Abstract
Background: The IALT, JBR.10, ANITA and Cancer and Leukemia Group B 9633 trials compared adjuvant chemotherapy with observation for patients with resected non-small-cell lung cancer (R-NSCLC). Data from the metastatic setting suggest high tumor class III beta-tubulin (TUBB3) expression is a determinant of insensitivity to tubulin-targeting agents (e.g. vinorelbine, paclitaxel). In 265 patients from JBR.10 (vinorelbine–cisplatin versus observation), high TUBB3 was an adverse prognostic factor and was associated (nonsignificantly) with ‘greater’ survival benefit from chemotherapy. We explored this further in additional patients from JBR.10 and the other three trials.
Patients and methods: TUBB3 immunohistochemical staining was scored for 1149 patients on the four trials. The original JBR.10 cut-off scores were used to classify tumors as TUBB3 high or low. The prognostic and predictive value of TUBB3 on disease-free survival (DFS) and overall survival (OS) was assessed by Cox models stratified by trial and adjusted for clinical factors.
Results: High TUBB3 expression was prognostic for OS [hazard ratio (HR) = 1.27 (1.07–1.51), P = 0.008) and DFS [HR = 1.30 (1.11–1.53), P = 0.001). TUBB3 was not predictive of a differential treatment effect [interaction P = 0.20 (OS), P = 0.23 (DFS)]. Subset analysis (n = 420) on vinorelbine–cisplatin gave similar results.
Conclusions: The prognostic effect of high TUBB3 expression in patients with R-NSCLC has been validated. We were unable to confirm a predictive effect for TUBB3.
Keywords: chemotherapy, meta-analysis, predictive value, prognostic, randomized trial, tubulin
introduction
Platinum-based adjuvant chemotherapy is routinely given to patients with resected stage II–IIIA non-small-cell lung cancer (NSCLC) on the strength of the results of the NCIC/US NCI Intergroup study JBR.10 [1], Adjuvant Navelbine International Trialist Association (ANITA) [2], Cancer and Leukemia Group B (CALGB) 9633 [3] and International Adjuvant Lung Cancer Trial (IALT) [4] randomized controlled trials. In JBR.10 and ANITA, the tubulin-targeting agent vinorelbine was given with cisplatin; CALGB 9633 used another tubulin-targeting agent, paclitaxel, with carboplatin; and in IALT, a variety of two-drug regimens were used including cisplatin plus one of vinorelbine, vinblastine, vindesine, or etoposide. A meta-analysis of the results of these and other similar trials demonstrated an absolute improvement in 5-year overall survival (OS) of 5% with the addition of chemotherapy to surgery [5]. It is likely that some patients do not derive any benefit from treatment, some may have been cured by surgery alone, and some may even be harmed by chemotherapy. Given the toxic effects of chemotherapy and the resource utilization required to administer the treatment, it would be helpful to identify those patients who are destined to benefit from adjuvant chemotherapy before its administration. Conversely, it would be helpful to identify those patients who will not benefit and to spare them from undergoing adjuvant chemotherapy. It would also be helpful to be able to identify which chemotherapy regimen is most likely to be effective for an individual patient.
Class III beta-tubulin (TUBB3) is an isotype of beta-tubulin that is normally found in neural [6], vascular endothelial [7] and other tissues [8], where (like other tubulin isotypes) it is a building block for microtubules. TUBB3 is overexpressed in NSCLC and other cancers [9]. Preclinical studies in lung cancer cell lines [10] and a variety of other cell types suggest that TUBB3 up-regulation confers resistance to paclitaxel [11, 12] and vinorelbine [13]. In the setting of advanced NSCLC, it has been reported that high TUBB3 expression correlates with shorter survival and reduced response to chemotherapy regimens that contain an anti-tubulin drug, including taxanes and vinorelbine [14–18].
Given these results, high TUBB3 expression was examined for its prognostic significance in early NSCLC and for its value in predicting benefit from adjuvant vinorelbine–cisplatin chemotherapy using tumor tissues from 265 of the 482 patients in the JBR.10 randomized trial [7]. Although not significant, the analysis suggested that TUBB3 is an adverse prognostic factor in JBR.10, particularly in patients treated with surgery alone although not in patients given adjuvant chemotherapy. Additionally, and in contrast to the studies in the preclinical and metastatic settings, high TUBB3 expression appeared to correlate with ‘greater’ likelihood of benefit from adjuvant chemotherapy, although we were not able to demonstrate a significant interaction. We felt that these results were of sufficient interest to justify further study. Other authors have since reported on the adverse prognostic significance of high TUBB3 expression in resected non-small-cell lung cancer (R-NSCLC) [19, 20]; however, these studies were not designed to determine the value of TUBB3 in predicting benefit from adjuvant chemotherapy.
In this article, we report the results of a cross-validation study of the prognostic and predictive value of TUBB3 expression in tumors from patients who participated in four randomized controlled trials of adjuvant chemotherapy versus observation following complete resection of NSCLC.
patients and methods
patients and tumor tissues
The validation cohort comprised all patients for whom paraffin-embedded tumor tissues were available from the ANITA, IALT and CALGB 9633 trials, as well as patients from JBR.10 that were not included in the previously published TUBB3 study [7] because their tumor tissues only became available after that study was completed. The patients from JBR.10 included in the previous TUBB3 study [7] are hereafter labeled as ‘JBR.10(1)’, and the JBR.10 patients included in the validation cohort are labeled ‘JBR.10(2)’. The IALT and JBR.10(2) tissues were available as tissue microarray slides, and the CALGB 9633 and ANITA tissues were available as whole mount sections. This study was conducted with the approval of the Alberta Cancer Board Research Ethics Board, Canada, in accordance with internationally accepted standards for research involving human subjects.
immunohistochemistry
Tumor tissue sections were received on glass slides, stained with an antibody to TUBB3 and scored using the method previously described in the JBR.10(1) study [7]. Briefly, the TUBB3 antibody (clone TUJ1 from the laboratory of Anthony Frankfurter) was applied following deparaffinization, rehydration and antigen retrieval. Chromogenic detection was with biotin–avidin followed by 3,3′-diaminobenzidine. TUBB3 expression was scored by two independent observers (PS and RL) using the same ‘H-score’ method employed in the JBR.10(1) TUBB3 study [7], generating a semiquantitative measure with a minimum possible score of 100 and a maximum of 300 based on the distribution of staining intensity among the malignant cells. Normal neurons and vascular endothelial cells served as strongly staining internal controls and tumor samples with known TUBB3 positivity served as external controls. Scores that were within 10% between the two observers were averaged, and scores that differed by >10% were resolved by consensus. Specimens were deemed nonassessable for TUBB3 expression if both observers felt that the amount of well-preserved and stained tumor tissue present on the slides was inadequate.
statistical analysis of the validation cohort
In the JBR.10(1) study [7], patients were classified as ‘TUBB3 high’ or ‘TUBB3 low’ based on the median H-score of 176. We rounded this score to 180 and applied it to the validation cohort: patients with TUBB3 H-scores of ≥180 were classified as TUBB3 high and patients with scores <180 as TUBB3 low. A logistic regression model stratified by trial was used to examine the correlation between TUBB3 and covariates. A Cox model stratified by trial and including the covariates of treatment assignment, sex, age, performance status, T stage, N stage, and histological subtype was used to examine the prognostic significance of TUBB3 expression on both OS and disease-free survival (DFS). The interaction between treatment assignment and TUBB3 status on OS and DFS was assessed in the models to determine the predictive value of TUBB3. Analyses were also carried out using distribution quartiles and test for trends. Overall, these analyses led to the same conclusion and only the analyses on the predictive value for the combined cohort are reported. A test for heterogeneity was used to compare the hazard ratio (HR) between trials.
subset analysis of the validation cohort
Since the JBR.10(1) TUBB3 study [7] looked only at patients treated with vinorelbine–cisplatin or observation, we repeated the analysis on the subset of patients in the validation cohort who were randomly allocated to vinorelbine–cisplatin or observation.
analysis of the validation cohort combined together with the previously published JBR.10(1) cohort
In an exploratory analysis, we pooled the data from the validation cohort with those of the patients from the JBR.10(1) TUBB3 study [7], with the previously published data reanalyzed using the same adjustments as in the cross-validation study. This dataset is hereafter referred to as the ‘combined cohort’. The subset of patients in the combined cohort who were randomly allocated to vinorelbine–cisplatin or observation was also analyzed.
Statistical significance was set at P = 0.05 using two-sided analysis.
results
patient characteristics
A comparison of the baseline patient characteristics for those patients included in the validation cohort versus those patients on the four trials for whom tumor tissue was not available is shown in Table 1. No significant differences were observed for age, sex, stage, performance status, histology, type of surgery, planned postoperative radiotherapy use, and proportion assigned to chemotherapy or observation.
Table 1.
Characteristics of patients in the validation cohort studied for TUBB3 expression, as compared with the patients in the four randomized trials without available tissue
| Characteristics | P value | Patients with slides (N = 1200) |
Patients without slides (N = 538) |
||
| n | % | n | % | ||
| Triala | <0.0001b | ||||
| ANITA | 152 | 13 | 54 | 10 | |
| IALT | 784 | 65 | 286 | 53 | |
| JBR.10(2) | 52 | 4 | 115 | 21 | |
| CALGB | 212 | 18 | 83 | 15 | |
| Radiotherapy planned | 0.11 | ||||
| No | 890 | 74 | 409 | 76 | |
| Yes | 310 | 26 | 129 | 24 | |
| Age category | 0.12 | ||||
| <50 | 189 | 16 | 101 | 19 | |
| 50–59 | 406 | 34 | 195 | 36 | |
| 60–69 | 468 | 39 | 186 | 35 | |
| ≥70 | 137 | 11 | 56 | 10 | |
| Sex | 0.94 | ||||
| Male | 955 | 80 | 417 | 78 | |
| Female | 245 | 20 | 121 | 22 | |
| Stage | 0.87 | ||||
| IA | 73 | 6 | 22 | 4 | |
| IB | 491 | 41 | 233 | 43 | |
| II | 340 | 28 | 173 | 32 | |
| III | 293 | 24 | 106 | 20 | |
| Unknown | 3 | <1 | 4 | <1 | |
| Performance status | 0.13 | ||||
| 0 | 650 | 54 | 283 | 53 | |
| 1 | 484 | 40 | 219 | 41 | |
| 2 | 62 | 5 | 34 | 5 | |
| Unknown | 4 | <1 | 2 | <1 | |
| Histology | 0.79 | ||||
| Squamous cell carcinoma | 566 | 47 | 253 | 47 | |
| Adenocarcinoma | 467 | 39 | 218 | 41 | |
| Other | 167 | 14 | 67 | 12 | |
| Type of surgery | 0.07 | ||||
| Pneumonectomy | 402 | 33 | 147 | 27 | |
| Other | 795 | 66 | 388 | 72 | |
| Unknown | 3 | <1 | 3 | <1 | |
| Treatment arm | 0.65 | ||||
| No chemotherapy | 595 | 49 | 275 | 50 | |
| Chemotherapy | 605 | 51 | 263 | 50 | |
The two populations were compared using a logistic regression stratified on trial.
Chi-square test.
TUBB3, class III beta-tubulin; CALGB, Cancer and Leukemia Group B.
immunohistochemistry
Among the 1200 patients in the validation cohort with tissue available, 1149 were assessable for TUBB3 expression. The mean TUBB3 H-score was 180 (standard deviation 71); the median was 170 (range 100–300). Using the cut-off of ≥180, 48% were TUBB3 high. After adjustment for covariates, there was no significant difference among trials in the proportion of TUBB3-high patients.
comparison of covariates in TUBB3-high versus-low patients
As shown in Table 2, in univariate analyses, TUBB3-high patients in the validation cohort were more likely to be female, younger, have non-squamous histology, and less likely to require pneumonectomy. No differences were seen with regard to stage or performance status. Only histology remained significantly associated with TUBB3 status in multivariate analysis (P < 0.0001).
Table 2.
Characteristics of patients with low versus high TUBB3 expression in the validation cohort (n = 1149)
| P valuea | β-tubulin low |
β-tubulin high |
|||
| n | % | n | % | ||
| Sex | 0.001 | 498 | 54 | 417 | 46 |
| Male | |||||
| Female | 97 | 41 | 137 | 59 | |
| Age (in years) | 0.008 (0.004) | 154 | 45 | 188 | 55 |
| <55 | |||||
| 55–64 | 251 | 54 | 211 | 46 | |
| >64 | 190 | 55 | 155 | 45 | |
| Stage | 0.53 (0.84) | ||||
| I | 261 | 49 | 273 | 51 | |
| II | 186 | 56 | 144 | 44 | |
| III | 146 | 52 | 136 | 48 | |
| Unknown | 2 | – | 1 | – | |
| N of TNM | 0.39 (0.98) | ||||
| 0 | 312 | 50 | 318 | 50 | |
| 1 | 161 | 57 | 120 | 43 | |
| 2 | 120 | 52 | 113 | 49 | |
| Unknown | 2 | – | 3 | – | |
| T of TNM | 0.95 (0.80) | ||||
| 1 | 71 | 55 | 57 | 45 | |
| 2 | 410 | 51 | 399 | 49 | |
| 3/4 | 112 | 54 | 97 | 46 | |
| Unknown | 2 | – | – | – | |
| Histology | <0.0001 | 385 | 71 | 158 | 29 |
| Squamous cell carcinoma | |||||
| Adenocarcinoma | 150 | 33 | 302 | 67 | |
| Other | 58 | 38 | 94 | 62 | |
| Unknown | 2 | – | |||
| Type of surgery | 0.002 | ||||
| Lobectomy/other | 363 | 48 | 395 | 52 | |
| Pneumonectomy | 230 | 59 | 158 | 41 | |
| Unknown | 2 | – | |||
| WHO PS | 0.95 | 319 | 52 | 298 | 48 |
| 0 | |||||
| 1/2 | 275 | 52 | 254 | 48 | |
| Unknown | 1 | – | 2 | – | |
Test for trend in parenthesis.
TUBB3, class III beta-tubulin; TNM, tumor–node–metastasis; WHO PS, World Health Organization performance status.
prognostic significance of TUBB3 expression
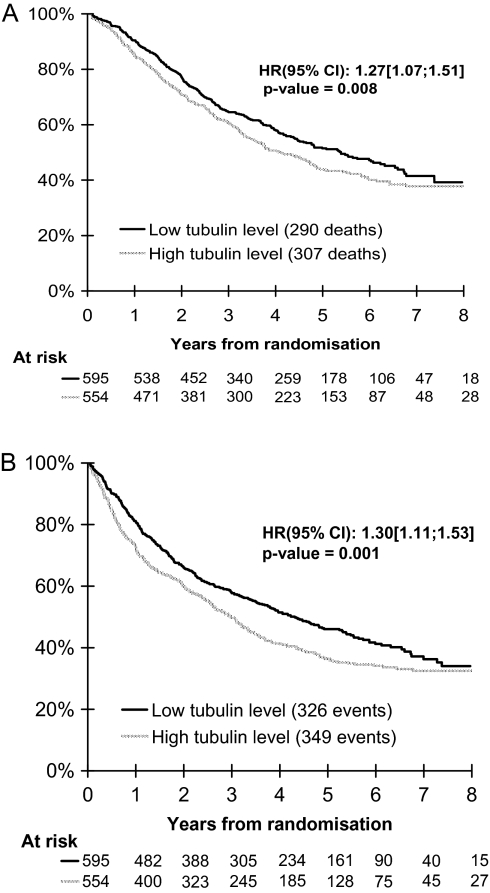
In Cox models, high TUBB3 expression in the validation cohort was an independent adverse prognostic factor for OS [HR = 1.27; 95% confidence interval (CI) 1.07–1.51; P = 0.008; Figure 1A] and DFS (HR = 1.30; 95% CI 1.11–1.53; P = 0.001; Figure 1B). There was no significant difference in these results among the four trials (test of heterogeneity, P = 0.71 for OS and 0.72 for DFS).
Figure 1.
Survival distributions for patients with TUBB3-high or -low tumors in the validation cohort of 1149 patients with R-NSCLC who participated in four clinical trials of adjuvant chemotherapy versus observation. The HRs, 95% CIs and P value estimations reported were obtained from adjusted Cox models stratified by trial. (A) Overall survival is inferior in TUBB3-high patients; (B) disease-free survival is inferior in TUBB3-high patients. TUBB3, class III β-tubulin; HR, hazard ratio; CI, confidence interval.
interaction between treatment assignment and TUBB3 status in predicting OS and DFS
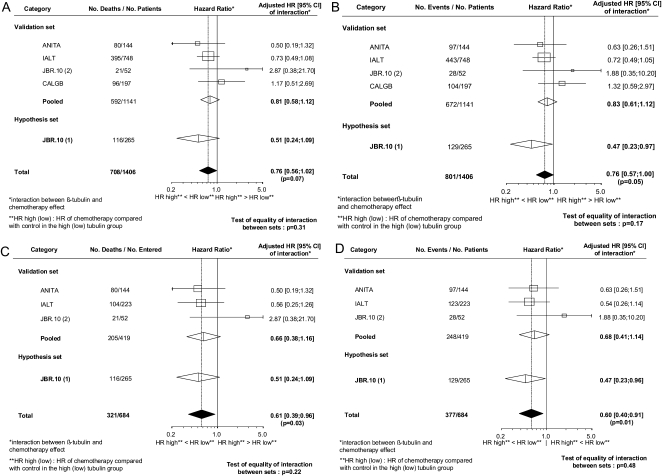
The adjusted HRs, 95% CIs and P values for the chemotherapy effect in TUBB3-high and TUBB3-low patients in the validation cohort are indicated in Table 3 for both OS and DFS. The point estimates of the HRs for chemotherapy versus observation were more favorable in TUBB3-high than in TUBB3-low patients for both OS and DFS. However, in Cox models, the interaction between treatment assignment and TUBB3 status was not statistically significant (test for interaction, P = 0.20 for OS and P = 0.23 for DFS). No heterogeneity among trials was seen for these results (test of heterogeneity, P = 0.42 for OS, Figure 2A; and 0.29 for DFS, Figure 2B). Similarly, no individual trial showed a significant interaction between TUBB3 and treatment assignment in predicting OS or DFS (Figure 2A and B).
Table 3.
Predictive value of TUBB3 for outcome in the entire validation cohort
| Chemotherapy group (no. of deaths/no. of patients)a | Control group (no. of deaths/no. of patients)a | Hazard ratio for event CT versus no CT (95% CI) | |
| Overall survival | |||
| β-tubulin low (n = 592) | 150/301 | 138/291 | 1.03 (0.81–1.30), P = 0.82 |
| β-tubulin high (n = 549) | 143/276 | 161/273 | 0.83 (0.66–1.04), P = 0.11 |
| Hazard ratio for event high versus low (95% CI) | 1.14 (0.90–1.45), P = 0.28 | 1.41 (1.11–1.79), P = 0.005 | Hazard ratio of interaction: 0.81 (0.58–1.12)b; test for interaction: β-tubulin × treatment, P = 0.20 |
| Disease-free survival | |||
| β-tubulin low (n = 592) | 166/301 | 158/291 | 0.97 (0.78–1.21), P = 0.77 |
| β-tubulin high (n = 549) | 162/276 | 184/273 | 0.80 (0.65–0.99), P = 0.04 |
| Hazard ratio for event high versus low (95% CI) | 1.19 (0.95–1.48), P = 0.14 | 1.43 (1.15–1.79), P = 0.002 | Hazard ratio of interaction: 0.83 (0.61–1.12)b; test for interaction: β-tubulin × treatment, P = 0.23 |
Eight patients are missing due to missing data on covariates.
Ratio of the hazard ratio of CT versus no CT for β-tubulin high divided by the one for β-tubulin low.
TUBB3, class III beta-tubulin; CI, confidence interval; CT, chemotherapy.
Figure 2.
Forest plots of predictive analysis results from individual trials included in the validation cohort for patients treated with chemotherapy versus observation according to TUBB3 status, along with the results from the patients in the previously published JBR.10(1) study [7]. HRs of the interaction between TUBB3 and chemotherapy effect for each trial, the validation set, the hypothesis set, and the overall population are reported with their 95% confidence intervals, the corresponding number of events and patients. The number of events/patients given for each line corresponds to the sum of the number of events/patients of the four following groups of the corresponding trial or group of trials of this line: control group with low tubulin; control group with high tubulin; chemotherapy group with low tubulin; chemotherapy group with high tubulin. The interaction HR is equal to the HR of chemotherapy effect (chemotherapy compared with control) for the high tubulin group (HR high) divided by the HR for the low tubulin group (HR low); if this ratio is equal to 1 then the effect of chemotherapy is the same for the two tubulin groups. In this case, there is no interaction between tubulin group and chemotherapy effect. For instance, in Figure 2A, the HR of 0.81 given for the pooled analysis of the validation set is the ratio of the HR of deaths given in the upper part of Table 3 left column): 0.83 divided by 1.03. (A) Overall survival for all patients. (B) Disease-free survival for all patients. (C) Overall survival for the subset randomly allocated to cisplatin–vinorelbine or observation. (D) Disease-free survival for the subset randomly allocated to cisplatin–vinorelbine or observation. TUBB3, class III β-tubulin; HR, hazard ratio.
subset analysis of patients treated with vinorelbine and cisplatin
In the subset of patients from trials or trial strata of the validation cohort comparing vinorelbine–cisplatin with observation (n = 420), high TUBB3 expression correlated with reduced OS (HR = 1.43; 95% CI 1.06–1.93; P = 0.02) and DFS (HR = 1.57; 95% CI 1.20–2.05; P = 0.001). The point estimates of the HRs for chemotherapy versus observation were again more favorable in TUBB3-high than in TUBB3-low patients for both OS and DFS (Table 4). However, the interaction between treatment assignment and TUBB3 in predicting outcome was not statistically significant (test for interaction, P = 0.15 for both OS and DFS).
Table 4.
Predictive value of TUBB3 for outcome in the subset of patients in the validation cohort randomly allocated to vinorelbine–cisplatin or observation
| Chemotherapy group (no. of deaths/no. of patients) | Control group (no. of deaths/no. of patients) | Hazard ratio for event CT versus no CT (95% CI) | |
| Overall survival | |||
| β-tubulin low (n = 230) | 59/121 | 44/109 | 1.14 (0.77–1.71), P = 0.51 |
| β-tubulin higha (n = 189) | 43/87 | 59/102 | 0.76 (0.51–1.13), P = 0.17 |
| Hazard ratio for event high versus low (95% CI) | 1.17 (0.78–1.76), P = 0.45 | 1.77 (1.16–2.69), P = 0.008 | Hazard ratio of interaction 0.66 (0.38–1.16)b; test for interaction: β-tubulin × treatment, P = 0.15 |
| Disease-free survival | |||
| β-tubulin low (n = 230) | 70/121 | 54/109 | 1.12 (0.78–1.61), P = 0.54 |
| β-tubulin higha (n = 189) | 53/87 | 71/102 | 0.76 (0.53–1.10), P = 0.15 |
| Hazard ratio for event high versus low (95% CI) | 1.30 (0.90–1.88), P = 0.17 | 1.90 (1.31–2.77), P = 0.0008 | Hazard ratio of interaction: 0.68 (0.41–1.14)b; test for interaction: β-tubulin × treatment, P = 0.15 |
One patient is missing due to missing data on covariates.
Ratio of the hazard ratio of CT versus no CT for β-tubulin high divided by the one for β-tubulin low.
TUBB3, class III beta-tubulin; CI, confidence interval; CT, chemotherapy.
exploratory analyses of the combined cohort
Given the parallel but nonsignificant findings in both the validation cohort and the previously published JBR.10(1) cohort [7], we opted to pool the two cohorts in an exploratory combined analysis. A total of 1406 patients were included in the combined cohort, with 684 patients in the vinorelbine–cisplatin versus observation subset. In this combined cohort, there was a trend for an interaction between treatment assignment and TUBB3 status [test for interaction: combined cohort, P = 0.07 for OS (Figure 2A) and P = 0.05, DFS (Figure 2B)]. When TUBB3 quartiles were used, the treatment effect did not vary significantly according to quartiles for OS (P for trend = 0.136) but there was borderline significance for DFS (P = 0.063). For OS, the treatment effect HRs for the different quartiles were 1.030, 0.996, 0.841, and 0.774; for DFS, the HRs for the different quartiles were 0.973, 0.919, 0.811, and 0.684.
In the vinorelbine–cisplatin subset, there was a significant interaction [P = 0.03 for OS (Figure 2C) and P = 0.01 for DFS (Figure 2D)], suggesting that TUBB3 may be a true predictive factor. When TUBB3 quartiles were used, the treatment effect did not vary significantly according to quartiles for OS (P for trend = 0.108) but significantly for DFS (P = 0.012). For OS, the HRs for the different quartiles were 0.949, 1.304, 0.760, and 0.663; for DFS, the HRs for the different quartiles were 0.948, 1.128, 0.871, and 0.471.
discussion
The results of the validation cohort are consistent with the results of the JBR.10(1) TUBB3 study [7]. In both JBR.10(1) and the validation cohort, high TUBB3 expression was a negative prognostic factor, particularly in untreated patients, correlating with inferior survival. The adverse prognostic significance of high TUBB3 expression has also been reported in metastatic NSCLC [7, 14–16]. The magnitude of the prognostic effect is moderate in our study. Further research is needed to explore the prognostic relevance of TUBB3 when included in a model with other biomarkers.
In both the JBR.10(1) TUBB3 study and the current study, TUBB3 expression was significantly greater in non-squamous subtypes of NSCLC. While the significance of this observation is not yet clear, it is another indication of biology varying with histology in NSCLC. Understanding these underlying differences in biology will eventually help to explain the prior observation that the effectiveness of specific chemotherapy drugs varies with histology [21].
We were unable to demonstrate a statistically significant interaction between treatment effect and TUBB3 status in the validation cohort. Therefore, we were unable to confirm that TUBB3 is a predictor of benefit from adjuvant chemotherapy in R-NSCLC. It is possible that both the JBR.10(1) cohort [7] and the validation cohort were underpowered to detect the predictive value of TUBB3, particularly in the vinorelbine–cisplatin subset where our exploratory pooled analysis yielded the strongest indication of a predictive effect. It is also possible that the heterogeneity in the chemotherapy regimens used among the included trials explained the small observed effect or that the predictive effect was very modest. Finally, a more precise method of TUBB3 protein expression quantitation or the use of a different TUBB3 H-score cut-off might have altered the results. It is unlikely that future trials will be conducted in this setting utilizing an observation arm.
As was seen in the JBR.10(1) study [7], the point estimates of the magnitude of DFS and OS benefit from adjuvant chemotherapy in the validation cohort were greater in patients with high versus low TUBB3 expression. This is contrary to what might have been expected based on the results from studies of metastatic NSCLC, where response rates to chemotherapy were ‘lower’ in patients with high TUBB3 expression [14–16]. Importantly, the reported studies in metastatic NSCLC have included relatively small numbers of patients, and none were randomized trials with an untreated control arm, limiting the conclusions that could be drawn. Thus, the inferior response rates reported in the metastatic setting may have been due to the negative ‘prognostic’ effect of high TUBB3 rather than true interaction with tubulin-targeting agents as suggested in the publications. We were not able to evaluate response in this study as all patients were treated in the adjuvant setting.
Our study is subject to the limitations of immunohistochemistry, which is recognized as being a standard method that is readily applicable in the clinic but which is also subject to some variability in both staining and interpretation. In the future, more objective methods of measuring tissue protein expression and function may become more practically applicable and further improve upon the approach we took here, allowing better biomarker analysis.
Our results suggest that TUBB3 cannot be used to select patients or regimens for the treatment of R-NSCLC with adjuvant chemotherapy. However, our results do suggest a possible role for TUBB3, warranting further exploration of this biomarker in prospectively planned studies. Its role in conjunction with other biomarkers is also of interest [22, 23]. It is unlikely that a single biomarker will explain all the variability in benefit from chemotherapy. Other biomarkers such as the excision repair cross complementation group 1 (ERCC1) protein and p53 are hypothesized to be of particular relevance to cisplatin chemotherapy, whereas TUBB3 is hypothesized to be particularly relevant to tubulin-targeting chemotherapy. We hope that future studies of the integration of these and possibly other markers might yield greater predictive value than each marker individually.
Further preclinical studies to better understand the role of TUBB3 in chemoresistance may be helpful in guiding future biomarker studies. Newer tubulin-targeting agents such as ixabepilone are hypothesized to act on TUBB3 and potentially to be of greater value in TUBB3-high tumors [24]. Finally, our results speak to the need for testing biomarkers in appropriate clinical settings, preferably in the context of a randomized clinical trial, to ensure that robust and reproducible conclusions can be drawn.
funding
Canadian Cancer Society; Ontario Cancer Research Network; Ligue Nationale Contre le Cancer; Sanofi-Aventis (unrestricted grants); USA National Cancer Institute (CA 31946 and 33601); Alberta Heritage Foundation for Medical Research; Alberta Cancer Foundation.
disclosure
FAS has done consulting work and has received honoraria from both GlaxoSmithKline and Pierre Fabre Oncology, two companies that have produced and/or distributed the chemotherapy drug vinorelbine. All remaining authors have declared no conflicts of interest.
Acknowledgments
Presented in part at the 2008 Annual Meeting of the American Society of Clinical Oncology and the 2009 World Conference on Lung Cancer.
References
- 1.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25):2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 2.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 3.Strauss GM, Herndon JE, 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26(31):5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 5.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 6.Katsetos CD, Herman MM, Mork SJ. Class III beta-tubulin in human development and cancer. Cell Motil Cytoskeleton. 2003;55(2):77–96. doi: 10.1002/cm.10116. [DOI] [PubMed] [Google Scholar]
- 7.Seve P, Lai R, Ding K, et al. Class III beta-tubulin expression and benefit from adjuvant cisplatin/vinorelbine chemotherapy in operable non-small cell lung cancer: analysis of NCIC JBR.10. Clin Cancer Res. 2007;13(3):994–999. doi: 10.1158/1078-0432.CCR-06-1503. [DOI] [PubMed] [Google Scholar]
- 8.Jouhilahti EM, Peltonen S, Peltonen J. Class III beta-tubulin is a component of the mitotic spindle in multiple cell types. J Histochem Cytochem. 2008;56(12):1113–1119. doi: 10.1369/jhc.2008.952002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seve P, Dumontet C. Is class III beta-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol. 2008;9(2):168–175. doi: 10.1016/S1470-2045(08)70029-9. [DOI] [PubMed] [Google Scholar]
- 10.Gan PP, Pasquier E, Kavallaris M. Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res. 2007;67(19):9356–9363. doi: 10.1158/0008-5472.CAN-07-0509. [DOI] [PubMed] [Google Scholar]
- 11.Kavallaris M, Burkhart CA, Horwitz SB. Antisense oligonucleotides to class III beta-tubulin sensitize drug-resistant cells to Taxol. Br J Cancer. 1999;80(7):1020–1025. doi: 10.1038/sj.bjc.6690507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamath K, Wilson L, Cabral F, Jordan MA. BetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem. 2005;280(13):12902–12907. doi: 10.1074/jbc.M414477200. [DOI] [PubMed] [Google Scholar]
- 13.Stengel C, Newman SP, Leese MP, et al. Class III beta-tubulin expression and in vitro resistance to microtubule targeting agents. Br J Cancer. 2010;102(2):316–324. doi: 10.1038/sj.bjc.6605489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seve P, Isaac S, Tredan O, et al. Expression of class III {beta}-tubulin is predictive of patient outcome in patients with non-small cell lung cancer receiving vinorelbine-based chemotherapy. Clin Cancer Res. 2005;11(15):5481–5486. doi: 10.1158/1078-0432.CCR-05-0285. [DOI] [PubMed] [Google Scholar]
- 15.Seve P, Mackey J, Isaac S, et al. Class III beta-tubulin expression in tumor cells predicts response and outcome in patients with non-small cell lung cancer receiving paclitaxel. Mol Cancer Ther. 2005;4(12):2001–2007. doi: 10.1158/1535-7163.MCT-05-0244. [DOI] [PubMed] [Google Scholar]
- 16.Rosell R, Scagliotti G, Danenberg KD, et al. Transcripts in pretreatment biopsies from a three-arm randomized trial in metastatic non-small-cell lung cancer. Oncogene. 2003;22(23):3548–3553. doi: 10.1038/sj.onc.1206419. [DOI] [PubMed] [Google Scholar]
- 17.Azuma K, Sasada T, Kawahara A, et al. Expression of ERCC1 and class III beta-tubulin in non-small cell lung cancer patients treated with carboplatin and paclitaxel. Lung Cancer. 2009;64(3):326–333. doi: 10.1016/j.lungcan.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Azuma K, Sasada T, Kawahara A, et al. Expression of ERCC1 and class III beta-tubulin in non-small cell lung cancer patients treated with a combination of cisplatin/docetaxel and concurrent thoracic irradiation. Cancer Chemother Pharmacol. 2009;64(3):565–573. doi: 10.1007/s00280-008-0907-3. [DOI] [PubMed] [Google Scholar]
- 19.Okuda K, Sasaki H, Dumontet C, et al. Expression of excision repair cross-complementation group 1 and class III beta-tubulin predict survival after chemotherapy for completely resected non-small cell lung cancer. Lung Cancer. 2008;62(1):105–112. doi: 10.1016/j.lungcan.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Koh Y, Jang B, Han SW, et al. Expression of class III beta-tubulin correlates with unfavorable survival outcome in patients with resected non-small cell lung cancer. J Thorac Oncol. 2010;5(3):320–325. doi: 10.1097/JTO.0b013e3181ce684f. [DOI] [PubMed] [Google Scholar]
- 21.Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6(1):64–70. doi: 10.1097/JTO.0b013e3181f7c6d4. [DOI] [PubMed] [Google Scholar]
- 22.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355(10):983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 23.Tsao MS, Aviel-Ronen S, Ding K, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25(33):5240–5247. doi: 10.1200/JCO.2007.12.6953. [DOI] [PubMed] [Google Scholar]
- 24.Dumontet C, Jordan MA, Lee FF. Ixabepilone: targeting betaIII-tubulin expression in taxane-resistant malignancies. Mol Cancer Ther. 2009;8(1):17–25. doi: 10.1158/1535-7163.MCT-08-0986. [DOI] [PubMed] [Google Scholar]