Abstract
Patient vital status generally is passively obtained by cancer registries, and no previous population-based studies have used extensive active follow-up to compute a more accurate overall survival rate for pancreatic cancer. Therefore, the authors used multiple active and passive follow-up methods to determine vital status and date of death for 1,954 pancreatic cancer patients diagnosed from 1995 to 1999 in a large population-based study in the San Francisco Bay Area, California. Survival rates were estimated by using Kaplan-Meier methods. Hazard ratios and 95% confidence intervals were estimated by using multivariable Cox proportional-hazards models. Vital status was confirmed for >99% of 1,954 patients. The overall 5-year survival rate was 1.3% and was greater in patients who were younger and who had localized disease, well-differentiated tumors, and surgical resection. Shorter survival was associated with older age at diagnosis, male sex, distant/metastatic disease, and poorly differentiated tumors. Longer survival was observed for Asian/Pacific Islanders compared with non-Hispanic whites and for any active treatment regardless of tumor stage. With an almost complete follow-up, the authors observed a low overall 5-year survival rate. Although the results provide further evidence of poor survival among patients with pancreatic cancer, the data also suggest that within-stage-of-disease patients survived somewhat longer with therapy.
Keywords: cohort studies, pancreatic neoplasms, survival
Pancreatic cancer is the fourth leading cause of cancer death in US men and women, with a similar number of deaths and new cases diagnosed each year (1). The typically advanced stage at diagnosis and lack of effective treatment modalities have contributed to the poor prognosis of this disease. Surveillance, Epidemiology, and End Results (SEER) data show an overall 5-year relative survival rate of 6% and a median survival of 3–6 months (2). Studies that have investigated pancreatic cancer outcomes have tended to be small clinical investigations among patients who have received surgery or other treatment modalities, with substantial exclusion criteria limiting the evaluated population (3–9). Larger population-based studies have tended to focus on survival in patient subgroups, for example, race and socioeconomic status, or on trends in survival in SEER regions (10–15). Although relevant to these select patient populations, results from these studies, including estimates of survival, are unlikely to be generalizable to the entire population of pancreatic cancer patients. Population-based studies that include all cases arising in a well-defined population would provide more accurate estimates of survival for the general population of pancreatic cancer patients. However, few population-based studies have focused on estimating overall survival rates and survival associated with demographic and prognostic clinical factors.
We used a combination of both active and passive methods to determine vital status and date of death for all incident pancreatic cancer patients identified as part of our population-based, case-control study of pancreatic cancer in the San Francisco Bay Area of California. We computed survival rates and duration for the total patient population, with additional consideration of demographic and clinical data already collected from SEER abstracts and in-person interviews.
MATERIALS AND METHODS
Study population
There were 1,972 patients newly diagnosed with pancreatic cancer from January 1, 1995, to December 31, 1999, in 6 San Francisco Bay Area counties with a total population of nearly 5,900,000 based on data from the 2000 US Census. Details about study design and methodology have been published for the large, population-based, case-control pancreatic cancer parent study that was the source for patients included in these survival analyses (16, 17). Briefly, patients were identified by using rapid case ascertainment and SEER registry data from the Northern California Cancer Center (now the Cancer Prevention Institute of California), were 21–85 years of age at diagnosis, and were residents of 1of 6 San Francisco Bay Area counties. Pancreatic cancer diagnoses were confirmed by participants’ physicians and by SEER abstracts. Eighteen patients in the study cohort were found not to have had pancreatic cancer per their clinicians, the patients themselves, or the patient’s pathology reports or medical records, reducing the total study population for this analysis to 1,954. This study was approved by the University of California San Francisco Committee on Human Research.
Data collection
Patient demographic and clinical information was obtained from SEER abstracts/registry data and from in-person interviews. Age at diagnosis, sex, race/ethnicity, disease stage, tumor site, tumor grade, and primary treatment were included among the data items that were collected for this patient population. For these analyses, age at diagnosis and race/ethnicity were grouped as <50, 50–59, 60–69, 70–79, and ≥80 years and as non-Hispanic white, Hispanic, black/African American, Asian/Pacific Islander, and other. Stage at diagnosis was determined by SEER Registry abstractors using pathologic or clinical data and classified as local (confined to the pancreas), regional (extension to surrounding organs/or regional lymph nodes), or distant disease (metastases). Primary treatment data obtained from SEER abstracts and in-person interviews were coded as surgical resection (Whipple or local resection), chemotherapy/radiation therapy, bypass/stent, other treatment, and no treatment/therapy unknown.
Vital status
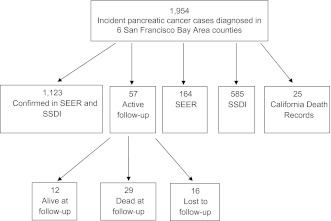
Patient vital status was determined by using multiple passive and active follow-up methods (Figure 1). The date of death was actively ascertained in the parent study as part of the patient recruitment process. Updated vital status and date of death also were obtained from SEER Registry and abstract data that largely use California vital statistics data and annual cross-linking to national vital statistics data. In addition, patient death and date of death were obtained from the Social Security Death Index and the California Death Records databases. When patient vital status was not available from the aforementioned sources, patients, their relatives, their treating physicians, and/or the treating hospitals were contacted to determine patient vital status. All patients were followed through December 31, 2008. “Survival time” was defined as the time from diagnosis to death or to date of last contact for patients who remained alive or who were lost to follow-up.
Figure 1.
Ascertainment and confirmation of vital status and date of death for 1,954 incident pancreatic cancer patients, 21–85 years of age at diagnosis in 1995–1999 in 6 San Francisco Bay Area counties of California. SEER, Surveillance, Epidemiology, and End Results; SSDI, Social Security Death Index.
Statistical analysis
Kaplan-Meier survival estimates were used to compare the survival rates of pancreatic cancer by age at diagnosis, sex, race/ethnicity, cancer stage, grade, tumor site, and primary treatment. The log-rank test was used to assess differences in Kaplan-Meier estimates. Cox proportional-hazards regression models were used to estimate the hazard ratios and 95% confidence intervals for the association between various demographic, tumor, and treatment factors and survival from pancreatic cancer, with adjustment for other covariates (18). Statistical tests were 2 sided and considered statistically significant for P < 0.05. Statistical analyses were conducted by using SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Follow-up
Of the 1,972 incident pancreatic cancer patients identified, 18 patients were excluded because of unconfirmed cancer diagnoses or diagnoses other than pancreatic adenocarcinoma, including pancreatitis and cancer in situ. By use of multiple methods, vital status and date of death were confirmed for >99% of these pancreatic cancer patients (Figure 1). The median follow-up for this patient population was 11.3 years (range: 9.0–15.9 years). Of the total 1,954 patients, there were 1,926 (98.6%) patients confirmed dead, 12 (0.6%) patients alive at last contact, and 16 (0.8%) lost to follow-up for whom vital status was unknown. For the 12 patients alive at last contact, pathology reports or medical records were reviewed to reconfirm their pancreatic cancer diagnosis. For the 16 patients who were lost to follow-up, 10 patients were foreign born, had metastatic disease at diagnosis, and were reported to have moved back to their home country upon receiving their pancreatic cancer diagnosis (based on information from the SEER abstract, their primary physician, or relatives).
Demographic and tumor/treatment characteristics
The distributions of patient demographic, tumor, and treatment characteristics are shown in Table 1. The median age at diagnosis was 70 years, with 21% of patients less than 60 years of age. Approximately 50% of patients were men, and 65% were non-Hispanic white. Nearly 75% had regional or distant disease at diagnosis, whereas only 7% had localized disease. Tumor grade (available for 40%) also indicated advanced disease at diagnosis, with approximately 19% of tumors moderately differentiated, 15% poorly differentiated, and only 6% characterized as well differentiated. Tumor location was available for 77% of patients, with the majority of tumors located in the head (53%), followed by tail (9%) or body (8%), of the pancreas. Most patients for whom the primary treatment was known did receive some form of treatment intervention, although only 11% of patients underwent surgical resection (Whipple or local resection), and 21% received radiation or chemotherapy.
Table 1.
Estimates of Median Survival in Months and 1-Year and 5-Year Survival by Demographic and Clinical Characteristics Among Population-based Pancreatic Cancer Patients, San Francisco Bay Area, California, 1995–1999
| Characteristics | No. | % | Survival, months |
1-Year Survival, % | 5-Year Survival, % | P Valuea | |
| Median | 95% CI | ||||||
| Total | 1,954 | 3.8 | 3.5, 4.1 | 15.1 | 1.3 | ||
| Age at diagnosis, years | |||||||
| <50 | 102 | 5 | 5.2 | 4.3, 7.0 | 23.0 | 6.6 | <0.0001 |
| 50–59 | 305 | 16 | 5.5 | 4.8, 6.0 | 20.7 | 2.0 | |
| 60–69 | 511 | 26 | 4.6 | 4.2, 5.4 | 17.6 | 1.4 | |
| 70–79 | 694 | 36 | 3.0 | 2.7, 3.6 | 14.5 | 0.9 | |
| ≥80 | 342 | 17 | 2.3 | 1.9, 2.7 | 5.3 | 0 | |
| Sex | |||||||
| Men | 960 | 49 | 3.9 | 3.5, 4.2 | 14.6 | 1.3 | 0.32 |
| Women | 994 | 51 | 3.8 | 3.4, 4.3 | 15.7 | 1.3 | |
| Race/ethnicity | |||||||
| Non-Hispanic white | 1,270 | 65 | 4.0 | 3.7, 4.4 | 15.3 | 1.6 | <0.0001 |
| Hispanic white | 113 | 6 | 3.3 | 2.4, 4.6 | 16.8 | 1.8 | |
| Black/African American | 198 | 10 | 3.8 | 3.0, 5.0 | 12.6 | 0.5 | |
| Asian/Pacific Islander/other | 233 | 12 | 4.8 | 4.2, 5.8 | 18.3 | 0.9 | |
| Unknown | 140 | 7 | 2.0 | 1.5, 2.6 | 10.3 | 0 | |
| Stage at diagnosis | |||||||
| Local | 130 | 7 | 7.7 | 6.3, 8.8 | 33.7 | 8.6 | <0.0001 |
| Regional | 524 | 27 | 7.4 | 6.7, 8.2 | 28.9 | 2.3 | |
| Distant | 916 | 47 | 2.7 | 2.4, 2.9 | 6.2 | 0.1 | |
| Unstaged/unknown | 384 | 20 | 3.0 | 2.6, 3.6 | 11.3 | 0.3 | |
| Tumor grade | |||||||
| Well differentiated | 111 | 6 | 9.0 | 6.3, 10.1 | 33.1 | 5.5 | <0.0001 |
| Moderately well differentiated | 53 | 3 | 7.6 | 5.4, 10.5 | 28.9 | 4.2 | |
| Moderately differentiated | 229 | 12 | 6.0 | 4.7, 7.1 | 28.0 | 3.6 | |
| Moderately/poorly differentiated | 73 | 4 | 5.2 | 4.6, 6.0 | 24.7 | 6.8 | |
| Poorly differentiated | 288 | 15 | 3.7 | 3.1, 4.2 | 12.0 | 0.4 | |
| Not graded/unknown | 1,200 | 60 | 3.0 | 2.8, 3.3 | 10.6 | 0.3 | |
| Tumor location | |||||||
| Head | 1,042 | 53 | 4.9 | 4.5, 5.4 | 19.7 | 2.2 | <0.0001 |
| Body | 164 | 8 | 3.9 | 3.0, 4.9 | 9.2 | 0 | |
| Tail | 169 | 9 | 3.0 | 2.3, 3.5 | 11.8 | 0 | |
| Head, body, tail | 134 | 7 | 3.9 | 2.8, 5.0 | 7.5 | 0 | |
| Not specified/unknown | 445 | 23 | 2.4 | 2.0, 2.7 | 10.2 | 0.7 | |
| Primary treatment | |||||||
| Surgical resection | 223 | 11 | 12.7 | 10.4, 15.0 | 50.8 | 10.8 | <0.0001 |
| Chemotherapy/radiation | 410 | 21 | 5.8 | 5.2, 6.3 | 16.6 | 0 | |
| Other treatmentb | 112 | 6 | 4.8 | 3.5, 5.7 | 18.2 | 0.9 | |
| Bypass/stent | 235 | 12 | 4.2 | 3.5, 5.1 | 12.3 | 0 | |
| No treatment | 147 | 8 | 0.5 | 0.4, 0.6 | 0 | 0 | |
| Treatment unknown | 827 | 42 | 2.6 | 2.3, 2.8 | 8.0 | 0.1 | |
Abbreviation: CI, confidence interval.
Log-rank test P value for H0: no difference in the probability of death among categories.
Primarily cholecystectomy, biliary drainage, ostomy, and other palliative procedures.
Survival
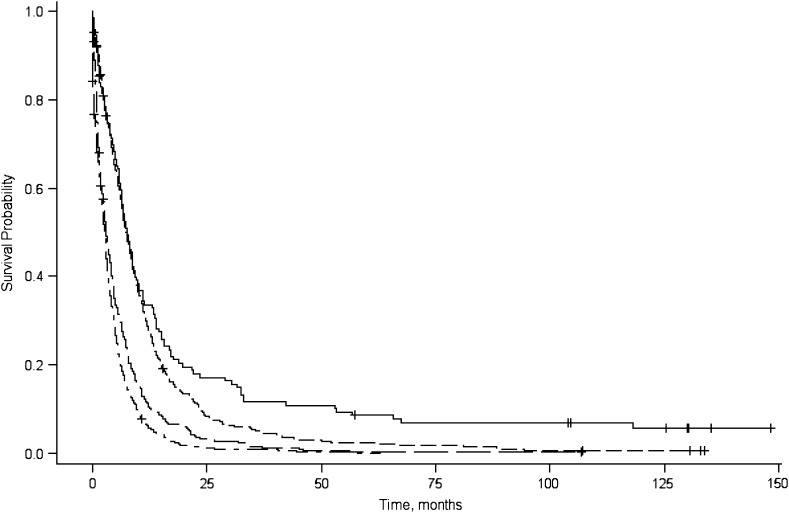
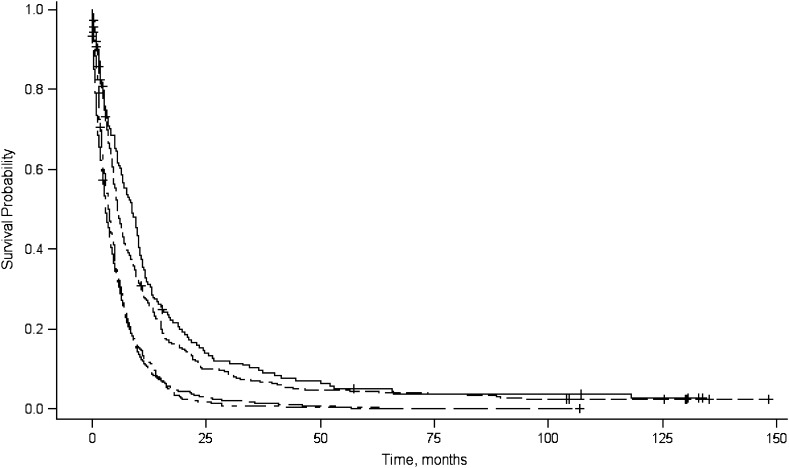
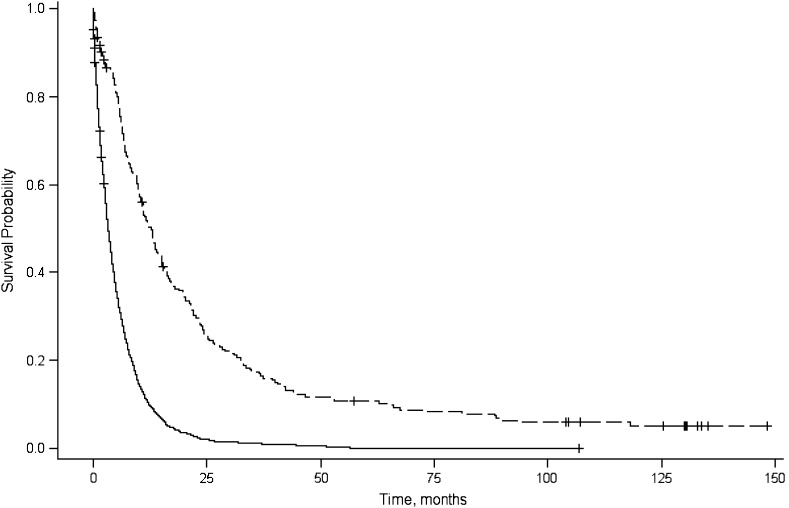
The median survival time and 1-year and 5-year relative survival rates are shown in Table 1. The median survival time for all patients was 3.8 months and shorter for patients diagnosed with distant disease (2.7 months), whereas for patients who had received surgical resection, the median survival was 12.7 months. Longer median survival times also were observed for patients with localized disease (7.7 months) and well-differentiated tumors (9.0 months). Plots of the product-limit estimates for survival are depicted by tumor stage (Figure 2), tumor grade (Figure 3), and surgical resection (Figure 4).
Figure 2.
Product-limit survival curves for survival probability (y-axis), by survival in months (x-axis), associated with tumor stage (local: solid line; regional: dashed line; distant: dash-dot-dash line; unknown: long dash-short dash line, San Francisco Bay Area, California, 1995–1999.
Figure 3.
Product-limit survival curves for survival probability (y-axis), by survival in months (x-axis), associated with tumor grade (well/moderate-well differentiation: solid line; moderate differentiation: dashed line; moderate-poor/poor differentiation: dash-dot-dash line; unknown differentiation: long dash-short dash line), San Francisco Bay Area, California, 1995–1999.
Figure 4.
Product-limit survival curves for survival probability (y-axis), by survival in months (x-axis), associated with surgical resection (no surgical: solid line; surgical: dashed line), San Francisco Bay Area, California, 1995–1999.
The overall 1-year and 5-year survival rates were 15.1% and 1.3%, respectively. The 5-year survival rate was higher in patients who were younger than 50 years of age (6.6%), or who had localized disease (8.6%) or well-differentiated tumor (5.5%), or who had undergone surgical resection (10.8%). A total of 24 patients (actual survival, 1.2%) survived 5 years or longer.
Long-term survivors, descriptive analysis
The median survival for patients who survived 5 years or longer (n = 24) was 104.1 months (range: 63–148 months; data not shown). There were 12 patients in this subgroup who were alive at last follow-up contact (median survival = 130.1 months; range: 104–148) and 12 who had died (median survival = 77.4 months; range: 63–118). A descriptive analysis showed that this group of 24 long-term survivors tended to be white (79%) and women (54%) and to have had tumors located in the head of the pancreas (87%)—factors consistent with those of the total patient population. Characteristics of patients who survived 5 years or longer that differed from those of less long-lived patients were age at diagnosis (median age = 61.5), tumor differentiation (62% with moderate- to well-differentiated tumors), disease extent (42% localized, 50% regional), and treatment (92% had a Whipple resection). Within the group of long-term survivors, further exploration showed that the factor that best distinguished those alive from those dead at last follow-up contact was disease extent, with 58% of those alive having had localized disease at diagnosis.
Prognostic factors for survival
Prognostic factors related to survival among the pancreatic cancer patients are shown in Table 2. In adjusted multivariable proportional-hazard models, factors associated with poor survival included older age at diagnosis (age ≥80 years: hazard ratio (HR) = 2.0, 95% confidence interval (CI): 1.6, 2.6), male sex (HR = 1.1, 95% CI: 1.0, 1.2), distant disease at diagnosis (HR = 2.0, 95% CI: 1.6, 2.5), and poorly differentiated tumor (HR = 1.5, 95% CI: 1.2, 1.9). Compared with non-Hispanic white patients, Asian patients had improved survival (HR = 0.75, 95% CI: 0.65, 0.87). The association between tumor site (head, body, tail) and survival was no longer observed after adjustment for other prognostic factors. Improved survival also was observed for patients who received any initial treatment, including surgical resection, chemotherapy/radiation, or bypass/stent (HR range: 0.38–0.79) compared with patients who received no treatment or for whom treatment was unknown. The association between treatment and survival was not modified by disease stage in stratified analyses (data not shown).
Table 2.
Hazard Ratios and 95% Confidence Intervals for Death Associated With Demographic and Prognostic Factors Among Population-based Pancreatic Cancer Patients, San Francisco Bay Area, California, 1995–1999
| Characteristics | No. of Patients | HRa | 95% CI | HRb | 95% CI |
| Age at diagnosis, years | |||||
| <50 | 103 | 1.0 | 1.0 | ||
| 50–59 | 307 | 1.2 | 0.96, 1.5 | 1.2 | 0.96, 1.5 |
| 60–69 | 513 | 1.3 | 1.1, 1.6 | 1.2 | 0.96, 1.5 |
| 70–79 | 696 | 1.6 | 1.3, 2.0 | 1.5 | 1.2, 1.9 |
| ≥80 | 342 | 2.3 | 1.8, 2.9 | 2.0 | 1.6, 2.6 |
| Sex | |||||
| Women | 998 | 1.0 | 1.0 | ||
| Men | 963 | 1.0 | 0.96, 1.2 | 1.1 | 1.0, 1.2 |
| Race/ethnicity | |||||
| Non-Hispanic white | 1,273 | 1.0 | 1.0 | ||
| Hispanic white | 114 | 1.0 | 0.87, 1.3 | 1.3 | 1.0, 1.5 |
| Black/African American | 201 | 1.0 | 0.89, 1.2 | 1.0 | 0.85, 1.2 |
| Asian//Pacific Islander/other | 233 | 0.91 | 0.79, 1.0 | 0.75 | 0.65, 0.87 |
| Unknown | 140 | 1.6 | 1.3, 1.8 | 1.1 | 0.89, 1.3 |
| Stage at diagnosis | |||||
| Local | 131 | 1.0 | 1.0 | ||
| Regional | 525 | 1.3 | 1.1, 1.6 | 1.2 | 0.96, 1.5 |
| Distant | 917 | 2.8 | 2.3, 3.4 | 2.0 | 1.6, 2.5 |
| Unstaged/unknown | 388 | 2.2 | 1.8, 2.7 | 1.2 | 0.96, 1.5 |
| Tumor grade | |||||
| Well differentiated | 112 | 1.0 | 1.0 | ||
| Moderately well differentiated | 53 | 1.1 | 0.78, 1.5 | 1.2 | 0.84, 1.7 |
| Moderately differentiated | 229 | 1.2 | 0.98, 1.6 | 1.3 | 1.0, 1.7 |
| Moderately/poorly differentiated | 73 | 1.2 | 0.90, 1.6 | 1.2 | 0.92, 1.7 |
| Poorly differentiated | 288 | 2.0 | 1.6, 2.5 | 1.5 | 1.2, 1.9 |
| Not graded/unknown | 1,206 | 2.1 | 1.7, 2.6 | 1.5 | 1.2, 1.8 |
| Tumor site | |||||
| Head | 1,044 | 1.0 | 1.0 | ||
| Body | 164 | 1.4 | 1.2, 1.6 | 1.1 | 0.90, 1.3 |
| Tail | 171 | 1.4 | 1.2, 1.6 | 1.1 | 0.97, 1.4 |
| Head, body, tail | 134 | 1.4 | 1.2, 1.7 | 1.1 | 0.88, 1.3 |
| Not specified/unknown | 448 | 1.6 | 1.4, 1.8 | 1.3 | 1.1, 1.4 |
| Primary treatment | |||||
| No treatment/unknown | 982 | 1.0 | 1.0 | ||
| Surgical resection | 223 | 0.26 | 0.22, 0.31 | 0.38 | 0.32, 0.46 |
| Chemotherapy/radiation therapy | 406 | 0.55 | 0.49, 0.62 | 0.59 | 0.52, 0.67 |
| Other treatmentc | 114 | 0.54 | 0.44, 0.65 | 0.61 | 0.50, 0.75 |
| Bypass/stent | 236 | 0.66 | 0.57, 0.76 | 0.79 | 0.68, 0.91 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Univariate analyses.
Multivariable analyses including all the factors presented in this table.
Includes mainly cholecystectomy, biliary drainage, ostomy, and other palliative procedures.
DISCUSSION
In this large, population-based study of nearly 2,000 pancreatic cancer patients in the San Francisco Bay Area, vital status was confirmed in >99% of patients (using active and passive follow-up methods), allowing us to compute an overall 5-year survival rate of 1.3% and a median survival of 3.8 months. The 5-year survival rate and median survival time were greater in patients who were younger, were diagnosed with localized disease, had well-differentiated tumors, and had received surgical resection. Independent prognostic factors for poor survival were consistent with published data and included older age, male sex, distant or metastatic disease, and poorly differentiated tumor. In contrast, longer survival was observed among Asian/Pacific Islanders and patients who received any active treatment at the time of diagnosis.
We computed a lower overall 5-year survival rate (1.3%) compared with that reported in several population-based studies that used the National Cancer Database (19) and SEER data (10, 20). Our 5-year survival rate also was lower for some patient subgroups including those diagnosed with localized disease and those who had undergone surgical resection, compared with rates reported from studies using SEER data and hospital or clinic-based data (19–21). These differences may be partly explained by effects inherent to survival analysis methods where actuarial survival rates from the Kaplan-Meier method can differ from the actual survival rate when censoring is present. In the analysis of pancreatic cancer survival data, this would result in an increase in the 5-year survival rate with increasing number of censored patients (22). Survival estimates also may be biased (higher) if the censoring (i.e., loss to follow-up) was correlated with patient death (e.g., poor survival and a large number of censored patients). Therefore, a possible explanation for our lower survival rate is that, unlike other studies, we used both active and passive follow-up of patients in our study, and it was nearly complete with less than 1% lost to follow-up. This allowed us to compute a “true” survival rate (actuarial survival rate of 1.3% vs. actual rate of 1.2%). Survival rate discrepancies also may be attributed to study design differences, as some studies excluded patients with a zero survival time (i.e., diagnosed at death or hours prior to death, whereas these patients were included in this study) and patients with missing demographic data or tumor/treatment characteristics. If these patients had shorter than average survival, their exclusion may have resulted in slight overestimates of the overall survival.
Similar to previous studies (11, 13, 19, 21, 23, 24), this study found that younger age, localized disease, well-differentiated tumors, and surgical resection were related to better survival and longer median survival time. Consistent with other studies (12, 25, 26), our study also found that surgical or other treatment factors were significantly related to improved survival. Because treatment is related to stage at diagnosis, we further evaluated treatment by disease stage. Results showed that, regardless of stage at diagnosis, patients who had undergone surgical resection or had received radiation/chemotherapy or other treatments had better survival compared with those who received no active treatment or whose treatment was unknown. This suggests that active initial treatment may prolong survival, even for patients with advanced disease.
Although there were few long-term survivors and even fewer patients alive at last follow-up contact, our descriptive analyses agree with the results from the few other recent studies that have described the population of patients who have lived 5 years or longer (27, 28). Our results also showed that tumor extent and differentiation, as well as having had a tumor resection, are key survival-related factors of this patient subgroup. More detailed clinical data were not available that would have allowed us to further define these groups (i.e., lymph-node involvement). Our results and those of other studies found that patients who survived at least 5 years tended to be somewhat younger than the overall population of pancreatic cancer patients. As early onset cancers often have a hereditary component and can be more aggressive, it is unclear whether these younger long-term survivors have fewer comorbidities and/or have other underlying/correlated factors that affected their prognosis.
This large, population-based study included all pancreatic cancer patients diagnosed from 1995 to 1999 in 6 San Francisco Bay Area counties. Our use of active follow-up (contact of physicians’ offices, hospitals, patients’ relatives, and patients) in addition to passive follow-up including SEER vital statistics data allowed for a more complete vital status and survival assessment. Our use of active follow-up also allowed us to further evaluate long-term survivors to confirm their pancreatic cancer diagnosis, something that has been shown to be critical in small clinic-based studies (27). Although we excluded few patients (n = 18) after follow-up, our data suggest that, in a population with few long-term survivors, misclassification of pancreatic cancer among survivors combined with loss to follow-up can impact the overall survival rates. Further, because clinical data were obtained mainly from the cancer registry, many patients were missing clinical prognostic data, although our results were consistent with those of most previously published studies. These data likely reflect the low heterogeneity in pancreatic cancer stage at diagnosis, the few effective treatment options, and the lack of “curative” treatments—each a factor that influences cancer survival.
In summary, a lower overall survival rate was observed among patients diagnosed between 1995 and 1999 in our large, population-based study compared with estimates based on registry statistics alone. This may be attributed to a nearly complete determination of vital status and dates of death as a direct result of our use of extensive active follow-up of all pancreatic cancer patients diagnosed in 6 counties of the San Francisco Bay Area of California. Our results suggest that the poor pancreatic cancer survival rates have changed little over time. Consistent with other studies, this study found that localized disease and active treatment were the best predictors of longer survival. Early detection and improved treatment strategies are needed to improve prognosis for this deadly disease.
Acknowledgments
Author affiliations: Department of Epidemiology and Biostatistics, School of Medicine, University of California San Francisco, San Francisco, California (Zhihong Gong, Elizabeth A. Holly, Paige M. Bracci).
This work was supported in part by National Cancer Institute grants CA59706, CA108370, CA109767, CA89726, and CA121846 (E. A. H.) and by the Rombauer Pancreatic Cancer Research Fund. The collection of cancer incidence data for the University of California San Francisco Study was supported by the California Department of Public Health as part of the statewide cancer-reporting program; the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U55/CCR921930-02 awarded to the Public Health Institute.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- HR
hazard ratio
- SEER
Surveillance
- Epidemiology
and End Results
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 3.Tsiotos GG, Farnell MB, Sarr MG. Are the results of pancreatectomy for pancreatic cancer improving? World J Surg. 1999;23(9):913–919. doi: 10.1007/s002689900599. [DOI] [PubMed] [Google Scholar]
- 4.Cleary SP, Gryfe R, Guindi M, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198(5):722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Mambrini A, Bassi C, Pacetti P, et al. Prognostic factors in patients with advanced pancreatic adenocarcinoma treated with intra-arterial chemotherapy. Pancreas. 2008;36(1):56–60. doi: 10.1097/mpa.0b013e31812e9672. [DOI] [PubMed] [Google Scholar]
- 6.Tani M, Kawai M, Terasawa H, et al. Prognostic factors for long-term survival in patients with locally invasive pancreatic cancer. J Hepatobiliary Pancreat Surg. 2007;14(6):545–550. doi: 10.1007/s00534-007-1209-6. [DOI] [PubMed] [Google Scholar]
- 7.Ghaneh P, Smith R, Tudor-Smith C, et al. Neoadjuvant and adjuvant strategies for pancreatic cancer. Eur J Surg Oncol. 2008;34(3):297–305. doi: 10.1016/j.ejso.2007.07.204. [DOI] [PubMed] [Google Scholar]
- 8.Yeo CJ, Sohn TA, Cameron JL, et al. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227(6):821–831. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16(4):836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau MK, Davila JA, Shaib YH. Incidence and survival of pancreatic head and body and tail cancers: a population-based study in the United States. Pancreas. 2010;39(4):458–462. doi: 10.1097/MPA.0b013e3181bd6489. [DOI] [PubMed] [Google Scholar]
- 11.Cress RD, Yin D, Clarke L, et al. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States) Cancer Causes Control. 2006;17(4):403–409. doi: 10.1007/s10552-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 12.Eloubeidi MA, Desmond RA, Wilcox CM, et al. Prognostic factors for survival in pancreatic cancer: a population-based study. Am J Surg. 2006;192(3):322–329. doi: 10.1016/j.amjsurg.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Zell JA, Rhee JM, Ziogas A, et al. Race, socioeconomic status, treatment, and survival time among pancreatic cancer cases in California. Cancer Epidemiol Biomarkers Prev. 2007;16(3):546–552. doi: 10.1158/1055-9965.EPI-06-0893. [DOI] [PubMed] [Google Scholar]
- 14.Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol. 2007;14(4):1320–1326. doi: 10.1245/s10434-006-9249-8. [DOI] [PubMed] [Google Scholar]
- 15.Artinyan A, Soriano PA, Prendergast C, et al. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10(5):371–376. doi: 10.1080/13651820802291233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holly EA, Eberle CA, Bracci PM. Prior history of allergies and pancreatic cancer in the San Francisco Bay area. Am J Epidemiol. 2003;158(5):432–441. doi: 10.1093/aje/kwg174. [DOI] [PubMed] [Google Scholar]
- 17.Holly EA, Chaliha I, Bracci PM, et al. Signs and symptoms of pancreatic cancer: a population-based case-control study in the San Francisco Bay area. Clin Gastroenterol Hepatol. 2004;2(6):510–517. doi: 10.1016/s1542-3565(04)00171-5. [DOI] [PubMed] [Google Scholar]
- 18.Cox DR. Regression models with life tables (with discussion) J R Stat Soc (B) 1972;34(2):187–220. [Google Scholar]
- 19.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189(1):1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 20.Shaib YH, Davila JA, El-Serag HB. The epidemiology of pancreatic cancer in the United States: changes below the surface. Aliment Pharmacol Ther. 2006;24(1):87–94. doi: 10.1111/j.1365-2036.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- 21.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237(1):74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gudjonsson B. Survival statistics gone awry: pancreatic cancer, a case in point. J Clin Gastroenterol. 2002;35(2):180–184. doi: 10.1097/00004836-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225(5):621–633. doi: 10.1097/00000658-199705000-00018. discussion 633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaib Y, Davila J, Naumann C, et al. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. population-based study. Am J Gastroenterol. 2007;102(7):1377–1382. doi: 10.1111/j.1572-0241.2007.01202.x. [DOI] [PubMed] [Google Scholar]
- 25.Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol. 2003;21(18):3409–3414. doi: 10.1200/JCO.2003.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Jefford M, Thursfield V, Torn-Broers Y, et al. Use of chemotherapy and radiotherapy in patients with pancreatic cancer in Victoria (2002–2003): a retrospective cohort study. Med J Aust. 2010;192(6):323–327. doi: 10.5694/j.1326-5377.2010.tb03531.x. [DOI] [PubMed] [Google Scholar]
- 27.Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247(3):456–462. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 28.Westgaard A, Larønningen S, Mellem C, et al. Are survival predictions reliable? Hospital volume versus standardisation of histopathologic reporting for accuracy of survival estimates after pancreatoduodenectomy for adenocarcinoma. Eur J Cancer. 2009;45(16):2850–2859. doi: 10.1016/j.ejca.2009.03.019. [DOI] [PubMed] [Google Scholar]