Abstract
Pessimism, a general tendency toward negative expectancies, is a risk factor for depression and also heart disease, stroke, and reduced cancer survival. There is evidence that individuals with higher lead exposure have poorer health. However, low socioeconomic status (SES) is linked with higher lead levels and greater pessimism, and it is unclear whether lead influences psychological functioning independently of other social factors. The authors considered interrelations among childhood and adult SES, lead levels, and psychological functioning in data collected on 412 Boston area men between 1991 and 2002 in a subgroup of the VA Normative Aging Study. Pessimism was measured by using the Life Orientation Test. Cumulative (tibia) lead was measured by x-ray fluorescence. Structural equation modeling was used to quantify the relations as mediated by childhood and adult SES, controlling for age, health behaviors, and health status. An interquartile range increase in lead quartile was associated with a 0.37 increase in pessimism score (P < 0.05). Low childhood and adult SES were related to higher tibia lead levels, and both were also independently associated with higher pessimism. Lead maintained an independent association with pessimism even after childhood and adult SES were considered. Results demonstrate an interrelated role of lead burden and SES over the life course in relation to psychological functioning in older age.
Keywords: depression, lead, metals, orientation, psychology, socioeconomic factors
Exposures to environmental toxicants, poor physical health, and psychosocial functioning often cluster in lower socioeconomic status (SES) groups (1–5). Numerous investigators have posited that SES-related risks leading to health disparities may aggregate and accumulate throughout life to influence well-being and disease risk (1, 6, 7). Specifically considering psychosocial functioning, it has been postulated that growing up in lower SES environments may lead to impaired development of stress regulatory systems and also negatively bias the processing of psychosocial information both early and later in life (1). This does not rule out an independent effect of adult SES. These experiences are then hypothesized to link social disadvantage with impaired psychosocial functioning across the life course (6). However, whether exposure to environmental toxicants, which may be more prevalent in socially disadvantaged environments, leads to poor psychosocial functioning independently of other effects of social disadvantage has not been fully determined (8, 9).
Bone lead is a marker of cumulative lead exposure, because most lead absorbed by individuals is stored in bone rather than eliminated (10). The risk of lead exposure varies with SES, so that older adults who grew up in low SES conditions are more likely to have lived in lead-contaminated environments, including dilapidated housing and near areas of high-lead gasoline (5, 11–13). In addition, children in low SES environments are more likely to attain less education as adults, have lower SES as adults, and live in residential areas and work in occupations with greater risk of lead exposure (2, 14). Researchers have found a link between lead and psychiatric symptoms in young adults and also in older men (15–17). For example, in the study population used for the present study, prior work found that tibia lead is associated with higher somatization and higher levels of general distress (15, 16). These studies controlled for adult SES by using educational attainment, which provides some reassurance that the relation between lead and poor psychological functioning is not an artifact. However, given the importance of early life environments in determining lead exposure and adult functioning (18), a stronger test of the relations would account for both childhood and adult SES.
Pessimism is a cognitive orientation (personality trait) characterized by generalized expectancy of negative outcomes (19, 20). It is associated with lower psychological well-being; for example, pessimism is a strong predictor of depressive symptomology even after accounting for other psychological factors (19). Pessimism has also been associated with a faster rate of cellular aging (21), as well as increased risk of chronic disease and premature mortality, the most compelling evidence of which has been related to cardiovascular disease (20, 22–26).
The current study expands upon earlier work by investigating the effect of cumulative lead on pessimism and the role of SES over the life course on the association between lead burden and pessimism. We hypothesized that cumulative lead would be related to increased pessimism, and that these effects would be maintained independently of childhood and adult SES. We further considered whether effects of childhood and adult SES on pessimism across the life course might be partly mediated by lead. As a secondary analysis, we investigated the relations among SES, tibia lead, and pessimism with a major mental disorder, depression, projected to globally be a leading cause of years of life with disability (27, 28). Given the known relation between pessimism and depression, we hypothesized that SES and lead would influence depression via pessimism.
MATERIALS AND METHODS
This study examined a subgroup of men in the VA Normative Aging Study, established in 1963 by the Veterans Administration (now the Department of Veterans Affairs). The cohort and subgroup of participants used in this research have been described elsewhere (29, 30). Briefly, the Normative Aging Study is a closed cohort of 2,280 male volunteers from the Greater Boston area. Men were screened at entry and enrolled if they had no chronic medical conditions. Since enrollment, participants have been reevaluated every 3–5 years by questionnaires and detailed on-site physical examinations.
Between 1991 and 1999, 797 participants had bone lead content measured by K-shell x-ray fluorescence (KXRF). Of these, 546 also completed a series of questionnaires including questions about optimism and pessimism between 1993 and 2002. The participants’ first KXRF and questionnaire measurements were used in these analyses. On average, lead measurements were taken 3 years prior to the pessimism measure. Of the 546 with assessment of pessimism, 414 also had information on SES and other control variables.
This study was approved by the human research committees of Brigham and Women’s Hospital and the Department of Veterans Affairs, and written informed consent was obtained from subjects at each visit.
Lead measurement
Bone lead was measured for 30 minutes at the mid-tibia shaft by using KXRF (ABIOMED, Inc., Danvers, Massachusetts). The tibia has been used for bone lead research because it consists primarily of cortical bone, and tibia lead has a long half-life (20–30 years) in the body. The technical specifications and validity of the instrument are described in detail elsewhere (31–33). Two tibia measures with estimated uncertainties greater than 10 μg/g (usually reflecting excessive patient movement during measurement) (29) were excluded (n = 412).
Psychological measurements
The Life Orientation Test was used to measure pessimism. This test is made up of 12 items: 4 assessing optimistic attitudes, 4 assessing pessimistic attitudes, and 4 filler questions. Respondents were asked to indicate their degree of agreement with each statement by use of a scale from 0 (strongly disagree) to 4 (strongly agree). In prior research, including work in the Normative Aging Study population, the Life Orientation Test exhibited significant stability over time with high test-retest correlation coefficients (34–37). Although the Life Orientation Test was designed to assess optimism as a bipolar dimension, it can also be separated into 2 subscales capturing optimism and pessimism as distinct constructs, with unique relations to health. Therefore, as recommended by many studies (19, 38–40), we considered optimism and pessimism as distinct constructs in the context of this research question. A mean of items assessing pessimistic attitudes was calculated to yield a pessimism score (higher scores indicate greater pessimism). A mean of items assessing optimism was calculated to yield an optimism score (higher scores indicate greater optimism). However, optimism was not associated with lead; thus, we did not pursue further analyses with optimism.
Depressive symptoms were evaluated by using the Brief Symptom Inventory, a 53-item validated questionnaire that assesses 9 primary symptom dimensions including depression (41). Respondents indicated their level of distress on a scale from 0 (not at all) to 4 (extremely). Depression was assessed as present when the depression score was 1 standard deviation above the mean for a normal population (i.e., >0.50) (16).
Socioeconomic measures
Four items, ascertained by questionnaire at the time of enrollment, were used to determine childhood SES: 1) paternal education (less than grammar school, grammar school, less than high school, high school, less than college, college, greater than college); 2) maternal education; 3) paternal occupation (unskilled, semiskilled, skilled/foreman, white collar, semiprofessional, professional/managerial); and 4) parental homeownership. Three items, ascertained by questionnaire at the early visits, were used to determine adult SES: 1) educational attainment (less than high school, high school, college, greater than college); 2) homeownership; and 3) occupation (white collar, blue collar). On the basis of a prior report in the Normative Aging Study, white collar (working in white collar occupations) and mixed collar (working in both blue and white collar occupations) were grouped together under white collar as these 2 groups appeared similar with respect to lead exposure (42).
Control variables
Control variables included age, health behaviors, and self-rated health, ascertained by questionnaire at the time pessimism was assessed. Health behaviors included pack-years of smoking; smoking status (never, former, current); physical activity (metabolic equivalent task (MET)-hours/week); and self-rated health (from 1 (poor) to 4 (excellent)). For physical activity, where concurrent values were missing, the measure closest to the pessimism assessment was used.
Statistical analysis
Descriptive statistics.
To address normality, pack-years of smoking and physical activity were log-transformed. To address extreme values of lead, we modeled continuous lead after removing extreme outliers using the extreme-studentized-deviation many-outlier method (43). Results are expressed per interquartile-range increase in lead (14 μg/g). We also modeled lead divided into quartiles as another way to address extreme outliers and to consider possible nonlinearities in relation to pessimism. We compared SES, age, pack-years, physical activity, and depression for those with and without lead or pessimism measure using chi-square and t tests. For these pessimism analyses, we dichotomized pessimism at the median into low (≤4) versus high (>4).
Structural equation modeling.
Structural equation modeling was used to estimate 1) the relation between lead and pessimism, accounting for the covariance between lead and adult SES; 2) direct and indirect relations between childhood and adult SES and lead in relation to pessimism; and 3) direct and indirect relations among childhood and adult SES, lead, pessimism, and depression.
On the basis of the relations identified in preliminary analyses, age and smoking were included in the equations predicting lead (44), physical activity was included in models predicting pessimism (45), and self-rated health was included in models predicting depression (46).
An index for childhood SES was created (47) as a linear combination of the variables paternal education, maternal education, paternal occupation, and parental homeownership, weighted by the factor score regression coefficients. Similarly, an index of adult SES was created based on educational attainment, homeownership, and occupation. Worth noting is that we also ran structural equation modeling SES as unmeasured latent variables and observed the same associations, but for simplicity we report only models using SES indices.
We used PROC CALIS in SAS, version 9.12, software (SAS Institute, Inc., Cary, North Carolina) to perform structural equation modeling analyses. These analyses used the maximum likelihood method of parameter estimation and were performed on the variance-covariance matrix (48, 49). As depression was a binary outcome, we also used maximum likelihood estimation for the sum of squares and cross-products matrix (50). Model fit was evaluated by using root mean square error of approximation (RMSEA), for which values closer to 0 indicate better fit, and the goodness-of-fit index and Bentler’s comparative fit index for which values closer to 1 indicate better fit (51, 52). Coefficients on the natural scale are reported; however, we also report the standardized coefficients (representing the number of standard deviation changes in the outcome per standard deviation change in exposure). All P values are 2 sided.
RESULTS
Those with and without a pessimism measure did not differ on childhood and adult SES, age, pack-years of smoking, physical activity, or depression. There were no differences between childhood or adult SES, pack-years of smoking, physical activity, or depression among subjects with or without lead measurement, but those with lead measurement tended to be younger than those without (results not shown).
The mean age of the sample was 68.3 years (standard deviation (SD), 6.37). Five lead values were excluded by the extreme-studentized-deviation method. The mean lead level was 20.6 (SD, 10.7). The mean pessimism level was 4.93 (SD, 2.69), and the mean depression score was 0.22 (SD, 0.39). Table 1 shows the characteristics of participants by higher versus lower pessimism. Compared with those having lower pessimism, those with higher pessimism tended to be older, to be less educated, to have more pack-years of smoking, to be less physically active, to have lower paternal and maternal education and paternal occupation, to have higher lead levels, and to be depressed.
Table 1.
Characteristics of Participants in a Subgroup of the VA Normative Aging Study, United States, between 1991 and 2002
| Characteristics | Low Pessimism (≤4)a (n = 211)b |
High Pessimism (>4) (n = 201)b |
P Valuec | ||
| % | Mean (SD) | % | Mean (SD) | ||
| Age, years | 67.6 (6.49) | 69.0 (6.16) | 0.004 | ||
| Education | <0.001 | ||||
| Less than high school | 2.37 | 5.97 | |||
| High school | 20.4 | 28.9 | |||
| More than high school | 77.3 | 66.2 | |||
| Homeownership | 0.89 | ||||
| Yes | 81.7 | 83.2 | |||
| No | 18.3 | 16.8 | |||
| Occupation | <0.001 | ||||
| Blue collar | 29.7 | 44.1 | |||
| White collar | 70.3 | 55.9 | |||
| Smoking status | 0.91 | ||||
| Current | 8.06 | 6.97 | |||
| Former | 60.7 | 61.7 | |||
| Never | 31.3 | 31.3 | |||
| Pack-years of smoking | 19.0 (22.2) | 24.0 (27.3) | 0.01 | ||
| Alcohol consumption | 0.22 | ||||
| <2 drinks/day | 82.5 | 75.1 | |||
| ≥2 drinks/day | 17.5 | 24.9 | |||
| Physical activity, MET-hours/week | 21.8 (23.9) | 15.5 (22.2) | <0.001 | ||
| Paternal education | 0.004 | ||||
| Less than high school | 57.8 | 70.9 | |||
| High school | 28.4 | 20.1 | |||
| More than high school | 13.7 | 8.99 | |||
| Maternal education | |||||
| Less than high school | 56.4 | 72.0 | <0.001 | ||
| High school | 34.3 | 23.8 | |||
| More than high school | 9.31 | 4.23 | |||
| Paternal occupation | 0.01 | ||||
| Unskilled/skilled labor | 70.1 | 81.5 | |||
| White collar/professional | 29.9 | 18.5 | |||
| Parental homeownership | 0.97 | ||||
| Yes | 43.5 | 36.6 | |||
| No | 56.5 | 63.4 | |||
| Tibia lead, μg/g | 19.6 (12.2) | 21.8 (10.9) | 0.04 | ||
| Depression (>0.50)d | <0.001 | ||||
| Yes | 12.2 | 27.4 | |||
| No | 87.8 | 72.6 | |||
Abbreviations: MET, metabolic equivalent task; SD, standard deviation; VA, Department of Veterans Affairs.
The cutpoint for high vs. low pessimism was defined by the median level.
The numbers varied for the covariates physical activity, homeownership, paternal education, maternal education, paternal occupation, and paternal homeownership, with the lowest available for low pessimism and homeownership (n = 202) and for high pessimism and maternal education (n = 189).
Derived by using chi-square and t tests.
Depression was assessed as present when the depression score was 1 SD above the mean for a normal population (i.e., >0.50). The numbers for depression were 205 for low pessimism and 197 for high pessimism.
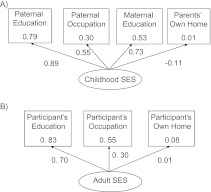
Childhood SES was strongly represented by paternal education (e.g., standardized coefficient as factor loading of 0.89; P < 0.05) (Figure 1A). Adult SES was primarily represented by educational attainment (Figure 1B). The greatest reliability of the measure of SES was for educational attainment, with a squared multiple correlation of 0.83; the lowest reliability measure was for parental homeownership, with a squared multiple correlation of 0.01.
Figure 1.
Relation of latent variables of childhood socioeconomic status (SES) (A) and adult SES (B) to manifest variables as squared multiple correlation values (inside box) and standardized coefficients (with arrows) in a subgroup of the VA Normative Aging Study, United States, between 1991 and 2002. VA, Department of Veterans Affairs.
For the model of relations among lead, adult SES, and pessimism, the RMSEA was 0.06, the goodness-of-fit index was 0.99, and the comparative fit index was 0.99, indicating acceptable model fit. For the model also accounting for childhood SES, the RMSEA was 0.02, the goodness-of-fit index was 0.99, and the comparative fit index was 1.00, indicating acceptable model fit. With depression added, the RMSEA was 0.02, the goodness-of-fit index, 0.99; and the comparative fit index, 0.99, indicating acceptable model fit.
Lead was positively related to pessimism (standardized coefficient = 0.10; P < 0.05). An interquartile-range increase in lead was related to a 0.37 increase in pessimism score (natural coefficient) (Table 2). This analysis also took into account the correlation (natural covariance of −2.88; standardized covariance of −0.26; P < 0.05) between lead and adult SES. In addition, higher adult SES was associated with lower pessimism; adult SES had a larger magnitude standardized coefficient of −0.18 (P < 0.05) than did lead with a standardized coefficient of 0.10 (P < 0.05).
Table 2.
Differences in Pessimism Associated With Adult and Childhood SES and Tibia Lead Using Natural Scale (Unstandardized) Units (95% Confidence Interval) Controlling for Age and Health Behaviors (n = 407) in a Subgroup of the VA Normative Aging Study, United States, between 1991 and 2002
| Variable | Differences in Pessimism |
|||
| Model 1 (Tibia and Adult SES) |
Model 2 (Tibia, Adult, and Childhood SES) |
|||
| Effect Estimate | 95% CI | Effect Estimate | 95% CI | |
| Tibia lead (per IQR increasea) | 0.37* | 0.07, 0.66 | 0.30 | 0.00, 0.61 |
| Adult SES (per 1-unit increase) | −0.47* | −0.68, −0.25 | −0.41* | −0.63, −0.19 |
| Childhood SES (per 1-unit increase) | −0.42* | −0.80, −0.04 | ||
| R2 | 0.09 | 0.10 | ||
Abbreviations: CI, confidence interval; IQR, interquartile range; SES, socioeconomic status; VA, Department of Veterans Affairs.
* P < 0.05 (using structural equation modeling).
IQR increase: 14 μg/g.
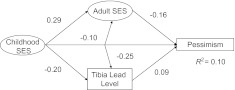
Figure 2 shows the results of modeling relations among childhood SES, adult SES, and cumulative lead on pessimism levels using standardized units. Taking into account both childhood and adult SES, the relation between lead levels and pessimism was attenuated (Table 2) but remained significant, particularly modeled as quartiles of lead (data not shown). The analysis also revealed that childhood SES was related to adult SES and lead levels and also independently to pessimism levels (Figure 2).
Figure 2.
Model of the interrelations of socioeconomic status (SES), tibia lead, and pessimism, controlling for age, pack-years of smoking, smoking status, and physical activity, with standardized coefficients derived by using structural equation modeling in a subgroup of the VA Normative Aging Study, United States, between 1991 and 2002. P < 0.05 for all relations (i.e., of childhood SES to adult SES, lead to pessimism, adult SES to pessimism, lead to pessimism, and lead to adult SES). VA, Department of Veterans Affairs.
In the relations among childhood SES, adult SES, and cumulative lead with pessimism levels and depression, there was no relation (i.e., independent of their effects on pessimism) between either SES over the life course or lead with depression. However, there was a strong relation between pessimism level and depression. A 1-unit increase in pessimism level was associated with an increased likelihood of being in the high depression group (odds ratio = 1.04, 95% confidence interval: 1.02, 1.05). For all models, covariates were significantly associated with the outcome (e.g., physical activity was significantly negatively associated with pessimism).
DISCUSSION
We considered the relation between cumulative lead and dispositional pessimism and also examined the role of SES over the life course in the lead-pessimism relation. Tibia lead predicted levels of pessimism even after accounting for childhood and adult SES. Moreover, older men experiencing low childhood SES and low adult SES had higher cumulative lead levels. In addition, childhood SES and adult SES were associated with pessimism independently of each other and of lead burden. More detailed modeling revealed that lead levels are partly explained by SES and explain some of the effect of SES on pessimism. Secondary analyses considered the association of depression with lead after accounting for childhood and adult SES but did not find direct effects. Only pessimism was directly (but very strongly) associated with depression. The strong relation between pessimism and depression, together with other findings from this study, suggests that lead is an important risk for poor psychosocial outcomes net of other risk factors with which it may cluster.
There is emerging research highlighting the importance of exposures across the life course on diseases later in life. Recent evidence indicates that low childhood SES is associated with poorer health in adulthood, over and above adult SES levels (1, 53–57). Chen et al. (58) found that, in children, low SES is associated with stress-related hormonal trajectories. These researchers hypothesized that SES may affect the way children interpret their social world. Low SES environments may be characterized by chronic stress coupled with fewer coping resources; individuals in these environments may adapt to their circumstances by being highly vigilant (59, 60). This coping strategy may, over time, solidify into a relatively stable attribute of expecting negative outcomes, that is, being pessimistic about the future (38, 60). It is interesting that there was a significant relation between childhood SES and pessimism even after accounting for adult SES and lead levels. This likely indicates other pathways by which childhood SES impacts adult cognitive orientation. It may also indicate that the developmental origins of pessimistic orientation are evident in childhood and have lasting effects into adulthood.
One explanation for the relation between SES and various mental and physical health outcomes has been the increased risk of environmental toxicant exposure with low SES status. Gump et al. (5, 8) found that, in children, blood lead was a significant mediator of the association between SES and vascular and hormonal responses to acute stress. There is also accumulating evidence in both human and animal studies that children in low SES environments are more vulnerable to the effects of a given level of lead (5, 61, 62), which may be influenced by circumstances related to SES such as increased stress and poorer nutrition and health care (9). For example, deficiencies in calcium or iron frequently observed in children in low SES environments can increase lead absorption and its effect on the brain (61).
We found adult SES and cumulative lead to be related. Adults with low educational attainment are more likely to work in blue collar jobs that tend to have greater lead exposure and to live in lead-rich environments. In another Normative Aging Study, Elreedy et al. (63) found a relation between individual and community-level low educational attainment and higher tibia lead levels. Also in the Normative Aging Study, Elmarsafawy et al. (42) found that blue collar workers had on average 5.5 μg/g higher tibia lead levels. Conversely, lead in childhood may affect adult SES through its inverse relation with cognitive achievement. Worth noting is that the effect of adult SES on pessimism, although attenuated after factoring in childhood SES, was still a significant predictor of lead and pessimism. Similarly, Ek et al. (64) found that adult achievement, independent of early relations and parental SES, was an important predictor of optimism.
As far as we know, this is the first study to investigate the association between cumulative lead and pessimism. Our findings of a positive association are congruent with findings of a relation between lead burden and depressive symptoms, particularly seen in occupational studies, which can be influenced by a pessimistic orientation (15, 65–70). In this study, we found no direct relation with depression but an indirect relation through lead’s association with pessimism, congruent with other studies finding an association between cumulative low-level lead and depression.
The etiologic mechanisms underpinning lead and emotional processing have not been fully elucidated (15). It is not clear if psychological problems are caused directly by lead-induced brain damage or are secondary to conditions surrounding its effect on cognitive performance (61). Poor academic performance could lead to loss of self-esteem, poor social development, and general expectation of negative outcomes (61). However, there is some indication that lead may affect the brain systems that regulate social/emotional functioning (61, 71). Physiologically, lead is thought to alter the functioning of the hypothalamic-pituitary-adrenal axis either directly or indirectly, the latter mediated by inducing alterations in neurochemical function (3). Lead may interfere with the release of neurotransmitters by mimicking or inhibiting calcium-mediated processes (3, 72–74).
There are some limitations to this study. Other work has found that pessimism among children predicted more parent-reported social and academic deficits (75). Pessimism could thus have affected cognitive functioning and thereby adult social and economic position and life choices that could increase lead exposure, although including measures of child social environment mitigates some of this concern. The role of genetics (heritability) versus environment (learning) on mental health status has been a matter of debate. Pessimism is characterized as a trait (76). Similar to other traits, it has a heritability factor of 27%, suggesting that it is also partially learned and elaborated over the life course (77, 78). Other research has found a graded association between optimism/pessimism and SES (22, 79) and patterning in optimism by changes in SES between childhood and adulthood (60), further providing evidence that while pessimism may be relatively stable in the absence of efforts to alter it (likely in low-income environments), it can be changed where resources are available (80). So although pessimism is characterized as a trait, it is one that is partially learned and therefore is unlikely to be fully elaborated prior to lead exposure.
Because there was little variability in depression scores (over 50% of the participants had a zero score resulting in a zero-inflation problem), we used depression as a binary variable, a more limited measure than is perhaps ideal (16). Also, interpretation of the results may be less clear where lead is divided into quartiles and distances between quartiles tend to vary. Other limitations include the determination of childhood SES based on retrospective recollection. However, studies have shown that objective information such as parent’s education and occupation can be measured this way reliably (14). Although we based childhood SES on a number of different factors, we did not have a measure of family income; however, we did account for homeownership, an index of wealth. In addition, it has been found that parental education and occupation are more important than family finances on outcomes such as cognitive functioning (14). We also had limited assessment of adult SES, basing it on educational attainment, occupational category, and homeownership. Finally, this study may have limited generalizability, being a male cohort that is 97% white. Despite these limitations, this study makes an important contribution in addressing the relation among psychosocial resources, a long-term environmental exposure, and SES over the life course.
In summary, we found that higher cumulative lead was associated with higher pessimism, taking into account childhood and adult SES as well as age and health behaviors. Childhood and adult SES were negatively related to lead and associated with greater pessimism independently of each other and lead burden; adult SES was the larger contributor to pessimism. These findings suggest that lead burden and adult SES can affect pessimistic orientation and ultimately depression in later adulthood.
Acknowledgments
Author affiliations: Department of Environmental Health, Boston University School of Public Health, Boston, Massachusetts (Junenette L. Peters); Department of Environment Health, Harvard School of Public Health, Boston, Massachusetts (Junenette L. Peters, Robert O. Wright, Marc G. Weisskopf, Joel Schwartz); Department of Society, Human Development, and Health, Harvard School of Public Health, Boston, Massachusetts (Laura D. Kubzansky, Ai Ikeda, Daniel Kim); Veterans Affairs Boston Healthcare System and Boston University Schools of Medicine and Public Health, Boston, Massachusetts (Avron Spiro III, David Sparrow); the Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts (Robert O. Wright, Marc G. Weisskopf); RAND Corporation, Boston, Massachusetts (Daniel Kim); School of Health Sciences, Purdue University, West Lafayette, Indiana (Linda H. Nie); and Department of Environmental Health Sciences, University of Michigan School of Public Health, Ann Arbor, Michigan (Howard Hu).
This research was supported by the National Institutes of Health (grants ES05257-06A1, ES014663, ES15172, ES00002, P20-MD000501, R01-ES07821, P42-ES05947, R01-AG02237, R29-AG07465, R01-AG18436) and the National Center for Research Resources General Clinical Research Centers program (grant M01RR02635); by the US Environmental Protection Agency (R832416); by a US Department of Veterans Affairs (VA) Merit Review and VA Research Career Scientist award to D. K.; by a National Heart, Lung, and Blood Institute Pathway to Independence award to D. K.; and by a Harvard School of Public Health Robert Wood Johnson Foundation grant to J. L. P. The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiological Research and Information Center of the Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC). The KXRF instrument used was developed by ABIOMED, Inc. (Danvers, Massachusetts) with National Institutes of Health grant support (grant ES03918-02).
Conflict of interest: none declared.
Glossary
Abbreviations
- KXRF
K-shell x-ray fluorescence
- RMSEA
root mean square error of approximation
- SD
standard deviation
- SES
socioeconomic status
References
- 1.Gianaros PJ, Horenstein JA, Hariri AR, et al. Potential neural embedding of parental social standing. Soc Cogn Affect Neurosci. 2008;3(2):91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Soc Sci Med. 1997;44(6):809–819. doi: 10.1016/s0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- 3.Virgolini MB, Chen K, Weston DD, et al. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicol Sci. 2005;87(2):469–482. doi: 10.1093/toxsci/kfi269. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill MS, Jerrett M, Kawachi I, et al. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003;111(16):1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gump BB, Reihman J, Stewart P, et al. Blood lead (Pb) levels: a potential environmental mechanism explaining the relation between socioeconomic status and cardiovascular reactivity in children. Health Psychol. 2007;26(3):296–304. doi: 10.1037/0278-6133.26.3.296. [DOI] [PubMed] [Google Scholar]
- 6.Adler NE, Boyce T, Chesney MA, et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49(1):15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 7.Marmot M, Shipley M, Brunner E, et al. Relative contribution of early life and adult socioeconomic factors to adult morbidity in the Whitehall II study. J Epidemiol Community Health. 2001;55(5):301–307. doi: 10.1136/jech.55.5.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gump BB, Reihman J, Stewart P, et al. Blood lead (Pb) levels: further evidence for an environmental mechanism explaining the association between socioeconomic status and psychophysiological dysregulation in children. Health Psychol. 2009;28(5):614–620. doi: 10.1037/a0015611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clougherty JE, Kubzansky LD. A framework for examining social stress and susceptibility to air pollution in respiratory health. Environ Health Perspect. 2009;117(9):1351–1358. doi: 10.1289/ehp.0900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu H, Shih R, Rothenberg S, et al. The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues. Environ Health Perspect. 2007;115(3):455–462. doi: 10.1289/ehp.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silbergeld EK. Preventing lead poisoning in children. Annu Rev Public Health. 1997;18:187–210. doi: 10.1146/annurev.publhealth.18.1.187. [DOI] [PubMed] [Google Scholar]
- 12.Baghurst PA, Tong S, Sawyer MG, et al. Sociodemographic and behavioural determinants of blood lead concentrations in children aged 11–13 years. The Port Pirie Cohort Study. Med J Aust. 1999;170(2):63–67. [PubMed] [Google Scholar]
- 13.Schnaas L, Rothenberg SJ, Flores MF, et al. Blood lead secular trend in a cohort of children in Mexico City (1987–2002) Environ Health Perspect. 2004;112(10):1110–1115. doi: 10.1289/ehp.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. J Gerontol B Psychol Sci Soc Sci. 2005;60(suppl 2):S93–S101. doi: 10.1093/geronb/60.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajan P, Kelsey KT, Schwartz JD, et al. Lead burden and psychiatric symptoms and the modifying influence of the delta-aminolevulinic acid dehydratase (ALAD) polymorphism: the VA Normative Aging Study. Am J Epidemiol. 2007;166(12):1400–1408. doi: 10.1093/aje/kwm220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhodes D, Spiro A, III, Aro A, et al. Relationship of bone and blood lead levels to psychiatric symptoms: the Normative Aging Study. J Occup Environ Med. 2003;45(11):1144–1151. doi: 10.1097/01.jom.0000094995.23808.7b. [DOI] [PubMed] [Google Scholar]
- 17.Bouchard MF, Bellinger DC, Weuve J, et al. Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in US young adults. Arch Gen Psychiatry. 2009;66(12):1313–1319. doi: 10.1001/archgenpsychiatry.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilman SE, Kawachi I, Fitzmaurice GM, et al. Socioeconomic status in childhood and the lifetime risk of major depression. Int J Epidemiol. 2002;31(2):359–367. [PubMed] [Google Scholar]
- 19.Chang EC, Maydeu-Olivares A, D’Zurilla TJ. Optimism and pessimism as partially independent constructs: relationship to positive and negative affectivity and psychological well-being. Person Individ Diff. 1997;23(3):433–440. [Google Scholar]
- 20.Roy B, Diez-Roux AV, Seeman T, et al. Association of optimism and pessimism with inflammation and hemostasis in the Multi-Ethnic Study of Atherosclerosis (MESA) Psychosom Med. 2010;72(2):134–140. doi: 10.1097/PSY.0b013e3181cb981b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donovan A, Lin J, Dhabhar FS, et al. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009;23(4):446–449. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubzansky LD, Sparrow D, Vokonas P, et al. Is the glass half empty or half full? A prospective study of optimism and coronary heart disease in the Normative Aging Study. Psychosom Med. 2001;63(6):910–916. doi: 10.1097/00006842-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Grossardt BR, Bower JH, Geda YE, et al. Pessimistic, anxious, and depressive personality traits predict all-cause mortality: the Mayo Clinic cohort study of personality and aging. Psychosom Med. 2009;71(5):491–500. doi: 10.1097/PSY.0b013e31819e67db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Räikkönen K, Matthews KA. Do dispositional pessimism and optimism predict ambulatory blood pressure during school days and nights in adolescents? J Pers. 2008;76(3):605–630. doi: 10.1111/j.1467-6494.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Räikkönen K, Matthews KA, Flory JD, et al. Effects of optimism, pessimism, and trait anxiety on ambulatory blood pressure and mood during everyday life. J Pers Soc Psychol. 1999;76(1):104–113. doi: 10.1037//0022-3514.76.1.104. [DOI] [PubMed] [Google Scholar]
- 26.Nabi H, Koskenvuo M, Singh-Manoux A, et al. Low pessimism protects against stroke: the Health and Social Support (HeSSup) prospective cohort study. Stroke. 2010;41(1):187–190. doi: 10.1161/STROKEAHA.109.565440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez AD, Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002–2030. Ann Trop Med Parasitol. 2006;100(5-6):481–499. doi: 10.1179/136485906X97417. [DOI] [PubMed] [Google Scholar]
- 28.Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 29.Hu H, Aro A, Payton M, et al. The relationship of bone and blood lead to hypertension. The Normative Aging Study. JAMA. 1996;275(15):1171–1176. [PubMed] [Google Scholar]
- 30.Cheng Y, Schwartz J, Sparrow D, et al. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension: the Normative Aging Study. Am J Epidemiol. 2001;153(2):164–171. doi: 10.1093/aje/153.2.164. [DOI] [PubMed] [Google Scholar]
- 31.Hu H, Milder FL, Burger DE. X-ray fluorescence measurements of lead burden in subjects with low-level community lead exposure. Arch Environ Health. 1990;45(6):335–341. doi: 10.1080/00039896.1990.10118752. [DOI] [PubMed] [Google Scholar]
- 32.Burger DE, Milder FL, Morsillo PR, et al. Automated bone lead analysis by K-x-ray fluorescence for the clinical environment. Basic Life Sci. 1990;55:287–292. doi: 10.1007/978-1-4613-1473-8_39. [DOI] [PubMed] [Google Scholar]
- 33.Hu H, Watanabe H, Payton M, et al. The relationship between bone lead and hemoglobin. JAMA. 1994;272(19):1512–1517. [PubMed] [Google Scholar]
- 34.Scheier MF, Carver CS. Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4(3):219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- 35.Schulz R, Tompkins CA, Rau MT. A longitudinal study of the psychosocial impact of stroke on primary support persons. Psychol Aging. 1988;3(2):131–141. doi: 10.1037//0882-7974.3.2.131. [DOI] [PubMed] [Google Scholar]
- 36.Mroczek DK, Spiro A, III, Aldwin CM, et al. Construct validation of optimism and pessimism in older men: findings from the Normative Aging Study. Health Psychol. 1993;12(5):406–409. doi: 10.1037//0278-6133.12.5.406. [DOI] [PubMed] [Google Scholar]
- 37.Achat H, Kawachi I, Spiro A, III, et al. Optimism and depression as predictors of physical and mental health functioning: the Normative Aging Study. Ann Behav Med. 2000;22(2):127–130. doi: 10.1007/BF02895776. [DOI] [PubMed] [Google Scholar]
- 38.Robb KA, Simon AE, Wardle J. Socioeconomic disparities in optimism and pessimism. Int J Behav Med. 2009;16(4):331–338. doi: 10.1007/s12529-008-9018-0. [DOI] [PubMed] [Google Scholar]
- 39.Robinson-Whelen S, Kim C, MacCallum RC, et al. Distinguishing optimism from pessimism in older adults: is it more important to be optimistic or not to be pessimistic? J Pers Soc Psychol. 1997;73(6):1345–1353. doi: 10.1037//0022-3514.73.6.1345. [DOI] [PubMed] [Google Scholar]
- 40.Kubzansky LD, Kubzansky PE, Maselko J. Optimism and pessimism in the context of health: bipolar opposites or separate constructs? Pers Soc Psychol Bull. 2004;30(8):943–956. doi: 10.1177/0146167203262086. [DOI] [PubMed] [Google Scholar]
- 41.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 42.Elmarsafawy SF, Tsaih SW, Korrick S, et al. Occupational determinants of bone and blood lead levels in middle aged and elderly men from the general community: the Normative Aging Study. Am J Ind Med. 2002;42(1):38–49. doi: 10.1002/ajim.10078. [DOI] [PubMed] [Google Scholar]
- 43.Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25(2):165–172. [Google Scholar]
- 44.Hu H, Payton M, Korrick S, et al. Determinants of bone and blood lead levels among community-exposed middle-aged to elderly men. The Normative Aging Study. Am J Epidemiol. 1996;144(8):749–759. doi: 10.1093/oxfordjournals.aje.a008999. [DOI] [PubMed] [Google Scholar]
- 45.Taylor WC, Baranowski T, Klesges LM, et al. Psychometric properties of optimism and pessimism: results from the Girls’ Health Enrichment Multisite Studies. Prev Med. 2004;38(suppl):S69–S77. doi: 10.1016/j.ypmed.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan GA, Roberts RE, Camacho TC, et al. Psychosocial predictors of depression. Prospective evidence from the Human Population Laboratory studies. Am J Epidemiol. 1987;125(2):206–220. doi: 10.1093/oxfordjournals.aje.a114521. [DOI] [PubMed] [Google Scholar]
- 47.Diamantopoulos A, Winklhofer HM. Index construction with formative indicators: an alternative to scale development. J Marketing Res. 2001;38(2):269–277. [Google Scholar]
- 48.Loehlin JC. Latent Variable Models: An Introduction to Factor, Path, and Structural Analysis. Mahwah, NJ: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- 49.Chuang HY, Schwartz J, Gonzales-Cossio T, et al. Interrelations of lead levels in bone, venous blood, and umbilical cord blood with exogenous lead exposure through maternal plasma lead in peripartum women. Environ Health Perspect. 2001;109(5):527–532. doi: 10.1289/ehp.01109527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kupek E. Log-linear transformation of binary variables: a suitable input for SEM. Struct Equ Modeling. 2005;12(1):28–40. [Google Scholar]
- 51.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 52.Hu L, Bentler P. Structural equation modeling. Concepts, issues, and applications. In: Hoyle RH, editor. Evaluating Model Fit. London, United Kingdom: Sage; 1995. pp. 76–99. [Google Scholar]
- 53.Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Ann N Y Acad Sci. 2010;1186:174–189. doi: 10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- 54.Galobardes B, Lynch JW, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- 55.Galobardes B, Davey Smith G, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16(2):91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 56.Gianaros PJ, Horenstein JA, Cohen S, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci. 2007;2(3):161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melchior M, Moffitt TE, Milne BJ, et al. Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. Am J Epidemiol. 2007;166(8):966–974. doi: 10.1093/aje/kwm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol Sci. 2010;21(1):31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- 59.Chen E, Matthews KA. Cognitive appraisal biases: an approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Ann Behav Med. 2001;23(2):101–111. doi: 10.1207/S15324796ABM2302_4. [DOI] [PubMed] [Google Scholar]
- 60.Heinonen K, Räikkönen K, Matthews KA, et al. Socioeconomic status in childhood and adulthood: associations with dispositional optimism and pessimism over a 21-year follow-up. J Pers. 2006;74(4):1111–1126. doi: 10.1111/j.1467-6494.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 61.Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126(pt 1):5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- 62.Bellinger DC. Lead neurotoxicity and socioeconomic status: conceptual and analytical issues. Neurotoxicology. 2008;29(5):828–832. doi: 10.1016/j.neuro.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elreedy S, Krieger N, Ryan PB, et al. Relations between individual and neighborhood-based measures of socioeconomic position and bone lead concentrations among community-exposed men: the Normative Aging Study. Am J Epidemiol. 1999;150(2):129–141. doi: 10.1093/oxfordjournals.aje.a009972. [DOI] [PubMed] [Google Scholar]
- 64.Ek E, Remes J, Sovio U. Social and developmental predictors of optimism from infancy to early adulthood. Soc Indic Res. 2004;69(2):219–242. [Google Scholar]
- 65.Baker EL, Feldman RG, White RF, et al. The role of occupational lead exposure in the genesis of psychiatric and behavioral disturbances. Acta Psychiatr Scand. 1983;67(suppl 303):38–48. doi: 10.1111/j.1600-0447.1983.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 66.Lindgren KN, Masten VL, Tiburzi MJ, et al. The factor structure of the Profile of Mood States (POMS) and its relationship to occupational lead exposure. J Occup Environ Med. 1999;41(1):3–10. doi: 10.1097/00043764-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz BS, Stewart WF, Bolla KI, et al. Past adult lead exposure is associated with longitudinal decline in cognitive function. Neurology. 2000;55(8):1144–1150. doi: 10.1212/wnl.55.8.1144. [DOI] [PubMed] [Google Scholar]
- 68.Fiedler N, Weisel C, Lynch R, et al. Cognitive effects of chronic exposure to lead and solvents. Am J Ind Med. 2003;44(4):413–423. doi: 10.1002/ajim.10287. [DOI] [PubMed] [Google Scholar]
- 69.Maizlish NA, Parra G, Feo O. Neurobehavioural evaluation of Venezuelan workers exposed to inorganic lead. Occup Environ Med. 1995;52(6):408–414. doi: 10.1136/oem.52.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lilis R, Fischbein A, Eisinger J, et al. Prevalence of lead disease among secondary lead smelter workers and biological indicators of lead exposure. Environ Res. 1977;14(2):255–285. doi: 10.1016/0013-9351(77)90037-8. [DOI] [PubMed] [Google Scholar]
- 71.Mendelsohn AL, Dreyer BP, Fierman AH, et al. Low-level lead exposure and cognitive development in early childhood. J Dev Behav Pediatr. 1999;20(6):425–431. doi: 10.1097/00004703-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Cory-Slechta DA, Garcia-Osuna M, Greenamyre JT. Lead-induced changes in NMDA receptor complex binding: correlations with learning accuracy and with sensitivity to learning impairments caused by MK-801 and NMDA administration. Behav Brain Res. 1997;85(2):161–174. doi: 10.1016/s0166-4328(96)00174-x. [DOI] [PubMed] [Google Scholar]
- 73.Weisskopf MG, Proctor SP, Wright RO, et al. Cumulative lead exposure and cognitive performance among elderly men. Epidemiology. 2007;18(1):59–66. doi: 10.1097/01.ede.0000248237.35363.29. [DOI] [PubMed] [Google Scholar]
- 74.Wright RO, Tsaih SW, Schwartz J, et al. Lead exposure biomarkers and mini-mental status exam scores in older men. Epidemiology. 2003;14(6):713–718. doi: 10.1097/01.EDE.0000081988.85964.db. [DOI] [PubMed] [Google Scholar]
- 75.Ey S, Hadley W, Allen DN, et al. A new measure of children’s optimism and pessimism: the Youth Life Orientation Test. J Child Psychol Psychiatry. 2005;46(5):548–558. doi: 10.1111/j.1469-7610.2004.00372.x. [DOI] [PubMed] [Google Scholar]
- 76.Carver CS, Scheier MF. Optimism, pessimism, and self regulation. In: Chang EC, editor. Optimism and Pessimism: Implications for Theory, Research, and Practice. Washington, DC: American Psychological Association; 2001. pp. 31–52. [Google Scholar]
- 77.Plomin R, Scheier MF, Bergerman CS, et al. Optimism, pessimism and mental health: a twin/adoption analysis. Pers Indiv Dif. 1992;13(8):921–930. [Google Scholar]
- 78.Caprara GV, Steca P, Alessandri G, et al. Positive orientation: explorations on what is common to life satisfaction, self-esteem, and optimism. Epidemiol Psichiatr Soc. 2010;19(1):63–71. doi: 10.1017/s1121189x00001615. [DOI] [PubMed] [Google Scholar]
- 79.Kubzansky LD, Wright RJ, Cohen S, et al. Breathing easy: a prospective study of optimism and pulmonary function in the Normative Aging Study. Ann Behav Med. 2002;24(4):345–353. doi: 10.1207/S15324796ABM2404_11. [DOI] [PubMed] [Google Scholar]
- 80.Kagan J. Behavioral inhibition as a temperamental category. In: Davidson RJ, Scherer K, Goldsmith HH, editors. Hanbook of Affective Sciences. New York, NY: Oxford University Press; 2002. [Google Scholar]