; 2010. pp. 143–193. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in the right temporo — parietal junction is not selective for theory of mind. Cerebral Cortex. 2008;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J. Cogn. Neurosci. 2005;17:1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about chartiable donation. Proceedings of the National Academcy of Sciences. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. Eur. J. Neurosci. 2003;17:2475–2480. doi: 10.1046/j.1460-9568.2003.02673.x. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Kathirgamanathan U, Humphreys GW. Seeing it my way: a case of a selective deficit in inhibiting self-perspective. Brain. 2005;128:1102–1111. doi: 10.1093/brain/awh464. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in "theory of mind”. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ. Daily family assistance and the psychological well being of adolescents from Latin American, Asian, and European backgrounds. Dev. Psychol. 2009a;45:1177–1189. doi: 10.1037/a0014728. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ. A longitudinal daily diary study of family assistance and academic achievement among adolescents from Mexican, Chinese, and European backgrounds. Journal of Youth and Adolescence. 2009b;38:560–571. doi: 10.1007/s10964-008-9391-7. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, Fuligni AJ. Gaining while giving: an fMRI study of the rewards of family assistance among White and Latino youth. Soc. Neurosci. 2010;5:508–518. doi: 10.1080/17470911003687913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuro-Image. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happe F, Falkai P, et al. Mind reading: neural mechanisms of theory of mind and self-perspective. NeuroImage. 2001;14:170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. NeuroImage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 http://afni.nimh.nih.gov/pub/dist/doc/manuals/AlphaSim.pdf.