Abstract
Background.
The recently developed non-invasive high-intensity focussed ultrasound (HIFU) technique for the destruction of parathyroid adenomas could also be of interest for the treatment of secondary hyperparathyroidism (SHP) in patients with chronic kidney disease (CKD). We conducted a pilot study using this method.
Methods.
Five chronic haemodialysis patients with severe SHP underwent one to three HIFU treatments, respectively. They had at least one or two enlarged parathyroid glands, which were accessible to this technique.
Results.
In Patients 1-I and 5-V, serum intact parathyroid hormone (iPTH) could be successfully reduced in the long run. In Patient 3-N, serum iPTH decreased dramatically down to the normal range but increased again subsequently. In Patients 2-E and 4-D, transient reductions in serum iPTH were also obtained but HIFU failed to correct SHP during follow-up. Serum total calcium and phosphorus decreased in four among the five patients, either transiently or permanently. Serum total alkaline phosphatases were reduced in four of five patients. Side effects included local oedema, transient impairment of vocal cord mobility and bitonal voice.
Conclusions.
HIFU treatment may be of help in controlling SHP in selected patients with CKD. Further experience is clearly needed.
Keywords: chronic kidney disease, high-intensity focussed ultrasound, parathyroid ablation, secondary hyperparathyroidism
Background
The successful management of severe forms of secondary hyperparathyroidism (SHP) in patients with chronic kidney disease (CKD) may prove impossible based on medical therapy alone owing to contraindications to drug treatment, medication intolerance and non-compliance. Even at present, some of these patients still require surgical parathyroidectomy [1, 2].
Alternative solutions to parathyroid surgery have long been sought. Percutaneous ethanol injection as a possible alternative is being successfully used in expert centres in Japan [3] but not in other countries [4–7].
High-intensity focussed ultrasound (HIFU) is a non-invasive ablative method based on the generation of extracorporeal ultrasound waves focussed on target tissues. The energy propagates through the skin without damaging it, up to the focal point where the temperature increases. HIFU has been recently shown to be a promising procedure for patients with primary hyperparathyroidism [8].
The aim of the present pilot study was to test the feasibility, safety and efficacy of the HIFU technique in five patients with CKD and severe SHP.
Materials and methods
Five patients with severe SHP agreed to undergo HIFU treatment. Table 1 shows their baseline clinical characteristics. All of them had end-stage renal disease and were on thrice-weekly stable haemodialysis treatment for at least 3 months. The serum PTH was >50 pmol/L and on ultrasonography (US), there was at least one or two enlarged parathyroid glands accessible to HIFU treatment, as shown in Table 2, with no contraindications to this method [8]. Accessibility of the gland to HIFU treatment was defined as follows: depth of the gland ≤23 mm from the posterior border of the gland to skin surface and location >2 mm laterally from the oesophagus or the carotid artery and/or >3 mm laterally from the trachea.
Table 1.
Baseline demographic characteristics
| Patient ID | Age (years) | Gender | Underlying nephropathy causes | CKD-associated complications | Haemodialysis vintage (years) |
| 1-I | 56 | M | Membranous glomerulonephritis | Soft tissue calcifications, anaemia | 7 |
| 2-E | 55 | F | ADPKDa and chronic pyelonephritis | Hypertension, anaemia | 5 |
| 3-N | 37 | M | Obstructive uropathy (Marion’s disease) | Multiple fractures (femoral neck, patella and wrist) | 19 |
| 4-D | 35 | M | ADPKD | None | 2 |
| 5-V | 42 | M | Interstitial nephritis | Hypertension, anaemia | 28 |
ADPKD, adult-dominant polycystic kidney disease.
Table 2.
Parathyroid glands location and volumes, number of HIFU treatments (per gland and per patient) and evolution of gland volumes compared to baselinea
| Patient ID | Number of glands at ultrasound | Gland location | HIFU treatments | Baseline gland volume (mL) | 6 mo post-last HIFU (mL) | 12 mo post-last HIFU (mL) | Change of volume at last follow-up (%) |
| 1-I | 2 | RI | HIFU1 + HIFU2 | 0.59 | 0.13 | 0.07 | −88.1 |
| LI | HIFU3 | 2.12 | 0.66 | 1.00 | −52.8 | ||
| 2-E | 2 | LI | HIFU1 | 1.05 | 0.42 | n.a. | −60.0 |
| RI | HIFU2 + HIFU3 | 0.95 | 0.75 | n.a. | −21.1 | ||
| 3-N | 1 | LS | HIFU1 + HIFU2 | 1.24 | 0.83 | 0.73 | −41.1 |
| 4-D | 1 | RI | HIFU1 | 0.91 | 0.25 | 0.54 | −40.6 |
| 5-V | 2 | RI | HIFU1 | 0.87 | 0.05 | n.a. | −94.3 |
| LI | No treatment | 0.28 | 0.25 | n.a. | n.a. |
RI, right inferior; LI, left inferior; LS, left superior; mo, month; HIFU1, first HIFU treatment; HIFU2, second HIFU treatment; HIFU3, third HIFU treatment; n.a., not available.
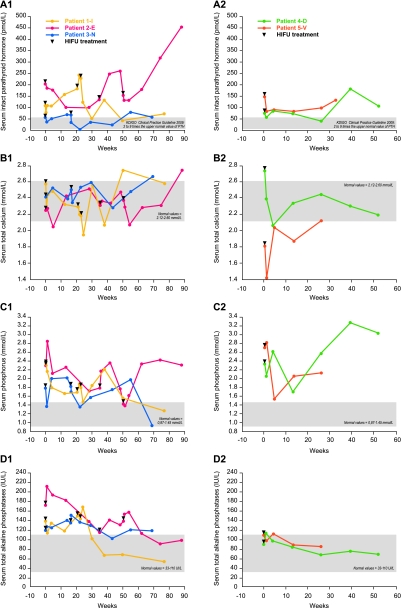
All the patients had high to very high serum intact parathyroid hormone (iPTH) levels (Figure 1), associated with high serum phosphorus and normal to high serum total calcium levels except for Patient 5-V who had slight hypocalcaemia. All patients were on long-term treatment with variable doses of oral calcitriol (comprised between 0.125 and 0.5 μg/day) and/or calcium carbonate (comprised between 600 and 4000 mg/day).
Fig. 1.
Serum biochemistry of all patients, separated in two groups: Group 1, Patients 1-I, 2-E and 3-N who received two or three HIFU treatments; Group 2, Patients 4-D and 5-V who received only one HIFU treatment, with a total follow-up till 90 weeks. (A–D) Graphs show changes in serum intact parathyroid hormone (A1 and A2), total calcium (B1 and B2), phosphorus (C1 and C2) and total alkaline phosphatase (D1 and D2) levels for the two groups after each HIFU treatment (black triangles).
The patients were investigated prior to the first HIFU treatment, 1 and 7 days, 1, 3, 6, 9 and 12 months post-therapy. In case of persistently elevated serum iPTH values or lack of parathyroid tumour shrinkage, a second HIFU treatment was performed at variable time points after the 1-month visit and follow-up offered as per initial therapy. Two HIFU treatments per gland were performed with a maximum of two glands treated per patient.
Biochemical parameters included serum values of iPTH (Elecsys®, Roche; reference range, 1.6–6.9 pmol/L), total calcium (reference range, 2.12–2.60 mmol/L), phosphorus (reference range, 0.87–1.45 mmol/L) and total alkaline phosphatases (reference range, 33–110 IU/L).
HIFU treatment was performed as reported recently [8], using the CE-marked HIFU system (TH-One; THERACLION, Paris, France). In brief, it included an electronics cabinet, an extracorporeal treatment head, a cooling unit and an ultrasound imaging scanner. US, including colour Doppler, was performed using a real-time 8-MHz transducer (Aloka, Prosound Alpha 7). The parathyroid volume was calculated according to the formula V = [a × b × c] × π/6, where a, b and c represent parathyroid gland dimensions. The patients were placed flat on their back, with the neck hyperextended. Conscious intravenous analgesia was administered using a combination of hypnotic agents with opioid and non-opioid analgesics. During therapy, pulse frequency, blood pressure, respiration rate and peripheral oxygen tension were monitored, and patients were asked to indicate any abnormal sensation. All HIFU treatments were performed by one physician (R.K.). The calculation of HIFU dose was based on treatment site depth. The total energy delivered was a function of the gland size.
Follow-up US was performed at 1, 3, 6, 9 and 12 months, irrespective of number of treatments. Alterations in gland size and vascularization were recorded.
The mobility of the vocal cords was assessed with indirect laryngoscopy before and after each HIFU treatment.
The study was approved by the local ethics committee, and written consent was obtained from each patient.
Results
Patients 1-I and 2-E received three HIFU treatments (HIFU1, HIFU2 and HIFU3), Patient 3-N received two HIFU treatments and Patients 4-D and 5-V received only one HIFU treatment each (see black triangles in Figure 1). Exposure parameters and treatment times for individual HIFU treatments are shown in Table 3.
Table 3.
Exposure parameters for each parathyroid gland to individual HIFU treatmentsa
| Average depth (mm) | Total exposure time (min) | Total HIFU delivered energy (kJ) | Mean HIFU power (W) | |
| Patient 1-I | ||||
| HIFU1, RI PT | 19.0 | 2.2 | 4.7 | 6.5 |
| HIFU2, RI PT | 16.0 | 2.2 | 5.1 | 3.0 |
| HIFU3, LI PT | 21.4 | 7.0 | 24.0 | 5.5 |
| Patient 2-E | ||||
| HIFU1, LI PT | 20.0 | 3.9 | 20.6 | 5.2 |
| HIFU2, RI PT | 18.0 | 8.9 | 19.9 | 7.9 |
| HIFU3, RI PT | 19.7 | 4.0 | 22.4 | 5.4 |
| Patient 3-N | ||||
| HIFU1, LS PT | 23.8 | 4.0 | 7.8 | 2.6 |
| HIFU2, LS PT | 22.6 | 9.0 | 15.3 | 5.0 |
| Patient 4-D | ||||
| HIFU1, RI PT | 21.8 | 3.1 | 8.6 | 3.7 |
| Patient 5-V | ||||
| HIFU1, RI PT | 19.7 | 2.1 | 11.0 | 5.9 |
The HIFU frequency was 3 MHz for all treatments. Total exposure time (duration of HIFU pulses per patient) and HIFU delivered energy [total delivered energy to parathyroid glands (PT) taking into account tissue attenuation] depend on treated PT volume. Due to pauses between pulses (cooling time, repositioning and pauses for other reasons), the mean power applied is quite low. RI, right inferior; LI, left inferior; LS, left superior.
Changes in biochemical parameters and gland volumes of the patients in response to one or more HIFU treatments are depicted in Figure 1 and Table 2, respectively. In Patient 1-I, serum iPTH decreased after HIFU1 and HIFU3, with transient reascensions after HIFU1 and HIFU2. Overall, there was a 60% decrease in iPTH from 112 pmol/L (baseline) to 45.1 pmol/L 6 months after last HIFU treatment (HIFU3). Serum total calcium decreased markedly after HIFU2 and HIFU3, and the control of serum phosphorus overall improved. The volume of the two treated glands decreased, respectively, down to 88% (from 0.59 to 0.07 mL) and to 53% (from 2.12 to 1.00 mL) 12 months after the last HIFU treatment (HIFU3).
In Patient 2-E, HIFU1 and HIFU3 also led to a decrease in serum iPTH but subsequently iPTH levels rose again, with the final value being higher than baseline (Figure 1). Serum total calcium decreased markedly after HIFU1 and HIFU3 to values slightly below normal. However, the control of serum phosphorus remained relatively poor. The volume of the first parathyroid gland treated decreased to ∼60% after HIFU1. Due to marked shrinkage of this first gland, it was decided to treat the other visible gland as well. In contrast, the volume of the second gland treated remained unchanged after HIFU2 but decreased after HIFU3, down to 21% from baseline.
In Patient 3-N, serum iPTH exhibited a maximum decrease from 59 to 38 pmol/L after HIFU1 and from 69 down to 4 pmol/L after HIFU2. Serum total calcium, phosphorus and total alkaline phosphatases remained essentially unchanged. A marked decrease of gland volume has been observed 3 months after last HIFU treatment. Thereafter, the volume remained constant.
In Patient 4-D, serum iPTH decreased from 72 to 56 pmol/L, and in Patient 5-V from 146 to 81 pmol/L after HIFU1. These two latter patients had no further HIFU treatments due to vocal cord mobility impairment for patient 4-D and road accident for patient 5-V. For both patients, a marked decrease in the volume of treated glands was observed. The volume reduction was 72 and 94% from baseline at 6 months post-HIFU1, however, with a subsequent reascension in Patient 4-D. In summary, serum total calcium, phosphorus levels and serum alkaline phosphatases decreased in four among the five patients, either transiently or permanently, but remained unchanged in Patient 3-N.
HIFU-related adverse events, all transient, consisted of mild subcutaneous oedema in three patients (for some days), of prolonged vocal cord mobility impairment in two patients (for 9 and 11 months, respectively), of prolonged bitonal voice in one patient (for 9 months) and of difficulty in swallowing water in one patient (for 3 weeks).
Discussion
Surgical removal of the parathyroid glands remains the ultimate treatment of the most severe forms of SHP in patients with CKD who fail to respond to or comply with medical treatment, including dietary phosphate restriction and optimal dialysis management in those with end-stage kidney disease or who do not tolerate it because of major adverse events [9]. This has also been recommended in the recent KDIGO guideline on the CKD-related mineral and bone disorder (CKD–MBD) [10].
Since most patients clearly prefer minimally invasive or non-invasive therapies, it appears legitimate to seek non-surgical methods for the physical destruction of hyperplastic parathyroid glands. The HIFU technique could be such an alternative option, as recently shown by us in patients with primary parathyroid adenomas [8]. HIFU might also be useful in CKD patients with SHP. However, the problem in such patients is that generally parathyroid hyperplasia is not limited to one gland, although the degree of hyperplasia may be highly variable from one gland to the other. We reasoned that in patients having only one or two greatly enlarged parathyroid glands, HIFU could be helpful in managing SHP.
The present pilot study in five cases with SHP represents the first experience with the HIFU technique in chronic haemodialysis patients. Our report illustrates the potential benefits, but also the limitations, of this method in the setting of CKD. A marked decrease of serum iPTH could be achieved in three patients after one or more (up to three) HIFU treatments applied to one or two accessible hyperplastic glands, down to the PTH range recommended by the recent KDIGO CKD–MBD guideline or even below [10]. Concomitantly, the control of serum calcium and phosphorus also was improved, although only transiently in most instances. In the long run, however, HIFU treatment was successful in only two patients. The main reasons for unsuccessful long-term PTH control in three of five patients probably included an extreme severity of SHP linked to the presence of remaining, undestroyed hyperplastic parathyroid glands, the impossibility to perform repeat HIFU treatments in some patients owing to side effects and the probable progression of parathyroid hyperplasia in non-treated glands. Of note, in patients with extremely severe SHP, the surgical removal of only one or two glands would also not be sufficient to correct parathyroid overfunction.
The presence of diffuse major parathyroid hyperplasia in three or more glands clearly is a limitation for the success of the HIFU method. The accessibility of the targeted gland for this type of therapy is another limitation. In any case, HIFU can only be considered, if proven effective by further study, as an adjunct therapy in patients with CKD and SHP since the primary causes of PTH oversecretion are not affected by this type of treatment. It could allow uraemic patients to respond more effectively to established medical therapy or reduce drug needs in that HIFU might greatly reduce the hyperplastic gland mass in some of them and thereby create a more favourable PTH secretory context. Clearly, even the entire destruction of one or two hyperplastic parathyroid glands in patients with CKD does not protect against progressive hyperplasia of the remaining glands if not accompanied by adequate medical treatment.
Side effects of HIFU treatment also have to be considered. Like with surgery, recurrent nerve palsy may occur but this complication should only be transient, as observed in two patients. Local subcutaneous oedema of short duration and without clinical significance was observed in three patients. There was no clear-cut association between the energy delivered to the glands and the occurrence of oedema or nerve palsy. The incidence of local side effects of HIFU treatment should decrease with improved selection concerning the size and location of parathyroid glands, possibly earlier treatment initiation and more extended experience with the maximal amount of energy needed for the destruction of the targeted parathyroid tissue.
The HIFU technique may be suitable for CKD patients with various degrees of SHP and hyperplastic parathyroid glands accessible to this technique, in those in whom medical treatment is unsuccessful, in patients in whom cinacalcet treatment is not possible or contraindicated and in those who refuse or have contraindications to parathyroid surgery.
In conclusion, the HIFU technique may enable a marked reduction of SHP in those uraemic patients in whom PTH oversecretion mainly stems from one or two HIFU accessible hyperplastic glands. In these patients, it could represent a non-surgical alternative to parathyroidectomy. More importantly, it might become a valuable adjunct therapy to medical treatment at earlier stages of SHP.
Acknowledgments
The study has been partly supported by Theraclion.
Conflict of interest statement. R.K. (MD, PhD) is consultant to, Theraclion. T.B.D. (MD) is an advisory board member and consultant for Theraclion. F.A. (PhD) and C.O. (MD) are employees of Theraclion.
References
- 1.Drüeke TB. Hyperparathyroidism in chronic kidney disease (Chapter 6) In: Singer F, editor. Diseases of Bone and Mineral Metabolism. South Dartmouth, MA: Endotext.org The Endocrine Source; 2009. http://www.endotext.org/parathyroid/parathyroid6/parathyroidframe6.htm (11 October 2011, date last accessed) [Google Scholar]
- 2.Cannata-Andía JB, Fernández-Martín JL, Zoccali C, et al. Current management of secondary hyperparathyroidism: a multicenter observational study (COSMOS) J Nephrol. 2008;21:290–298. [PubMed] [Google Scholar]
- 3.Fukagawa M, Kitaoka M, Tominaga Y, et al. Guidelines for percutaneous ethanol injection therapy of the parathyroid glands in chronic dialysis patients. Nephrol Dial Transplant. 2003;18(Suppl 3):iii31–iii33. doi: 10.1093/ndt/gfg1008. [DOI] [PubMed] [Google Scholar]
- 4.de Barros Gueiros JE, Chammas MC, Gerhard R, et al. Percutaneous ethanol (PEIT) and calcitrol (PCIT) injection therapy are ineffective in treating severe secondary hyperparathyroidism. Nephrol Dial Transplant. 2004;19:657–663. doi: 10.1093/ndt/gfg586. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham J, Danese M, Olson K, et al. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005;68:1793–1800. doi: 10.1111/j.1523-1755.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 6.Drüeke TB, Ritz E. Treatment of secondary hyperparathyroidism in CKD patients with cinacalcet and/or vitamin D derivatives. Clin J Am Soc Nephrol. 2009;4:234–241. doi: 10.2215/CJN.04520908. [DOI] [PubMed] [Google Scholar]
- 7.Cozzolino M, Galassi A, Pasho S, et al. Preventive measures and new pharmacological approaches of calcium and phosphate disorders. Contrib Nephrol. 2008;161:234–239. doi: 10.1159/000130696. [DOI] [PubMed] [Google Scholar]
- 8.Kovatcheva RD, Vlahov JD, Shinkov AD, et al. High-intensity focused ultrasound to treat primary hyperparathyroidism: a feasibility study in four patients. AJR Am J Roentgenol. 2010;195:830–835. doi: 10.2214/AJR.09.3932. [DOI] [PubMed] [Google Scholar]
- 9.Sarfati E, Drueke TB. Surgical management of secondary hyperparathyroidism. In: Olgaard K, Silver J, Salusky IB, editors. The Spectrum of Mineral and Bone Disorders in Chronic Kidney Disease. 2nd edn. Oxford, UK: Oxford University Press; 2010. pp. 543–559. [Google Scholar]
- 10.Kidney Disease-Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]