Abstract
Low circulating concentrations of 25-hydroxyvitamin D (25(OH)D) are associated with adverse health outcomes in diverse populations. However, 25(OH)D concentrations vary seasonally with varying exposure to sunlight, so single measurements may poorly reflect long-term 25(OH)D exposure. The authors investigated cyclical trends in average serum 25(OH)D concentrations among 2,298 individuals enrolled in the Cardiovascular Health Study of community-based older adults (1992–1993). A sinusoidal model closely approximated observed 25(OH)D concentrations and fit the data significantly better than did a mean model (P < 0.0001). The mean annual 25(OH)D concentration was 25.1 ng/mL (95% confidence interval: 24.7, 25.5), and the mean peak-trough difference was 9.6 ng/mL (95% confidence interval: 8.5, 10.7). Male sex, higher latitude of study site, and greater physical activity levels were associated with larger peak-trough difference in 25(OH)D concentration (each P < 0.05). Serum concentrations of intact parathyroid hormone and bone-specific alkaline phosphatase also varied in a sinusoidal fashion (P < 0.0001), inversely to 25(OH)D. In conclusion, serum 25(OH)D varies in a sinusoidal manner, with large seasonal differences relative to mean concentration and laboratory evidence of biologic sequelae. Single 25(OH)D measurements might not capture overall vitamin D status, and the extent of misclassification could vary by demographic and behavioral factors. Accounting for collection time may reduce bias in research studies and improve decision-making in clinical care.
Keywords: alkaline phosphatase, parathyroid hormone, seasons, vitamin D
Measurement of circulating 25-hydroxyvitamin D (25(OH)D) concentration has garnered widespread interest in clinical medicine and research (1–3). A person’s concentration of 25(OH)D is widely accepted as a marker of total vitamin D intake from cutaneous synthesis and dietary consumption, its 2 biologic sources, because it rises proportionally to ultraviolet light exposure and dietary supplementation (1–3). Low 25(OH)D concentration has become increasingly common in modern society because of decreased outdoor activity, increased sunscreen use, increasing adiposity, and changes in foods consumed (4). Moreover, a low 25(OH)D concentration has been associated with increased risks of numerous chronic diseases, including cancer, cardiovascular disease, and autoimmune diseases, such as type 1 diabetes mellitus and multiple sclerosis (2, 3). These associations are supported by biologic evidence from animal-experimental studies, which demonstrate clear effects of vitamin D that relate to these disease processes.
Circulating 25(OH)D concentrations vary substantially within individuals and populations over the calendar year and are highest at the end of summer and lowest at the end of winter (5–8). This variation is likely due to seasonal differences in exposure to ultraviolet light, the main source of vitamin D for most people. However, current recommendations for using 25(OH)D concentrations to determine need for and dose of vitamin D supplements do not account for time of measurement (1–3, 9). As a result, clinical decisions regarding initiation and dosage of year-long vitamin D supplementation are likely to be heavily influenced by time of ascertainment, which is often arbitrary. In addition, for clinical research, current analytic methods to account for seasonal 25(OH)D variation may result in bias toward or away from the null (10).
Further understanding of seasonal variation in 25(OH)D concentration is needed to improve methods of ascertaining vitamin D status. Therefore, in the present study, we evaluated seasonal variation in 25(OH)D levels in a community-based population at risk of low 25(OH)D concentrations (older adults), focusing on the pattern and extent of variation, determinants of variation, and the extent to which use of a single 25(OH)D measurement may misclassify year-long 25(OH)D exposure.
MATERIALS AND METHODS
Study participants
The Cardiovascular Health Study (CHS), a prospective, community-based cohort study, was designed to examine risk factors for the development and progression of cardiovascular disease in people 65 years of age or older (11). Participants were recruited from 4 communities in the United States: Forsyth County, North Carolina (latitude north 36°6'); Sacramento County, California (latitude north 38°35'); Washington County, Maryland (latitude north 39°38'); and Pittsburgh, Pennsylvania (latitude north 40°27'). Subjects who were not institutionalized and who were expected to remain in the area for at least 3 years were selected from Medicare eligibility lists. Persons who were wheelchair-bound in the home or who were receiving hospice treatment, radiation therapy, or chemotherapy for cancer were excluded. The original CHS cohort of 5,201 participants was enrolled in 1989–1990, with an additional 687 predominantly black participants enrolled in 1992–1993. We measured serum 25(OH)D concentrations at the 1992–1993 study visit for CHS participants who had no clinical evidence of cardiovascular disease at that time and who had available frozen serum (n = 2,312) (12, 13). The present study was restricted to whites and blacks because of extremely low numbers of other races (n = 14); this resulted in 2,298 participants for this analysis.
Laboratory measurements
Fasting serum was collected from CHS participants at the 1992–1993 study visit and stored at −70°C. Time of collection was classified as calendar month. Total 25(OH)D (including 25-hydroxyvitamins D2 and D3) levels were measured from previously unthawed serum using high performance liquid chromatography-tandem mass spectrometry on a Quattro Micro mass spectrometer (Waters Corporation, Milford, Massachusetts) at the University of Washington Clinical Nutrition Research Unit (Seattle, Washington). The interassay coefficient of variation was less than 3.4%, and the working range was 1–200 ng/mL. Calibration of the assay was confirmed by using the National Institute of Standards and Technology control material SRM-972 (14). It has been shown that 25(OH)D is stable for long periods of time at −70°C (15). Intact serum parathyroid hormone (PTH) levels were measured by using the Unicel DxI 800 immunoassay system (Beckman Coulter, Inc., Brea, California) (second generation 2-site immunoassay; coefficient of variation, 6.7%; working range: 1–3,500 pg/mL). The Unicel DxI 800 immunoassay system (Beckman Coulter, Inc.) was also used to measure bone-specific alkaline phosphatase (BAP; ostease immunoassay). Serum calcium and phosphorus were measured using the UniCel DxC 880i Synchron Access clinical system (Beckman Coulter, Inc.). Calcium values were not adjusted for albumin levels.
Covariate data
Covariates were ascertained at the 1992–1993 CHS study visit. Age, sex, race (white or black), and overall health status were defined by self-report. Medication inventories were completed by CHS staff using participants’ prescription and nonprescription medication bottles (11). Total physical activity was quantified in kilocalories expended per week by using validated questionnaires that assessed a broad range of common activities (16). Having diabetes was defined as use of insulin or oral hypoglycemic agents and/or having a fasting blood glucose level of 126 mg/dL or higher (17). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Serum cystatin C was measured using a BNII nephelometer (Dade Behring, Deerfield, Illinois) that used a particle-enhanced immunonephelometric assay (N Latex Cystatin-C) and was used to estimate glomerular filtration rate (GFR) using the equation: GFR = 76.7 × (cystatin C)−1.19 (18). To further evaluate whether genetic differences might influence variation in 25(OH)D, we assessed genotypes for 4 single nucleotide polymorphisms that were previously associated with 25(OH)D concentration in a genome-wide association study (19). Each of these single nucleotide polymorphisms is located in or near a gene known to be involved in vitamin D metabolism: vitamin D-binding protein (rs2282679), CYP2R1 (25-alpha hydroxylase, rs10741657), 7-dehydrocholesterol reductase (rs12785878), and CYP24A1 (24-alpha hydroxylase, rs6013897). Genotypes were called using Illumina BeadStudio software (Illumina, Inc., San Diego, California) on the basis of single nucleotide polymorphisms measured using the Illumina 370CNV BeadChip system, with number of imputed (estimated) minor alleles categorized as less than 0.5, 0.5–1.5, or greater than 1.5.
Statistical methods
The distribution of mean 25(OH)D levels was plotted by calendar month. On the basis of observed data and published reports that 25(OH)D levels followed a sinusoidal pattern, we fit a cosinor model to the data (5, 6). In the cosinor model, the dependent variable (25(OH)D) is modeled as a sine wave characterized by phase shift (location of peak and trough levels on the time axis), height (vertical shift of the sine wave), and amplitude (maximum variation of the sine wave from its mean height) (20). This model can be used to derive annual mean 25(OH)D concentrations (mean height) and estimate the magnitude of seasonal variation (amplitude). Moreover, this model can be used to assess independent determinants of mean 25(OH)D and its seasonal variation.
In the cosinor model, the time variable t (month) is transformed as cos(t) and sin(t), which are then fit as predictors of 25(OH)D in a linear model. The resulting coefficients of the cos(t) and sin(t) predictors are then transformed to give the amplitude and phase shift of the sinusoidal curve. The present article reports the total peak-trough difference, which is equal to twice the amplitude. The annual mean is the intercept term of the model. In adjusted models, covariates are centered at their study means so that the reported annual mean 25(OH)D concentration is standardized to mean covariate values of the study population.
Adjustment for other covariate effects on the yearly mean is accomplished by adding terms to the regression model, as in standard regression. These adjustments correspond to shifting the sinusoidal curve up or down. Covariate effects on the phase shift and amplitude of the sinusoidal curve are modeled by including interactions between the cos(t) and sin(t) terms with the covariate.
Improvement in model fit between nested models was assessed by using likelihood ratio tests and adjusted R2 terms. Standard errors and confidence intervals for the amplitude parameter were calculated by applying the Delta method because amplitude is a function of several covariates in the regression model (21). A multivariate Wald test was used to test the null hypothesis that amplitudes do not differ by covariate level. Model-based standard errors for regression parameters were used, which assume that errors are homoscedastic and normally distributed. Model-checking revealed no noticeable departures from this assumption.
To assess potential misclassification of year-long 25(OH)D concentrations by a single 25(OH)D measurement, the coefficients from a cosinor model were used to predict annual mean 25(OH)D concentration for each participant. This model was adjusted for age, sex, race, study site, physical activity level, diabetes, estimated GFR, and interactions of sex, study site, and physical activity with time to best predict the annual mean 25(OH)D concentration while maintaining a parsimonious model. Concordance was then assessed by cross-classifying the deficient/sufficient 25(OH)D concentration (<20 ng/mL or ≥20 ng/mL) (1) as determined by each participant’s measured value versus his/her predicted mean annual value.
Parallel cosinor models were created for PTH, BAP, calcium, and phosphorus. The biomarkers PTH and BAP were log-transformed to yield residuals that were more normally distributed and because of the belief that these markers may vary seasonally on a multiplicative scale. Unlike 25(OH)D, calcium, and phosphorus, PTH and BAP tend to vary on a multiplicative scale rather than an additive one; log transformation transforms these variables to an additive scale for analysis. Values for these biomarkers are presented as geometric means, and the peak-trough difference thus represents the ratio of the peak to the trough of the geometric means.
Analyses were done using STATA, version 10.0 (StataCorp LP, College Station, Texas) and R, version 2.9.2 (R Foundation for Statistical Computing, Vienna, Austria) running on a Mac computer using OS X (Apple, Inc., Cupertino, California). No adjustment to confidence intervals or P values was made for multiple comparisons.
RESULTS
Study population
All participants were older than 65 years of age, and 976 were 75 years of age or older (43%, Table 1). A total of 333 participants were black (14.5%), and 1,603 were women (70%). Distributions by age, sex, and clinical site did not vary substantially by season of measurement, but relatively few black participants (n = 18, 3%) were assessed during summer months. The mean level of 25(OH)D was 25.3 ng/mL (standard deviation, 10.4), of which 23.2 ng/mL (standard deviation, 10.0) was 25-hydroxyvitamin D3. Levels of 25-hydroxyvitamin D2 were detectable for 522 participants (23%).
Table 1.
Annual Mean 25-Hydroxyvitamin D Concentration and Mean Peak-Trough Difference in 25-Hydroxyvitamin D Concentration Among 2,298 Participants in the Cardiovascular Health Study, 1992–1993a
| Characteristic | No. of Subjects | % | 25(OH)D Annual Meanb, ng/mL | 95% CI | 25(OH)D Mean Peak-Trough Difference, ng/mL | 95% CI |
| Age, years | ||||||
| <75 | 1,322 | 57.5 | 25.5 | 25.0, 26.0 | 8.4 | 6.9, 9.8 |
| ≥75 | 976 | 42.5 | 24.5 | 23.9, 25.1 | 6.7 | 5.1, 8.4 |
| P for difference | 0.013 | 0.133 | ||||
| Race | ||||||
| White | 1,965 | 85.5 | 26.1 | 25.7, 26.6 | 7.6 | 6.5, 8.7 |
| Black | 333 | 14.5 | 19.1 | 17.8, 20.5 | 6.6 | 2.5, 10.7 |
| P for difference | <0.0005 | 0.652 | ||||
| Study site | ||||||
| Forsyth County, North Carolina | 682 | 29.7 | 25.4 | 24.7, 26.1 | 5.5 | 3.4, 7.5 |
| Sacramento County, California | 552 | 24.0 | 26.4 | 25.6, 27.1 | 6.9 | 4.8, 9.0 |
| Washington County, Maryland | 560 | 24.4 | 24.2 | 23.4, 25.0 | 8.4 | 6.3, 10.6 |
| Pittsburgh, Pennsylvania | 504 | 21.9 | 24.4 | 23.5, 25.2 | 10.3 | 7.9, 12.6 |
| P for difference | <0.0005 | 0.014 | ||||
| Sex | ||||||
| Female | 1,603 | 69.8 | 24.0 | 23.6, 24.5 | 6.8 | 5.5, 8.1 |
| Male | 695 | 30.2 | 27.6 | 26.9, 28.3 | 9.4 | 7.4, 11.3 |
| P for difference | <0.0005 | 0.026 | ||||
| Body mass indexc | ||||||
| <25 | 884 | 38.5 | 26.7 | 26.0, 27.3 | 7.5 | 5.8, 9.2 |
| 25–30 | 948 | 41.3 | 25.0 | 24.4, 25.6 | 7.9 | 6.3, 9.6 |
| >30 | 466 | 20.3 | 22.5 | 21.6, 23.3 | 6.3 | 3.9, 8.7 |
| P for difference | <0.0005 | 0.531 | ||||
| Estimated glomerular filtration rate, mL/minute/1.73 m2 | ||||||
| >60 | 1,892 | 82.3 | 25.2 | 24.8, 25.7 | 7.6 | 6.4, 8.8 |
| ≤60 | 406 | 17.7 | 24.7 | 23.7, 25.6 | 8.3 | 5.6, 10.9 |
| P for difference | 0.299 | 0.639 | ||||
| Diabetes | ||||||
| No | 2,036 | 88.6 | 25.5 | 25.0, 25.9 | 7.3 | 6.1, 8.4 |
| Yes | 262 | 11.4 | 22.8 | 21.6, 23.9 | 8.8 | 5.7, 11.9 |
| P for difference | <0.0005 | 0.347 | ||||
| Physical activity, kcal/week | ||||||
| <510 | 767 | 33.5 | 23.4 | 22.7, 24.0 | 4.4 | 2.6, 6.3 |
| 510–1,530 | 761 | 33.2 | 24.8 | 24.1, 25.4 | 7.8 | 6.0, 9.6 |
| >1,530 | 761 | 33.2 | 27.0 | 26.3, 27.7 | 8.7 | 6.8, 10.6 |
| P for difference | <0.0005 | 0.004 | ||||
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D.
Models were adjusted for the main effect of age, race, sex, and study site.
Annual means were centered to reflect study population values for age, race, sex, and study site.
Weight (kg)/height (m)2.
25-Hydroxyvitamin D
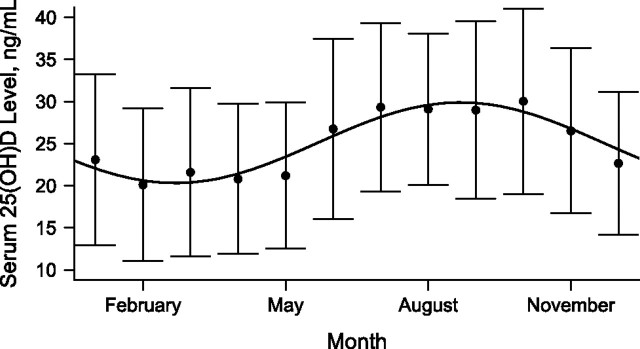
The monthly population mean 25(OH)D concentrations followed a sinusoidal pattern (Figure 1). The lowest 25(OH)D concentrations occurred in March and peak concentrations occurred in September. The cosinor model fit observed data significantly better than did a mean model, which included adjustments only for yearly mean 25(OH)D concentration (P < 0.0001, adjusted R2 = 0.111). Adding the main effects of age, sex, race, site, BMI, estimated GFR, diabetes mellitus, and physical activity level to the cosinor model (allowing vertical shift of the sine wave by covariate status) further improved the model fit (P < 0.0001, adjusted R2 = 0.225). Further addition of interactions of these covariates with time (allowing for differences in amplitude and phase shift of the sine wave by covariate status) improved the model fit to a smaller extent (P = 0.004, adjusted R2 = 0.231).
Figure 1.
Fitted sinusoidal model for observed serum 25-hydroxyvitamin D (25(OH)D) concentration superimposed on plot of observed monthly mean (standard deviation) values for 25-hydroxyvitamin D concentration among 2,298 participants in the Cardiovascular Health Study, 1992–1993. Bars, 1 standard deviation.
In the unadjusted cosinor model, the mean 25(OH)D concentration over the calendar year was 25.1 ng/mL (95% confidence interval: 24.7, 25.5), and the estimated peak-trough difference in the population 25(OH)D concentration was 9.6 ng/mL (95% confidence interval: 8.5, 10.7). In adjusted models, male sex and greater physical activity were each associated with higher annual mean 25(OH)D concentrations (upward shift of the sign wave) and greater peak-trough differences in 25(OH)D concentrations (greater sine wave amplitude) (Table 1 and Figure 2A). Being black and having a greater BMI, an older age, and diabetes were all associated with lower mean 25(OH)D concentrations but not with a difference in the amplitude of seasonal variation (Table 1 and Figure 2B). Mean 25(OH)D concentrations were highest in Sacramento County, California, and peak-trough differences showed a strong direct correlation with study site latitude (lowest in Forsyth County, North Carolina (latitude north 36°6′); highest in Pittsburgh, Pennsylvania (latitude north 40°27′)).
Figure 2.
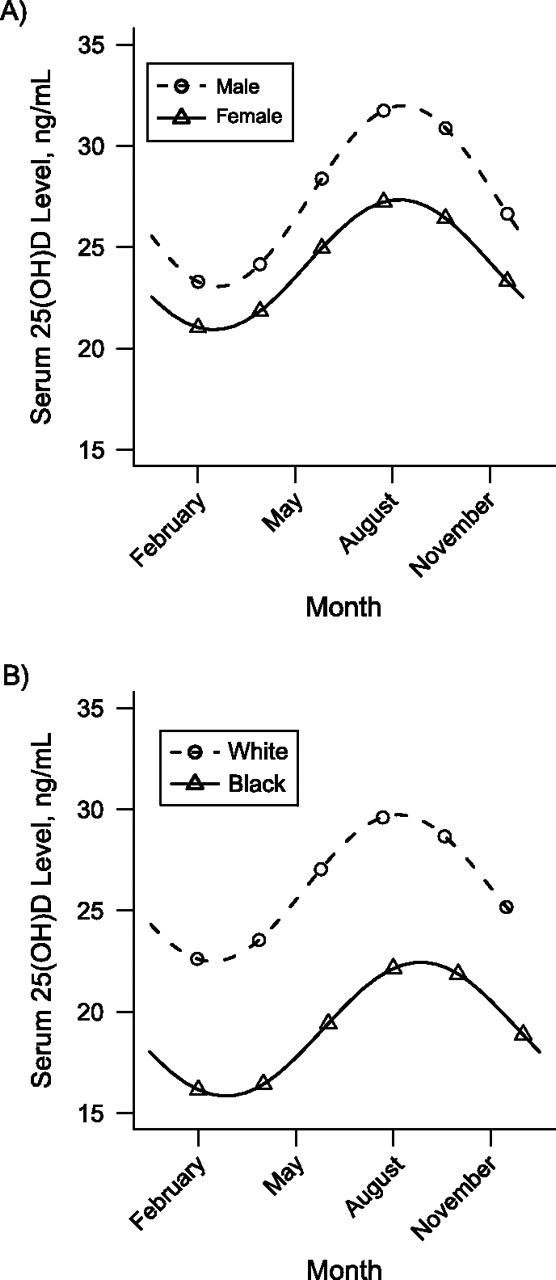
Modeled mean serum concentrations of 25-hydroxyvitamin D (25(OH)D) over the course of the calendar year by sex and race, adjusted for the main effects of age and clinical site, Cardiovascular Health Study, 1992–1993. A) Compared with women, men had higher mean 25(OH)D concentrations throughout the year and greater seasonal variation. B) Compared with black participants, white participants had higher mean 25(OH)D concentrations throughout the year but no difference in seasonal variation.
Other biomarkers
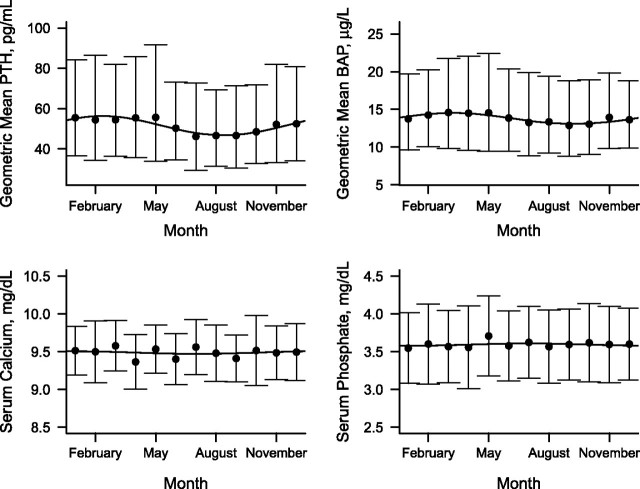
PTH and BAP concentrations also exhibited seasonal variation (Figure 3; each P value comparing the cosinor model with the mean model < 0.0001). Compared with 25(OH)D, the magnitude of variation was somewhat smaller, and peak and trough concentrations occurred in opposite seasons, with trough levels in February–March and peak levels in August–September.
Figure 3.
Fitted sinusoidal model for observed serum concentrations of parathyroid hormone (PTH), bone-specific alkaline phosphatase (BAP), calcium, and phosphate, each superimposed on plot of observed monthly mean (standard deviation) values, Cardiovascular Health Study, 1992–1993. PTH and BAP are presented on the geometric mean (log) scale. Bars, 1 standard deviation.
The peak geometric mean PTH concentration was estimated to be 1.20 times higher than its trough (95% confidence interval: 1.15, 1.26). Annual geometric mean PTH concentrations were higher for participants who were older, black, female, and more obese and who had lower GFRs (Appendix Table 1). The geometric mean PTH also varied by geographic location but not by physical activity level. Seasonal variation in PTH (peak-trough difference) differed only by sex (greater for men).
The peak geometric mean BAP concentration was estimated to be 1.11 times higher than its trough (95% confidence interval: 1.06, 1.16). Geometric mean BAP concentrations were higher for participants who were black, female, and more obese, and these concentrations also varied by geographic location (Appendix Table 1). Seasonal variation in BAP (peak-trough difference) was not statistically different by any of the covariates tested. Serum calcium and phosphorus concentrations did not differ by season (Figure 3; each P value comparing the cosinor model with the mean model > 0.25).
Genetic effects
Genetic variations in the 4 single nucleotide polymorphisms previously associated with the 25(OH)D concentration were associated with annual mean 25(OH)D concentrations but not with seasonal 25(OH)D variations (Appendix Table 2). Analyses of genetic data included only white participants to reduce the risk of population stratification.
Reclassification
Classification of the measured 25(OH)D concentration and the estimated annual mean 25(OH)D concentration differed for 9% of participants (Table 2). A total of 16% of the participants with a 25(OH)D level of 20 ng/mL or higher during summer months had an estimated annual mean 25(OH)D level less than 20 ng/mL, and 8% of participants with a 25(OH)D level less than 20 ng/mL during winter months had an estimated annual mean 25(OH)D of 20 ng/mL or higher.
Table 2.
Classification of 25-Hydroxyvitamin D Concentrations Comparing Single Measured Values With Estimated Annual Mean Values Among 2,298 Participants in the Cardiovascular Health Study, 1992–1993
| Measured 25(OH)D by Season | No. of Participants by Estimated Annual Mean 25(OH)D, ng/mL | Concordance, % | |
| <20 | ≥20 | ||
| All seasons, ng/mL | |||
| <20 | 684 | 60 | 92 |
| ≥20 | 136 | 1,409 | 91 |
| Summer, ng/mL | |||
| <20 | 106 | 0 | 100 |
| ≥20 | 88 | 469 | 84 |
| Winter, ng/mL | |||
| <20 | 240 | 41 | 92 |
| ≥20 | 0 | 296 | 100 |
Abbreviation: 25(OH)D, 25-hydroxyvitamin D.
DISCUSSION
In the present large, community-based study of older adults, the serum 25(OH)D concentration varied in a sinusoidal pattern throughout the calendar year, and the extent of this variation (peak-trough difference = 9.6 ng/mL) was large compared with the annual mean concentration (25.1 ng/mL). As a result, use of single 25(OH)D measurements without accounting for time of collection led to misclassification of estimated long-term vitamin D exposure, particularly when 25(OH)D was measured during summer or winter months. The main implication of these findings is that analytic approaches that accurately account for collection time, perhaps based on the sinusoidal model presented here, might more accurately inform clinical decisions regarding year-long vitamin D supplementation and reduce bias when the 25(OH)D concentration is used to assess health risk in clinical research studies.
In many prior epidemiology studies, investigators have observed seasonal variation in 25(OH)D concentrations (5–8). Sherman et al. (5) and Bolland et al. (6) suggested that this variation might be best captured using a sine function. The present work builds on those observations by demonstrating the robust nature of a cosinor model in a large, community-based population with racial diversity by using this model to demonstrate clinical characteristics that affect seasonal variation in 25(OH)D concentration and by applying this model to complementary circulating makers of mineral metabolism.
As in other studies, the mean levels of 25(OH)D varied by age, race, sex, geographic location, BMI, presence of diabetes mellitus, and physical activity level (4, 6, 7, 22). In addition, the extent of seasonal variation in 25(OH)D varied by age, sex, geographic location, and physical activity level but not by race or BMI. Thus, younger participants, men, and participants who reported more physical activity had higher mean 25(OH)D concentrations throughout the calendar year (upward shift of the 25(OH)D sine wave) and greater seasonal variation in 25(OH)D (larger peak-trough difference), and participants in more northern study sites had larger seasonal 25(OH)D variations. Across these clinical characteristics, differences in 25(OH)D were accentuated during summer compared with winter. This pattern can be fully explained by differences in ultraviolet light exposure.
In contrast, black participants (compared with white participants) and more obese participants had lower 25(OH)D concentrations throughout the calendar year (downward shift of the 25(OH)D sine wave) but no statistically significant differences in seasonal variation in 25(OH)D (roughly parallel 25(OH)D sine waves over the calendar year). Skin pigmentation directly affects the amount of vitamin D produced in skin upon a given dose of ultraviolet radiation (23). As a result, we expected seasonal 25(OH)D variation to be smaller among black participants because of a smaller increase in summer vitamin D synthesis. Lack of a racial difference in seasonal 25(OH)D variation is thus a pertinent negative finding that should be confirmed or disproved in future studies. In particular, the relatively few black participants in whom 25(OH)D was measured during summer might have limited our ability to detect a racial difference in seasonal variation in the present study. More obese persons were previously observed to have less seasonal variation in 25(OH)D concentration, perhaps because of adipose tissue buffering of circulating levels or decreased summer sunlight exposure (6, 22). In the present study, a BMI of 30 or higher was associated with slightly less 25(OH)D variation, but this difference was small and not statistically significant.
Classification of 25(OH)D status differed using single measured 25(OH)D concentrations compared with estimated annual mean 25(OH)D concentrations. This approach posits that mean long-term 25(OH)D exposure is most relevant for health (10). It is also possible that the yearly 25(OH)D concentration nadir affects health, and comparing single measured 25(OH)D concentrations and estimated annual nadir 25(OH)D concentrations results in even more differential classification of 25(OH)D status (6). Thus, whether mean or nadir 25(OH)D concentrations are important, seasonal variation meaningfully impacts classification. The Institute of Medicine recently called for additional study of non-bone vitamin D actions to advise public health policy regarding vitamin D supplementation and fortification (1). Accurate classification of 25(OH)D concentration is important for such studies.
Mean serum concentrations of PTH and BAP varied in a sinusoidal fashion reciprocal to 25(OH)D concentration. PTH secretion is suppressed by vitamin D, and the elevated PTH concentration is often viewed as a marker of vitamin D insufficiency (9). BAP is secreted from osteoblasts and is a biomarker of bone turnover. Insufficient vitamin D is a known risk factor for osteoporosis, which is characterized by high bone turnover. Therefore, reciprocal relations with PTH and BAP suggest that seasonal changes in 25(OH)D may have biologic effects.
The present study confirmed that PTH concentrations tend to be higher for people who are older and/or black and/or who have lower GFRs (5, 24, 25). Mean annual PTH concentrations were higher among women, and the seasonal PTH variation was greater in men, which could be explained by differences in mean 25(OH)D and 25(OH)D variation, respectively. BAP concentrations were higher for participants who were female, black, and/or obese. These associations may reflect increased bone turnover related to lower 25(OH)D levels.
Common genetic polymorphisms previously associated with 25(OH)D concentration in a large meta-analysis (19) were strongly associated with the annual mean 25(OH)D concentration but not with the amount of seasonal variation in 25(OH)D concentration. Based on biologic action, genetic variability in 7-dehydrocholesterol reductase, which is involved in cutaneous vitamin D synthesis, may have been expected to influence seasonal variation in 25(OH)D. Our results suggest that examined genetic polymorphisms affect vitamin D metabolism downstream from cutaneous vitamin D synthesis and, thus, the association with annual mean but not the amount of seasonal variation.
The main limitation of the present study is that variation in 25(OH)D and other biomarkers was assessed in cross-sectional analyses rather than by using multiple longitudinal measurements within individuals. Estimates of seasonal 25(OH)D variation relative to mean 25(OH)D concentrations from this study are still likely to be valid because mean seasonal 25(OH)D variation on the population level is likely to be equal or similar to the mean seasonal 25(OH)D variation of the individuals who comprise it. However, this study is unable to estimate the specific distribution of seasonal 25(OH)D variation for individuals. To validate any approach estimating year-long 25(OH)D exposure for an individual, investigators should first measure seasonal variation in 25(OH)D within individuals over time. It would also be useful to test whether randomly timed measurements of 25(OH)D concentration, season-specific 25(OH)D measurements, or a validated estimate of year-long 25(OH)D exposure most closely associates with adverse health outcomes. In addition, the present study included only older adults from 4 US communities, which limited external validity. Further limitations include no available information on dietary or supplemental sources of vitamin D and no direct measure of sun exposure; this analysis used physical activity as a maker for sun exposure, but not all physical activities occur outdoors, and use of sun protection varies widely.
The present study represents a first step toward more accurately classifying long-term vitamin D sufficiency. Ultimately, it may be useful to develop and validate equations that estimate year-long 25(OH)D exposure from single 25(OH)D measurements to better guide decisions for vitamin D supplementation in clinical practice and to reduce misclassification bias in clinical research. Further studies that seek to improve classification of vitamin D status can draw upon the cosinor model developed in this study and should take into account the clinical characteristics observed to be related to 25(OH)D concentration and its seasonal variation.
Acknowledgments
Author affiliations: Division of Biostatistics, College of Public Health, Ohio State University, Columbus, Ohio (Abigail B. Shoben); Kidney Research Institute, Division of Nephrology, Departments of Medicine and Epidemiology, University of Washington, Seattle, Washington (Bryan Kestenbaum, Ian H. de Boer); Department of Biostatistics, University of Washington, Seattle, Washington (Gregory Levin); Department of Laboratory Medicine, University of Washington, Seattle, Washington (Andrew N. Hoofnagle); and Cardiovascular Health Research Unit, Departments of Medicine and Epidemiology, University of Washington, Seattle, Washington (Bruce M. Psaty, David S. Siscovick).
The research reported in this article was supported by contract numbers N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133 and grant numbers U01 HL080295 and R01 HL087652 from the National Heart, Lung, and Blood Institute, with an additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support came from National Institutes of Health grants R01 HL084443, R01AG027002, R01HL096875, and 1KL2RR025015. DNA handling and genotyping were supported in part by National Center for Research Resources grant M01-RR00425 to the Cedars-Sinai General Clinical Research Center Genotyping Core and National Institute of Diabetes and Digestive and Kidney Diseases grant DK063491 to the Southern California Diabetes Endocrinology Research Center.
A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Conflict of interest: none declared.
Glossary
Abbreviations
- BAP
bone-specific alkaline phosphatase
- BMI
body mass index
- CHS
Cardiovascular Health Study
- GFR
glomerular filtration rate
- 25(OH)D
25-hydroxyvitamin D
- PTH
parathyroid hormone
Appendix Table 1.
Annual Geometric Mean and Peak:Trough Ratios in Parathyroid Hormone and Bone Alkaline Phosphatase Concentrations Among 2,298 Participants in the Cardiovascular Health Study, 1992–1993a
| Characteristic | Parathyroid Hormone |
Bone Alkaline Phosphatase |
||||||
| Annual Geometric Meanb, pg/mL | 95% CI | Peak:Trough Ratio | 95% CI | Annual Geometric Meanb, μg/L | 95% CI | Peak:Trough Ratio | 95% CI | |
| Age, years | ||||||||
| <75 | 49.6 | 48.5, 50.7 | 1.16 | 1.08, 1.24 | 13.6 | 13.3, 13.9 | 1.11 | 1.04, 1.18 |
| ≥75 | 53.7 | 52.3, 55.2 | 1.18 | 1.10, 1.27 | 14.0 | 13.7, 14.3 | 1.08 | 1.01, 1.15 |
| P for difference | <0.0005 | 0.668 | 0.065 | 0.528 | ||||
| Race | ||||||||
| White | 50.7 | 49.7, 51.7 | 1.17 | 1.11, 1.23 | 13.7 | 13.4, 13.9 | 1.09 | 1.04, 1.14 |
| Black | 54.9 | 51.6, 58.5 | 1.14 | 0.95, 1.36 | 14.7 | 13.9, 15.6 | 1.17 | 1.04, 1.32 |
| P for difference | 0.016 | 0.805 | 0.011 | 0.266 | ||||
| Study site | ||||||||
| Forsyth County, North Carolina | 51.1 | 49.5, 52.8 | 1.25 | 1.15, 1.37 | 14.0 | 13.6, 14.4 | 1.08 | 0.99, 1.17 |
| Sacramento County, California | 54.6 | 52.7, 56.6 | 1.15 | 1.05, 1.27 | 13.2 | 12.7, 13.6 | 1.10 | 1.01, 1.20 |
| Washington County, Maryland | 49.0 | 47.3, 50.8 | 1.14 | 1.03, 1.25 | 13.6 | 13.1, 14.0 | 1.06 | 0.97, 1.16 |
| Pittsburgh, Pennsylvania | 50.5 | 48.7, 52.4 | 1.11 | 1.00, 1.23 | 14.5 | 14.0, 15.0 | 1.12 | 1.02, 1.23 |
| P for difference | <0.0005 | 0.271 | <0.0005 | 0.829 | ||||
| Sex | ||||||||
| Female | 52.2 | 51.1, 53.2 | 1.10 | 1.04, 1.17 | 14.2 | 13.9, 14.4 | 1.10 | 1.04, 1.16 |
| Male | 49.6 | 48.1, 51.2 | 1.32 | 1.21, 1.44 | 12.9 | 12.5, 13.3 | 1.05 | 0.97, 1.14 |
| P for difference | 0.01 | 0.001 | <0.0005 | 0.399 | ||||
| Body mass indexc | ||||||||
| <25 | 48.3 | 46.9, 49.6 | 1.21 | 1.12, 1.31 | 13.6 | 13.2, 13.9 | 1.12 | 1.04, 1.20 |
| 25–30 | 50.9 | 49.6, 52.3 | 1.13 | 1.05, 1.21 | 13.7 | 13.4, 14.0 | 1.10 | 1.03, 1.18 |
| >30 | 58.6 | 56.4, 60.9 | 1.13 | 1.01, 1.26 | 14.4 | 13.9, 15.0 | 1.03 | 0.93, 1.13 |
| P for difference | <0.0005 | 0.337 | 0.013 | 0.375 | ||||
| Estimated glomerular filtration rate, mL/minute/1.73 m2 | ||||||||
| ≥60 | 49.8 | 48.9, 50.8 | 1.16 | 1.10, 1.23 | 13.7 | 13.5, 13.9 | 1.10 | 1.05, 1.16 |
| <60 | 59.1 | 56.6, 61.6 | 1.24 | 1.11, 1.38 | 14.1 | 13.6, 14.6 | 1.02 | 0.92, 1.12 |
| P for difference | <0.0005 | 0.309 | 0.203 | 0.143 | ||||
| Diabetes | ||||||||
| No | 51.5 | 50.5, 52.4 | 1.16 | 1.10, 1.22 | 13.7 | 13.5, 13.9 | 1.07 | 1.02, 1.12 |
| Yes | 49.9 | 47.4, 52.6 | 1.21 | 1.04, 1.39 | 14.1 | 13.4, 15.0 | 1.21 | 1.05, 1.41 |
| P for difference | 0.27 | 0.631 | 0.301 | 0.118 | ||||
| Physical activity, kcal/week | ||||||||
| <510 | 52.7 | 51.1, 54.4 | 1.14 | 1.05, 1.25 | 14.0 | 13.6, 14.4 | 1.08 | 1.00, 1.17 |
| 510–1,530 | 51.0 | 49.5, 52.5 | 1.11 | 1.02, 1.21 | 13.9 | 13.5, 14.2 | 1.03 | 0.95, 1.11 |
| >1,530 | 50.5 | 48.9, 52.2 | 1.24 | 1.13, 1.35 | 13.6 | 13.2, 14.0 | 1.15 | 1.07, 1.25 |
| P for difference | 0.144 | 0.189 | 0.364 | 0.099 | ||||
Abbreviation: CI, confidence interval.
Models were adjusted for the main effect of age, race, sex, and study site.
Annual means were centered to reflect study population values for age, race, sex, and study site.
Weight (kg)/height (m)2.
Appendix Table 2.
Annual Mean and Mean Peak-Trough Differences in 25-Hydroxyvitamin D Among 1,790 White Participants in the Cardiovascular Health Study, 1992–1993a
| No. of Minor Alleles per SNP | No. of Subjects | % | 25-Hydroxyvitamin D, ng/mL |
|||
| Annual Meanb | 95% CI | Mean Peak-Trough Difference | 95% CI | |||
| rs2282679 | ||||||
| <0.5 | 899 | 50.2 | 27.7 | 27.1, 28.3 | 6.7 | 5.1, 8.4 |
| 0.5–1.5 | 740 | 41.3 | 25.3 | 24.6, 25.9 | 9.1 | 7.2, 11.0 |
| >1.5 | 151 | 8.4 | 22.0 | 20.5, 23.6 | 8.3 | 3.9, 12.7 |
| P for difference | <0.0005 | 0.188 | ||||
| rs10741657 | ||||||
| <0.5 | 727 | 40.6 | 25.4 | 24.7, 26.1 | 6.9 | 5.0, 8.4 |
| 0.5–1.5 | 809 | 45.2 | 26.3 | 25.6, 25.9 | 8.3 | 6.4, 10.1 |
| >1.5 | 254 | 14.2 | 28.6 | 27.5, 23.6 | 8.7 | 5.5, 12.7 |
| P for difference | <0.0005 | 0.48 | ||||
| rs12785878 | ||||||
| <0.5 | 973 | 54.4 | 26.7 | 26.1, 27.4 | 7.7 | 6.0, 9.4 |
| 0.5–1.5 | 683 | 38.3 | 25.8 | 25.0, 26.5 | 7.4 | 5.4, 9.3 |
| >1.5 | 134 | 7.5 | 25.2 | 23.5, 26.8 | 8.5 | 4.1, 12.9 |
| P for difference | 0.054 | 0.895 | ||||
| rs6013897 | ||||||
| <0.5 | 1,103 | 61.6 | 26.6 | 26.0, 27.2 | 6.9 | 5.3, 8.4 |
| 0.5–1.5 | 601 | 33.6 | 25.9 | 25.1, 26.7 | 8.6 | 6.5, 10.7 |
| >1.5 | 86 | 4.8 | 23.7 | 21.6, 25.8 | 10.1 | 4.7, 15.5 |
| P for difference | 0.017 | 0.281 | ||||
Abbreviations: CI, confidence interval; SNP, single nucleotide polymorphism.
Models were adjusted for the main effect of age, sex, and study site.
Annual means were centered to reflect study population values for age, sex, and study site.
References
- 1.Ross AC, Taylor CL, Yaktine AL, et al., editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Rosen CJ. Clinical practice: vitamin D insufficiency. N Engl J Med. 2011;364(3):248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 4.Looker AC, Pfeiffer CM, Lacher DA, et al. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman SS, Hollis BW, Tobin JD. Vitamin D status and related parameters in a healthy population: the effects of age, sex, and season. J Clin Endocrinol Metab. 1990;71(2):405–413. doi: 10.1210/jcem-71-2-405. [DOI] [PubMed] [Google Scholar]
- 6.Bolland MJ, Grey AB, Ames RW, et al. The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am J Clin Nutr. 2007;86(4):959–964. doi: 10.1093/ajcn/86.4.959. [DOI] [PubMed] [Google Scholar]
- 7.de Boer IH, Ioannou GN, Kestenbaum B, et al. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2007;50(1):69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Jorde R, Sneve M, Hutchinson M, et al. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171(8):903–908. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Jacobs EJ, McCullough ML, et al. Comparing methods for accounting for seasonal variability in a biomarker when only a single sample is available: insights from simulations based on serum 25-hydroxyvitamin D. Am J Epidemiol. 2009;170(1):88–94. doi: 10.1093/aje/kwp086. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 12.Kestenbaum B, Katz R, de Boer IH, et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58(14):1433–1441. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson-Cohen C, Katz R, Hoofnagle AN, et al. Mineral metabolism markers and the long-term risk of hip fracture: the Cardiovascular Health Study. J Clin Endocrinol Metab. 2011;96(7):2186–2193. doi: 10.1210/jc.2010-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008;88(2):511S–512S. doi: 10.1093/ajcn/88.2.511S. [DOI] [PubMed] [Google Scholar]
- 15.Agborsangaya C, Toriola AT, Grankvist K, et al. The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutr Cancer. 2010;62(1):51–57. doi: 10.1080/01635580903191460. [DOI] [PubMed] [Google Scholar]
- 16.Robinson-Cohen C, Katz R, Mozaffarian D, et al. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med. 2009;169(22):2116–2123. doi: 10.1001/archinternmed.2009.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care. 2007;30(suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikulich SK, Zerbe GO, Jones RH, et al. Comparing linear and nonlinear mixed model approaches to cosinor analysis. Stat Med. 2003;22(20):3195–3211. doi: 10.1002/sim.1560. [DOI] [PubMed] [Google Scholar]
- 21.van Belle G, Fisher LD, Heagerty PJ, et al. Biostatistics: A Methodology for the Health Sciences. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2004. [Google Scholar]
- 22.Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 23.Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57(4):588–593. doi: 10.1016/j.jaad.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone level is associated with body mass index. The 5th Tromsø study. Eur J Endocrinol. 2004;151(2):167–172. doi: 10.1530/eje.0.1510167. [DOI] [PubMed] [Google Scholar]
- 25.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]