Abstract
Insulin-like growth factor (IGF)-I is important in the acquisition and maintenance of both soft and hard tissues. Skeletal remodeling requires energy and recent work has demonstrated that bone can influence insulin sensitivity and thereby regulate metabolic processes. New insights from mouse models into the role of IGF-binding proteins (IGFBPs) as more than mere depots for the IGFs has reignited investigations into the metabolic targets influenced by the IGF regulatory system and the pathways that link bone to adipose tissue. Although there remains continued uncertainty about the relative balance between the effects of circulating vs tissue IGF-I actions, the role of the IGFBPs has been redefined both as modulators of IGF-I action and as independent signaling factors. This review highlights several recent findings that shed new light on the physiologic role of the IGF regulatory system and its influence on skeletal and fat metabolism.
Introduction
The Insulin-like growth factor (IGF) regulatory system consists of IGFs (IGF-I and IGF-II), Type I and Type II IGF receptors, and regulatory proteins including IGF-binding proteins (IGFBP-1-6) and the acid-labile subunit (ALS) [Rosen et al., 1994]. The ligands in this system (i.e. the IGFs) are potent mitogens, and in some circumstances differentiation factors, that are bound in the circulation and interstitial fluid as binary (to IGFBPs) or ternary complexes (IGF-ALS-IGFBP-3, or-5) with little free IGF-I or –II. IGF bio-availability is regulated by the interaction of these molecules at the receptor level; hence changes in any component of the system will have profound effects on the biologic activity of the ligand. The IGFBPs have a particularly important role in regulating IGF-I access to its receptor since their binding affinity exceeds that of the IGF receptors. What makes the IGF system unique is that the IGFBPs are regulated in a cell-specific manner at the pericellular micro-environment such that small changes in their concentrations could strongly influence the mitogenic activity of IGF-I [Firth and Baxter, 2002; Hwa et al., 1999; Jones and Clemmons, 1995].
In addition to the ubiquitous expression of the IGFs in virtually all tissues, these growth factors also circulate in high concentrations. The main source of circulating IGF-I in mammals is the liver and its role as an endocrine mediator of growth hormone has been established for half a century. Although nearly 80% of the circulating IGF-I comes from hepatic sources, bone and fat both synthesize IGF-I and these tissues may contribute to the total circulating pool. The expression diversity of IGF-I and the IGFBPs in a tissue specific manner coupled with a large circulating pool adds several levels of complexity to our understanding of the role of the IGF-I/IGFBP system in metabolic homeostasis. However, there is little doubt that IGF-I signaling is requisite for proper skeletal accrual and adipose tissue development. On the other hand, the function of the IGFBPs and ALS in skeletal and adipose tissue development still remains unclear. Notwithstanding, emerging evidence points to both tissues as secretory organs that work in concert through endocrine, autocrine and paracrine signaling pathways to fine tune metabolic status. IGF-I is clearly one such factor. In this review, we will discuss the interrelationship of skeletal and adipose tissue through the IGF-I/IGFBPs regulatory system.
Role of IGF-I in adipogenesis
Adipogenesis is regulated by multiple cascades that involve many transcriptional regulators. The first step begins with lineage commitment of pluripotent mesenchymal stem cells, followed by the expansion of preadipocytes [Rosen and MacDougald, 2006; Rosen and Spiegelman, 2000]. Differentiation of preadipocytes into adipocytes has been extensively studied using preadipocyte cell lines, such as 3T3-L1 cells and 3T3-F442A cells. In early adipogenesis, preadipocytes undergo clonal expansion, which is followed by the cessation of cell cycle, and entry into terminal differentiation. The essential components of this network are the transcription factors, Cebpb and Pparg, which initiate a cascade of other factors that enhance differentiation of adipocytes. PPAR-gamma is a critical transcription factor for adipogenesis as evidenced by the finding that adipogenesis is completely blocked in Pparg-deficient mouse embryonic fibroblasts. PPAR-gamma dimerizes with Retinoic X receptor (RXR) alpha and initiates transcription by binding to the promoter of target genes. Furthermore, the transcriptional activity of PPAR-gamma is also determined by its ligand, and the recruitment of specific co-activators or co-repressors.
Not surprisingly, several growth factors have been reported to be involved in adipogenesis. In respect to IGF-1 signaling, accumulating evidence demonstrates the positive effect of IGF-1 on adipogenesis in vitro [Christoffersen et al., 1998; Smith et al., 1988] although in a context specific manner. Boney et al. reported that MAPK activation by IGF-I is markedly suppressed in differentiating 3T3-L1 cells compared to proliferating 3T3-L1 pre-adipocytes; inhibition of MAPK activation by a MEK inhibitor enhanced adipogenesis in 3T3-L1 cells. Interestingly, MAPK activation was not suppressed in response to epidermal growth factor (EGF), which has been shown to be an inhibitor for adipogenesis in differentiating preadipocytes, suggesting that impairment of MAPK activation by IGF-I is specific and critical for adipogenesis [Boney et al., 2000; Boney et al., 1998]. In addition, IGF-1 has been implicated in the regulation of the clonal expansion of 3T3-L1 cells [Siddals et al., 2002]. Because the switch from a proliferation phase to a terminal differentiation phase is accompanied by cell cycle cessation, down-regulation of MAPK could be the key process during adipogenesis. Interestingly, we found that IGF-1 expression during 3T3-L1 adipogenesis was decreased in early adipogenesis coincident with the period when 3T3-L1 cells stop their cell cycle, followed by a marked up-regulation during very late adipogenesis.
Signaling through IRS-1 and -2 also plays an important role in adipogenesis. Embryonic fibroblasts lacking either IRS-1 or IRS-2, which have impaired PI3K activity, displayed reduced adipogenesis with lower expression of Pparg and Cebpa vs control cells, and adipogenic capacity was completely blocked in IRS-1/IRS-2 deficient cells [Miki et al., 2001]. Unlike IGF-I induced MAPK activity, IRS-1 phosphorylation by IGF-I is maintained throughout adipogenesis [Boney et al., 1998]. Thus, IGF-1 signaling has a dual role during adipogenesis, and fine tuning of IGF-1 signaling is critical for proper adipogenesis. In vivo evidence supports the importance of IGF-I signaling in adipogenesis. First, IGF-I is expressed in adipose tissue, and could influence adipocyte metabolism and adipogenesis in a autocrine/paracrine manner [Villafuerte et al., 2000]. Second, several genetically altered mouse models of IGF-I signaling provide clues to understanding the role of IGF-1 in adipogenesis. For example, reduction of IGF-I receptor expression in adipose tissue has been shown to result in a smaller volume of adipose tissue [Holzenberger et al., 2001]. Akt1 and Akt2 double knockout mice (Akt-DKO mice) lack the lipid-containing brown adipocytes in brown adipose tissue [Peng et al., 2003]. In addition, mouse embryonic fibroblast from Akt-DKO mouse failed to show adipogenesis with impaired induction of Pparg [Peng et al., 2003]. In our mouse model harboring a point mutation in the IRS-1 gene, which results in a truncated mutation of IRS-1 and marked loss of Akt phosphorylation by IGF-I, there is growth retardation, and reduced amounts of adipose tissue and marrow adiposity [DeMambro et al.].
Pref-1 (preadipocyte factor-1) is a member of the epidermal growth factor-like family of proteins and is abundantly expressed in preadipocytes, whereas its expression decreases during the process of adipogenesis [Sul, 2009]. Pref-1 has been shown to suppress adipogenesis. Zhang et al. reported that IGF-I could bypass the suppressive effect of Pref-1 on adipogesis [Zhang et al., 2003]. Forced over-expression of either soluble form or full-length Pref-1 blocked MDI (IBMX, insulin and dexamethasone)-induced adipogenesis of 3T3-L1 cells, but IGF-I antagonized the suppressive effect of Pref-1 in a dose dependent manner.
Adipocytes produce a variety of secretory factors, acting on neighbor cells in a paracrine/autocrine manner. Obesity is now recognized as a condition with adipocyte hypertrophy and increased number of adipocytes. Because the expression profile of secretory factors in hypertrophic adipocytes is different from those in adipocytes of normal size, it is not surprising that factors produced by hypertrophic adipocytes can influence and regulate the number of adipocytes. This occurs by promoting the proliferation and differentiation of preadipocytes to meet the excess demand for energy storage. Marques et al. reported that IGF-I secreted from adipose tissue of rodents fed a high-fat diet (HFD) stimulated proliferation of preadipocytes[Marques et al., 2000]. These authors collected the conditioned media (CM) from inguinal adipose tissue from rats fed a HFD or a low-fat diet (LFD), and analyzed the effect of the CM on preadipocyte proliferation. CM from HFD-fed rat showed greater proliferation capacity vs LFD-fed rat, and this effect was attenuated when IGF-I was depleted from the CM [Marques et al., 2000]. Taken together, it can be concluded that the signaling cascade exerted by IGF-I, especially the IRS-1/PI3K/Akt pathway, is critical for adipogenesis, and impairment of this signaling pathway results in a defect in adipose tissue formation.
Recent updates of the role of IGF-1 on skeleton
There is clinical evidence that serum IGF-1 concentrations have a positive correlation with skeletal mass. For example, peak bone acquisition at puberty is accompanied by the dramatic increase in IGF-I serum levels [Kawai and Rosen, 2009]. Human cohort studies in adults also have revealed the positive association between serum IGF-I levels and bone mass in women [Garnero et al., 2000; Langlois et al., 1998]. Recent advances in molecular biology and development of genetically modified mouse have expanded our understanding of the role of IGF-1 in skeleton. Global deletion of IGF-I in mice showed postnatal growth retardation [Liu et al., 1993] although most of the mice died perinatally. IGF-I heterozygous mice on CD-1 background displayed reduced serum IGF-I levels, altered body weight and low cortical bone mineral density [He et al., 2006]. Similarly, IGF-IR knockout mice showed organ hypoplasia, delayed skeletal calcification, severe growth retardation by 45% and invariably die postnatally due to respiratory dysfunction [Liu et al., 1993]. Additionally, Akt1 and Akt2 double knock out mice showed severely delayed skeletal development [Peng et al., 2003]. Thus, IGF-I signaling is clearly an important factor in skeletal development.
One of the complexities in understanding the role of IGF-I in the skeleton lies in the presence of both circulating and locally produced IGF-I. Circulating IGF-1 functions mainly in an endocrine manner, whereas IGF-1 produced in the skeletal environment functions in a paracrine/autocrine manner. One of the challenges in this field has been to clarify the differential roles of circulating and local IGF-I. Several genetically altered animal models have been developed to address this issue. Osteoblast-specific IGF-I transgenic mouse showed increased bone mineral density and increased trabecular bone volume despite normal levels of circulating IGF-1 [38], but cortical bone volume was not altered. In line with this, osteoblast-specific deletion of IGF-IR showed reduced trabecular bone volume [39]. Thus, local IGF-I signaling is requisite for proper skeletal acquisition. Yakar et al. generated a liver-specific IGF-I deficient mice (LID mice) using an albumin promoter and examined the role of IGF-I produced by liver on the skeleton [40, 41]. Although serum IGF-I levels was reduced by 75% in LID mice, LID mice exhibited relatively normal development with only a 6% reduction in femur length and body weight. Of note the skeletal phenotype included a marked reduction in cortical bone volume and periosteal circumference despite the lack of trabecular bone phenotype [42]. To further analyze the role of circulating IGF-I on skeletal mass, these same authors crossed LID mouse with ALS knockout mice which have a similar reduction in serum IGF-I level by 65% and low cortical bone volume. The double knockout mice revealed a reduction in serum IGF-I by 85–90%, despite normal expression of skeletal IGF-I, and reduced cortical bone volume. These mice also exhibited severe growth retardation with disordered growth plates. To better understand the role of circulating IGF-I, two independent groups generated transgenic mice expressing IGF-I in liver in an IGF-I null background. Stratikopoulos et al. generated a mouse model carrying IGF-I cDNA in an Igf1 null background which is regulated by a native promoter/enhancer of IGF-I gene only in liver and showed that endocrine IGF-I contributed approximately 30% to the adult mouse body size [Stratikopoulos et al., 2008]. Elis et al. generated Igf1 transgenic mice under the transthyretin promoter in an Igf1 null background (KO-HIT (Hepatic IGF-1 transgenic mice)) and analyzed the skeletal phenotype in detail [Elis et al.]. KO-HIT, with elevated serum IGF-I but no autocrine/paracrine IGF-I, exhibited approximately 3-fold increase in serum IGF-I levels. Body weight of KO-HIT mice was similar to that of controls up to 16 weeks but these mice had shorter femorae at 4 weeks of age and exhibited catch-up growth by 8 weeks of age. These data suggested that locally expressed IGF-I is important in the regulation of body length during first the 4 weeks of life but circulating IGF-I can compensate for the loss of local IGF-I after that time. In respect to the skeletal phenotype, KO-HIT mice exhibited shorter femorae and smaller bone size at 4 weeks of age, but then showed a marked increase in these parameters. Thus, circulating IGF-I can compensate for the loss of locally produced IGF-I in a developmentally stage- specific manner.
Role of IGFBPs in adipose tissue
IGF binding proteins (IGFBPs) are members of a highly conserved family and modulate IGF-1 action by regulating the access of IGF-I to its receptor in the pericellular environment. The biological effects of the IGFBPs are very dependent on their concentration relative to IGF and are tissue specific [Firth and Baxter, 2002; Hwa et al., 1999; Jones and Clemmons, 1995]. Generally, IGFBPs have been shown to be negative regulators for IGF-I signaling. In support of this notion, the Igfbp1 transgenic mouse gained less weight, and showed reduced fat accumulation and adipocyte size vs wild type mice under a sucrose-enriched diet [Rajkumar et al., 1999]. IGF-I action on the proliferation and differentiation of preadipocytes in vitro was impaired in preadipocytes from the Igfbp1 transgenic mouse [Rajkumar et al., 1999]. Furthermore, IGFBP-1 has been shown to inhibit IGF-I-induced clonal expansion of 3T3-L1 cells [Siddals et al., 2002]. Thus, IGFBP-1 is likely functioning as a negative regulator for IGF-I during adipogenesis. Similarly, Igfbp2 transgenic mice were protected from age-related glucose intolerance and obesity. In addition, these mice were also protected from high-fat diet induced obesity and insulin-resistance [Wheatcroft et al., 2007]. In vitro, IGFBP-2 blocked adipogenesis by antagonizing IGF-I, but not insulin, with reduced expression of Pparg and aP2. Recently, Hedbacker et al. reported the antidiabetic effects of IGFBP-2. These authors showed that leptin induced Igfbp2 expression in the liver, which is insulin independent, and that adenoviral transduction of IGFBP-2 in liver improved insulin sensitivity in ob/ob mouse. The antidiabetic effects of IGFBP-1 and -2 are also evident from human epidemiological studies [Ahmed et al., 2007; Hu et al., 2009; Rajpathak et al., 2008; Sandhu et al., 2002]. Sandhu et al. performed a cohort study with 615 normo- glycaemic adults (45–65 years), and analyzed the role of IGF-I and IGFBP-1 in the development of glucose intolerance. After 4.5 years follow-up, the authors found that the participants with higher IGF-I concentrations exhibited a reduced risk for the development of glucose intolerance or type 2 diabetes compared with those who had lower concentrations of IGF-I and that this inverse association was independently modified by IGFBP-1, suggesting the important role of IGF-I and IGFBP-1 in glucose homoeostasis [Sandhu et al., 2002]. Rajpathak et al. analyzed the association of IGFBP-1 with glucose intolerance in 922 adults (≥65 years old) and reported that high serum IGFBP-1 levels were associated with a reduced risk for the impaired glucose tolerance and fasting glucose. [Rajpathak et al., 2008]. Hu et al. also examined serum IGF-I, IGFBP-1, and IGFBP-2 from 625 adults (≥70 years), and demonstrated that higher IGFBP-1 and -2 were significantly associated with reduced fasting insulin, fasting glucose, and adiposity, [Hu et al., 2009]. Thus, IGFBP-1 and -2 could be important determinants of glucose metabolism and adiposity in part by enhancing IGF-I action.
In addition to their role as transport proteins for the IGFs, the IGFBPs may have IGF-I binding independent functions [Firth and Baxter, 2002]. Interestingly, IGFBPs possess several functional motifs including heparin-binding domains and RGD sequences. In addition, some of the IGFBPs exhibit intracellular localization, suggesting the possibility that IGFBPs influence glucose metabolism and adiposity in a manner independent of IGF-I action. Chan et al. reported that IGFBP-3 suppressed 3T3-L1 adipogenesis independent of IGF-I binding [Chan et al., 2009]. Unlike IGFBP-2, IGFBP-3 blocked 3T3-L1 adipogenesis induced by insulin, and an IGFBP-3 mutant, which has a markedly reduced affinity for IGF-I, also exhibited a similar effect in wild-type cells, suggesting that the effect of IGFBP-3 on blocking adipogenesis is independent of IGF-I binding. Interestingly, these authors showed that IGFBP-3 binding to retinoid X is a critical step in this inhibition. Wild type IGFBP-3, not a mutant IGFBP-3 lacking RXRα binding ability, suppressed adipogenesis of 3T3-L1 cells, and inhibited PPARgamma dimerization with RXRα, resulting in impaired PPARgamma transcriptional activity [Chan et al., 2009]. IGFBP-2 has been also shown to be localized intracellularly, but the precise role of this localization remains to be clarified [Miyako et al., 2009; Russo et al., 1994; Terrien et al., 2005].
Role of IGFBPs in skeleton
IGF-I bioactivity is modulated by the IGFBPs (IGFBP1-6) and their role in skeletal acquisition has been also analyzed using genetically altered mice. IGFBPs primarily work as an inhibitor for IGF-I and IGFBP-2 also blocks IGF-I binding to its receptor. In vitro, IGFBP-2 inhibits IGF-I stimulated bone cell proliferation, bone collagen synthesis and bone formation. Over-expression of Igfbp2 in vivo has been demonstrated to exhibit reduced bone mass and inhibit growth hormone (GH)-stimulated linear growth in GH transgenic mice, possibly by antagonizing GH/IGF-I axis [Hoeflich et al., 2001]. Human studies also have demonstrated the inverse relationship between serum IGFBP-2 levels and bone mass [Amin et al., 2004; Nakaoka et al., 2001; van den Beld et al., 2003]. Amin et al. reported that IGFBP-2 levels increased with age, and that serum IGFBP-2 levels showed negative association with bone mineral density both in men and women [Amin et al., 2004]. van den Beld et al showed that higher serum IGFBP-2 levels were associated with higher degree of disability, a lower physical performance, muscle strength, bone mineral density of proximal femur [van den Beld et al., 2003]. Despite these studies, there is also growing evidence of an anabolic effect of IGFBP-2 on the skeleton. Khosla et al. reported that IGFBP-2 stimulated bone formation in association with IGF-II in patients with hepatitis C-associated osteosclerosis [Conover et al., 2002; Khosla et al., 1998]. Additionally, we have recently examined the bone phenotype of Igfbp2−/− mice. These mice had a gender and compartment specific skeletal phenotype. Igfbp2−/− females had increased cortical thickness with a greater periosteal circumference, whereas male Igfbp2−/− males had reduced cortical bone size. Trabecular bone volume was reduced by 20% in male Igfbp2−/− whereas trabecular bone was not affected in female Igfbp2−/− mice. In addition, PTEN expression was up-regulated in osteoblasts isolated from bone marrow of Igfbp2−/− mice compared to controls. Because PTEN regulates IGF-I signaling in a negative direction, IGF-I signaling may be impaired in Igfbp2−/− mice.
IGFBP-3 is the predominant IGFBP forming a tertiary complex with IGF-I and ALS. Like other IGFBPs, IGFBP-3 can also inhibit IGF-I actions. Consistently, skeletal phenotypes of Igfbp3 transgenic mice demonstrated impaired cortical and trabecular bone mineral density and reduced trabecular connectivity and size. These changes are possibly caused by the antagonistic effect of IGFBP-3 on IGF-I signaling [Silha et al., 2003]. IGFBP-4 is one of the most abundant IGFBPs synthesized by bone cells. Targeted over-expression of Igfbp4 in bone using the human osteocalcin promoter results in postnatal growth retardation, altered bone turnover and low bone mass [Zhang et al., 2003]. Igfbp4−/− mice also have modest growth retardation at birth and reduced bone mineral density. IGFBP-5 transgenic mice under the control of osteocalcin promoter showed decreased bone volume with low bone formation [Devlin et al., 2002]. These lines of evidence demonstrate that physiological levels of IGFBPs have an anabolic effect on skeleton at least in part through targeting IGF-I to the skeletal micro-environment.
Conclusion
The IGF-I/IGFBPs system plays an important role regulating bone mass and adipose tissue homeostasis. Since obesity and osteoporosis have become serious medical issues, development of new therapeutic interventions for these diseases are required. Recent advances in this field suggest that the IGF/IGFBP system is involved in the pathogenesis of these diseases and provides new insights into their physiological role. Initially there were very high expectations for the use of recombinant IGF-I in various neuromuscular and skeletal disorders. However, pharmacologically, IGF-I has not met those expectations and has been used therapeutically only in some individuals with growth failure, particularly those who are growth hormone resistant. Ironically, the IGFBPs may prove to be more useful in the treatment of insulin resistance, diabetes, and osteoporosis than IGF-I. And pharmacologic blockade of the IGF receptors is now a recognized strategy in combined clinical trials of several different agents for malignant neoplasms. Further understanding of the IGF/IGFBP system is likely to shed new light on both the origin of metabolic diseases and their treatment.
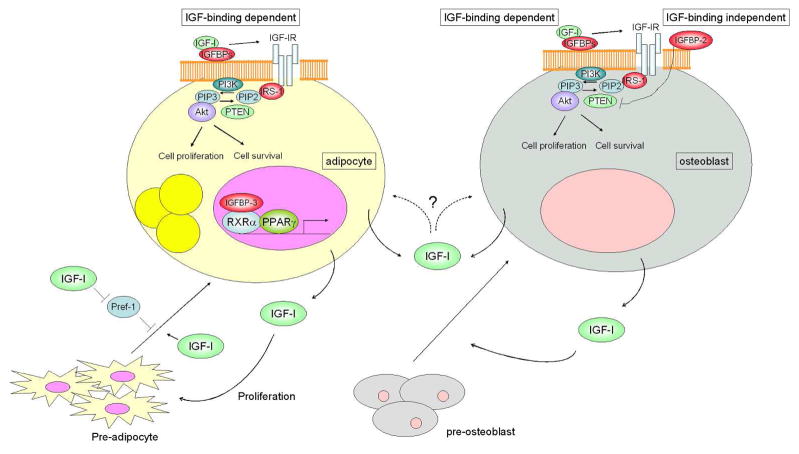
Figure 1. Schematic model for IGF-I and IGFBPs action on adipocytes and osteoblasts.
IGFBPs determine the IGF-I accessibility to its receptor through IGF-I binding, while IGFBPs may also possess IGF-I binding independent action. For example, IGFBP-2 suppresses PTEN expression in osteoblastic cells and IGFBP-3 binds to RXRα and regulates PPARγ transcriptional activity in adipocytic cells. Circulating IGF-I has a significant impact on adipose and skeletal development, whereas locally produced IGF-I also functions in an autocrine/paracrine manner. IGF-I stimulates adipogenesis in part through the activation of PI3K pathway and also enhances the maturation of pre-osteoblasts into osteoblasts.
References
- Ahmed RL, Thomas W, Schmitz KH. Interactions between insulin, body fat, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2007;16:593–7. doi: 10.1158/1055-9965.EPI-06-0775. [DOI] [PubMed] [Google Scholar]
- Amin S, Riggs BL, Atkinson EJ, Oberg AL, Melton LJ, 3rd, Khosla S. A potentially deleterious role of IGFBP-2 on bone density in aging men and women. J Bone Miner Res. 2004;19:1075–83. doi: 10.1359/JBMR.040301. [DOI] [PubMed] [Google Scholar]
- Boney CM, Gruppuso PA, Faris RA, Frackelton AR., Jr The critical role of Shc in insulin-like growth factor-I-mediated mitogenesis and differentiation in 3T3-L1 preadipocytes. Mol Endocrinol. 2000;14:805–13. doi: 10.1210/mend.14.6.0487. [DOI] [PubMed] [Google Scholar]
- Boney CM, Smith RM, Gruppuso PA. Modulation of insulin-like growth factor I mitogenic signaling in 3T3-L1 preadipocyte differentiation. Endocrinology. 1998;139:1638–44. doi: 10.1210/endo.139.4.5920. [DOI] [PubMed] [Google Scholar]
- Chan SS, Schedlich LJ, Twigg SM, Baxter RC. Inhibition of adipocyte differentiation by insulin-like growth factor-binding protein-3. Am J Physiol Endocrinol Metab. 2009;296:E654–63. doi: 10.1152/ajpendo.90846.2008. [DOI] [PubMed] [Google Scholar]
- Christoffersen CT, Tornqvist H, Vlahos CJ, Bucchini D, Jami J, De Meyts P, Joshi RL. Insulin and insulin-like growth factor-I receptor mediated differentiation of 3T3-F442A cells into adipocytes: effect of PI 3-kinase inhibition. Biochem Biophys Res Commun. 1998;246:426–30. doi: 10.1006/bbrc.1998.8637. [DOI] [PubMed] [Google Scholar]
- Conover CA, Johnstone EW, Turner RT, Evans GL, John Ballard FJ, Doran PM, Khosla S. Subcutaneous administration of insulin-like growth factor (IGF)-II/IGF binding protein-2 complex stimulates bone formation and prevents loss of bone mineral density in a rat model of disuse osteoporosis. Growth Horm IGF Res. 2002;12:178–83. doi: 10.1016/s1096-6374(02)00044-8. [DOI] [PubMed] [Google Scholar]
- DeMambro VE, Kawai M, Clemens TL, Fulzele K, Maynard JA, Marin de Evsikova C, Johnson KR, Canalis E, Beamer WG, Rosen CJ, Donahue LR. A novel spontaneous mutation of Irs1 in mice results in hyperinsulinemia, reduced growth, low bone mass and impaired adipogenesis. J Endocrinol. 204:241–53. doi: 10.1677/JOE-09-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RD, Du Z, Buccilli V, Jorgetti V, Canalis E. Transgenic mice overexpressing insulin-like growth factor binding protein-5 display transiently decreased osteoblastic function and osteopenia. Endocrinology. 2002;143:3955–62. doi: 10.1210/en.2002-220129. [DOI] [PubMed] [Google Scholar]
- Elis S, Courtland HW, Wu Y, Rosen CJ, Sun H, Jepsen KJ, Majeska RJ, Yakar S. Elevated serum levels of IGF-1 are sufficient to establish normal body size and skeletal properties, even in the absence of tissue IGF-1. J Bone Miner Res. doi: 10.1002/jbmr.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–54. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Garnero P, Sornay-Rendu E, Delmas PD. Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet. 2000;355:898–9. doi: 10.1016/s0140-6736(99)05463-x. [DOI] [PubMed] [Google Scholar]
- He J, Rosen CJ, Adams DJ, Kream BE. Postnatal growth and bone mass in mice with IGF-I haploinsufficiency. Bone. 2006;38:826–35. doi: 10.1016/j.bone.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Hoeflich A, Nedbal S, Blum WF, Erhard M, Lahm H, Brem G, Kolb HJ, Wanke R, Wolf E. Growth inhibition in giant growth hormone transgenic mice by overexpression of insulin-like growth factor-binding protein-2. Endocrinology. 2001;142:1889–98. doi: 10.1210/endo.142.5.8149. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Hamard G, Zaoui R, Leneuve P, Ducos B, Beccavin C, Perin L, Le Bouc Y. Experimental IGF-I receptor deficiency generates a sexually dimorphic pattern of organ-specific growth deficits in mice, affecting fat tissue in particular. Endocrinology. 2001;142:4469–78. doi: 10.1210/endo.142.10.8461. [DOI] [PubMed] [Google Scholar]
- Hu D, Pawlikowska L, Kanaya A, Hsueh WC, Colbert L, Newman AB, Satterfield S, Rosen C, Cummings SR, Harris TB, Ziv E. Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: the Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2009;57:1213–8. doi: 10.1111/j.1532-5415.2009.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–87. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kawai M, Rosen CJ. Insulin-like growth factor-I and bone: lessons from mice and men. Pediatr Nephrol. 2009;24:1277–85. doi: 10.1007/s00467-008-1040-6. [DOI] [PubMed] [Google Scholar]
- Khosla S, Hassoun AA, Baker BK, Liu F, Zein NN, Whyte MP, Reasner CA, Nippoldt TB, Tiegs RD, Hintz RL, Conover CA. Insulin-like growth factor system abnormalities in hepatitis C-associated osteosclerosis. Potential insights into increasing bone mass in adults. J Clin Invest. 1998;101:2165–73. doi: 10.1172/JCI1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, Kiel DP. Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab. 1998;83:4257–62. doi: 10.1210/jcem.83.12.5308. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Marques BG, Hausman DB, Latimer AM, Kras KM, Grossman BM, Martin RJ. Insulin-like growth factor I mediates high-fat diet-induced adipogenesis in Osborne-Mendel rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R654–62. doi: 10.1152/ajpregu.2000.278.3.R654. [DOI] [PubMed] [Google Scholar]
- Miki H, Yamauchi T, Suzuki R, Komeda K, Tsuchida A, Kubota N, Terauchi Y, Kamon J, Kaburagi Y, Matsui J, Akanuma Y, Nagai R, Kimura S, Tobe K, Kadowaki T. Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol Cell Biol. 2001;21:2521–32. doi: 10.1128/MCB.21.7.2521-2532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyako K, Cobb LJ, Francis M, Huang A, Peng B, Pintar JE, Ariga H, Cohen P. PAPA-1 Is a nuclear binding partner of IGFBP-2 and modulates its growth-promoting actions. Mol Endocrinol. 2009;23:169–75. doi: 10.1210/me.2008-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaoka D, Sugimoto T, Kaji H, Kanzawa M, Yano S, Yamauchi M, Sugishita T, Chihara K. Determinants of bone mineral density and spinal fracture risk in postmenopausal Japanese women. Osteoporos Int. 2001;12:548–54. doi: 10.1007/s001980170075. [DOI] [PubMed] [Google Scholar]
- Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–65. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar K, Modric T, Murphy LJ. Impaired adipogenesis in insulin-like growth factor binding protein-1 transgenic mice. J Endocrinol. 1999;162:457–65. doi: 10.1677/joe.0.1620457. [DOI] [PubMed] [Google Scholar]
- Rajpathak SN, McGinn AP, Strickler HD, Rohan TE, Pollak M, Cappola AR, Kuller L, Xue X, Newman AB, Strotmeyer ES, Psaty BM, Kaplan RC. Insulin-like growth factor-(IGF)-axis, inflammation, and glucose intolerance among older adults. Growth Horm IGF Res. 2008;18:166–73. doi: 10.1016/j.ghir.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ, Donahue LR, Hunter SJ. Insulin-like growth factors and bone: the osteoporosis connection. Proc Soc Exp Biol Med. 1994;206:83–102. doi: 10.3181/00379727-206-43726. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–71. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- Russo VC, Edmondson SR, Mercuri FA, Buchanan CR, Werther GA. Identification, localization, and regulation of insulin-like growth factor binding proteins and their messenger ribonucleic acids in the newborn rat olfactory bulb. Endocrinology. 1994;135:1437–46. doi: 10.1210/endo.135.4.7523098. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet. 2002;359:1740–5. doi: 10.1016/S0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- Siddals KW, Westwood M, Gibson JM, White A. IGF-binding protein-1 inhibits IGF effects on adipocyte function: implications for insulin-like actions at the adipocyte. J Endocrinol. 2002;174:289–97. doi: 10.1677/joe.0.1740289. [DOI] [PubMed] [Google Scholar]
- Silha JV, Mishra S, Rosen CJ, Beamer WG, Turner RT, Powell DR, Murphy LJ. Perturbations in bone formation and resorption in insulin-like growth factor binding protein-3 transgenic mice. J Bone Miner Res. 2003;18:1834–41. doi: 10.1359/jbmr.2003.18.10.1834. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Wise LS, Berkowitz R, Wan C, Rubin CS. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J Biol Chem. 1988;263:9402–8. [PubMed] [Google Scholar]
- Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A. The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci U S A. 2008;105:19378–83. doi: 10.1073/pnas.0809223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol. 2009;23:1717–25. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrien X, Bonvin E, Corroyer S, Tabary O, Clement A, Henrion Caude A. Intracellular colocalization and interaction of IGF-binding protein-2 with the cyclin-dependent kinase inhibitor p21CIP1/WAF1 during growth inhibition. Biochem J. 2005;392:457–65. doi: 10.1042/BJ20050517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Beld AW, Blum WF, Pols HA, Grobbee DE, Lamberts SW. Serum insulin-like growth factor binding protein-2 levels as an indicator of functional ability in elderly men. Eur J Endocrinol. 2003;148:627–34. doi: 10.1530/eje.0.1480627. [DOI] [PubMed] [Google Scholar]
- Villafuerte BC, Fine JB, Bai Y, Zhao W, Fleming S, DiGirolamo M. Expressions of leptin and insulin-like growth factor-I are highly correlated and region-specific in adipose tissue of growing rats. Obes Res. 2000;8:646–55. doi: 10.1038/oby.2000.83. [DOI] [PubMed] [Google Scholar]
- Wheatcroft SB, Kearney MT, Shah AM, Ezzat VA, Miell JR, Modo M, Williams SC, Cawthorn WP, Medina-Gomez G, Vidal-Puig A, Sethi JK, Crossey PA. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56:285–94. doi: 10.2337/db06-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Noohr J, Jensen CH, Petersen RK, Bachmann E, Teisner B, Larsen LK, Mandrup S, Kristiansen K. Insulin-like growth factor-1/insulin bypasses Pref-1/FA1-mediated inhibition of adipocyte differentiation. J Biol Chem. 2003;278:20906–14. doi: 10.1074/jbc.M300022200. [DOI] [PubMed] [Google Scholar]
- Zhang M, Faugere MC, Malluche H, Rosen CJ, Chernausek SD, Clemens TL. Paracrine overexpression of IGFBP-4 in osteoblasts of transgenic mice decreases bone turnover and causes global growth retardation. J Bone Miner Res. 2003;18:836–43. doi: 10.1359/jbmr.2003.18.5.836. [DOI] [PubMed] [Google Scholar]