Introduction
Much attention has focused on elucidating how activated myofibroblasts and mesenchymal stem cells (MSCs) distally recruited from bone marrow contribute to breast cancer progression. Even though adipose tissue is the most abundant stromal constituent in the breast and a rich source of mesenchymal stem-like cells, very little is known about the involvement of this resident stem cell population in mammary carcinogenesis. We hypothesize that multipotent adipose-derived stromal cells (ASCs) directly alter the breast microenvironment favoring the transition from pre-malignancy to malignancy.
Adipose tissue is composed predominantly of lipid-storing adipocytes and critical for providing energy, insulation and mechanical cushion. Adipocytes are supported by fine connective tissue interspersed with endothelial cells, macrophages and stromal cells, the latter of which have been traditionally referred to as ‘pre-adipocytes’ but are now typically called ASCs. Since adipose tissue is the primary extra-gonadal source of estrogen and the majority of breast carcinomas (~60%) express estrogen receptors (ER), most studies investigating the role of fat in mammary carcinogenesis have focused on hormone-dependent mechanisms. However, data generated from multiple disciplines are now converging to reveal that adipose tissue, and in particular ASCs, are far more complex than once appreciated. As we learn more about ASCs and consider these new data in the context of previous pathological, epidemiological and experimental observations, a new working hypothesis emerges: breast-derived ASCs play a critical role in mammary cancer development. Here, we present an integrative hypothesis uniquely focusing on early breast carcinogenesis and discuss its potential global implications in terms of prevention, early detection and stromal-based targeted therapies.
‘Pre-adipocyte’ is a misnomer
Stromal cells within breast adipose tissue have traditionally been called ‘pre-adipocytes’, based on observations that these cells can accumulate lipid droplets in vitro as well as revert to a more-fibroblastic appearance [1]. However, it was first reported in 2002 [2] that ASCs from abdominal tissue are not limited to adipocytic differentiation. In fact, they can also differentiate along osteogenic, chondrogenic, endothelial, and perhaps even neural cell lineages. While most studies have used ASCs derived from abdominal sources, it appears that ASCs from other fat depots also contain an abundance of multipotent stromal cells [2-5]. To date, very little is known regarding the role of ASCs in mammary carcinogenesis, and the few studies that have been conducted have predominantly relied on ASCs from non-breast sources, including abdominal [4, 7-8], peri-renal [5] and pelvine [5] adipose tissues. A recent study [6] describes the differential proteomic expression profiles between ligament, dental pulp and bone marrow progenitor cells, revealing that post-natal MSCs can differ depending on their site of origin. In humans, adipose tissue is located beneath the skin (subcutaneous fat), around internal organs (visceral fat), in bone marrow (yellow bone marrow) and in breast tissue. Since adipose depots in different parts of the body have different biochemical profiles, we feel it is important to use breast-derived ASCs when investigating the effects of ‘resident’ stem cells on mammary carcinogenesis.
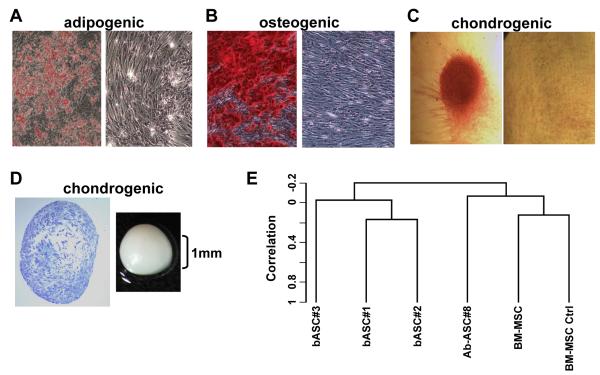
We have isolated ASCs from three reduction mammoplasty specimens. These stromal cells readily differentiate along adipogenic, osteogenic and chondrogenic lineages in vitro (Figure 1 A-D) and exhibit similar gene expression profiles (Figure 1E) and an indistinguishable immunophenotype (CD29+, CD73+, CD105+, CD14−, CD31−, CD45−) from ASCs from abdominoplasty specimens and bone marrow (BM)-MSCs. But notable gene expression differences were observed between mesenchymal stem-like cells from different sources, with abdominal ASCs being more similar to BM-MSCs and breast-derived ASCs being more similar amongst themselves when compared to abdominal ASCs (Figure 1E). A comparison of the gene expression profiles of 3 breast ASCs with 2 BM-MSCs identified 79 unique probe sets with numerous genes implicated in cell growth, matrix deposition and remodeling, and angiogenesis being expressed at significantly higher levels in the local/breast ASCs relative to BM-MSCs, suggesting that these resident stem-like cells may play a unique role in mammary carcinogenesis.
Figure 1. Breast-derived ASCs exhibit multipotent mesenchymal differentiation.
Breast ASCs were cultured in media to trigger adipogenic, osteogenic and chondrogenic lineages and then histochemically stained, as described previously [2], with: (A) Oil Red O to detect lipid droplets; (B) Alizarin red to detect extracellular calcium; and (C) Safranin O to detect glycosoaminoglycans where the micromass was removed for microscopic analysis. Undifferentiated breast ASCs were included as negative controls (right panels of A-C). (D) Cartilage micromass (gross image on right) and toludine staining of the sectioned micromass (left). (E) Dendrogram from unsupervised hierarchical clustering using centered correlation and average linkage. Four micrograms of total RNA from each sample was used to generate double-stranded cDNA using a 24-mer oligodeoxy-thymidylic acid primer with a T7 RNA polymerase promoter site added to the 3′ end [19]. Second strand cDNA synthesis, cleanup, and biotinylation were conducted according to a standard protocol. Ten μg of fragmented cRNA were then hybridized on the GeneChip® Human Genome U133A 2.0 (HG-U133A 2.0) array and analyzed as described previously [20]. Note: b: breast; Ab: abdominal; and the number appearing after the respective cell cultures reflect individual cell isolations from different donors.
Adipose tissue is the most abundant, yet least studied, stromal constituent in the breast
Collectively a number of histological and epidemiological observations suggest that adipose tissue contributes to breast carcinogenesis. Primary mammary cancers tend to develop in close proximity to adipose tissue because it is the most abundant stromal constituent in the breast. It has long been recognized that age is a risk factor for developing breast cancer [9], and perhaps not coincidentally, adiposity of breast tissue increases with increasing age. Extensive epidemiological studies have revealed that being overweight/obese is a risk factor for the development of postmenopausal breast cancer and excess abdominal fat by itself is a cancer risk factor [10]. While most of the breast tumors developing in these women expressed ER, this does not discount the possibility that increased adiposity also contributes to the development of breast cancer via hormone independent pathways.
Since BM-MSCs promote breast cancer progression, we should not ignore the presence of stem-like mesenchymal cells in local/breast fat depots
Mesenchymal stem cells have been described in the cancer stroma of breast carcinoma specimens. It is often assumed that these adult stem cells have been distally recruited from the bone marrow in response to breast soluble factors, since BM-MSCs efficiently home to breast cancers [11] and MSCs have been detected in peripheral blood, albeit at low numbers [12]. In response to breast tumor soluble mediators in vitro, BM-MSCs adopt a carcinoma-associated fibroblast (CAF)-like phenotype [13], suggesting that these distally recruited stem cells comprise at least a fraction of the heterogeneous population of breast cancer-associated myofibroblasts. Data generated from xenograft [14] and transgenic [15] mouse models have provided compelling in vivo data that BM-MSCs facilitate the development of cancer by promoting late stage events such as metastasis and angiogenesis. Based on the striking similarities we (Figure 1) and others [16] have discovered between BM-MSCs and breast-derived ASC, it was not surprising that three recent studies similarly report that ASCs (abdominal- and/or breast-derived) promote the growth, invasion and metastasis of fully malignant breast epithelial cells [4-5, 8].
One could argue that once late-stage breast cancer develops, the chances of effectively targeting a genomically unstable lesion and delivering a drug or small molecule through the typical dense cancer-associated stroma are problematic. If fibrosis (defined as a pathologic entity characterized by the proliferation of stroma with the obliteration of mammary ducts and acini [17]) is associated with an increased incidence of breast cancer development [18] and experimental data indicate that BM-MSCs are predominantly recruited late to the tumor microenvironment [15], what is the tissue source of myofibroblasts during early mammary carcinogenesis? We predict local/breast fat depots. This novel concept has been formulated based on data relating to breast cancer risk factors, the stepwise progression of mammary carcinogenesis, and our growing appreciation of the biology of breast-derived ASCs. Breast density, a radiologic phenomenon resulting from less fat than glandular and connective tissue, is associated with a four- to six-fold increased risk for developing breast cancer [18]. Scientists disagree on the underlying causes of breast density, but heredity, hormonal, and dietary factors are likely involved. If breast density and fibrosis are both associated with less fat and more connective tissue, we reason that resident/breast ASCs are chemoattracted toward mammary glandular elements where they trigger a desmoplastic response (Figure 2) rather than differentiating along an adipocytic lineage (i.e., maintaining fat reserves). Our following Affymetrix data and confirmatory qRT-PCR and western blotting results (data not shown) reveal that undifferentiated breast ASCs (1) exhibit a number of features consistent with CAFs; (2) constitutively express multiple matrix metalloproteinases, which together with cysteine proteases could degrade interstitial matrix components, permitting easier access to mammary ducts and lobules; and (3) constitutively express several chemokines receptors, which could theoretically guide these stromal cells from local fat stores into the parenchyma, triggering a cascade of events leading to fibrosis.
Figure 2. Working hypothesis schematic illustrating involvement of breast-derived ASCs in the induction of fibrosis.
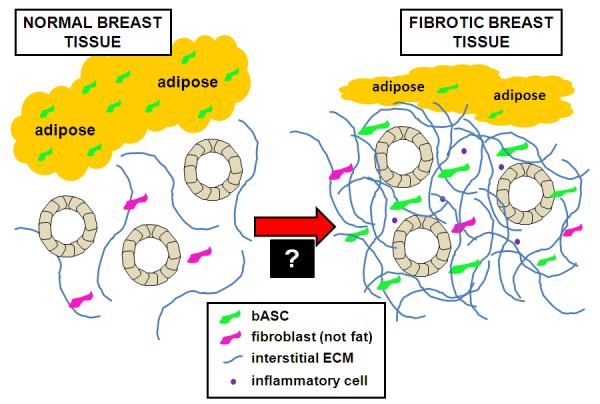
Normal breast tissue consists of glandular structures supported by a fine fibrotic stroma and an abundance of adipose tissue (on left). We propose that in response to a soluble mediator(s) derived from pre-malignant mammary epithelial cells, breast ASCs are chemoattracted from local fat stores toward the parenchyma, where they trigger a desmoplastic response characterized by their transdifferentiation into myofibroblasts, inflammation and a dense connective tissue (on right). Overall, this would result in reduced adiposity, increased connective tissue (fibrosis), and a microenvironment that could stimulate epithelial cell growth and cause genetic alterations while disrupting normal tissue homeostasis. Critical to better understanding early stage mammary carcinogenesis is validating involvement of breast ASCs in the development of fibrosis and then elucidating the mediators leading to the exodus of breast ASCs from local fat stores and altering the differentiation of these resident stem cells (as signified by the “black box” at the transition between the normal and fibrotic microenvironments).
To begin to study the involvement of local, breast ASCs in fibrosis, we established explant cultures from focal fibrotic lesions from two reduction mammoplasty specimens. We then compared the immunophenotype, differentiation potential, and expression of stem cell-associated genes in the human mammary stromal cells from fibrotic lesions with breast ASCs, reasoning that if ASCs migrate from local fat stores into the parenchyma, at least some of the phenotypic properties would be stable. The mammary stromal cells and breast ASCs had an identical immunophenotype as we fully anticipated since abdominal ASCs, BM-MSCs and BJ foreskin fibroblasts all share the same surface expression profiles (manuscript submitted for publication). More surprising, however, was our discovery that mammary stromal cells derived from fibrotic lesions were able to differentiate along osteogenic and adipocytic lineages, and they expressed a similar level of stem cell-associated genes (SOX2+, NANOG+, OCT-4+ and hTERT−) as breast ASCs (data not shown). To date, the only difference we have observed between these two cell populations are their morphologies: the stromal cells from fibrotic lesions have a more elongated appearance suggestive of myofibroblasts. Collectively, our comparative analysis is certainly consistent with a lineage relationship between stromal cells from mammary fat and those within fibrotic lesions in benign breast tissue.
Translational Implications for breast ASCs promoting breast carcinogenesis
If local/breast ASCs contribute to fibrosis, and the resultant dense breast tissue is a risk factor for breast cancer [18], it is critical to better understand what triggers this early fibrotic change in the breast microenvironment. We are confident that defining the underlying mechanism(s) of breast ASC-induced fibrosis will lead to the identification of new targeted therapies for early stage breast cancer. Since breast density reduces radiographic sensitivity and therefore can mask the detection of early lesions, targeted therapy aimed at minimizing tissue density could provide an attractive early intervention strategy especially for individuals at high risk of developing early onset breast cancer. Studies relating to resident breast ASCs and mammary cancer are still in their infancy. Here, we present a working hypothesis implicating breast ASCs in the early stages of mammary carcinogenesis. Our data, together with recent findings implicating ASCs in breast cancer progression, strongly suggest that resident stem cells in local fat depots can no longer be ignored. While we may discover (and fully anticipate) that some of the stromal effects of breast ASCs on mammary epithelial cells will mimic those of closely related BM-MSCs, it is naïve to think that mesenchymal stem-like cells being recruited from different microenvironments will behave identically. To date, the involvement of MSCs in mammary carcinogenesis, regardless of their site of origin, has focused almost exclusively on their effects on fully malignant (including metastatic) breast epithelial cells. The focus must now shift to the early stages of breast cancer if we hope to design stromally-based targeted therapies to manage (and ideally to prevent) the growing number of early diagnosed lesions without the toxicities associated with standard chemo- and radio-therapeutic regimens.
Acknowledgements
We thank Peter Ma, Patrick Sachs and Michael Frances (Department of Pathology, Virginia Commonwealth University) for their assistance in the isolation of breast-derived adipose stromal cells. We are appreciative of the flow cytometry help from Frances White and Julie Farnsworth (Massey Cancer Center Flow Cytometry Core Lab; supported by Massey Cancer Center NIH Grant P30CA16059). Microscopy was performed at the Virginia Commonwealth University Department of Anatomy & Neurobiology Microscopy Facility, supported, in part, with funding from NIH-NINDS Center core grant 5P30NS047463. Due to reference limits, we regret that we were unable to cite all relevant studies in the literature.
Grant Support This work was supported by a National Institutes of Health KO1 CA105050-01A131, a Department of Defense BCRP Concept Award (BC085416), and the Department of Pathology at Virginia Commonwealth University (to LWE). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or DOD.
Abbreviations
- ASCs
adipose stromal cells
- BM-MSCs
bone marrow-derived mesenchymal stem cells
- CAF
carcinoma-associated fibroblasts
- ER
estrogen receptor
- MSCs
mesenchymal stem cells
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
References
- 1.Manabe Y, Toda S, Miyazaki K, Sugihara H. Mature adipocytes, but not pre-adipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal interactions. J Pathology. 2003;201:221–228. doi: 10.1002/path.1430. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Ashijian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells-basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 4.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745–2755. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muehlberg FL, Song Y-H, Krohn A, et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30:589–597. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 6.Mrozik KM, Zilm PS, Bagley C, et al. Proteomic characterization of mesenchymal stem cell-like populations derived from ovine periodontal ligament, dental pulp and bone marrow: analysis of differentially expressed proteins. Stem Cells Dev. 2010 doi: 10.1089/scd.2009.0446. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Sun B, Roh KH, Park JR, et al. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11:289–298. doi: 10.1080/14653240902807026. [DOI] [PubMed] [Google Scholar]
- 8.Pinilla S, Eckard A, Khalek FJ Abdul, et al. Tissue resident stem cells produce CCl5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Letters. 2009;284:80–85. doi: 10.1016/j.canlet.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Dumitrescu RG, Cotarla I. Understanding breast cancer risk—where do we stand in 2005? J Cell Mol Bio. 2005;9:208–221. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleary MP, Grossman ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. JNCI. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 12.Kuznetsov SA, Mankani MH, Gronthos S, et al. Circulating skeletal stem Cells. J Cell Biol. 2001;153:1133–1140. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra PJ, Mishra PJ, Humeniuk R, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4330. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 15.Ishii G, Sangai T, Oda T, et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Comm. 2003;309:232–240. doi: 10.1016/s0006-291x(03)01544-4. [DOI] [PubMed] [Google Scholar]
- 16.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1304. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 17.Sklair-Levy M, Samuels TH, Catzavelos C, Hamilton P, Shumak R. Stromal fibrosis of the breast. Am J Roentgenol. 2001;177:573–577. doi: 10.2214/ajr.177.3.1770573. [DOI] [PubMed] [Google Scholar]
- 18.White J. Breast density and cancer risk: what is the relationship? JNCI. 2000;92:433. doi: 10.1093/jnci/92.6.443. [DOI] [PubMed] [Google Scholar]
- 19.Elmore LW, Di X, Dumur CI, Holt SE, Gewirtz DA. Evasion of a single-step, chemotherapy-induced senescence in breast cancer cells: implications for treatment response. Clin Cancer Res. 2005;11:2637–2643. doi: 10.1158/1078-0432.CCR-04-1462. [DOI] [PubMed] [Google Scholar]
- 20.Dumur CI, Ladd AC, Wright HV, et al. Genes involved in radiation therapy response in head and neck cancers. Laryngoscope. 2009;119:91–101. doi: 10.1002/lary.20005. [DOI] [PMC free article] [PubMed] [Google Scholar]