Abstract
This report addresses recent advances in our understanding of the role of the dorsolateral (DLS), dorsomedial (DMS), and ventral striatum in behavioral responses to alcohol, including alcohol craving in abstinent alcoholics, and alcohol consumption and withdrawal in rat, mouse and nonhuman primate models. Recent data are discussed showing that reduced neuronal activity as well as dysfunctional connectivity between the ventral striatum and the dorsolateral prefrontal cortex is associated with alcohol craving and impairment of new learning processes in abstinent alcoholics. Emerging results show that, within the DLS of mice and nonhuman primates withdrawn from alcohol after chronic exposure, glutamatergic transmission in striatal projection neurons (medium spiny neurons, MSNs) is increased, while GABAergic transmission is decreased. Glutamatergic transmission in DMS MSNs is also increased in ethanol withdrawn rats. Ex vivo or in vivo ethanol exposure and withdrawal causes a long-lasting increase in NR2B subunit-containing NMDA receptor activity in the DMS, contributing to ethanol-drinking. Analyses of neuronal activation associated with alcohol withdrawal and site-directed lesions implicate the rostroventral caudate putamen (rvCP), a ventrolateral segment of the DMS, in genetically determined differences in risk for alcohol withdrawal in mice. The influence of the identified risk factor on alcohol withdrawal may be involved in physical association of the multi-PDZ domain protein, MPDZ, with 5-HT2C receptors and/or NR2B within the rvCP. Taken together, these studies increase our understanding of the role of dopaminergic, glutamatergic and GABAergic signaling within different regions of the striatum in alcohol craving, consumption, dependence and withdrawal in humans and animal models.
Keywords: PET, fMRI, electrophysiology, self-administration, Fyn kinase, c-Fos
The striatum, a subcortical part of the forebrain, is the major input station of the basal ganglia. It receives glutamatergic inputs from virtually the whole of the cortical mantle and, to a lesser degree from the thalamus, as well as dopaminergic inputs from the ventral midbrain, and serotonergic inputs from the dorsal raphe nucleus (Harber, 1986). The striatal projection neurons, also called medium spiny neurons (MSNs), constitute more than 95% of striatal neurons (Graveland & DiFiglia, 1985). These neurons send GABAergic efferent projections to the globus pallidus and substantia nigra, and through these connections the striatum influences all other basal ganglia regions to ultimately regulate cortical and subcortical targets. As the entry site of information flow into the basal ganglia, the striatum regulates a variety of brain functions, including action learning and performance, cognition, and emotion. Alcohol (ethanol) alters the function of striatal circuits in multiple ways, which may contribute to acute intoxication, alcohol seeking, dependence and withdrawal. Thus, a number of groups have begun to investigate alcohol actions in the striatum to gain a better understanding of alcohol actions and alcohol use disorders, with the ultimate goal of developing improved therapeutic strategies.
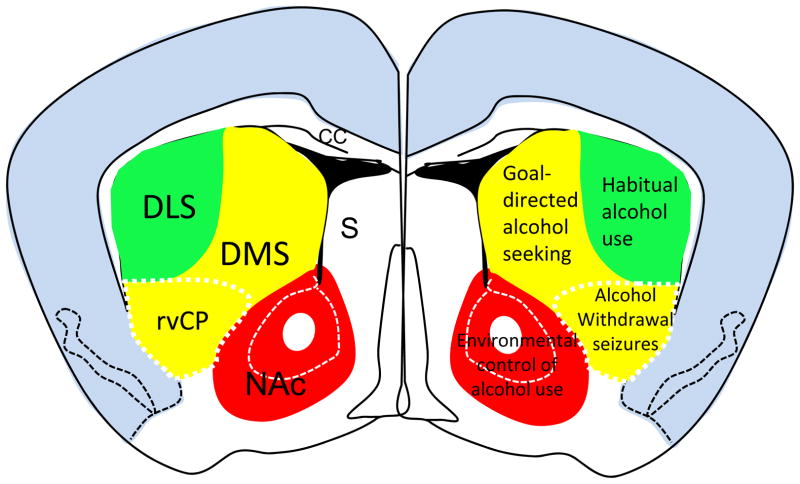
On the basis of connectivity and function, the striatum can be separated into at least three distinct parts: the sensorimotor dorsolateral striatum (DLS, or putamen in primates), the associative dorsomedial striatum (DMS, or the caudate nucleus in primates) and the limbic ventral striatum (also known as the nucleus accumbens, NAc). Growing evidence indicates regional-specificity of alcohol actions on the striatum. The DLS has key roles in habit formation, and may participate in the development of habitual alcohol use. The DMS participates in control of goal-directed actions (Balleine & O’Doherty, 2010), and thus may influence goal-directed alcohol seeking. The ventral striatum participates in cue- and environmental conditioning of actions, and appears to have important roles in environmental control of alcohol drinking and relapse (Figure 1). This review will discuss recent progress in elucidating the role of different regions and neuronal populations within the striatum in behavioral responses to alcohol including craving, self-administration and withdrawal. Our results arising from studies using human subjects and animal models (rats, mice and nonhuman primates) are compared and discussed. These data include imaging studies in abstinent alcoholics. Additionally, neurophysiological analyses using rat, mouse and nonhuman primate models assess the function of MSNs in the DLS and DMS following acute or chronic ethanol exposure as well as withdrawal to elucidate the differential roles of these two regions and the mechanisms involved. New insights are gained as to the neural circuit substrates involved in alcohol withdrawal using robust mouse models. Taken together, the work presented demonstrates the role of the striatum in response to alcohol and the importance of bridging research across rodent, nonhuman primate, and human studies.
Figure 1.
Schematic diagram of striatal subregions in relation to alcohol-associated behaviors. The locations of dorsolateral striatum (DLS), dorsomedial striatum (DMS), and ventral striatum (also known as nucleus accumbens of NAc) are illustrated in the left side of a mouse brain. Alcohol-associated behaviors controlled by the individual striatal subregions are illustrated in the right side. Other abbreviations: cc, corpus callosum; S, septum.
CHRONIC EXPOSURE TO ETHANOL ALTERS NEUROTRANSMISSION ONTO MEDIUM SPINY NEURONS OF THE MONKEY AND MOUSE STRIATUM
Addiction has been viewed as a maladaptive type of habit learning characterized by a transition from recreational to addictive use with a diminished control over drug seeking and reduced pleasure from the drug’s biological reward. It has been hypothesized that this transition represents a shift from prefrontal cortical to striatal control over drug use behavior and a progression from ventral to dorsal domains of the striatum (Everitt and Robins, 2005; Vollstädt-Klein et al., 2010). With overtraining of a task, there is a shift in behavioral control from goal-directed actions (caudate/DMS) to habits (putamen/DLS). This shift in striatal control of actions is thought to be accelerated by drugs of abuse such as alcohol.
On the molecular level, ethanol exerts direct and indirect effects on several voltage-gated ion channels and neurotransmitter receptor systems. The best-characterized ethanol-induced effects are on the glutamatergic and GABAergic systems due to chronic alcohol exposure altering the balance between inhibitory and excitatory neurotransmission.
Ethanol exposure has been shown to have opposite effects on glutamatergic and GABAergic neurotransmission as well as opposite effects within the same neurotransmitter system with acute versus chronic exposure. Acute ethanol exposure inhibits the glutamatergic system by decreasing NMDA receptor-activated currents and to a lesser extent AMPA and kainate receptors (reviewed by Crews et al., 1996; Kumari & Ticku, 2000; Woodward, 2000; Vengeliene et al., 2008). The GABAergic system, on the other hand, is enhanced by acute ethanol exposure by increasing GABA release and potentiating GABAA receptor-mediated currents (reviewed by Crews et al., 1996; Vengeliene et al., 2008; Dopico & Lovinger, 2009; Kumar et al., 2009). However, the potency of ethanol differs depending on receptor subunit combination, cell viability, cell types, and posttranslational processing. Chronic ethanol exposure enhances the glutamatergic system while concomitantly decreasing the GABAergic system. Chronic ethanol increases the release of glutamate and increases postsynaptic receptors, specifically NMDA receptors (reviewed by Crews et al., 1996; Kumari & Ticku, 2000; Vengeliene et al., 2008). On the other hand chronic exposure to ethanol has been shown to decrease GABAA receptor number, alter the expression of certain GABAA receptor subunits, and change receptor trafficking (reviewed by Crews et al., 1996; Vengeliene et al., 2008; Kumar et al., 2009)..
The above evidence of ethanol-induced changes in neurotransmission has been performed in heterologous cell lines and in brain areas such as the cortex, hippocampus and cerebellum. The effect of ethanol on striatal neurotransmission, in particular within the putamen/DLS, has not been fully explored. Therefore, Drs. Cuzon Carlson and Lovinger have examined the effect of chronic intermittent ethanol exposure on passive membrane properties, glutamatergic, and GABAergic neurotransmission onto MSNs in two animal models. In the first model, cynomolgus monkeys (Macaca fascicularis) were trained to orally self-administer 4% ethanol for 22 hours/day for a total of 33 months, interspersed with three 28-day abstinences from ethanol (Vivian et al., 2001). This paradigm elicited average blood ethanol concentrations (BECs) of 75–308 mg/dl. After the last 28 days of withdrawal, monkeys were subjected to necropsy for harvesting tissue and organs (Daunais et al., 2010). In the second model, C57BL/6J strain mice were exposed to ethanol via a vapor chamber (Becker and Lopez, 2004) for 16 hours/day, four days/week, for a total of four weeks. Mice were sacrificed three days after their last exposure to ethanol. Mice in this paradigm had average BECs between 175–200 mg/dl. Control monkeys and mice were exposed to the same diet and daily routines as their ethanol-exposed counterparts. The use of two models permitted combining the benefits of these models while mitigating the caveats. Mouse models, including inbred strains, are useful due to their known genetic background, proclivity for reproduction, minimal expense, and their potential for genetic manipulation. However, it is difficult to train mice to drink ethanol at a level or pattern similar to human alcoholics, and their relatively short life spans preclude the study of long-term effects of ethanol exposure over a time frame ranging from several months to years. Non-human primates, in particular macaque monkeys, have many characteristics that make them useful for ethanol research, such as 95% gene homology with humans (Hacia et al., 1998), similar comparative anatomy and physiology, similar ethanol absorption and metabolism (Green et al., 1999) and the propensity to self-administer large quantities of ethanol orally (Meisch and Stewart, 1994).
The membrane properties of putamen/DLS MSNs of control and ethanol-exposed monkeys and mice were examined to determine if intrinsic MSN excitability is altered by prolonged ethanol intake. In both monkey and mouse MSNs, there were no ethanol-induced changes in cell size as evidenced by similar whole-cell capacitance measurements between control and ethanol MSNs. Overall, MSNs from ethanol-exposed monkeys and mice exhibited more depolarized resting membrane potentials and action potential thresholds at more depolarized levels. When the MSN membrane potential was set at levels above the action potential threshold, the frequency of action potentials was greater in putamen/DLS MSNs of ethanol-exposed monkeys and mice compared to controls. These findings indicate increased neuronal excitability in MSNs of the putamen/DLS from chronic ethanol-exposed monkeys and mice.
Chronic ethanol exposure decreased the inter-event interval of glutamatergic miniature excitatory postsynaptic currents (mEPSCs) recorded in monkey putamen/DLS MSNs but not of mouse DLS MSNs, suggesting either increased presynaptic release of glutamate and/or an increased number of glutamatergic synapses in the ethanol-drinking monkeys. In agreement with an increased number of glutamatergic synapses, diolystic analysis of spine density of MSNs of the monkey putamen suggested that ethanol-drinking individuals demonstrate an increase in spine density compared to controls. However, in both monkey and mouse MSNs, there were no differences in mEPSC amplitude, area, rise time, and decay time between control and ethanol-exposed subjects, suggesting a lack of change in postsynaptic responsiveness to glutamate.
In both animal models, GABAA receptor-mediated miniature inhibitory postsynaptic currents (mIPSCs) recorded from putamen/DLS MSNs of ethanol-exposed subjects displayed longer interevent intervals compared to their control counterparts. This finding suggests a decrease in GABA release frequency and/or decreased number of GABAergic synapses. With regard to postsynaptic changes, mIPSCs recorded from putamen MSNs of ethanol-exposed monkeys displayed decreases in amplitude and area, but no changes in rise time and decay time. These postsynaptic mIPSC changes were not observed in MSNs recorded in the DLS of ethanol-exposed mice. This finding suggests that postsynaptic changes in the MSNs of the monkey putamen are most likely due to a decrease in receptor number rather than a change in receptor subunit composition since changes in subunit composition would be expected to change the shape of mIPSCs.
In comparing the two animal models of chronic intermittent ethanol exposure, Drs. Cuzon Carlson and Lovinger observed several similarities, such as ethanol-induced increases in neuronal excitability, decrease in GABAergic mIPSC frequency and no change in mEPSC postsynaptic parameters. However there were several differences in the findings between the two animal models, in particular the increased mEPSC frequency and decrease in mIPSC amplitude that were observed in the monkey but not the mouse DLS. These differences could be due to intrinsic differences between monkeys and mice that may be hard to address with future experiments comparing these two models. It is also possible that these discrepancies are due to differences in the duration of ethanol exposure suggesting that ethanol-induced changes in the GABAergic system occurs before those in the glutamate system in the putamen/DLS. It has been suggested that passive drug administration does not activate habit systems to the same extent as self-administration (Jacobs et al., 2003). Therefore the differences observed in the MSNs of the monkey and mouse DLS may be due to the differences in ethanol administration between the two models. Further studies with mouse drinking models should address this difference. Despite these observed differences, the data from Drs. Cuzon Carlson and Lovinger suggest that chronic ethanol exposure causes changes in the neurotransmission onto MSNs in the putamen/DLS, which ultimately can lead to an increase in the output from the putamen/DLS and contribute to habitual behaviors of alcoholism.
LONG-LASTING ADAPTATIONS OF NR2B SUBUNIT-CONTAINING NMDA RECEPTORS IN THE DORSOMEDIAL STRIATUM PLAY A CRUCIAL ROLE IN ALCOHOL CONSUMPTION IN RATS
The NMDA-type glutamate receptor (NMDAR), consisting of obligatory NR1 subunits and regulatory NR2(A–D) subunits, is a ligand-gated ion channel (Cull-Candy et al., 2001). The activity of the channel is regulated, in part, via phosphorylation of the intracellular tails of the subunits (Ron, 2004). The main NR2 subunits in the central nervous system (CNS) are NR2A and NR2B (Monyer et al., 1994), and phosphorylation of the NR2B subunit by the protein tyrosine kinase Fyn results in the enhancement of channel activity (Ron, 2004; Salter and Kalia, 2004). One of the major targets of ethanol in the CNS is the NMDAR system, and the activity of the NMDARs is inhibited by ethanol (Lovinger et al., 1989; Ron and Wang, 2008). However, in addition to the acute inhibitory actions of ethanol on channel activity, selective brain regions also exhibit ethanol-mediated enhancement of NR2B-NMDAR function (Yaka et al., 2003; Roberto et al., 2004; Wang et al., 2007, Wang et al., 2010; Kash et al., 2009). For example, acute exposure of slices containing the dorsal striatum (DS) to, and withdrawal from, ethanol induces long-term facilitation (LTF) of NMDAR-mediated excitatory postsynaptic currents (NMDAR-EPSCs) (Wang et al., 2007). Furthermore, the ethanol-induced LTF in the DS is mediated by NR2B-containing NMDARs and requires Fyn (Wang et al., 2007). Biochemical studies suggested that acute ex vivo and in vivo exposure to ethanol results in Fyn activation and NR2B phosphorylation in the DS (Wang et al., 2007). Together, these findings imply that acute ethanol-mediated Fyn phosphorylation of NR2B subunits leads to LTF of NR2B-NMDAR activity in the DS.
The DS can be divided into two subregions, the DMS and the DLS. Using slice electrophysiology, Wang et al. (2010) first examined whether ethanol-mediated LTF of NMDAR activity was induced distinctly in the DMS or the DLS. Notably, acute ex vivo exposure of rat striatal slices to, and withdrawal from, 40 mM of ethanol induces LTF of NMDAR-EPSCs in the DMS but not the DLS. The differential sensitivity of the NMDAR to ethanol in DMS and DLS was not due to differences in the protein level of the NR2 subunit, or in the sensitivity of the channel to an NR2B specific inhibitor (Wang et al., 2010).
To determine whether ethanol-mediated LTF of NMDAR function occurs in vivo, Drs. Wang and Ron examined if exposure of rats to, and withdrawal from, ethanol induces upregulation of NMDAR activity in the DMS. Sprague-Dawley rats were systemically administered saline or 2 g/kg of ethanol daily for 7 days, and NMDAR activity was measured in DMS neurons from striatal slices prepared 16 hours after the last administration. The NMDA-induced current and NMDAR-EPSCs were significantly greater in DMS neurons from ethanol-treated rats than those from saline-treated rats. When the administration period was increased from 7 to 14 days, the upregulation of NMDAR activity in the DMS was observed even 40 hours after the last administration (Wang et al., 2010). Together, these results suggest that repeated in vivo exposure of animals to, and withdrawal from, ethanol leads to a long-lasting increase in synaptic NMDAR activity. If the increase in NMDAR function induced by in vivo exposure to ethanol is similar to the mechanism that underlies LTF of NMDAR function ex vivo, then it should also be mediated by the NR2B subunit. To test this possibility, rats were treated with saline or 2 g/kg of ethanol for 7 days and striatal slices were prepared 16 hrs after the last treatment to measure NMDAR-EPSCs in the presence of a NR2B-NMDAR antagonist, Ro 25–6981. Bath application of Ro 25-6981 produced a greater inhibition of NMDAR-EPSCs in DMS slices from ethanol-treated rats than those from saline-treated rats. This result suggests that the contribution of NR2B-NMDARs to the activity of the channel is increased after repeated ethanol administration and withdrawal. The same paradigm resulted in long-lasting increases in the activity of Fyn and the phosphorylation level of synaptosomal NR2B subunits (Wang et al., 2010).
Using the intermittent-access 20% ethanol 2-bottle-choice drinking paradigm (Carnicella et al., 2009), Wang and colleagues tested the involvement of long-lasting upregulation of NMDAR activity in the DMS in mechanisms that contribute to the development and/or maintenance of ethanol-drinking behaviors. After stable high levels of ethanol intake was established (5–6 g/kg/24 hrs and 20–30 mM of blood ethanol concentration measured 30 min after the start of the ethanol session), DMS slices were prepared 16 hrs or 9 days after the last ethanol-drinking session to measure NMDAR activity. The NMDA-induced current and the NMDAR-EPSCs were greater in ethanol-drinking rats than in control water-drinking rats, 16 hrs and even 9 days after the last ethanol session. These results suggest that voluntarily intermittent consumption of ethanol produces a very stable and long-lasting increase in the activity of the NMDAR. Biochemical measurements conducted in parallel revealed that the phosphorylation level of the NR2B subunits was also increased in the DMS, 16 hrs after the ethanol drinking session. These findings suggest that repeated sessions of voluntarily excessive consumption of ethanol and withdrawal leads to a long-lasting increase in the phosphorylation of NR2B subunits which, in turn, increases the function of synaptic NR2B-containing channels (Wang et al., 2010).
Wang and colleagues lastly examined the possible behavioral consequences of the ethanol-mediated upregulation of NMDAR activity upon excessive ethanol consumption and withdrawal. Rats were first trained to drink high levels of ethanol in the intermittent-access 2-bottle choice drinking paradigm described above, and then to press a lever to self-administer a solution of 20% ethanol (Wang et al., 2010). Intra-DMS infusion of a NR2B-NMDAR selective antagonist, ifenprodil, or 4-Amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo [3,4-D] pyrimidine (PP2) which blocks Fyn kinase, produced a significant decrease in ethanol, but not sucrose self-administration. Interestingly, intra-DLS infusion of ifenprodil or PP2 did not significantly alter ethanol self-administration. These results suggest that the upregulation of Fyn and NR2B-NMDARs in the DMS that are induced by repeated intermittent exposure to ethanol contribute to ethanol-drinking behaviors (Wang et al., 2010).
Taken together, these results suggest that the ethanol-mediated neuroadaptations in the Fyn/NR2B-NMDAR pathway in the DMS contributes to the maladaptive synaptic plasticity and abnormal learning that lead to and/or maintain excessive alcohol consumption.
THE ROSTROVENTRAL CAUDATE PUTAMEN AFFECTS RISK FOR ALCOHOL WITHDRAWAL IN MICE
Withdrawal is a hallmark of alcohol physiological dependence, and is thought to constitute a motivational force that sustains alcohol use/abuse and may contribute to relapse in dependent individuals (Koob and Le Moal, 2008). Although no animal model duplicates alcohol dependence in humans, models for specific factors, including withdrawal, are useful for identifying potential genetic and neural determinants of liability in humans. Using robust animal models, Buck and colleagues have identified a quantitative trait locus (QTL) and gene (Mpdz) on chromosome 4 with large affects on alcohol and barbiturate withdrawal in mice (Buck et al., 1997, 1999; Fehr et al., 2002; Shirley et al., 2004). Using c-Fos mapping as a high-resolution histological marker of neuronal stimulation, Chen and colleagues found that chromosome 4 congenic and DBA/2J background strain mice differ significantly in neuronal activation associated with alcohol withdrawal within the basal ganglia, and particularly in regions associated with limbic function (Chen et al., 2008), including the rostroventral caudate putamen (rvCP) (Chen and Buck, 2010). The rvCP, located ventral to the dorsolateral caudate putamen and lateral to the NAc, is a ventrolateral segment of the DMS (Figure 1). In contrast, the NAc core and shell, dorsolateral caudate putamen, and the medial segment of the dorsomedial caudate putamen did not show genotype-dependent neuronal activation associated with withdrawal in this model (Chen et al., 2008).
Within the basal ganglia circuit, the substantia nigra pars reticulata (SNr) also shows significant differential neuronal activation associated with withdrawal that is influenced by chromosome 4 QTL status. Particularly, the caudolateral SNr (clSNr) plays a critical role in withdrawal following acute and repeated alcohol exposure as well as barbiturate withdrawal (Chen et al., 2008; Chen et al., 2011). Neuroanatomic studies demonstrate the existence of direct projections from the rvCP to the clSNr. Unilateral injection of a retrograde tract tracer (fluorogold) restricted to the clSNr resulted in a high density of labeled neurons within the rvCP, with little or no labeling detected in the NAc. This projection is corroborated by anterograde tract tracing, with injection of Texas Red conjugated dextran amine into the rvCP resulting in substantial labeling of terminal arborizations and varicosities within the clSNr (Chen and Buck, 2010). These studies demonstrate that the clSNr receives strong monosynaptic projections from rvCP neurons in mice. This rvCP-clSNr projection also exists in nonhuman primates (Goldman-Rakic, 1990).
Lesion studies support the role of both the rvCP and clSNr in withdrawal, measured using the handling-induced convulsions in mice. Bilateral lesions of the rvCP significantly increased alcohol withdrawal severity compared to sham-lesioned controls, indicating that, overall, rvCP activity has an inhibitory effect on alcohol withdrawal (Chen and Buck, 2010). Moreover, bilateral lesions of the clSNr significantly reduced withdrawal severity following acute or repeated exposures to alcohol as well as barbiturate withdrawal (Chen et al., 2010). Taken together, these results indicate that disruption of the inhibitory striatonigral pathway results in disinhibition of the SNr and more severe withdrawal, while reduced SNr activity is associated with less severe withdrawal. Lesions of the rvCP also disrupt the indirect striatopallidal pathway. If the striatopallidal pathway were also involved in withdrawal, then one might predict that disruption of this excitatory pathway could result in decreased SNr activity and reduced withdrawal severity; however, this was not observed after lesioning the rvCP. Additionally, bilateral lesions of the subthalamic nucleus, a key part of this indirect pathway, did not influence alcohol withdrawal convulsions (Chen et al., 2008), suggesting that the subthalamic nucleus and/or the associated indirect pathway may not have a major impact on alcohol withdrawal convulsions in mice.
In addition to its critical role in withdrawal convulsions, the rvCP may contribute to additional signs associated with the withdrawal syndrome. Neuroimaging studies in humans and pharmacological microinjection analyses in nonhuman primates show that the striatal ventral putamen, the primate counterpart of the rvCP, is associated with depression, anxiety, apathy, stereotypy and hypoactivity (Hasler et al., 2007; Mah et al., 2007; Worbe et al., 2009), which are frequently observed in the alcohol withdrawn subjects. Neuroimaging studies also suggest that this part of the striatum may be associated with alcohol craving (Heinz et al., 2005; Olbrich et al., 2006). The ventral putamen receives projections from the amygdala (Russchen & Price, 1984), which is implicated in ethanol and barbiturate withdrawal (Chen et al., 2009, and unpublished data), and has a prominent role in alcohol preference (Dhaher et al., 2008). Notably, alcohol withdrawal convulsions and preference/consumption are significantly genetically correlated in mice (Metten & Crabbe, 2005). Future work will be needed to assess the role of neurons within the rvCP, as well as projections to and from this region, in withdrawal and consumption of alcohol, barbiturates and related drugs.
The multi-PDZ domain protein (MPDZ, also called MUPP1) is the protein product of Mpdz, a quantitative trait gene found to underlie the phenotypic effects of the chromosome 4 QTL on ethanol and barbiturate withdrawal convulsions (Shirley et al., 2004). Recently, its human homolog (MPDZ) has also been implicated in alcohol dependence (Karpyak et al., 2009, Tabakoff et al., 2009). MPDZ has no apparent intrinsic activity and is thought to exert its effects by regulating the function of other proteins with which it physically associates. These include 5HT2C receptors (Becamel et al., 2001, Parker et al., 2003) and SynGAP, a postsynaptic density GTPase activating protein that complexes MPDZ with Ca+2-calmodulin kinase and the NMDAR NR2B subunit in vitro and in vivo (Kim et al., 2005, Krapivinsky et al., 2004). Immunohistochemical studies demonstrate that virtually all c-Fos-immunoreactive neurons detected in the rvCP of alcohol withdrawn animals express MPDZ and 5-HT2C receptors, and that approximately 30% of these also express SynGAP. Selective blockade of 5-HT2C transmission increases neuronal activation within the striatum (De Deurwaerdere et al., 2010), so it is plausible that MPDZ influences neural activation associated with withdrawal in the rvCP via regulation of 5-HT2C receptor mediated transmission. Stimulation of striatal NMDAR increases GABA release within the SNr (Fantin et al., 2007; Morari et al., 1998). NMDAR containing NR2B preferentially regulate the striatonigral neurons whereas those containing the NR2A subunit preferentially regulate the striatopallidal neurons (Fantin et al., 2007, 2008). Notably, the activities of NR2B-NMDARs are increased in the DMS, but not in the DLS, in ethanol-dependent rats withdrawn from ethanol (Wang et al., 2010, see above). It is therefore plausible that MPDZ regulates NR2B-NMDAR dependent signaling in striatonigral projection neurons within the rvCP of the DMS in alcohol withdrawn animals.
In summary, these findings implicate the rvCP of the DMS, as well as brain regions that project to and from the rvCP, in withdrawal in mice. These observations raise the possibility that MPDZ may regulate transmission mediated by 5-HT2C and/or NR2B-NMDA receptors in rvCP neurons. Future work will be needed to directly assess the impact of MPDZ on 5-HT2C and/or NR2B-NMDA receptor function in the rvCP and its potential role in ethanol and drug dependence and withdrawal.
THE VENTRAL STRIATUM AND ITS IMPLICATION IN REWARD PROCESSING AND LEARNING DYSFUNCTIONS IN HUMAN ALCOHOLICS
One common factor contributing to addictive disorders like alcoholism is the enduring consumption of the drug in spite of diverse negative consequences. Dependent patients seem to have difficulties to integrate information about positive and negative consequences of their actions and to adapt their future behavior accordingly. The question is why? Dopaminergic neurotransmission has long been implicated in the acquisition and maintenance of alcohol dependence, particularly because it is known that all drugs of abuse induce dopamine release in the ventral striatum, one of the core regions of the reward circuitry, and it has been suggested that this plays an eminent role in the attribution of incentive salience to formerly neutral but now alcohol-associated stimuli (Robinson & Berridge, 1993; Schultz et al., 1997). Such phasic dopamine release is thought to reinforce especially those behaviors which elicited the release (Di Chiara, 1995). Thus, dopamine’s role in the acquisition of alcohol dependence had already been attributed to its effect on Pavlovian conditioning and Pavlovian-to-Instrumental-Transfer (Robbins & Everitt, 1996).
In detoxified alcohol-dependent patients, brain imaging studies with positron emission tomography (PET) revealed a reduction of availability and sensitivity of central dopamine D2-receptors, which may reflect a compensatory down-regulation and which was correlated with lifetime alcohol intake (Heinz et al., 1996, Volkow et al., 1996). Further PET studies showed that a low dopamine synthesis capacity measured with 3,4-Dihydroxy-6-fluoro-DL-phenylananine (F-DOPA) PET was specifically correlated with alcohol craving in detoxified alcohol-dependent patients who also displayed reduced dopamine D2 receptor availability in the ventral striatum including the nucleus accumbens (Heinz et al., 2004; 2005). During detoxification and early abstinence, dopamine dysfunction may further be augmented by reduced intra-synaptic dopamine release: studies in rats showed that extracellular dopamine concentrations decreased rapidly during detoxification (Rossetti et al., 1992) and a PET study showed that dopamine release following acute amphetamine administration was significantly reduced in detoxified alcohol-dependent individuals, indicating that presynaptic dopamine storage capacity is reduced during early abstinence (Martinez et al., 2005). Interestingly, this dopamine dysfunction during early alcohol abstinence appears to be associated with an increased neuronal response to drug-associated stimuli: in a multimodal imaging study combining PET and functional magnetic resonance imaging (fMRI), ventral striatal dopamine D2 receptor down-regulation was not only correlated with the severity of alcohol craving but also with increased processing of alcohol-associated cues in the anterior cingulate and medial prefrontal cortex (Heinz et al., 2004). These brain areas are part of the attention network and an increased activation in these regions during the processing of alcohol cues has clinically been associated with an increased prospective relapse risk (Grüsser et al., 2004). Such dysfunction of dopaminergic neurotransmission during early abstinence could interfere with two processes: 1) the acquisition of new stimulus-reward-associations and 2) the extinction of well-learned, existing stimulus-reward-associations. The first effect is based on dopamine’s role in encoding a prediction error (PE): according to this hypothesis, dopamine is released whenever an incoming reward exceeds the predicted reward so that the positive difference between the actually received minus the expected reward is reflected in dopaminergic firing. This finding was also supported by human neuroimaging studies that showed activity in the ventral striatum, a target area of dopaminergic midbrain neurons, correlated with PE (e.g. O’Doherty et al., 2003). Animal experiments suggested that conditioned cues that indicate the positive value of an upcoming reward can acquire the same capacity to elicit a short, phasic increase in dopaminergic firing, because their appearance is unpredicted and therefore exceeds the individual’s expectation, while a reward that arrives exactly as predicted by the previous conditioned cue will no longer elicit dopamine release, because the difference between the incoming and the expected reward is zero (Schultz et al., 1997). On that note, a human study by Knutson et al. (2001) showed that in healthy volunteers, presentation of a salient stimulus that has reliably been shown to predict reward evokes a phasic activation of the ventral striatum.
In this context, it has been suggested that in alcohol dependence alcohol-associated cues require the same positive motivational value of the anticipated drug itself (Heinz, 2002). Dysfunction of such dopamine-driven mechanisms should impair the acquisition of new conditioned responses or the attribution of salience to newly learned stimuli which are presented unexpectedly and indicate the availability of (non-alcohol) reward. Indeed, studies in alcohol-dependent patients showed that ventral striatal activation elicited by newly learned, previously neutral but now reward-predicting stimuli (salient for the availability of monetary reward) was reduced in the ventral striatum of alcohol-dependent patients compared with healthy controls (Beck et al., 2009; Wrase et al., 2007). This reduced activation of the ventral striatum was furthermore correlated with the severity of alcohol craving. In a probabilistic reversal learning paradigm, in which positive and negative reinforcement drives learning performance, alcohol-dependent patients displayed reduced learning speed, which was correlated with dysfunctional connectivity between the ventral striatum and the dorsolateral prefrontal cortex (dlPFC), an area of higher decision making (Park et al., 2010) (Figure 2). In these detoxified patients, the more impaired this outcome-dependent frontostriatal connectivity modulation, the higher was the reported craving. The dorsolateral prefrontal cortex has been suggested to integrate motivational information and cognitive information (Sakagami and Watanabe, 2007), functions that indeed appear to be based in part on interactions with the striatum and other subcortical structures (Cools et al., 2007). A recent study has confirmed volume reductions in the dlPFC of patients with alcohol dependence (Makris et al., 2008). On the other hand, as described above, neuronal and subjective responses to well-learned, e.g., alcohol-associated stimuli was increased among detoxified alcohol-dependent patients and correlated with the reduction of ventral striatal dopamine D2 receptor availability (Heinz et al., 2004, 2005). In these patients, persisting and increased responses to alcohol-associated cues even in settings that are completely devoid of alcohol (e.g., lying in the scanner and being in a detoxification program) may be due to impaired extinction processes, and it has been suggested that a negative prediction error, i.e. the non-occurrence of reward after it has been predicted, is encoded by a brief cessation of dopaminergic firing rates (Schultz et al., 1997). Dysfunction of dopaminergic neurotransmission may thus interfere with a mechanism, which can otherwise encode the information that a conditioned stimulus is no longer followed by reward, and this impairment should contribute to the persisting over attribution of incentive salience to alcohol-associated cues (Heinz et al., 2010). Therefore, impairment of new learning processes and of extinction of drug-associated reactions may both contribute to the so-called “hijacking of the reward system” and bias patients to focus their attention on alcohol-related stimuli, which can thus elicit relapse.
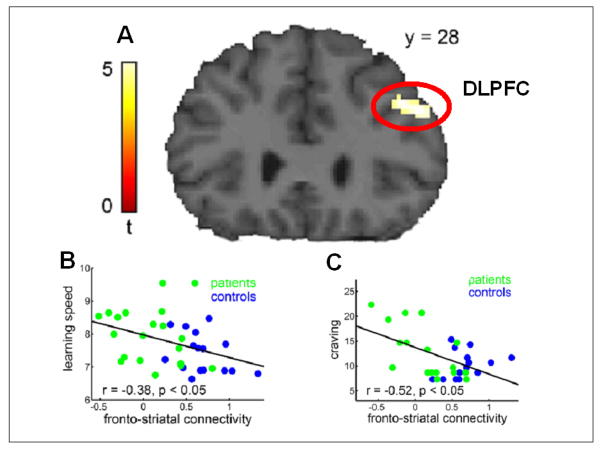
Figure 2.
Impaired frontostriatal connectivity in alcohol-dependent patients (see Park et al., 2010).
A: A functional connectivity analysis with ventral striatum as seed region revealed an impaired connectivity with the dorsolateral prefrontal cortex (DLPFC) during a reversal learning paradigm.
B: A multiple regression analysis showed a negative relationship between learning speed and the frontostriatal connectivity modulation: The smaller the feedback-related connectivity modulation, the more trials were required to learn the rule.
C: The greater the self-reported craving for alcohol the less pronounced was the connectivity modulation.
SUMMARY AND FUTURE DIRECTIONS
Recordings in the DLS MSNs of chronic alcohol exposed mice and long-term ethanol-drinking monkeys demonstrated several similarities, such as ethanol-induced increases in neuronal excitability, decrease in GABAergic mIPSC frequency and no change in mEPSC postsynaptic parameters. Although there were observed differences such an increase in glutamatergic mEPSC frequency, suggesting differences in ethanol induced changes in AMPA receptor mediated currents that was observed in the monkey model but not the mouse, future studies should explore ethanol-induced changes in NMDA receptor mediated currents in the DLS. It will be important to determine whether ethanol’s regulation of the NMDA receptor plays a role in the consistent observation of increased striatal excitability in rodents and nonhuman primates. Future studies will be needed that examine the effects of chronic intermittent ethanol exposure on neurotransmission in the DMS MSNs of rodents and monkeys. Preliminary data suggests that MSNs from these two regions have different baseline neurotransmission and that acute exposure to the same concentration of ethanol differentially affects the two regions. The enhanced striatal excitability may partially result from increased activities of the NMDA receptor, which is shown to contribute to “UP state” firing (Pomata et al., 2008). Measurement of NMDAR function in rat DMS neurons reveals that ex vivo or in vivo exposure to, and withdrawal from, ethanol results in a long-lasting upregulation of NR2B-NMDAR activity. Since the activity of NMDARs contributes to “UP state” firing of MSNs (Pomata et al., 2008) and is essential for the induction of long-term potentiation (LTP) of AMPA receptor (AMPAR)-mediated synaptic transmission (Partridge et al., 2000), it will be of interest to determine whether the ethanol-mediated facilitation of NMDARs alters “UP state” firing and/or AMPAR-LTP in the DMS neurons, which may underlie the development and maintenance of excessive ethanol intake. To complement these proposed studies in rodents, the use of a long-term ethanol self-administration model in non-human primates will allow for the correlation of individual ethanol intake and ethanol addicted behaviors with changes in neurotransmission that may be hard to accomplish in rodents due to the difficulty in training rodents to drink ethanol at a level, pattern or span of time similar to that of human alcoholics. The ventral part of the caudate-putamen, a part of the DMS, is an understudied region of the striatum. New findings demonstrate that the rvCP is crucially involved in alcohol withdrawal in mice, with an important role in genetically determined differences in risk for withdrawal. Futures studies will be needed to assess the potential role of the rvCP beyond withdrawal convulsions, and to elucidate the molecular mechanisms by which withdrawal is affected (e.g., involving MPDZ and its regulation of 5HT2C and/or NR2B-NMDA receptors). Human studies support the notion that dopaminergic dysfunction in the ventral striatum is involved in the impairment of both learning processes and the extinction of drug-associated reactions. Moreover, imaging studies provide evidence that disrupted functional coupling between ventral striatum and dorsolateral prefrontal cortex is associated with core symptoms of addiction. Neurobiological research may help to reduce the stigma of addiction by revealing brain-related changes, which help to explain why addicted individuals often relapse against their declared intention. Taken together, our findings offer new insights into the molecular and neural mechanisms involved in alcohol craving, self-administration, dependence and withdrawal.
Acknowledgments
The work described in this review was supported by the division of Intramural Clinical and Basic Research NIAAA, AA13510, AA13641 (VCCC, DML); R01AA014366, MH13438 and funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (DR); AA011114, AA10760, DA05228, and the Department of Veterans Affairs (KJB); the German Research Foundation (Deutsche Forschungsgemeinschaft, HE2597/4-3 and 7-3) and the Bernstein Center for Computational Neuroscience Berlin (Bundesministerium für Bildung und Forschung Grants 01GQ0411 and 01GS08159) (AH).
References
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becamel C, Figge A, Poliak S, Dumuis A, Peles E, Bockaert J, Lubbert H, Ullmer C. Interaction of Serotonin 5-Hydroxytryptamine Type 2C Receptors with PDZ10 of the Multi-PDZ Domain Protein MUPP1. J Biol Chem. 2001;276(16):12974–12982. doi: 10.1074/jbc.M008089200. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hägele C, Knutson B, Heinz A, Wrase J. Ventral Striatal Activation During Reward Anticipation Correlates with Impulsivity in Alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28(12):1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Buck KJ, Metten P, Belknap JK, Crabbe JC. Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J Neurosci. 1997;17(10):3946–55. doi: 10.1523/JNEUROSCI.17-10-03946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Buck KJ. Rostroventral caudate putamen involvement in ethanol withdrawal is influenced by a chromosome 4 locus. Genes Brain Behav. 2010;9(7):768–76. doi: 10.1111/j.1601-183X.2010.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Kozell LB, Buck KJ. Substantia nigra pars reticulata is crucially involved in barbiturate and ethanol withdrawal in mice. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2010.10.025. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Kozell LB, Hitzemann R, Buck KJ. Involvement of the limbic basal ganglia in ethanol withdrawal convulsivity in mice is influenced by a chromosome 4 locus. J Neurosci. 2008;28(39):9840–9. doi: 10.1523/JNEUROSCI.1713-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Reilly MT, Kozell LB, Hitzemann R, Buck KJ. Differential activation of limbic circuitry associated with chronic ethanol withdrawal in DBA/2J and C57BL/6J mice. Alcohol. 2009;43(6):411–20. doi: 10.1016/j.alcohol.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D’Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Morrow AL, Criswell H, Breese G. Effects of ethanol on ion channels. Int Rev Neurobiol. 1996;39:283–367. doi: 10.1016/s0074-7742(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Kraft RA, Davenport AT, Burnett EJ, Maxey VM, Szeliga KT, Rau AR, Flory GS, Hemby SE, Kroenke CD, Grant KA, Friedman DP. MRI-guided dissection of the nonhuman primate brain: a case study. Methods. 2010;50(3):199–204. doi: 10.1016/j.ymeth.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcohol Clin Exp Res. 2008;32:197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdere P, Le Moine C, Chesselet MF. Selective blockade of serotonin 2C receptor enhances Fos expression specifically in the striatum and the subthalamic nucleus within the basal ganglia. Neurosci Lett. 2010;469(2):251–5. doi: 10.1016/j.neulet.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38(2):95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Lovinger DM. Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev. 2009;61:98–114. doi: 10.1124/pr.108.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1491. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fantin M, Marti M, Auberson YP, Morari M. NR2A and NR2B subunit containing NMDA receptors differentially regulate striatal output pathways. J Neurochem. 2007;103(6):2200–11. doi: 10.1111/j.1471-4159.2007.04966.x. [DOI] [PubMed] [Google Scholar]
- Fantin M, Auberson YP, Morari M. Differential effect of NR2A and NR2B subunit selective NMDA receptor antagonists on striato-pallidal neurons: relationship to motor response in the 6-hydroxydopamine model of parkinsonism. J Neurochem. 2008;106:957–968. doi: 10.1111/j.1471-4159.2008.05439.x. [DOI] [PubMed] [Google Scholar]
- Fehr C, Shirley RL, Belknap JK, Crabbe JC, Buck KJ. Congenic Mapping of Alcohol and Pentobarbital Withdrawal Liability Loci to a <1 Centimorgan Interval of Murine Chromosome 4: Identification of Mpdz as a Candidate Gene. J Neurosci. 2002;22(9):3730–8. doi: 10.1523/JNEUROSCI.22-09-03730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HJ, Yang L, Faingold CL. Role of the amygdala in ethanol withdrawal seizures. Brain Res. 2007;1141:65–73. doi: 10.1016/j.brainres.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985;236(4):454–76. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327(1–2):307–11. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- Green KL, Szeliga KT, Bowen CA, Kautz MA, Azarov AV, Grant KA. Comparison of ethanol metabolism in male and female cynomolgus macaques (Macaca fascicularis) Alcohol Clin Exp Res. 1999;23(4):611–616. [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S. http://www.ncbi.nlm.nih.gov/pubmed?term=%22Klein%20S%22%5BAuthor%5D.; Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Haber SN. Neurotransmitters in the human and nonhuman primate basal ganglia. Hum Neurobiol. 1986;5(3):159–68. [PubMed] [Google Scholar]
- Hacia JG, Makalowski W, Edgemon K, Erdos MR, Robbins CM, Fodor SP, Brody LC, Collins FS. Evolutionary comparisons using high-density oligonucleotide arrays. Nat Genet. 1998;18(2):155–158. doi: 10.1038/ng0298-155. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27(23):6313–9. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A. Dopaminergic dysfunction in alcoholism and schizophrenia--psychopathological and behavioral correlates. Eur Psychiatry. 2002;17(1):9–16. doi: 10.1016/s0924-9338(02)00628-4. [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14(1):108–18. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Beck A, Mir J, Grüsser SM, Grace AA, Wrase J. Alcohol Craving and Relapse Prediction: Imaging Studies. In: Kuhn CM, Koob GF, editors. Advances in the Neuroscience of Addiction. CRC Press; 2010. pp. 127–162. [PubMed] [Google Scholar]
- Heinz A, Dufeu P, Kuhn S, Dettling M, Gräf K, Kürten I, Rommelspacher H, Schmidt LG. Psychopathological and behavioral correlates of dopaminergic sensitivity in alcohol dependent patients. Arch Gen Psychiatry. 1996;53:1123–1128. doi: 10.1001/archpsyc.1996.01830120061011. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162(8):1515–20. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser SM, Flor H, Braus DF, Buchholz HG, Gründer G, Schreckenberger M, Smolka MN, Rösch F, Mann K, Bartenstein P. Correlation between dopamine D-2 receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, DeVries J, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends in Pharmacol Sci. 2003;24(11):566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Karpyak VM, Kim JH, Biernacka JM, Wieben ED, Mrazek DA, Black JL, Choi DS. Sequence variations of the human MPDZ gene and association with alcoholism in subjects with European ancestry. Alcohol Clin Exp Res. 2009;33:712–721. doi: 10.1111/j.1530-0277.2008.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, 2nd, Conrad KL, Colbran RJ, Winder DG. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2009;34:2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosi. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3113–23. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozell LB, Hitzemann R, Buck KJ. Acute alcohol withdrawal is associated with c-Fos expression in the basal ganglia and associated circuitry: C57BL/6J and DBA/2J inbred mouse strain analyses. Alcohol Clin Exp Res. 2005;29(11):1939–48. doi: 10.1097/01.alc.0000187592.57853.12. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43:563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Ticku MK. Regulation of NMDA reeptors by ethanol. Prog Drug Res. 2000;54:152–189. [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Mah L, Zarate CA, Jr, Singh J, Duan YF, Luckenbaugh DA, Manji HK, Drevets WC. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol Psychiatry. 2007;61(6):765–75. doi: 10.1016/j.biopsych.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Meisch RA, Stewart RB. Ethanol as a reinforcer: A review of laboratory studies of non-human primates. Behav Pharmacol. 1994;5:425–440. [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morari M, Sbrenna S, Marti M, O’Connor WT, Bianchi C, Fuxe K, Beani L. Evidence for a striatal NMDA receptor modulation of nigral glutamate release. A dual probe microdialysis study in the awake freely moving rat. Eur J Neurosci. 1998;10(5):1716–22. doi: 10.1046/j.1460-9568.1998.00176.x. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Olbrich HM, Valerius G, Paris C, Hagenbuch F, Ebert D, Juengling FD. Brain activation during craving for alcohol measured by positron emission tomography. Aust N Z J Psychiatry. 2006;40(2):171–8. doi: 10.1080/j.1440-1614.2006.01765.x. [DOI] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosi. 2010;30(22):7749–53. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LL, Backstrom JR, Sanders-Bush E, Shieh BH. Agonist-induced phosphorylation of the serotonin 5-HT2C receptor regulates its interaction with multiple PDZ protein 1. J Biol Chem. 2003;278(24):21576–83. doi: 10.1074/jbc.M210973200. [DOI] [PubMed] [Google Scholar]
- Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84(3):1422–9. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- Pomata PE, Belluscio MA, Riquelme LA, Murer MG. NMDA receptor gating of information flow through the striatum in vivo. J Neurosci. 2008;28(50):13384–9. doi: 10.1523/JNEUROSCI.4343-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6(2):228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving–an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Ron D. Signaling cascades regulating NMDA receptor sensitivity to ethanol. Neuroscientist. 2004;10:325–336. doi: 10.1177/1073858404263516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Wang J. The NMDA receptor and alcohol addiction. In: AMV, editor. Biology of the NMDA receptor. Boca Raton: CRC Press; 2008. pp. 59–77. [Google Scholar]
- Rossetti ZL, Melis F, Carboni S, Gessa GL. Dramatic depletion of mesolimbic extracellular dopamine after withdrawal from morphine, alcohol or cocaine - a common neurochemical substrate for drug-dependence. Ann N Y Acad Sci. 1992;654:513–516. doi: 10.1111/j.1749-6632.1992.tb26016.x. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Price JL. Amygdalostriatal projections in the rat. Topographical organization and fiber morphology shown using the lectin PHA-L as an anterograde tracer. Neurosci Lett. 1984;47:15–22. doi: 10.1016/0304-3940(84)90379-3. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Topographic intermingling of striatonigral and striatopallidal neurons in the rhesus monkey. J Comp Neurol. 1990;297(3):359–76. doi: 10.1002/cne.902970304. [DOI] [PubMed] [Google Scholar]
- Shirley RL, Walter NA, Reilly MT, Fehr C, Buck KJ. Mpdz is a quantitative trait gene for drug withdrawal seizures. Nat Neurosci. 2004;7(7):699–700. doi: 10.1038/nn1271. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Printz M, Flodman P, Hodgkinson C, Goldman D, Koob G, Richardson HN, Kechris K, Bell RL, Hubner N, Heinig M, Pravenec M, Mangion J, Legault L, Dongier M, Conigrave KM, Whitfield JB, Saunders J, Grant B, Hoffman PL. Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biol. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154(2):299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): Long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25(8):1087–1097. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsivec alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105(10):1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Baup N, Grabli D, Chaigneau M, Mounayar S, McCairn K, Feger J, Tremblay L. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex. 2009;19(8):1844–56. doi: 10.1093/cercor/bhn214. [DOI] [PubMed] [Google Scholar]
- Woodward JJ. Ethanol and NMDA receptor signaling. Crit Rev Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–94. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J Neurosci. 2003;23:3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]