Abstract
Theobroma cacao is an economically important tree of several tropical countries. Its genetic improvement is essential to provide protection against major diseases and improve chocolate quality. We discovered and mapped new expressed sequence tag-single nucleotide polymorphism (EST-SNP) and simple sequence repeat (SSR) markers and constructed a high-density genetic map. By screening 149 650 ESTs, 5246 SNPs were detected in silico, of which 1536 corresponded to genes with a putative function, while 851 had a clear polymorphic pattern across a collection of genetic resources. In addition, 409 new SSR markers were detected on the Criollo genome. Lastly, 681 new EST-SNPs and 163 new SSRs were added to the pre-existing 418 co-dominant markers to construct a large consensus genetic map. This high-density map and the set of new genetic markers identified in this study are a milestone in cocoa genomics and for marker-assisted breeding. The data are available at http://tropgenedb.cirad.fr.
Keywords: Theobroma cacao, genetic map, SNP, molecular marker
1. Introduction
Theobroma cacao L. is a diploid species (2n = 2x = 20) with a small genome ranging in size from 411 to 494 Mb.1 According to Cheesman,2 its centre of origin is at the lower eastern equatorial slopes of the Andes.
Theobroma cacao is grown as a major cash crop that provides income to 14 million small-scale farmers in more than 50 tropical countries. However, cocoa production is markedly affected by a number of major diseases caused by several Phytophthora species, or by Moniliophthora perniciosa and Moniliophthora roreri. Several sources of disease resistance have been identified and the search for sustainable disease resistance by cumulating the different resistance genes is one of the major challenges facing T. cacao-breeding programmes.3 The quality of chocolate is another important trait in cocoa breeding, and consumer demand for high-quality chocolate is increasing. A better understanding of the molecular and genetic bases of these traits is a key goal of cocoa genetic research.
High-density genetic maps are essential tools for trait genetic studies. Several molecular marker types have been developed in T. cacao in recent decades: restriction fragment length polymorphism (RFLP), microsatellites or simple sequence repeats (SSRs), random amplified polymorphic DNA, amplified fragment length polymorphism and isozymes.4–6 Among them, only RFLP, SSR and single-nucleotide polymorphism (SNP) are co-dominant markers, and therefore more powerful for genetic analyses. Compared with RFLP, the advantage of SSR and SNP markers is that they can be revealed using high-throughput technologies with scant amounts of DNA. Semagn et al.7 made a detailed comparison of the characteristics of each kind of marker. A high-density cocoa linkage map enriched with SSR genomic markers, including only co-dominant markers, was developed by Pugh et al.8 More recently, that map was supplemented with 114 EST-SSRs.9
In recent years, the use of SNP markers has substantially increased in plant genetics such as in Arabidopsis,10 grapevine,11 wheat,12 and also a few woody perennial species.13–15 SNP is one of the most abundant types of DNA sequence polymorphism and the SNP markers are suitable for large-scale genome analysis using high-throughput automated genotyping techniques. SNPs have been used to construct high-resolution genetic maps16,17 or to trace evolution, particularly in the human genome, using large-scale SNP datasets.18,19 Knowledge of nucleotide substitution dynamics is an important basis for molecular evolutionary studies, phylogeny reconstruction and natural selection studies.20,21 Transitions are generally observed with higher frequencies than transversions. During natural selection, transitions are better tolerated because they generate more likely synonymous mutations in protein-coding sequences than transversions.22–25
Of existing SNP markers, EST-SNPs (i.e. SNPs located within a gene expressed sequence) are of particular interest for studying functional genetic diversity and identifying candidate genes as the functional base of quantitative trait loci (QTLs). EST-SNPs have been developed for numerous plant models such as melon,26,27 Brassica rapa,28 barley,29 poplar,14 and sugarcane30 to detect QTLs for many traits and facilitate the selection of resistant and productive plants. In T. cacao, a few SNPs were detected in ESTs from expression libraries representing T. cacao/M. perniciosa interactions.31
In our study, we discovered and mapped several hundred EST-SNP markers detected in an exhaustive collection of cocoa ESTs32 homologous to genes with a known function. These SNP markers were supplemented by 163 new SSR markers to construct a very high-density genetic map suitable for large-scale genetic studies.
2. Materials and Methods
2.1. Plant material
SNP polymorphisms were screened in a collection of diverse germplasm representing the major part of the T. cacao diversity and two existing mapping populations denominated UPA402 × UF676 and F2.
The collection of diverse germplasm consisted of 249 genotypes from various genetic groups and geographical origins (Table 1). Most of these accessions are maintained at the International Cocoa Genebank (ICG) at the Cocoa Research Unit (CRU), University of the West Indies, Trinidad and Tobago.
Table 1.
Theobroma cacao genotypes of various geographical origins used to screen the polymorphism of the 1536 GoldenGate SNP panel
| Accession collection group name | Number of genotypes | Geographical origin |
|---|---|---|
| AMAZ | 2 | Ecuador |
| APA | 1 | Colombia |
| Nacional | 3 | Ecuador |
| Criollo | 14 | Mexico-Belize |
| EBC | 4 | Colombia |
| Trinitario | 28 | Trinidad |
| GU | 12 | French Guiana |
| IMC | 19 | Peru |
| LCTEEN | 46 | Ecuador |
| MORONA | 3 | Peru |
| NANAY | 49 | Peru |
| PARINARI | 40 | Peru |
| POUND | 6 | Peru |
| SC | 5 | Colombia |
| SCAVINA | 8 | Peru |
| Amelonado type | 3 | Brazil |
| SPEC | 6 | Colombia |
| Total | 249 |
The UPA402 × UF676 mapping population consisted of 264 individuals derived from a cross of two unrelated heterozygous tree accessions; UPA402, an Upper Amazon Forastero from Peru, and UF676, a Trinitario (Forastero × Criollo hybrid) selected in Costa Rica. This progeny was maintained by Centre National de Recherche Agronomique (CNRA) in Bingerville and Divo, Côte d'Ivoire. It was used to establish genetic map as a reference in our laboratory.4,6,8,9 We mapped the new SSR and SNP markers in this population.
The F2 second progeny of 132 individuals was obtained by selfing a hybrid between two heterozygous parents: Scavina 6, an Upper Amazon Forastero collected in Peru, and ICS1, a Trinitario selected in Trinidad. This progeny was produced by Comissão Executiva do Plano da Lavoura Cacaueira (CEPLAC) at Itabuna, Brazil.
2.1.1. Genotypes used for EST-SNP detection and selection
Most of the ESTs screened for SNPs had been obtained from the contrasting genotypes Scavina 6, an upper Amazon Forastero genotype from Peru and ICS1, a Trinitario selected in Trinidad, a hybrid between a Criollo from Central America and a Forastero from Lower Amazonia of Brazil.
These two genotypes, which represent the three distinct genetic origins, Upper Amazon Forastero, Lower Amazon Forastero, and Criollo, were also the parents of the F2 population from Brazil used to map SNPs.
Eleven other genotypes were involved in the construction of the cDNA libraries and SNP identification: B97-CC2, a Criollo from Belize, P7, IMC47, UPA 134, Upper Amazon Forastero genotypes from Peru, Jaca, an Upper Amazon Forastero from Brazil, GU255V, collected in French Guiana, B240 and 33–49, two Nacional genotypes from Ecuador, UF676, UF273, two Trinitario, and seedlings from a hybrid selected in Papua New Guinea.
SSRs were screened in three T. cacao genotypes: the two parents of the reference map (UPA402 and UF676), and the sequenced Criollo genotype (B97-61/B2).
2.2. DNA extraction and purification
Genomic DNA was extracted according to a protocol using MATAB buffer already described for the isolation of genomic DNA.6 DNA was resuspended with 1 ml of TE (10 mM Tris–HCl and 1 mM EDTA, pH 8.0).
DNA was purified with the Nucleobond® PC 20 kit (Macherey-Nagel, Cat. No. 740.571.100) with the modification that steps 1 and 2 were omitted and the DNA was purified directly after its isolation. A 1 ml mixture composed of 200 µl of crude DNA (20 µg DNA maximum), 450 µl water and 350 µl S3 buffer + RNAse (buffers provided with the kit) was passed through the column (step 3). This solution was homogenized on a rocking table for at least 1 h. After precipitation of the eluate with an equal volume of isopropyl alcohol, the pellet was resuspended in 70 µl of TE.
The quality and quantity of DNA were first checked on 0.8% agarose gel, compared with a standard range, and then the Quant-iT™ PicoGreen® dsDNA Assay from Invitrogen™ was used. A quality test was performed for each sample by amplifying microsatellite markers in a PCR mixture with a high DNA concentration (100 ng DNA in a 10 μl reaction volume). The purification step was repeated when the amplification failed.
2.3. In silico SNP discovery and verification
A collection of 149 650 ESTs (EMBL accession number CU469588 to CU633156), corresponding to 48 594 unigenes, was produced after sequencing 56 cDNA libraries constructed from material collected from different organs, genotypes, and under different environmental conditions.32
SNPs were detected in silico and quality checked using the QualitySNP pipeline,33 as reported in Argout et al.32 and in ESTtik (http://esttik.cirad.fr).
QualitySNP uses quality information related to each EST and a haplotype-based strategy to predict reliable SNPs. In order to detect SNPs in known homologous coding sequences, we selected contigs displaying a significant similarity with proteins from a non-redundant protein sequence database (NR), with entries from GenPept, Swissprot, PIR, PDF, PDB, and NCBI RefSeq, and as described in Argout et al.32
2.4. Validation of SNPs via golden gate assay
A total of 30–50 ng of genomic DNA per plant was used for Illumina SNP genotyping with the Illumina BeadArray platform at the French National Genotyping Centre (CNG, CEA-IG, Evry, France), according to the GoldenGate Assay manufacturer's protocol. Three 3-day assays were carried out to genotype the progeny samples for the 1536 SNP set revealed by the GoldenGate assay (Supplementary Table S1). The protocol was similar to that briefly described by Hyten et al.34 except for the number of oligonucleotides involved in a single DNA reaction, thus comprising 4608 custom oligos assembled in the oligo pooled assays (OPA) designed by Illumina Inc. Raw hybridization, intensity data processing, clustering, and genotype calling were performed using the genotyping module in the BeadStudio/GenomeStudio package (Illumina, San Diego, CA, USA). Illumina has developed a self-normalization algorithm that relies on information contained in each array, as described by Akhunov et al.35
The clustering and genotype calling of each of the 1536 SNP markers were checked for their conformity and correct genotype distribution using known homozygous and heterozygous genotypes, included in the collection of diverse genotypes, as standards.
2.5. SSR in silico discovery and genotyping
The MIcroSAtellite identification tool (MISA http://pgrc.ipk-gatersleben.de/misa) was used to perform SSR searches, and primers were designed with Primer3 software.36
Primers flanking microsatellite loci were designed at each end of the scaffolds to orient and anchor them to the genetic map.
SSRs identified in the scaffolds were mapped only on the reference map established from the UPA402 × UF676 cross.
A total of 409 primer pairs (Supplementary Table S2) were defined in the 100 larger non-anchored scaffolds using Primer3 software36 and screened for their ability to segregate in the UPA402 × UF676 progeny.
For a given SSR locus, the forward primer was designed with a 5′-end M13 tail (5′-CACGACGTTGTAAAACGAC-3′). PCR amplifications were performed in a Mastercycler ep384 thermocycler (Eppendorf, Germany) with 5 ng of purified DNA in a 10 μl final volume of buffer containing 10 mM Tris–HCl (pH 8), 50 mM KCl, 0.001% glycerol, 2.0 mM MgCl2, 0.08 μM of the M13-tailed forward primer, 0.1 μM of the reverse primer, 200 μM of dNTP, 1 U of Taq DNA polymerase (Life Technologies, USA), 0.1 μM of M13 primer-fluorescent dye 6-FAM™, NED®, VIC®, or PET® (Applied Biosystems, CA, USA). The DNA and buffer were distributed in 384 plates using a Biomek NX automatic pipetting robot (Beckman Coulter, CA, USA). The touchdown PCR programme used was as follows: initial denaturation at 95°C for 5 min, followed by 10 cycles at 95°C for 45 s, Tm of 56–46°C (−1°C/cycle) for 1 min, and 72°C for 1 min 30 s. After these cycles, an additional round of 25 cycles were performed at 95°C for 45 s, Tm of 50°C for 1 min, and 72°C for 1 min, with a final elongation step at 72°C for 30 min.
PCR products were diluted specifically for each dye and pooled for multiplex SSR genotyping (revealing two SSRs having different sizes of amplified product per dye). A mixture of 15 µl of Hi-Di™ formamide (Applied Biosystems) and 0.12 µl of size marker GeneScan™ 600-LIZ-Size® Standard V2.0. (Applied Biosystems) was added to 2 μl of the diluted PCR pool. This pool was then analysed using the ABI 3500xL automatic sequencer (Applied Biosystems).
Images were analysed using Genemapper 4.0 software (Applied Biosystems) and exported as a data table.
2.6. Genetic mapping
The UPA402 × UF676 population was the result of a cross between two heterozygous cocoa clones, UPA402 (♀) an Upper-Amazon Forastero and UF676 (♂) a Trinitario. In this case, there were three segregation possibilities: loci that were homozygous for one parent and heterozygous for the other, segregation (1:1), and those that segregated in both parents (1:2:1 or 1:1:1:1).
Segregations were checked for goodness-of-fit to the expected Mendelian ratio using a chi-square test at significance levels of 0.05 and 0.01.
Individual and consensus maps were constructed using Joinmap software, version 4.0.37
Joinmap is able to combine data of several segregation types to construct a consensus genetic map. Here we used population type CP for the UPA402 × UF676 map, and population type F2 for the F2 population from Brazil. A lod score of 6 was used to identify 10 linkage groups (LGs) independently for each map. A consensus genetic map was established from the two distinct genetic maps. The corresponding groups were associated in pairs with JoinMap software. The Kosambi mapping function, with a lod score of 5 and a jump threshold of 3, was used to convert recombination frequencies into map distances.38
This consensus map combined the new EST-SNPs and genomic SSRs defined from the scaffolds, in addition to the previously mapped markers.9 This map contained only markers with a known nucleotide sequence.
3. Results
3.1. Identification of SNPs and development of the golden gate assay
The assembly made from the 149 650 T. cacao EST sequences (see Materials and Methods) generated 12692 T. cacao contigs. The number of ESTs per contig ranged from 2 to 5102. To detect good quality in silico SNPs, we assumed that contigs with more than 100 members contained paralogous sequences.13,39 We therefore first selected 4818 contigs that contained at least 4 but no more than 100 EST members. A total of 5246 SNPs were identified in silico in 2012 contigs.
We selected 4150 in silico SNPs detected in 1834 contigs that had a significant BlastX annotation similarity with known proteins of the NCBI non-redundant protein sequence database (NR) with entries from GenPept, Swissprot, PIR, PDF, PDB, and NCBI RefSeq, and as described in Argout et al.32
3.2. SNP performance and quality
The set of 4150 in silico SNPs was selected in the EST contigs and the SNP-harbouring sequences were then submitted to Illumina for processing using the Illumina® Assay Design Tool (ADT). ADT generates scores for each SNP that can range from 0 to 1; SNPs with scores >0.6 have a high probability of being converted into a successful genotyping assay. In the set of 4150 submitted SNPs, 83.5% showed a high conversion success rate (>0.6), 9.2% showed a moderate conversion success rate (between 0.4 and 0.6), and 7.3% showed either a low conversion success rate or no score. A total of 1536 SNP sites having ADT scores >0.4 and without any other SNPs within the adjacent 60 bp was selected for the OPA design (Supplementary Table S1).
3.3. Analysis of base changes
One thousand and forty-four in silico SNPs (68%) were transitions and 462 (32%) were transversions (Table 2). This ratio of transition/transversion SNPs tallies with the results observed in other plant species, where transition SNPs are always more frequent than transversion SNPs.
Table 2.
Nucleotide substitution types of the 1536 selected in silico SNPs
| Types | Number of SNPs | Percentage | Percentage | |
|---|---|---|---|---|
| A ↔ C | 126 | 8 | Transversion | 32 |
| A ↔ T | 128 | 8 | ||
| C ↔ G | 112 | 7 | ||
| T ↔ C | 126 | 8 | ||
| T ↔ G | 612 | 40 | Transition | 68 |
| A ↔ G | 432 | 29 | ||
3.4. SNP polymorphism
From the 1536 SNPs, 841 (55%) with a non-ambiguous polymorphic pattern across accessions were retained as true and verified SNPs and denominated TcSNP. Of the rest, 113 (7%) failed to be genotyped, 436 (28%) had a monomorphic pattern, and 146 (10%) were polymorphic but did not show any clear fluorescent pattern suitable for reliable genotype classification.
Of the 841 polymorphic SNPs, 461 segregated in the mapping population (UPA402 × UF676) and could be mapped on the reference map. Five hundred and thirty-one were polymorphic and mapped on the F2 population map. Two hundred and thirty-nine SNP markers were segregating in both maps, thus enabling construction of a consensus map between them.
3.5. SSR polymorphism
A high-density genetic map is a key tool to order the scaffold assembly needed to generate a complete cocoa genome sequence. SSR markers were defined in the largest non-anchored scaffolds in order to improve anchoring of the T. cacao genome assembly provided by the International Cocoa Genome Sequencing consortium1 on the genetic map.
From the 409 screened SSRs (Supplementary Table S2), 163 were polymorphic for the UPA402 × UF676 progeny and could be mapped.
The new SSR markers defined from scaffolds were named mTcCIR450 to mTcCIR613 to extend the previously identified SSR marker series; mTcCIR 1 to mTcCIR 2918 from genomic DNA and mTcCIR 292 to mTcCIR 4479 from ESTs.
3.6. Individual genetic linkage maps
3.6.1. Map of the UPA402 × UF676 population
A new set of 624 markers with their corresponding sequences, including 461 EST-SNP and 163 new SSR markers located on scaffolds of the genome assembly,1 were added to the reference map (Fig. 1, Supplementary Table S3).
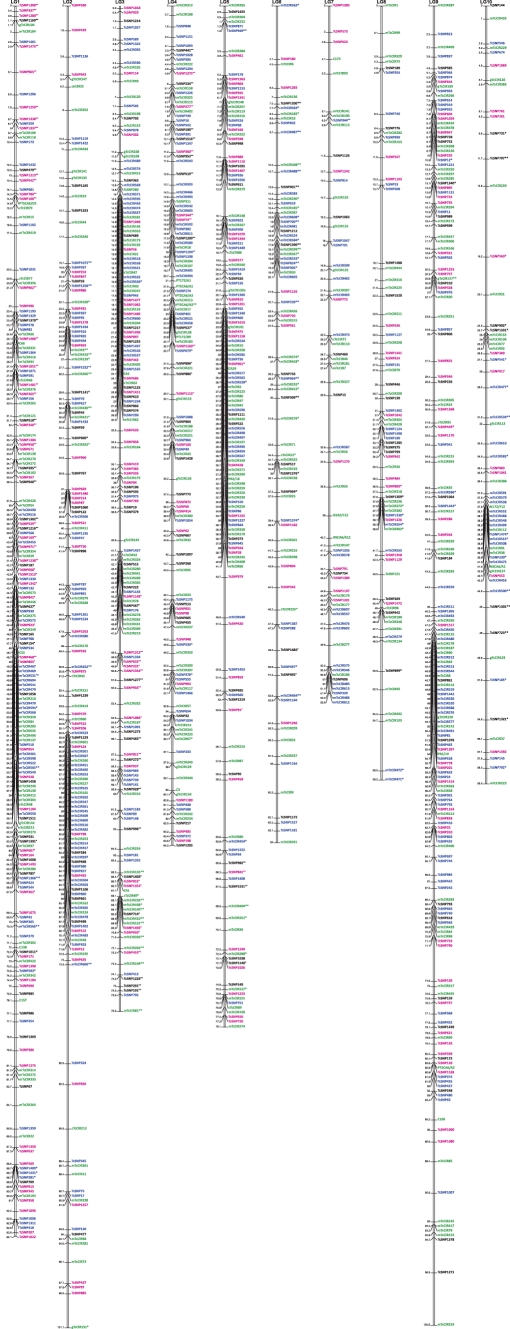
Figure 1.
Genetic map constructed from an F1 progeny of 264 individuals (located in CNRA, Côte d'Ivoire) belonging to the UPA402 × UF676 cross. This map consists of 1043 markers of a known DNA sequence (461 SNPs, 524 SSRs, and 58 RFLPs), spanning 752 cM. The average distance between two markers is 0.7 cM. The new markers added to this map are printed in red.
The new UPA402 × UF676 map contained 1043 markers, including 461 EST-SNPs, 524 SSRs and 58 RFLPs (Table 3). Of the 1043 markers, 571 corresponded to gene markers. The length of this map was 751.7 cM having an average distance of 0.7 cM between adjacent markers.
Table 3.
Distribution of each marker type in the LGs of the reference map (UPA402 × UF676)
| LG | Length (cM) | Total number of markers | Average distance between markers (cM) | SNP | RFLP | Genomic SSR | SSR from scaffold | EST-SSR |
|---|---|---|---|---|---|---|---|---|
| LG1 | 90.3 | 150 | 0.6 | 69 | 8 | 33 | 22 | 18 |
| LG2 | 97.5 | 126 | 0.8 | 55 | 6 | 26 | 28 | 11 |
| LG3 | 74.4 | 126 | 0.6 | 57 | 6 | 33 | 18 | 14 |
| LG4 | 75.6 | 120 | 0.6 | 61 | 11 | 27 | 14 | 8 |
| LG5 | 77.4 | 121 | 0.6 | 55 | 6 | 34 | 15 | 11 |
| LG6 | 62.7 | 73 | 0.9 | 28 | 2 | 19 | 12 | 12 |
| LG7 | 48.9 | 51 | 1.0 | 11 | 6 | 16 | 16 | 2 |
| LG8 | 60.3 | 64 | 0.9 | 30 | 1 | 17 | 5 | 11 |
| LG9 | 103.2 | 154 | 0.7 | 78 | 6 | 34 | 17 | 20 |
| LG10 | 61.3 | 54 | 1.1 | 17 | 6 | 12 | 16 | 3 |
| Total | 751.7 | 1043 | 0.7 | 461 | 58 | 251 | 163 | 110 |
SNP, single-nucleotide polymorphism; RFLP, restriction fragment polymorphism; SSR, simple sequence repeat; EST, expressed sequence tag.
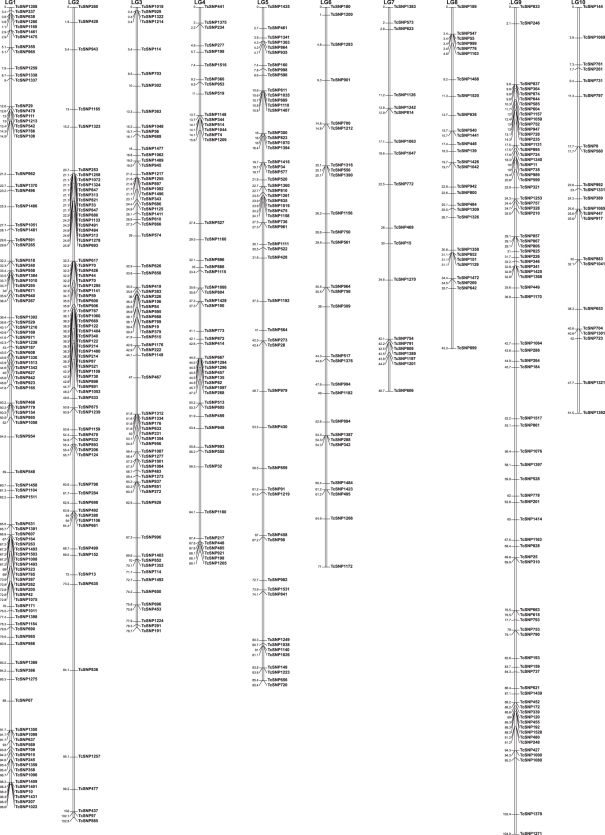
Skewed segregation was observed for 118 markers (11.3%). The skewed markers were mainly located in LGs 2, 3, 6 and 10, as is shown in Fig. 3.
Figure 3.
Consensus map of (UPA402 × UF676) and F2 progenies. Markers segregating in both progenies are indicated in black, those segregating only in (UPA402 × UF676) are printed in green (previously mapped markers) and blue (newly mapped markers). Markers segregating only in the F2 progeny are printed in pink. This consensus map consists of 1262 markers of a known DNA sequence, and it has a length of 734 cM. The average distance between two markers is 0.6 cM. Among the 1262 markers, 810 correspond to markers defined in expressed genes. Significant skewed segregations are indicated by asterisks (*P< 0.05, **P< 0.01) or dots (F2 population).
3.6.2. Map of the F2 population
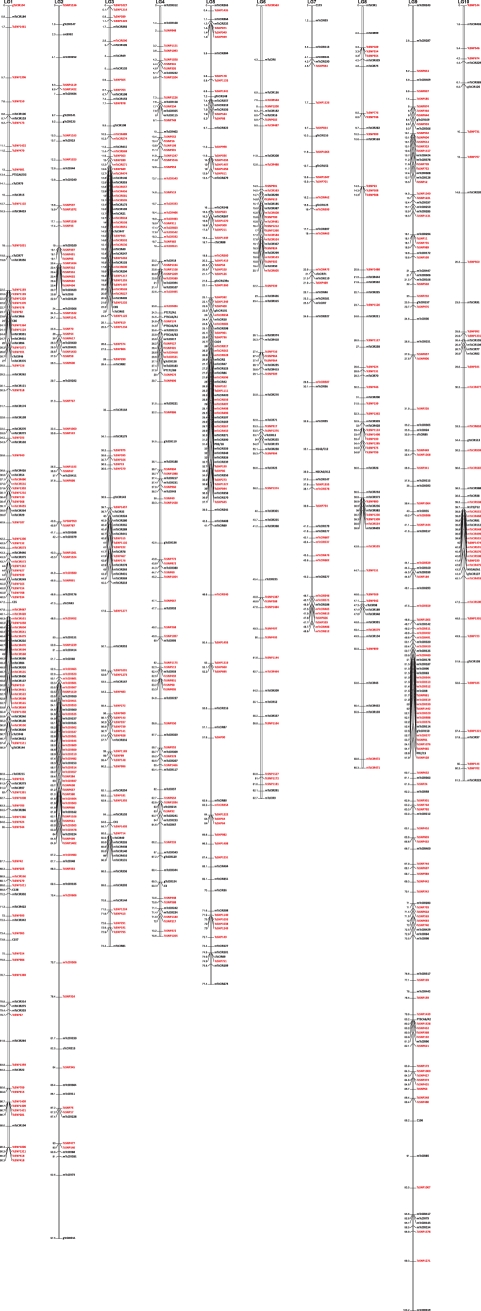
The F2 map (Fig. 2, Supplementary Table S4) contained 531 EST-SNP markers. This map had a total length of 753.9 cM, with an average distance of 1.4 cM between neighboring markers. The marker density varied, with an average distance between neighboring markers ranging from 0.9 cM in LG 9 to 2.7 cM in LG7 (Table 4).
Figure 2.
Genetic map constructed from an F2 progeny of 132 individuals (located at CEPLAC, Brazil) obtained by selfing of a single (Scavina 6 × ICS1) selected hybrid. This map consists of 531 SNP markers, spanning 754 cM. The average distance between two markers is 1.4 cM.
Table 4.
Distribution of SNP markers in the LGs of the F2 map (SCA6 × ICS1) selfing
| LG | Length (cM) | Number of SNP markers | Average distance between markers (cM) |
|---|---|---|---|
| LG1 | 98.6 | 104 | 0.9 |
| LG2 | 102.8 | 74 | 1.4 |
| LG3 | 78.7 | 73 | 1.1 |
| LG4 | 69.1 | 49 | 1.4 |
| LG5 | 85.4 | 56 | 1.5 |
| LG6 | 71.0 | 28 | 2.5 |
| LG7 | 48.7 | 19 | 2.6 |
| LG8 | 43.3 | 28 | 1.5 |
| LG9 | 104.9 | 78 | 1.3 |
| LG10 | 51.5 | 22 | 2.3 |
| Total | 753.9 | 531 | 1.4 |
SNP, single-nucleotide.
Skewed segregation was observed in 97 markers (18.3%). The skewed markers were mainly located in LGs 1, 3 and 4, as is shown in Fig. 3.
3.7. Consensus genetic linkage map
Two hundred and thirty-nine SNP markers were mapped in both populations.
The complete consensus map (Table 5) contained 1262 codominant markers including 681 EST-SNPs, 523 SSRs (163 scaffold-tagged-SSRs, 110 EST-SSRs, 250 SSRs from genomic DNA) and 58 RFLPs including 14 resistance gene analogues (Rgenes-RFLPs), arranged in 10 LGs corresponding to the haploid chromosome number of T. cacao (Fig. 3, Supplementary Table S5). Among the 1262 markers, 65% were gene-based markers, including SNPs, SSRs and RFLPs.
Table 5.
Distribution of each marker type in the LGs of the consensus genetic map
| LG | Length (cM) | Total number of markers | Average distance between markers (cM) | SNP | RFLP | Genomic SSR | SSR from scaffold | EST-SSR |
|---|---|---|---|---|---|---|---|---|
| LG1 | 77.1 | 201 | 0.4 | 120 | 8 | 33 | 22 | 18 |
| LG2 | 101.1 | 156 | 0.6 | 85 | 6 | 26 | 28 | 11 |
| LG3 | 76.9 | 162 | 0.5 | 91 | 6 | 33 | 18 | 14 |
| LG4 | 64.2 | 135 | 0.5 | 75 | 11 | 27 | 14 | 8 |
| LG5 | 78.1 | 147 | 0.5 | 81 | 6 | 34 | 15 | 11 |
| LG6 | 64 | 81 | 0.8 | 36 | 2 | 19 | 12 | 12 |
| LG7 | 52.6 | 62 | 0.8 | 22 | 6 | 16 | 16 | 2 |
| LG8 | 59.2 | 73 | 0.8 | 39 | 1 | 17 | 5 | 11 |
| LG9 | 100.9 | 182 | 0.6 | 106 | 6 | 33 | 17 | 20 |
| LG10 | 59.5 | 63 | 0.9 | 26 | 6 | 12 | 16 | 3 |
| Total | 733.6 | 1262 | 0.6 | 681 | 58 | 250 | 163 | 110 |
SNP, single nucleotide polymorphism; RFLP, restriction fragment polymorphism; SSR, simple sequence repeat; EST, expressed sequence tag.
The total map length was 733.6 cM, i.e. slightly shorter than previously constructed maps (782.8 cM for Pugh et al.8 and 779.2 cM for Fouet et al.9). The average distance between adjacent markers on this map was 0.6 cM, and thus shorter than the 1.3 cM of the map of Fouet et al.9
The number of mapped loci varied substantially between LGs on the consensus map; from 63 in LG10 to 201 in LG1. The average distance between two markers in the different LGs ranged from 0.4 cM in LG1 to 0.9 cM in LG10.
In total, 844 new markers (681 SNP markers and 163 SSR defined in scaffolds) were mapped. These new markers were well distributed over all chromosomes allowing to fill some gaps in the previous maps, for example on chromosome 10.
4. Discussion
A large set of EST-SNP markers was generated and mapped in T. cacao. New SSR markers were added to these SNPs, providing an efficient tool for high-throughput genotyping of cocoa populations.
SSR markers are multiallelic and well adapted for fine analysis of population diversity structure.40–43 In T. cacao, an average number of 5.8 alleles per SSR was observed by Loor Solorzano44 after genotyping a collection of genetic resources of various genetic origins, and with a maximum of 15 alleles revealed by one SSR (mTcCIR322). This is not the case for SNPs that are only biallelic, but a higher number of SNP markers (several thousands) can be easily revealed at once using high-throughput technologies.
We used our new SNP and SSR markers to construct a very high-density genetic map. Sixty-five per cent of the markers were from within genes and the average distance between adjacent markers was 0.6 cM.
Several chromosome regions include markers with skewed segregations, particularly on LG 1, LG 3, LG 4, and LG 6. The region on LG 4 includes the locus for self-incompatibility previously identified by Crouzillat et al.5 The gameto-sporophytic incompatibility system existing in T. cacao45,46 could possibly explain the segregation distortion on this LG 4 region. Other factors which could explain segregation distortion, such as chromosome rearrangements in banana47 which are responsible for highly skewed marker segregations, have not been reported in T. cacao.
This high-density genetic map can be used as a major tool for efficient genome-wide association studies (GWASs) in T. cacao populations. This method, first applied in human and animal genetics,48–51 was also found to be highly effective for studying the determinism of useful traits in plants,52–56 particularly in cocoa with the analysis of some recent hybrid populations.8,57,58 GWAS is an alternative to QTL analyses in cross progenies for the purpose of studying genetic control of phenotypic traits in cocoa.
GWASs can be carried out on unrelated genetic resources such as wild or cultivated populations or germplasm collections. Large cocoa germplasm collections are maintained in many countries and characterized for useful traits. Two international cocoa collections are hosted at the International Cocoa Genebank, Trinidad (ICG,T, preserving 2300 accessions),59 and at the Centro Agronomico Tropical de Investigacion y Ensenanza, Turrialba, Costa Rica (CATIE, preserving 1150 accessions).60 The markers identified here will now certainly facilitate such GWASs, providing added-value to this wide characterization work, thus boosting knowledge on the genetic determinants of useful cocoa traits.
Another benefit of this large set of mapped markers is the possible integration of molecular information in conventional cocoa breeding schemes using marker-assisted selection (MAS).
In cocoa, few MAS experiments are currently underway.3,61 The efficiency of MAS in selecting P. palmivora-resistant cocoa plants has been reported by Lanaud et al.3
Until now, MAS studies have mainly been focused on traits controlled by a small number of genes, using only markers close to QTLs. However, such methods are of limited use for traits that are determined by a large number of genes of small effects.
Substantial genome-wide molecular data can now be generated at lower cost by high-throughput technologies, such as SNP genotyping. This progress has paved the way for the development of new methods to predict genotype value via MAS. The genome-wide selection or genomic selection (GS) method was recently successfully applied in animal or plant breeding62–66 and allows to predict phenotypes using all marker information.
The integration of molecular markers in cocoa recurrent breeding programmes67–72 could be facilitated by the GS approach in order to accelerate genetic gains. The GS strategy seems particularly suitable for the selection of multigenic traits such as yield and disease resistance. Cumulating a large number of resistance alleles is one of the main objectives of cocoa breeding for sustainable cocoa resistance. The large set of available SNP markers could facilitate the selection of resistant and high yielding cocoa trees via GS approaches enabling the use of all genome regions tagged by SNP markers, even those with very small effects.
The search for candidate genes underlying trait variation is another major challenge for plant biologists, with the aim of gaining further insight into the mechanisms underlying trait variation, and producing tools to efficiently screen and exploit genetic resources.
The consensus map produced in this work has been used efficiently for anchoring an assembly of T. cacao Criollo genome sequences, and for constituting pseudomolecules.1 Recently, two different cocoa varieties, i.e. Criollo1 and Forastero from the Lower Amazon region (http://www.cacaogenomedb.org/), were sequenced, with 28 798 and 35 000 annotated genes, respectively. These sequences will greatly facilitate the identification of candidate genes, allowing integration of both genetic and genomic (functional and structural) data. Overall, about 300 QTLs or marker/trait associations have already been identified in T. cacao. High-throughput genotyping associated with a high marker density will facilitate fine mapping of genes involved in trait variation (with GWAS or classical QTL analyses conducted on large progenies), thus allowing to refine the QTL position in the genome, while facilitating the search for candidate genes in corresponding genome sequences. Several functional studies have already been conducted in cocoa, focused mainly on genes generally expressed in specific physiological conditions or metabolisms.73 It will be now possible to focus more specifically on the expression of genes directly responsible for trait variation after candidate gene validation.
Analysing genome evolution during domestication processes or adaptation to climate change can also help us to identify key genes underlying adaptive traits.74 Loss of diversity generally occurs during genome evolution, and some genes are selectively involved in natural selection or domestication. A large set of SNPs defined in expressed genes, such as those identified in this study, provides a key tool for identifying selection signatures or adaptive substitutions, and then highlighting candidate genes potentially involved in the adaptation60 or domestication processes and their corresponding molecular functions.75 All SNPs reported in this paper were identified in orthologous genes or gene families, thus facilitating comparative genomic approaches, and benefiting from gene knowledge accumulated in other species to accelerate cocoa breeding.
5. Availability
Information on the consensus linkage map, molecular markers, and primers are available in the Map Study ‘SSR_SNP_consensus_map’ of the cocoa module of TropGeneDB database (http://tropgenedb.cirad.fr).
Supplementary Data
Supplementary Data are available at www.dnaresearch.oxfordjournals.org.
Funding
We thank the Region Languedoc-Roussillon, Valrhona and the Fondation Agropolis for their financial support in this work.
Supplementary Material
Acknowledgements
We acknowledge Chantal Hamelin for TropGeneDB support. We thank Peter Biggins for improving the English in this paper, and the reviewers for their useful comments and editing job of this paper.
References
- 1.Argout X., Salse J., Aury J.M., et al. The genome of Theobroma cacao. Nat. Genet. 2011;43:101–8. doi: 10.1038/ng.736. [DOI] [PubMed] [Google Scholar]
- 2.Cheesman E.E. Notes on the nomenclature, classification and possible relationships of cocoa populations. Trop. Agricult. 1944;21:144–59. [Google Scholar]
- 3.Lanaud C., Fouet O., Clément D., et al. A meta-QTL analysis of disease resistance traits of Theobroma cacao L. Mol. Breed. 2009;24:361–74. [Google Scholar]
- 4.Lanaud C., Risterucci A.M., Ngoran A.K.J., et al. A genetic-linkage map of Theobroma cacao L. Theor. Appl. Genet. 1995;91:987–93. doi: 10.1007/BF00223910. [DOI] [PubMed] [Google Scholar]
- 5.Crouzillat D., Lerceteau E., Petiard V., et al. Theobroma cacao L.: a genetic linkage map and quantitative trait loci analysis. Theor. Appl. Genet. 1996;93:205–14. doi: 10.1007/BF00225747. [DOI] [PubMed] [Google Scholar]
- 6.Risterucci A.M., Grivet L., N'Goran J.A.K., Pieretti I., Flament M.H., Lanaud C. A high-density linkage map of Theobroma cacao L. Theor. Appl. Genet. 2000;101:948–55. [Google Scholar]
- 7.Semagn K., Bjornstad A.A., Ndjiondjop M.N. An overview of molecular marker methods for plants. Afr. J. Biotechnol. 2006;5:2540–68. [Google Scholar]
- 8.Pugh T., Fouet O., Risterucci A.M., et al. A new cacao linkage map based on codominant markers: development and integration of 201 new microsatellite markers. Theor. Appl. Genet. 2004;108:1151–61. doi: 10.1007/s00122-003-1533-4. [DOI] [PubMed] [Google Scholar]
- 9.Fouet O., Allegre M., Argout X., et al. Structural characterization and mapping of functional EST-SSR markers in Theobroma cacao. Tree Genet. Genomes. 2011;99:1–19. [Google Scholar]
- 10.Schmid KJ R.S.T. Large-scale identification and analysis of genome-wide single-nucleotide polymorphisms for mapping in Arabidopsis thaliana. Genome Res. 2003;13:1250–7. doi: 10.1101/gr.728603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lijavetzky D., Cabezas J.A., Ibanez A., Rodriguez V., Martinez-Zapater J.M. High throughput SNP discovery and genotyping in grapevine (Vitis vinifera L.) by combining a re-sequencing approach and SNPlex technology. BMC Genomics. 2007;8:424. doi: 10.1186/1471-2164-8-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berard A., Le Paslier M.C., Dardevet M., et al. High-throughput single nucleotide polymorphism genotyping in wheat (Triticum spp.) Plant Biotechnol. J. 2009;7:364–74. doi: 10.1111/j.1467-7652.2009.00404.x. [DOI] [PubMed] [Google Scholar]
- 13.Dantec L.L., Chagne D., Pot D., et al. Automated SNP detection in expressed sequence tags: statistical considerations and application to maritime pine sequences. Plant Mol. Biol. 2004;54:461–70. doi: 10.1023/B:PLAN.0000036376.11710.6f. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B., Zhou Y., Zhang L., Zhuge Q., Wang M.X., Huang M.R. Identification and validation of single nucleotide polymorphisms in poplar using publicly expressed sequence tags. J. Integr. Plant Biol. 2005;47:1493–9. [Google Scholar]
- 15.Pavy N., Parsons L.S., Paule C., MacKay J., Bousquet J. Automated SNP detection from a large collection of white spruce expressed sequences: contributing factors and approaches for the categorization of SNPs. BMC Genomics. 2006;7:174. doi: 10.1186/1471-2164-7-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho R.J., Mindrinos M., Richards D.R., et al. Genome-wide mapping with biallelic markers in Arabidopsis thaliana. Nat. Genet. 1999;23:203–7. doi: 10.1038/13833. [DOI] [PubMed] [Google Scholar]
- 17.Hoskins R.A., Phan A.C., Naeemuddin M., et al. Single nucleotide polymorphism markers for genetic mapping in Drosophila melanogaster. Genome Res. 2001;11:1100–13. doi: 10.1101/gr.178001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian C., Plenge R.M., Ransom M., et al. Analysis and application of European genetic substructure using 300 K SNP information. PLoS Genet. 2008;4:e4. doi: 10.1371/journal.pgen.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novembre J., Johnson T., Bryc K., et al. Genes mirror geography within Europe. Nature. 2008;456:98–101. doi: 10.1038/nature07331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z., Yoder A.D. Estimation of the transition/transversion rate bias and species sampling. J. Mol. Evol. 1999;48:274–83. doi: 10.1007/pl00006470. [DOI] [PubMed] [Google Scholar]
- 21.Swofford D.L., Olsen G.J., Waddell P.J., Hillis D.M. Phylogeny reconstruction. Mol. Syst. 1990;411:501. [Google Scholar]
- 22.Brown W.M., Prager E.M., Wang A., Wilson A.C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J. Mol. Evol. 1982;18:225–39. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- 23.Gojobori T., Li W.H., Graur D. Patterns of nucleotide substitution in pseudogenes and functional genes. J. Mol. Evol. 1982;18:360–9. doi: 10.1007/BF01733904. [DOI] [PubMed] [Google Scholar]
- 24.Wakeley J. Substitution-rate variation among sites and the estimation of transition bias. Mol. Biol. Evol. 1994;11:436–42. doi: 10.1093/oxfordjournals.molbev.a040124. [DOI] [PubMed] [Google Scholar]
- 25.Wakeley J. The excess of transitions among nucleotide substitutions: new methods of estimating transition bias underscore its significance. Trends Ecol. Evol. (Amst.) 1996;11:158–62. doi: 10.1016/0169-5347(96)10009-4. [DOI] [PubMed] [Google Scholar]
- 26.Morales M., Roig E., Monforte A.J., Arus P., Garcia-Mas J. Single-nucleotide polymorphisms detected in expressed sequence tags of melon (Cucumis melo L.) Genome. 2004;47:352–60. doi: 10.1139/g03-139. [DOI] [PubMed] [Google Scholar]
- 27.Deleu W., Esteras C., Roig C., et al. A set of EST-SNPs for map saturation and cultivar identification in melon. BMC Plant Biol. 2009;9 doi: 10.1186/1471-2229-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li F., Kitashiba H., Inaba K., Nishio T. A Brassica rapa linkage map of EST-based SNP markers for identification of candidate genes controlling flowering time and leaf morphological traits. DNA Res. 2009;16:311–23. doi: 10.1093/dnares/dsp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kota R., Varshney R., Prasad M., Zhang H., Stein N., Graner A. EST-derived single nucleotide polymorphism markers for assembling genetic and physical maps of the barley genome. Funct. Integr. Genomics. 2008;8:223–33. doi: 10.1007/s10142-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 30.Cordeiro G.M., Eliott F., McIntyre C.L., Casu R.E., Henry R.J. Characterisation of single nucleotide polymorphisms in sugarcane ESTs. Theor. Appl. Genet. 2006;113:331–43. doi: 10.1007/s00122-006-0300-8. [DOI] [PubMed] [Google Scholar]
- 31.Lima L.S., Gramacho K.P., Carels N., et al. Single nucleotide polymorphisms from Theobroma cacao expressed sequence tags associated with witches’ broom disease in cacao. Genet. Mol. Res. 2009;8:799–808. doi: 10.4238/vol8-3gmr603. [DOI] [PubMed] [Google Scholar]
- 32.Argout X., Fouet O., Wincker P., et al. Towards the understanding of the cocoa transcriptome: production and analysis of an exhaustive dataset of ESTs of Theobroma cacao L. generated from various tissues and under various conditions. BMC Genomics. 2008 doi: 10.1186/1471-2164-9-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang J., Vosman B., Voorrips R.E., van der Linden C.G., Leunissen J.A. QualitySNP: a pipeline for detecting single nucleotide polymorphisms and insertions/deletions in EST data from diploid and polyploid species. BMC Bioinformatics. 2006;7:438. doi: 10.1186/1471-2105-7-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyten D.L., Song Q., Choi I.-Y., et al. High-throughput genotyping with the GoldenGate assay in the complex genome of soybean. Theor. Appl. Genet. 2008;116:945–52. doi: 10.1007/s00122-008-0726-2. [DOI] [PubMed] [Google Scholar]
- 35.Akhunov E., Nicolet C., Dvorak J. Single nucleotide polymorphism genotyping in polyploid wheat with the Illumina GoldenGate assay. Theor. Appl. Genet. 2009;119:507–17. doi: 10.1007/s00122-009-1059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozen S., Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 37.Van Ooijen J.W. Joinmap, Software for the Calculation of Genetic Linkage Maps. The Netherlands: Wageningen; 2006. [Google Scholar]
- 38.Kosambi D.D. The estimation of map distance from recombination values. Ann. Eugen. 1944;12:172–5. [Google Scholar]
- 39.Batley J., Barker G., O'Sullivan H., Edwards K.J., Edwards D. Mining for single nucleotide polymorphisms and insertions/deletions in maize expressed sequence tag data. Plant Physiol. 2003;132:84–91. doi: 10.1104/pp.102.019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D., Mischke S., Johnson E.S., Phillips-Mora W., Meinhardt L. Molecular characterization of an international cacao collection using microsatellite markers. Tree Genet. Genomes. 2008;5:1–10. [Google Scholar]
- 41.Lachenaud P., Zhang D. Genetic diversity and population structure in wild stands of cacao trees (Theobroma cacao L.), in French Guiana. Ann. For. Sci. 2008;65:7. [Google Scholar]
- 42.Zhang D., Arevalo-Gardini E., Mischke S., Zúñiga-Cernades L., Barreto-Chavez A., Del Aguila J.A. Genetic diversity and structure of managed and semi-natural populations of cocoa (Theobroma cacao) in the Huallaga and Ucayali Valleys of Peru. Ann. Bot. 2006;98:647–55. doi: 10.1093/aob/mcl146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motamayor J.C., Lachenaud P., da Silva E Mota J.W., et al. Geographic and genetic population differentiation of the Amazonian chocolate tree (Theobroma cacao L.) PLoS ONE. 2008;3:e3311. doi: 10.1371/journal.pone.0003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loor Solorzano R.G. Contribution à l’étude de la domestication de la variété Nacional d'Equateur: recherche de la variété native et de ses ancêtres sauvages. 2007 PhD Thesis, Montpellier SUPAGRO- ED SIBAGHE- spécialité: biologie intégrative des plantes. [Google Scholar]
- 45.Knight R., Rogers H. Incompatibility in Theobroma cacao. Heredity. 1955;9:69–77. [Google Scholar]
- 46.Cope F.W. Incompatibility in Theobroma cacao. Nature. 1958;181:279. [Google Scholar]
- 47.Fauré S., Noyer J.L., Horry J.P., Bakry F., Lanaud C., Gońzalez de León D. A molecular marker-based linkage map of diploid bananas (Musa acuminata) Theor. Appl. Genetics. 1993;87:517–26. doi: 10.1007/BF00215098. [DOI] [PubMed] [Google Scholar]
- 48.Blott S., Kim J.-J., Moisio S., et al. Molecular dissection of a quantitative trait locus: a phenylalanine-to-tyrosine substitution in the transmembrane domain of the bovine growth hormone receptor is associated with a major effect on milk yield and composition. Genetics. 2003;163:253–66. doi: 10.1093/genetics/163.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cardon L.R., Bell J.I. Association study designs for complex diseases. Nat. Rev. Genet. 2001;2:91–9. doi: 10.1038/35052543. [DOI] [PubMed] [Google Scholar]
- 50.Liu P., Wang Y., Vikis H., et al. Candidate lung tumor susceptibility genes identified through whole-genome association analyses in inbred mice. Nat. Genet. 2006;38:888–95. doi: 10.1038/ng1849. [DOI] [PubMed] [Google Scholar]
- 51.Meuwissen T.H.E., Karlsen A., Lien S., Olsaker I., Goddard M.E. Fine mapping of a quantitative trait locus for twinning rate using combined linkage and linkage disequilibrium mapping. Genetics. 2002;161:373–9. doi: 10.1093/genetics/161.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y.-M., Mao Y., Xie C., Smith H., Luo L., Xu S. Mapping quantitative trait loci using naturally occurring genetic variance among commercial inbred lines of maize (Zea mays L.) Genetics. 2005;169:2267–75. doi: 10.1534/genetics.104.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aranzana M.J., Kim S., Zhao K., et al. Genome-wide association mapping in Arabidopsis identifies previously known flowering time and pathogen resistance genes. PLoS Genet. 2005;1:e60. doi: 10.1371/journal.pgen.0010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jannoo N., Grivet L., Dookun A., D'hont A., Glaszmann J.C. Linkage disequilibrium among modern sugarcane cultivars. Theor. Appl. Genet. 1999;99:1053–60. [Google Scholar]
- 55.Wei X., Jackson P.A., McIntyre C.L., Aitken K.S., Croft B. Associations between DNA markers and resistance to diseases in sugarcane and effects of population substructure. Theor. Appl. Genet. 2006;114:155–64. doi: 10.1007/s00122-006-0418-8. [DOI] [PubMed] [Google Scholar]
- 56.Agrama H.A., Eizenga G.C., Yan W. Association mapping of yield and its components in rice cultivars. Mol. Breed. 2007;19:341–56. [Google Scholar]
- 57.Marcano M., Pugh T., Cros E., et al. Adding value to cocoa (Theobroma cacao L.) germplasm information with domestication history and admixture mapping. Theor. Appl. Genet. 2007;114:877–84. doi: 10.1007/s00122-006-0486-9. [DOI] [PubMed] [Google Scholar]
- 58.Marcano M., Morales S., Hoyer M.T., et al. A genomewide admixture mapping study for yield factors and morphological traits in a cultivated cocoa (Theobroma cacao L.) population. Tree Genet. Genomes. 2009;5:329–37. [Google Scholar]
- 59.Iwaro A.D., Bekele F.L., Butler D.R. Evaluation and utilisation of cacao (Theobroma cacao L.) germplasm at the International Cocoa Genebank, Trinidad. Euphytica. 2003;130:207–21. [Google Scholar]
- 60.IBPGR Working Group on Genetics Resources of Cocoa. Genetic Resources of Cocoa. Rome, Italy: 1981. p. 25. [Google Scholar]
- 61.Schnell R.J., Kuhn D.N., Brown J.S., et al. Development of a marker assisted selection program for cacao. Phytopathology. 2007;97:1664–9. doi: 10.1094/PHYTO-97-12-1664. [DOI] [PubMed] [Google Scholar]
- 62.Meuwissen T.H., Hayes B.J., Goddard M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics. 2001;157:1819–29. doi: 10.1093/genetics/157.4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heffner E.L., Sorrells M.E., Jannink J.L. Genomic selection for crop improvement. Crop Sci. 2009;49:1–12. [Google Scholar]
- 64.Bernardo R., Yu J. Prospects for genomewide selection for quantitative traits in maize. Crop Sci. 2007;47:1082. [Google Scholar]
- 65.Wong C.K., Bernardo R. Genomewide selection in oil palm: increasing selection gain per unit time and cost with small populations. Theor. Appl. Genet. 2008;116:815–24. doi: 10.1007/s00122-008-0715-5. [DOI] [PubMed] [Google Scholar]
- 66.Jannink J.L., Lorenz A.J., Iwata H. Genomic selection in plant breeding: from theory to practice. Brief. Funct. Genomic. 2010;9:166. doi: 10.1093/bfgp/elq001. [DOI] [PubMed] [Google Scholar]
- 67.Clement D., Eskes A.B., Sounigo O., N'Goran J. Amélioration génétique du cacaoyer en Côte d'Ivoire-Présentation d'un nouveau schéma de selection. Proceedings of the 11th International Cocoa Research Conference; 1993. pp. 18–24. [Google Scholar]
- 68.Paulin D., Eskes A.B. Le cacaoyer: stratégies de sélection. Plant. Recherche Dével. 1995;2:5–18. [Google Scholar]
- 69.Pires J.L., Monteiro W.R., Pinto L.R.M., Figueira A., Yamada M.M., Ahnert D. A proposal for cocoa breeding. Proceedings of the 12th International Cocoa Research Conference; 1996. pp. 17–23. [Google Scholar]
- 70.Iwaro A.D., Singh V., Bharath S.M., Perez C., Ali L., Butler D.R. Germplasm enhancement for resistance to black pod disease – final stages and achievements. Proceedings of the 16th International Cocoa Research Conference; 2010. pp. 493–499. [Google Scholar]
- 71.Tahi M., Lachenaud P., N'Goran J., et al. Second cycle de selection recurrente du cacaoyer (Theobroma cacao L.) en Cote d'Ivoire: bilan a mi-parcours et propositions de sorties varietales. Proceedings of the 16th International Cocoa Research Conference; 2010. pp. 3–12. [Google Scholar]
- 72.Lopes U.V., Monteiro W.R., Pires J.L., Clement D., Yamada M.M., Gramacho K.P. Cacao breeding in Bahia, Brazil—strategies and results. Crop Breed. Appl. Biotechnol. 2011;1:73–81. [Google Scholar]
- 73.Micheli F., Guiltinan M., Gramacho K.P., et al. Functional genomics of Cacao. In: Jean-Claude K., Michel D., editors. Advances in Botanical Research. vol. 55. Londres: Academic Press; 2010. pp. 119–77. [Google Scholar]
- 74.Doebley J.F., Gaut B.S., Smith B.D. The molecular genetics of crop domestication. Cell. 2006;127:1309–21. doi: 10.1016/j.cell.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Nielsen R. Molecular signatures of natural selection. Annu. Rev. Genet. 2005;39:197–218. doi: 10.1146/annurev.genet.39.073003.112420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.