Abstract
In England and Wales, the National Institute for Health and Clinical Excellence (NICE) has provided guidance [technology appraisals (TAs) 130, 186, 195, 198 and 225] on the use of biologic drugs for the treatment of RA. This is based on an analysis of efficacy, safety and cost-effectiveness, and has resulted in a complex management pathway that restricts freedom to prescribe biologics according to their licensed indications. Specifically, TNF antagonists are the only class of biologics that can be used first line in DMARD-inadequate responders, and only in patients with a persistent 28-joint DAS score of ≥5.1. Alternative biologic agents are denied to those with contraindications to anti-TNF drugs and are also not supported following intolerance to TNF antagonists. Rituximab is the only class of biologic permitted after TNF antagonist inefficacy, in the absence of a contraindication to its use, whereas abatacept and tocilizumab are licensed and may be a more efficacious choice at this stage in some patient groups. Furthermore, for patients who demonstrate sequential inadequate responses, treatment is restricted to one TNF antagonist, rituximab and tocilizumab, whereas abatacept is only a permitted choice when rituximab is contraindicated or has been withdrawn because of an adverse event. In this review, we discuss the treatment algorithm published by NICE, and suggest alternatives where perceived deficiencies exist.
Keywords: rheumatoid arthritis, biologic agents, guidelines, TNF antagonists, adalimumab, etanercept, certolizumab pegol, rituximab, abatacept, tocilizumab
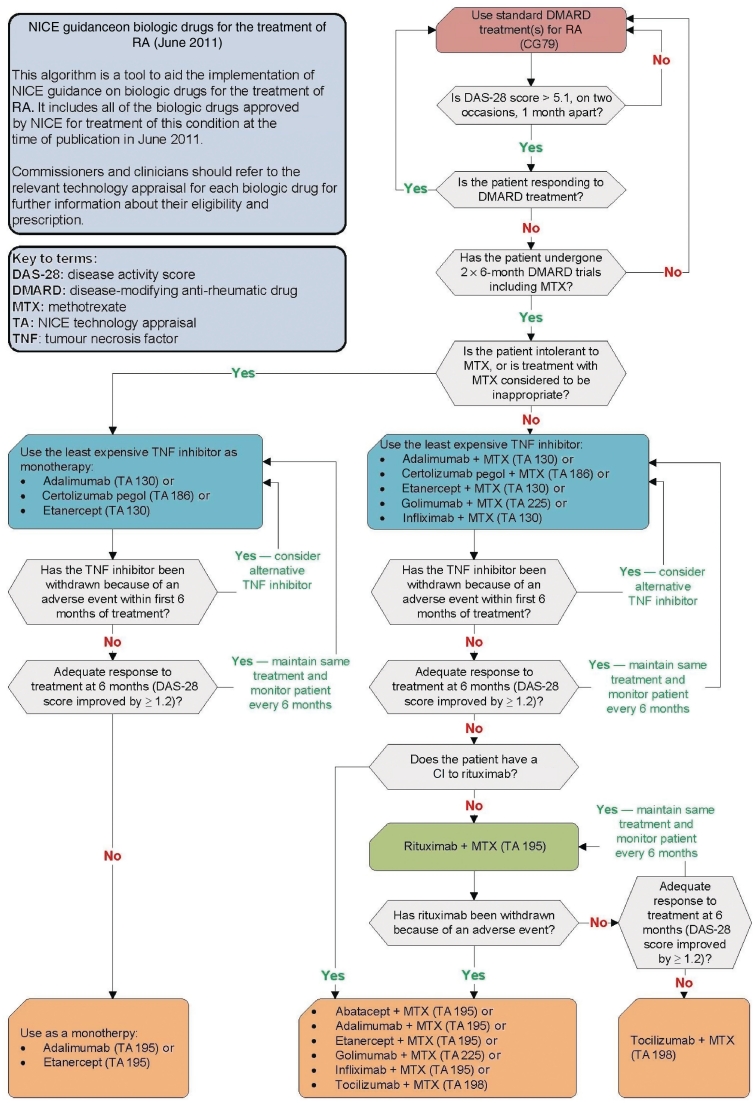
Patients with RA and their physicians have been extremely fortunate over the last decade in having four classes of biologic agents licensed to combat the disease. This has led to a seismic shift in RA management resulting in disease remission, or at least low disease activity, as the therapeutic goal [1, 2]. However, in England and Wales the ability to deliver evidenced-based practice has been restricted by the National Institute for Health and Clinical Excellence (NICE) following the publication of five technology appraisals (TAs 130, 186, 195, 198 and 225) providing guidance on the use of biologic drugs based on an analysis of efficacy, safety and cost-effectiveness [3–6]. The result is a complex management pathway that the majority of health commissioners in England and Wales insist rheumatologists follow (Fig. 1). Internationally, while not binding, these guidelines may also carry substantial influence. In this review we will attempt to navigate this algorithm and suggest alternatives where perceived deficiencies exist. Table 1 lists biologic agents according to licensed and NICE-approved status in different clinical scenarios.
Fig. 1.
Algorithm illustrating NICE guidance on biologic drugs for the treatment of RA. NICE (2011) algorithm: ‘rheumatoid arthritis’. www.nice.org.uk. Reproduced with permission from NICE. Algorithm was accurate at the time of publication CI: contraindication.
Table 1.
Biologic agents for RA listed according to licensed and NICE-approved status in different clinical scenarios
| MTX tolerant |
MTX intolerant |
|||
|---|---|---|---|---|
| Scenario | NICE approved | Licensed options | NICE approved | Licensed options |
| DMARD-IR | Adalimumab, certolizumab pegol, etanercept, golimumab, infliximab | Adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, abatacept, tocilizumab | Adalimumab, certolizumab pegol, etanercept | Adalimumab, certolizumab pegol, etanercept, tocilizumab |
| DMARD-IR, anti-TNF contraindication | Abatacept, tocilizumab | Tocilizumab | ||
| Anti-TNF intolerant | Alternative from adalimumab, certolizumab pegol, etanercept, golimumab, infliximab | Adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, abatacept, rituximab, tocilizumab | Alternative from adalimumab, certolizumab pegol, etanercept | As DMARD-IR |
| Anti-TNF ineffective | Rituximab | As anti-TNF intolerant | Alternative from adalimumab, etanercept | As DMARD-IR |
| Anti-TNF ineffective, rituximab contraindication | Adalimumab, etanercept, infliximab, abatacept, tocilizumab | As DMARD-IR | Alternative from adalimumab, etanercept | As DMARD-IR |
| Rituximab intolerant | As anti-TNF ineffective, rituximab contraindication | As DMARD-IR | Not applicable | Not applicable |
| Rituximab ineffective | Tocilizumab | As DMARD-IR | Not applicable | Not applicable |
Initial biologic therapy
The decision to initiate a biologic drug has been addressed by leading rheumatology societies worldwide (e.g. BSR, EULAR, ACR). A common consensus is that a biologic should be started in patients who fail to achieve a 28-joint DAS (DAS-28) < 3.2 after treatment with traditional DMARDs [7–11]. This contrasts with NICE TA 130 (October 2007) that recommends anti-TNF therapies be started in patients who have a persistently elevated DAS-28 > 5.1, after failure of two DMARDs (including MTX, unless contraindicated) taken over a minimum time of 6 months each. This remains the current standard of practice in England and Wales, being at variance with many published guidelines and standards in the European Union and the USA, which emphasize the importance of not delaying the start of biologic therapy when required [1, 2]. The implication for patients with a DAS-28 between 3.2 and 5.1 is that improvement in disease activity may only be achieved by continuing traditional DMARDs and CSs. However, data from the Early Rheumatoid Arthritis Network, a prospective inception cohort of early RA in England and Wales, has shown that only 27% of patients with DAS-28 3.2–5.1 achieve a DAS-28 < 3.2 at Year 2 and 35% at Year 3 in routine care using conventional DMARDs [12]. Furthermore, this data set has also shown that patients with a DAS persistently in this range fair poorly, with sustained and substantial disability as documented by poor scores of function. For those living in England and Wales the time has surely come to pay greater attention to this patient group with a persistent DAS-28 of 3.2–5.1 despite conventional therapies, and permit the use of biologics in order to achieve remission, or at least low disease activity, according to current principles of best practice [1, 2, 11].
MTX intolerance
It is clear that outcomes are improved if TNF antagonists are co-prescribed with MTX [13, 14]. As such, NICE concurs with other guidelines that MTX remains the cornerstone DMARD for RA. However, in routine care >30% of patients receive biologics as monotherapy [15, 16]. In those patients who are MTX intolerant, NICE allows the use of adalimumab, etanercept (TA 130) and certolizumab (TA 186) as monotherapy, in keeping with drug licences. In practice, clinicians may choose to co-prescribe an alternative DMARD to MTX, on the assumption that the benefit is not drug specific, but rather an effect of overall immunomodulation. An alternative first-line biologic may be tocilizumab, as this is licensed without MTX in DMARD-inadequate responder (IR) patients. There is randomized controlled trial (RCT) evidence for the use of tocilizumab as monotherapy [17] and in combination with non-MTX DMARDs [18]. Tocilizumab is therefore a legitimate choice as a first-line biologic in patients where MTX is not tolerated, but is not currently recommended under NICE guidelines.
Contraindications to TNF antagonists
Where contraindications to TNF antagonists exist, NICE provides no alternative first-line biologic option. The summary of product characteristics (SmPC) for anti-TNF drugs list active tuberculosis or other severe infection including sepsis or risk of sepsis, active infection including chronic or localized infection, and opportunistic infection, as well as moderate to severe heart failure [New York Heart Association (NYHA) Class III/IV] as contraindications to their use. Clinicians must also balance the risk/benefit of a range of other conditions listed as precautions to the use of TNF antagonists, such as history of malignancy, blood dyscrasias, demyelinating disease, chronic obstructive pulmonary disease, vasculitis, as well as viral and alcoholic hepatitis. These disorders are sufficiently common that a significant proportion of patients will either be excluded from receiving TNF antagonists, or treated with trepidation. In this situation, an alternative biologic should be available, as risk/benefit analysis may confer a preference over anti-TNF drugs. Both abatacept and tocilizumab are licensed for first-line biologic use (rituximab is not) on the basis of trial evidence in DMARD-IR patients [19–21]. They may therefore be legitimately considered as alternative first-line biologics, especially where relative and absolute contraindications to TNF antagonists exist. At present this is not recommended under NICE guidelines.
Intolerance to first TNF antagonist within 6 months
In this situation, NICE (TAs 130 and 186) allows a switch within anti-TNF class. However, NICE provides no guidance for patients who have responded but develop an adverse event after the first 6 months of therapy. These patients should also be allowed to switch to an alternative TNF antagonist. Furthermore, there is no guidance if the patient is also intolerant to the second or third anti-TNF agent. Arguably these patients are effectively at the first biologic stage, because intolerance has denied them the opportunity of a therapeutic response to TNF antagonists. Evidence exists that such patients may respond to a different class of biologic, including abatacept, rituximab and tocilizumab. Abatacept and tocilizumab are licensed in DMARD and anti-TNF-IR patients, and rituximab is licensed for patients who have been intolerant to one or more TNF antagonists. Therefore, they may all be used legitimately at this juncture. Of these, tocilizumab can be given as monotherapy in MTX-intolerant patients. Nevertheless, none of these options are currently recommended by NICE guidelines.
Poor efficacy of first anti-TNF drug in MTX-intolerant patients
In this situation, NICE (TA 195) supports a within-class switch to either adalimumab or etanercept monotherapy. Certolizumab is not covered by TA 195, but would be an acceptable alternative. Thus there is then no guidance if these too are ineffective. Although NICE allows a switch to another biologic class in this situation in MTX-tolerant patients (TAs 195 and 198), there is no allowance for MTX-intolerant patients. A legitimate alternative would be tocilizumab, which is licensed for monotherapeutic use in MTX-intolerant patients and has proven efficacy in anti-TNF-IR patients; however, in the key TNF-IR trial, all patients were co-prescribed MTX [22].
Poor efficacy of first anti-TNF drug in MTX-tolerant patients
NICE (TA 195) supports rituximab alone as the second-line biologic option, assuming no contraindications exist to its use. This is a logical and appropriate course, especially for patients who are seropositive for RF and/or anti-citrullinated protein antibodies ACPAs. However, rituximab is not unique among the biologics in working well in this cohort. In contrast, EULAR guidelines recommend a choice at this stage between all classes of licensed biologics [10], which allows clinicians freedom to use biomarker and other characteristics to individualize therapy. Rituximab has proven efficacy in anti-TNF-IR patients [23]; however, some uncertainty exists over future treatment options if hypogammaglobulinaemia develops following repeat cycles. As such, guidance to use rituximab as the only second-line biologic is constrictive, outside the approach adopted by EULAR, and likely to lead to certain patients not being treated with the most appropriate class of biologic at this stage. This is especially true of those patients who are both RF and ACPA negative, where the evidence (mainly from MTX-IR RCTs) suggests that this group does not benefit as much as those patients who possess these antibodies [23–25].
Poor efficacy of first anti-TNF drug in MTX-tolerant patients where rituximab is contraindicated
NICE allows a switch within the anti-TNF class or the use of abatacept (TA 195) or tocilizumab (TA 198). NICE TA 195 refers to the rituximab SmPC for definitions that would contraindicate its use, these being active, severe infection, the severely immunocompromised and those with severe heart failure (NYHA Class IV) or severe uncontrolled cardiac disease. As previously mentioned, in clinical practice there is hesitancy to prescribe rituximab to antibody-negative patients, given the lower likelihood of clinical benefit [23–25]. Thus seronegativity for RF should allow access to another biologic class with a potentially higher likelihood of response. NICE provides no guidance regarding therapeutic options if the first-choice second-line biologic agent is ineffective or not tolerated. There is no reason to assume, however, that a further switch between classes would not yield benefit in the rituximab-contraindicated population.
Intolerance to rituximab
NICE allows switching to an alternative anti-TNF (TA 195) or abatacept (TA 195) or tocilizumab (TA 198) if rituximab is not tolerated, but gives no further guidance if this next option is not tolerated or ineffective. There is no evidence to suggest that a further between-class switch (fourth-line biologic) would be ineffective or not tolerated. The treatment alternatives at this stage are limited and patients in this situation should have an opportunity to receive a trial of all licensed biologics, particularly as our current ability to match a biologic with an individual cannot exclude the chance of response.
Rituximab inefficacy
Despite the absolute absence of evidence, NICE supports a switch to tocilizumab alone if rituximab is inefficacious (TA 198). This contrasts with the rituximab-intolerant population, where a second anti-TNF agent or abatacept are also allowed. This is inconsistent guidance, and the choice of either a switch back to a second TNF antagonist or abatacept or tocilizumab should be permitted for rituximab-IR patients.
At present, if a patient is MTX tolerant but fails to respond to each biologic in turn (with no contraindications or intolerance), the NICE algorithm (Fig. 1) sequences anti-TNF followed by rituximab and then tocilizumab, with no option to use abatacept, or to switch within anti-TNF class. This guidance, if adhered to, is contrary to the evidence base [26, 27], and will deny patients all options to achieve a state of low disease activity or remission.
For patients who develop intolerance to biologics, the NICE algorithm (Fig. 1) is also inconsistent. At the anti-TNF stage the patient cannot move to another class of biologic, despite both abatacept and tocilizumab being licensed for DMARD-IR patients with good evidence for efficacy [19–21]. At the rituximab stage a patient can progress to abatacept or tocilizumab if intolerant, yet may only be treated with tocilizumab if rituximab is ineffective. These are anomalies that are not supported by data and prevent the use of the right drug at the right time.
Individualizing biologic therapy
Treatment algorithms as well as cost-effectiveness will be transformed by the ability to match an individual patient to a biologic, based on the mode of action and likely tolerability. The risk of infection is particularly important, as all classes are associated with increased risk, with differences in half-life governing the speed of elimination. The rates of serious infections quoted in the SmPC for each biologic agent are shown in Table 2. The following highlights the benefits and drawbacks of abatacept, rituximab and tocilizumab as therapeutic options in relation to TNF antagonists.
Table 2.
Biologic agent comparisons, incidence of serious infections
| Biologic drug | Speed of onset | Half-life | Incidence of serious infections | Contraindicated in heart failure |
|---|---|---|---|---|
| Adalimumab | Fast | 14 (10–20) days | 4.3 per 100 patient-years vs 3.0 per 100 patient-years in placebo and active control-treated patients | Yesa |
| Certolizumab pegol | Fast | 14 days | 6.0 per 100 patient-years vs 2.0 per 100 patient-years placebo | Yesa |
| Etanercept | Fast | 4 days (70–132 hours) | 6.3% of RA patients treated for up to 48 months | Nob |
| Golimumab | Fast | 9–15 days | 5 per 100 patient-years vs 6 per 100 patient-years for control patients (1 year data) | Yesa |
| Infliximab | Fast | 8–9.5 days | Data not quoted in SmPC | Yesa |
| Rituximab | Slow | 20.8 (8.5-35.9) days | Approximately 4 per 100 patient-years | Yesc |
| Abatacept | Slow | 13 (8-25) days | 2.87 per 100 patient-years; 1.8% vs 1.0% of placebo-treated | No |
| Tocilizumab (8 mg/kg) | Fast | 13 days | 5.3 per 100 patient-years vs 3.9 per 100 patient-years in placebo + DMARD group | No |
Source: SmPC. aNYHA Grade III and IV; bnot contraindicated but SmPC advises caution; cNYHA Grade IV.
Abatacept
There is extensive RCT evidence for efficacy of abatacept in MTX-IR and TNF-IR RA patients, including those seropositive and seronegative for RF, and it is licensed in combination with MTX in both MTX-IR and TNF-IR patients [20, 21, 26, 28]. Infusion reactions are rare, and the onset of action is slower than TNF antagonists, but incremental benefit is reported beyond 1 year of treatment. As with all biologics, abatacept is contraindicated in severe and uncontrolled infection. The rate of serious infections quoted in the SmPC is modest (Table 2) and consistent with expectations based on RA cohorts treated with conventional DMARDs. This may relate to mode of action, as abatacept modulates T-cell co-stimulation without depleting or completely inhibiting T cells. Thus, for patients with an increased risk of sepsis, the benefit/risk profile of abatacept appears to be favourable, with the possible exception of those aged >65 years, where the incidence of serious infection is reported to be higher than those <65 years (SmPC). Reassuringly in RCTs, no increased autoantibody- cardiovascular- or malignancy-related adverse events over that expected in an RA population are reported, and abatacept is not contraindicated in patients with heart failure.
Rituximab
There is extensive RCT evidence for efficacy of rituximab in MTX-IR and TNF-IR RA patients [23, 29], although it is only licensed in TNF-IR patients in combination with MTX. Rituximab appears particularly suited to patients with B-cell-driven disease, including autoantibody positivity (RF, ACPA, ANA), hypergammaglobulinaemia, nodules and features of secondary SS. Rituximab is contraindicated in patients with NYHA Class IV heart failure or severe uncontrolled cardiac disease, and evidence suggests that it is less suited to seronegative patients [23–25]. The duration of each rituximab infusion and high frequency of infusion-related reactions, including a cytokine release syndrome accompanied by hypotension and bronchospasm in 10% of the patients, places particular responsibility on clinicians. The rate of serious infection quoted in the SmPC is similar to TNF antagonists (Table 2). Hypogammaglobulinaemia is an unknown concern with respect to the safety of rituximab in the long term or after switching to another biologic agent or traditional DMARD. Similarly, long-term B-cell depletion, in some patients lasting for years, is of unknown consequence for the patient and the safety of future therapies. The inability to predict or reverse B-cell depletion provides some hesitancy to commit a patient to rituximab, especially when the other biologic classes may be used with similar efficacy and greater flexibility in the face of toxicity, including shorter half-life.
Tocilizumab
There is extensive RCT evidence for the efficacy of tocilizumab in MTX-IR and TNF-IR RA patients, including those seropositive and seronegative for RF and as a monotherapeutic agent [17–19, 22]. Tocilizumab is licensed in both MTX-IR and TNF-IR patients, may be used without MTX, and the onset of action is similar to TNF antagonists. Tocilizumab appears particularly suited to patients with features of IL-6-driven disease, including high CRP, anaemia of chronic disease, systemic involvement and fatigue. The rate of serious infection quoted in the SmPC and recent meta-analysis is similar to TNF antagonists (Table 2) [30]. However, inhibition of CRP and neutropenia in some patients (3.4%) requires vigilance, as signs and symptoms of sepsis may be diminished. Gastrointestinal perforation in the presence of diverticular disease has been reported, and tocilizumab should be used with particular caution in these patients. Thus the benefit/risk profile of tocilizumab with respect to infection does not appear to be any more favourable than TNF antagonists. Reassuringly, in RCTs no increased autoantibody, cardiovascular- or malignancy-related adverse events have been reported, and tocilizumab is not contraindicated in patients with heart failure. Regular monitoring of lipids, hepatic enzymes, neutrophils and platelets is required, and this may influence the benefit/risk analysis in some patients.
In conclusion, the optimal sequencing of biologic agents is currently unknown. Until patient profiling with robust biomarkers becomes available, treatment decisions remain guided by the licensed indications and the extensive evidence base of efficacy in varied situations. This must be balanced with the likely risk of toxicity with each class of biologic. The current NICE TAs restrict this process, not least in preventing the use of biologics in patients with a DAS-28 above a minimal acceptable target of 3.2 but <5.1, and in constraining choices particularly for MTX-intolerant patients (anti-TNF agents only) or serial IR patients (no switching within anti-TNF class or use of abatacept). This review provides an analysis of the situations where alternatives arise and has suggested a legitimate course of action in each case. Our goal as rheumatologists remains one of achieving remission in all RA patients in order to maximize physical, psychological, and economic outcomes.

Disclosure statement: P.D.W.K. has received departmental support for service and research from Sanofi-Aventis, Schering Plough (now MSD) and Wyeth (now Pfizer) and has received advisory fees and unrestricted educational grants from Abbott, Bristol Myers Squibb, Napp, Roche/Chugai, Schering Plough/MSD and UCB. A.J.K.Ö. has received support from (including attendance at conferences), undertakes clinical trials and acts as a consultant to Roche, Chugai, Schering-Plough/MSD, Abbott, Wyeth/Pfizer, BMS, GSK, MerckSorono and UCB. J.D. is the Chairman of the British Society for Rheumatology working group on Standards, Audit and Guidelines and is also a Trustee of Arthritis Research UK. C.D. has previously acted as a consultant to Novartis. The Department of Rheumatology at the Royal Derby Hospital where C.D. works has received sponsorship from Wyeth (now Pfizer), Abbott, Roche and Schering Plough (now MSD), Napp and Astra Zeneca Pharmaceuticals for support of clinical meetings, and unrestricted grants and payments for services to support an ultrasound machine, an anti-TNF audit clerk, research nurses and research and audit projects.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.Kiely PDW, Brown AK, Edwards CJ, et al. Contemporary treatment principles for early rheumatoid arthritis: a consensus statement. Rheumatology. 2009;48:765–72. doi: 10.1093/rheumatology/kep073. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NICE TA 130. http://www.nice.org.uk/nicemedia/live/11867/37914/37914.pdf.
- 4.NICE TA 186. http://www.nice.org.uk/nicemedia/live/12808/47544/47544.pdf.
- 5.NICE TA 195. http://www.nice.org.uk/nicemedia/live/13108/50413/50413.pdf.
- 6.NICE TA 198. http://www.nice.org.uk/nicemedia/live/13100/50391/50391.pdf.
- 7.Irish guidelines. http://www.isr.ie/_fileupload/File/ISR%20Guidelines%20A4%20(3).pdf.
- 8.Fautrel B, Pham T, Mouterde G, et al. Recommendations of the French Society for Rheumatology regarding TNFalpha antagonist therapy in patients with rheumatoid arthritis. Joint Bone Spine. 2007;74:627–37. doi: 10.1016/j.jbspin.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 10.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biologic disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deighton C, Hyrich K, Ding T, et al. BSR and BHPR rheumatoid arthritis guidelines on eligibility criteria for the first biological therapy. Rheumatology. 2010;49:1197–9. doi: 10.1093/rheumatology/keq006a. [DOI] [PubMed] [Google Scholar]
- 12.Kiely P, Walsh D, Williams R, Young A for the Early Rheumatoid Arthritis Network. Outcome in RA patients with continued conventional therapy for moderate disease activity. The Early Rheumatoid Arhtritis Network (ERAN) Rheumatology. 2011;50:926–31. doi: 10.1093/rheumatology/keq406. [DOI] [PubMed] [Google Scholar]
- 13.Klareskog L, van der Heidje D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compatred with each treatment alone in patients with rheumatoid arthritis: double blind randomised controlled trial. Lancet. 2004;363:675–81. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 14.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early aggressive rheumatoid arthritis who have not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 15.Hyrich KL, Watson KD, Lunt M, Symmons DPM on behalf of BSR Biologics Register. Changes in disease characteristics and response rates among patients in the United Kingdom starting anti-tumour necrosis factor therapy for rheumatoid arthritis between 2001 and 2008. Rheumatology. 2011;50:117–23. doi: 10.1093/rheumatology/keq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yazici Y, Shi N, John A. Utilization of biologic agents in rheumatoid arthritis in the United States: analysis of prescribing patterns in 16,752 newly diagnosed patients and patients new to biologic therapy. Bull NYU Hosp Joint Dis. 2008;66:77–85. [PubMed] [Google Scholar]
- 17.Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy (TOWARD) study. Arthritis Rheum. 2008;58:2968–80. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 19.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–97. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 20.Kremer JM, Genant HK, Moreland LW, et al. Effects of abatacept in patients with methotrexate-resistant active Rheumatoid arthritis. A randomized trial. Arthritis Rheum. 2008;58:953–63. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 21.Schiff M, Keiserman M, Codding C, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emery P, Keystone E, Tony H-P, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-TNF biologics: results from a 24-week multicentre randomised placebo controlled trial. Ann Rheum Dis. 2008;67:1516–23. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen SB, Emery P, Greenwald MW, et al. for the REFLEX Trial Group. Rituximab for rheumatoid arthritis refractory to anti–tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 24.Silverman GJ, Schwartzman S, Townsend M, et al. Identification of biomarkers for enhanced benefit to rituximab in rheumatoid arthritis: role of autoantibodies and inflammatory markers. Arthritis Rheum. 2009;60(Suppl. 10):1680. [Google Scholar]
- 25.Isaacs J, Olech E, Tak P, et al. Autoantibody-positive rheumatoid arthritis (RA) patients (PTS) have enhanced clinical response to rituximab when compared to seronegative patients. Ann Rheum Dis. 2009;68(Suppl. 3):442. [Google Scholar]
- 26.Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumour necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 27.Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicenter, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210–21. doi: 10.1016/S0140-6736(09)60506-7. [DOI] [PubMed] [Google Scholar]
- 28.Buch MH, Saleem B, Das S, et al. Abatacept therapy is associated with minimal attrition rate irrespective of rheumatoid factor status in patients with highly resistant rheumatoid arthritis. Ann Rheum Dis. 2010;69(Suppl. 3):378. [Google Scholar]
- 29.Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment. Results of a phase IIb randomised, double-blind, placebo-controlled, dose ranging trial. Arthritis Rheum. 2006;54:1390–400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 30.Campbell L, Chen C, Bhagat SS, et al. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology. 2011;50:552–62. doi: 10.1093/rheumatology/keq343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.