Abstract
Organic isothiocyanates (ITCs), which are characterized by the presence of an –N=C=S group, are among the most extensively studied cancer chemopreventive agents and show highly promising chemopreventive activities. Numerous studies have shown that ITCs can inhibit both carcinogenesis and cancer growth in a variety of animal models. Many cruciferous vegetables, which are commonly consumed by humans, are rich sources of these compounds. Of particular interest are their high bioavailability, their shared metabolic profile and their ability to target a wide array of cancer-related cellular proteins. This review is focused on discussing the molecular basis of these intriguing properties of ITCs, with a particular emphasis on the concept that cellular uptake and metabolism of ITCs and at least some of their major chemopreventive activities are all initiated through direct reaction of the carbon atom of the –N=C=S group of the ITCs with cysteine sulfhydryl groups of glutathione (GSH) and of proteins. This knowledge deepens our understanding about the biological activities of ITCs and may facilitate further research and development of these compounds for cancer prevention and treatment.
Introduction
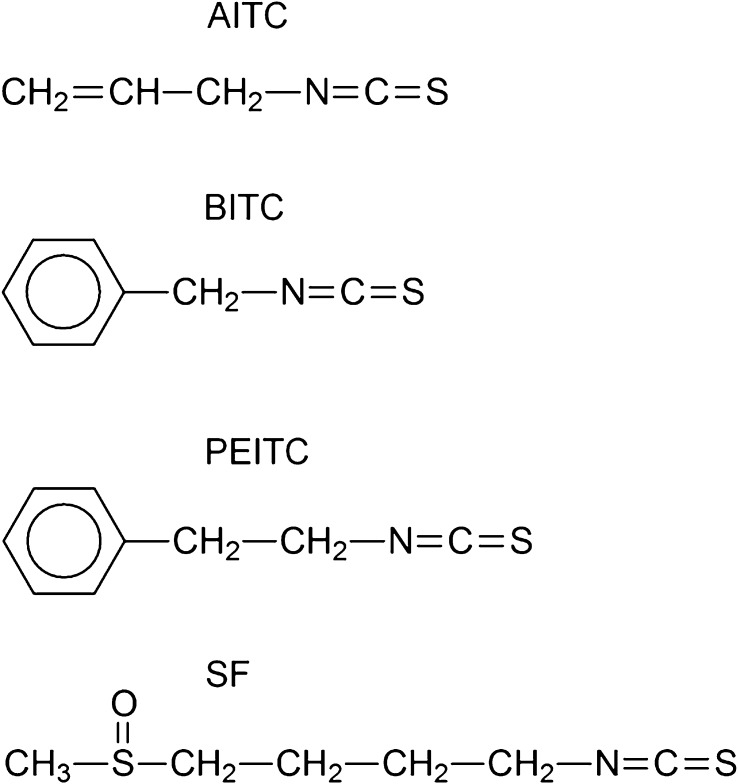
Organic isothiocyanates (ITCs), particularly allyl isothiocyanate (AITC), benzyl isothiocyanate (BITC), phenethyl isothiocyanate (PEITC) and sulforaphane (SF) (see Figure 1 for chemical structures), are among the most extensively studied cancer chemopreventive agents. Numerous studies have shown that ITCs can inhibit carcinogenesis and cancer growth in a wide variety of animal models, by inhibiting carcinogen activation, promoting carcinogen detoxification, inducing cell cycle arrest, activating apoptosis and/or inhibiting cancer cell invasion and metastasis. ITCs are known to modulate a large number of important cancer-related proteins, including but not limited to inhibition of cytochrome P450 (CYP) enzymes, induction of Phase 2 enzymes via activation of NF-E2-related factor-2 (Nrf2), inhibition of histone deacetylases (HDACs), inhibition of membrane drug transporters, modulation of cell cycle regulators and Bcl-2 family proteins, activation of caspases, downregulation of α-/β-tubulins and/or inhibition of tubulin polymerization, downregulation of vascular endothelial growth factor (VEGF) and its receptor and inhibition of nuclear factor kappa B (NF-κB), activator protein-1 (AP-1), mitogen-activated protein kinase kinase kinase (MEKK1), signal transducer and activator of transcription factor 3 (STAT3) and Toll-like receptor 4 (TLR4). Many reviews on ITCs have been published, some of which are cited here (1–11). Most of the activities mentioned above are shared by different ITCs. Moreover, studies have also shown that ITCs are significantly more toxic to transformed or malignant cells than normal cells (12–17). The human relevance of the above-described findings is underscored by the fact that many cruciferous vegetables, which are commonly consumed by humans, are rich sources of these compounds, such as mustard seed and horseradish root for AITC (18), garden cress for BITC (19), watercress for PEITC (20) and broccoli and broccoli sprout for SF (21,22). Human exposure to ITCs via dietary consumption is undoubtedly frequent and wide spread. ITCs are stored as inert glucosinolate precursors in plants and are released through a myrosinase-catalyzed hydrolysis reaction upon plant tissue damage (23). Dietary ITCs are efficiently absorbed in vivo, and oral bioavailability of these compounds may reach ≥80% (5,9,10). In vivo, ITCs are primarily metabolized through the mercapturic acid pathway and excreted in urine mainly as N-acetylcysteine (NAC) conjugates (20,24–31).
Fig. 1.
Chemical structures of AITC, BITC, PEITC and SF.
An intriguing question about the multifaceted chemopreventive mechanisms of ITCs is how these compounds are able to modulate so many diverse cellular targets. It is also important to understand why different ITCs share similar chemopreventive mechanisms and metabolic profiles. This review article is focused on addressing these questions. All ITCs are characterized by the presence of an –N=C=S group, the central carbon of which is electrophilic and readily reacts with nucleophiles. Indeed, the almost universal ability of the central carbon of ITCs and their metabolites to undergo successive nucleophilic additions with vicinal dithiols, forming five-membered cyclic thiocarbonyl products with release of the nitrogen atom as an amine, is the chemical basis of a widely known assay, namely the cyclocondensation assay, for measurement of ITCs and their metabolites in plants and biological specimens (32). The central concept I intend to convey in this article is that cellular uptake and metabolism of ITCs and at least some of their chemopreventive mechanisms are all initiated through direct reaction of the carbon atom of the –N=C=S group of ITCs with the cysteine sulfhydryl groups of glutathione (GSH) and proteins. The side chains of ITCs may play secondary roles, by influencing the electrophilicity of the –N=C=S group, altering the access to the reactive carbon through steric effects and controlling the lipophilicity of the whole molecule. To date, AITC, BITC, PEITC and SF have been the most commonly and most extensively studied ITCs; most of the data reviewed herein were generated with these compounds.
ITC metabolism via conjugation with the cysteine thiol of GSH
As mentioned above, ITCs are primarily metabolized through the mercapturic acid pathway in vivo. An initial reaction between the –N=C=S group of ITCs and the cysteine sulfhydryl group of GSH (γ-glutamylcysteinylglycine), which takes place spontaneously but is enhanced by glutathione S-transferase (GST), gives rise to the corresponding conjugates (Figure 2). In a previous study, in which the catalytic properties of four human GSTs, including A1-1, M1-1, M4-4 and P1-1, were examined with AITC, BITC, PEITC, SF and 10 other ITCs, we showed that all ITCs were substrates of the GSTs and that, of the four GSTs, M1-1 and P1-1 were generally the most efficient catalysts and M4-4 was the least effective (33). The conjugates then undergo successive enzymatic modifications on the GSH moiety, forming cysteinylglycine-, cysteine- and finally NAC-conjugates, which are excreted in the urine. Approximately 72% of a single oral dose of SF was detected in the urine as an NAC conjugate in rats in 24 h (26), and nearly 60% of a single oral dose of broccoli sprout ITCs (mainly SF) was disposed in 8 h urine in humans (34). Further studies of SF in humans showed the following percentages of urinary SF and its metabolites: ∼7% as SF, <1% as GS–SF conjugate, <1% as SF–cysteinylglycine conjugate, ∼28% as SF–cysteine conjugate and ∼65% as SF–NAC conjugate (27,28). Urinary-free SF detected in the above-mentioned studies probably was generated from its metabolites that are known to be unstable and to dissociate to SF (35). In rats given a single oral dose of 14C-labeled AITC, >90% of the dose was absorbed, nearly 80% of the dose was recovered in the urine (mainly within 24 h) and ∼80% of the radioactivity in the urine existed as the NAC conjugate (29,30). The NAC conjugate is also the major metabolite of AITC in humans, as ∼50% of the dose was recovered in the urine as NAC conjugate within 10–12 h (31,36). Similar results were obtained in humans who ingested BITC (25). Interestingly, in mice and several other species, the major urinary metabolites of ITCs are cyclic mercaptopyruvate conjugates, rather than NAC–ITC, presumably resulting from transamination of ITC–cysteine conjugates (37,38).
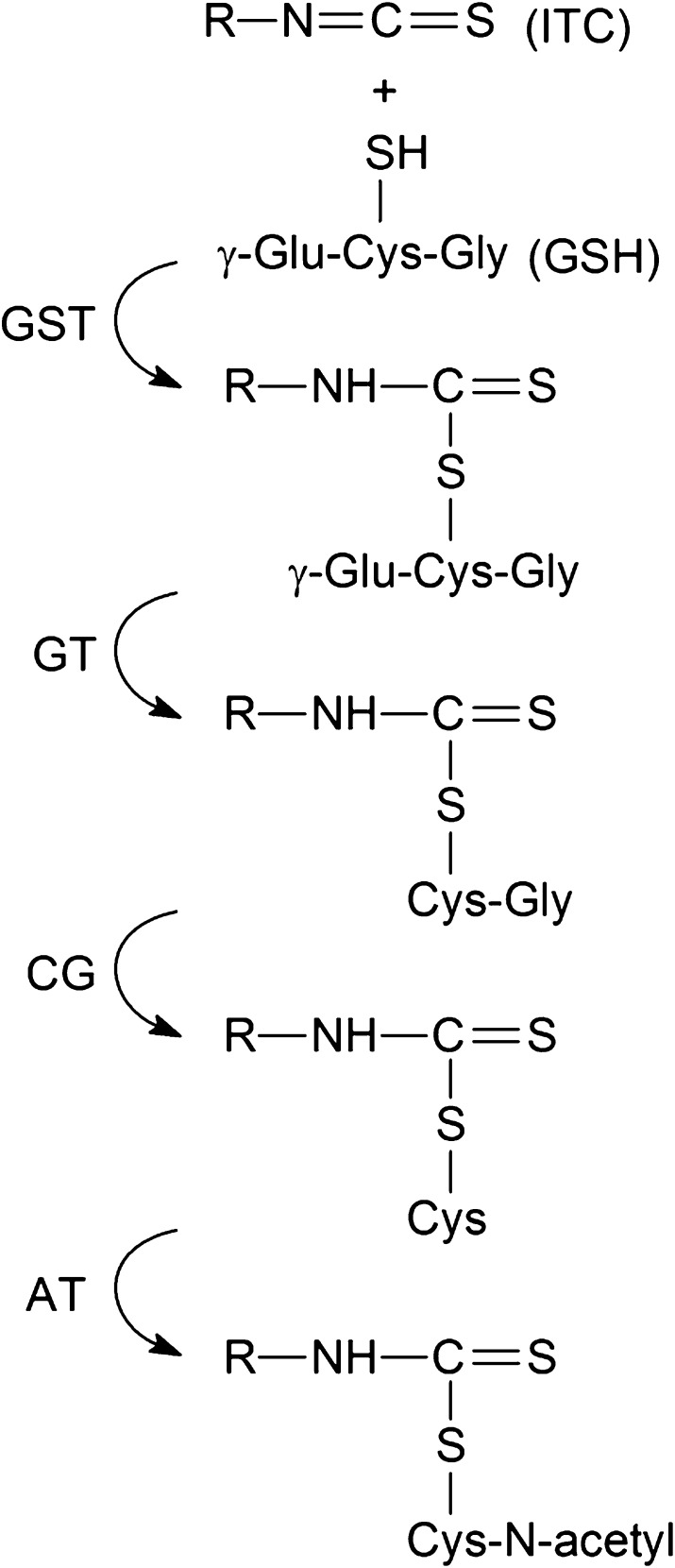
Fig. 2.
ITC metabolism via the mercapturic acid pathway. ITCs first react with GSH to form GS conjugates, which are promoted by glutathione S-transferase (GST). The conjugates undergo successive enzymatic modifications, first by γ-glutamyltranspeptidase (GT) to form cysteinylglycine–ITC conjugates, then by cysteinylglycinase (CG) to form the cysteine–ITC conjugates and finally by N-acetyltransferase (AT) to form the NAC–ITC conjugates.
The NAC conjugates of ITCs and the other conjugates formed in the mercapturic acid pathway are biologically relevant. They serve as carriers of the ITCs, as these conjugates are unstable and readily dissociate to their parent compounds or undergo exchange reactions with free thiols (35,39,40). The cancer chemopreventive activities of these conjugates closely resemble those of their parent compounds (41–43). However, little is known about the bioactivity of the cyclic mercaptopyruvate conjugates mentioned above.
Cellular uptake of ITC via reaction with cysteine thiols of intracellular GSH and proteins
We previously showed that ITCs rapidly accumulated in human and animal cells, with the peak intracellular ITC accumulation reached within 0.5–3 h of exposure and up to 100- to 200-fold over the extracellular ITC concentration or up to millimolar levels (44–47). ITCs apparently penetrate cells by diffusion but once inside the cells, they are rapidly conjugated via their –N=C=S group with intracellular thiols, mainly GSH (Figure 3) (45). Indeed, initial uptake rates of AITC, BITC, PEITC and SF in human breast cancer MCF-7 cells closely correlated with the non-enzymatic second-order rate constants of their conjugation reactions with GSH, and the initial uptake rates of the ITCs also increased linearly with an increase in cellular GST activity (46). Not surprisingly, exposure of cells to ITCs, especially at high concentrations, leads to rapid and marked depletion of GSH (45). The critical importance of GSH in cellular ITC uptake may be attributed primarily to its high intracellular concentrations (0.2–10 mM in animal and human cells and tissues) (48). ITCs also bind to proteins in cells via reaction with cysteine sulfhydryl groups, as documented in the examples described below, and may bind to proteins directly or via thiol exchange reactions of their metabolites (Figure 3). Protein binding became more significant with more lipophilic ITCs or when cells were incubated with ITCs at higher concentrations or for longer times (45,49). However, Mi et al. (49) showed that neither SF nor PEITC underwent significant binding to DNA or RNA in cells. Cellular uptake of both BITC and SF was almost completely blocked when the ITCs were added to culture medium together with GSH, cysteine or NAC at 100-fold excess (45). In the above-described experiment, ITCs in the culture media were presumably rapidly conjugated with the thiols that were present at large excess, thus blocking ITC accumulation in cells. This was indeed confirmed in a later study, using [14C]PEITC, [14C]SF and NAC (50). These results provide further support to the notion that reaction of the –N=C=S group with intracellular thiols, particularly GSH, drives rapid ITC accumulation in cells. These results have also led Mi et al. (50) to suggest that use of NAC or other thiols as antioxidants in ITCs studies may lead to wrong conclusions since the thiols may simply block intracellular uptake of ITCs, rather than working as antioxidants. We subsequently showed that high cellular accumulation of ITCs was followed by rapid membrane transporter-mediated export (half was exported in ∼1 h), involving multidrug resistance-associated protein-1 and P-glycoprotein-1 (Pgp-1) (47,51). However, whereas SF upon accumulation in human prostate cancer LNCaP cells was exported almost exclusively as the GSH conjugate (47), AITC, BITC and PEITC upon accumulation in human leukemia HL60 cells were exported as both GSH conjugates (36–60%) and cysteinylglycine conjugates (21–40%) (51). Moreover, in human bladder cancer RT4 cells exposed to BITC, although the GSH conjugate was the predominant form accumulated, ∼5% of accumulated BITC existed as the cysteine conjugate (41). These results show that ITCs accumulated as GSH conjugates in a cell may be further metabolized via the mercapturic acid pathway in the same cell. Interestingly, Morris et al. reported that BITC and PEITC as well as several other ITCs, but not AITC and SF, at 10–100 μM concentrations significantly inhibited Pgp-, multidrug resistance-associated protein-1- and breast cancer resistance protein-mediated cellular export of several anticancer agents possibly via direct inhibition of the ATPase of the transporters (52–54), suggesting a possibility for combination of an ITC with anticancer agents for enhanced cancer therapy. However, it is not known if ITCs inhibit their own cellular export.
Fig. 3.
Cellular uptake and elimination of ITCs and intracellular protein modification. ITCs (R-N=C=S) are believed to enter a cell by diffusion, but once in cell are rapidly accumulated via conjugation with intracellular thiols, primarily GSH but also proteins. The GSH conjugates may be further metabolized via the mercapturic acid pathway (‘R-NH-C(=S)-SR1’ stands for these metabolites), which are expelled from the cell via membrane transporters. The conjugates may modify cellular proteins via exchange reactions with cysteine sulfhydryl groups. ITCs and their thiol conjugates may also bind to certain proteins via reaction with amino groups and may also cause protein thiol oxidation by stimulating cellular reactive oxygen species production.
ITCs target cancer-related proteins via reaction with cysteine thiols
Cytochrome P450 enzymes
Many chemical carcinogens are metabolically activated in vivo, most notably by CYP enzymes. ITCs and particularly arylalkyl ITCs have been shown to rapidly suppress the catalytic activities of CYP enzymes, including CYP1A1, 1A2, 2A6, 2A13, 3A4, 2B1, 2B6, 2D6 and 2E1, via competitive-, non-competitive-, uncompetitive- or mechanism-based inhibition with in vitro Ki detected at low micromolar concentrations. The inhibition of CYP enzymes by ITCs and the ensuing prevention of metabolic activation of carcinogens, DNA damage and tumorigenesis have been previously reviewed (3,4,55). The effectiveness of achieving cancer prevention with ITCs by inhibiting specific CYP enzymes was most clearly demonstrated in animals treated with the tobacco-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). For example, Chung et al. showed that NNK oxidation metabolism, likely mediated by CYP 2A1 and 2B1 or related forms, was significantly inhibited in liver and lung microsomes prepared from A/J mice fed 3 μmol of PEITC/g diet (56) and showed in another study that NNK-induced lung tumor incidence and multiplicity decreased by 97 and 70%, respectively, in A/J mice fed four daily doses of 25 μmol PEITC/mouse before a single NNK dose (57). The –N=C=S group of ITC appears to be essential for inhibition of CYP enzymes and carcinogenesis. Jiao et al. (58) showed that while 1-dodecyl ITC strongly inhibited NNK metabolism and NNK-induced lung tumorigenesis, replacing its –N=C=S group with a hydroxyl group abolished the inhibitory activities. Moreover, Conaway et al. (59) showed that conjugation of ITCs with GSH, cysteine or NAC reduced their inhibitory activities against CYPs by as much as 18-fold and also showed that the dissociation rates of ITC conjugates (GSH, cysteine and NAC conjugates) determined their activity as inhibitors of CYP enzymes (35). Although specific cysteine residues of CYP enzymes that may be targeted by ITCs have not been identified, Hollenberg et al. (60) have shown that a single amino acid residue per molecule of CYP2B1 was bound by [14]BITC (60).
The Nrf2–Keap1 complex
Nrf2 is a transcription factor that is ubiquitously expressed, though maintained at low basal levels, and plays a critical role in stimulating the expression of genes involved in many aspects of cytoprotection, most notably genes encoding the carcinogen-detoxifying Phase 2 enzymes, such as GST and NAD(P)H:quinone oxidoreductase-1. Nrf2 works primarily by binding as a heterodimer with Maf or other partners to a cis-acting DNA regulatory element, namely the antioxidant response element, which is located in the upstream regions of its target genes. Nrf2 is activated by many chemical agents and has been found to be essential for SF and other chemopreventive agents to prevent cancer and other diseases in animal models (61–64). Nrf2 protein upon synthesis is rapidly degraded by the 26S proteasome in unstimulated cells (65). Kelch-like ECH-associated protein 1 (Keap1), also known as Nrf2 repressor, is crucial for the rapid turnover of Nrf2 and functions as an adaptor for Nrf2 ubiquitination by a Cul-3-dependent ubiquitin ligase complex (66,67). Chemical alkylation or oxidation of specific cysteine residues of Keap1 disrupts Keap1-mediated Nrf2 ubiquitination, leading to Nrf2 accumulation/activation, which in turn leads to increased transcription of antioxidant response element-regulated genes and increased cytoprotection (68). ITCs activate Nrf2 in cultured cells at low micromolar concentrations (69,70). Focusing on SF, Dinkova-Kostova et al. (71) showed that there was a direct reaction of the ITC with multiple sulfhydryl groups of Keap1, and Hong et al. (72) reported that 25 of the 27 cysteines of human Keap1 were directly bound by SF in a concentration-dependent manner. The biological significance of most of these bindings remains unknown, but Zhang and Hannink (73) showed that Cys273 and Cys288, but not Cys77, Cys171, Cys257 and Cys297, were required for Keap1-dependent Nrf2 ubiquitination and inhibition of Nrf2 ubiquitination by SF in transient gene transfection experiments, whereas Cys151 is required for the escape of Nrf2 from Keap1-mediated ubiquitination.
Nrf2 activation by SF via Keap1 modification leads to profound cellular changes. Microarray analysis of small intestines of mice treated with SF at 9 μmol/mouse/day for 7 days showed induction of numerous Nrf2-regulated genes by SF, including Phase 2 genes and genes encoding cellular reduced nicotinamide adenine dinucleotide phosphate-regenerating enzymes, xenobiotic-metabolizing enzymes, antioxidants and biosynthetic enzymes of the GSH and glucuronic acid conjugation pathways (74). In several in vivo carcinogenesis models, Nrf2 activation was essential for the chemopreventive activity of SF (61,64,75).
α-/β-Tubulin
Numerous studies have reported the inhibitory effects of ITCs on the survival and proliferation of both animal and human cancer cells, which were almost always associated with cell cycle arrest and activation of apoptosis (6). We have recently shown that AITC at 15–30 μM arrests human bladder cancer cells exclusively in mitosis and causes mitotic catastrophe via Bcl-2 protein phosphorylation and that AITC stimulates the ubiquitination and degradation of α-/β-tubulin (76). Given the essential importance of α-/β-tubulin and microtubule in mitosis, the tubulins may be key targets of AITC in the induction of mitotic arrest and mitotic catastrophe. AITC was found to bind to multiple cysteine residues of α-tubulin (Cys127, Cys347 and Cys376) and β-tubulin (Cys12, Cys239, Cys303 and Cys354), but our study also indicated that the binding was reversible (76). It remains to be determined if AITC binding to the cysteine residues or to a particular cysteine residue of the tubulins is responsible for their increased ubiquitination and degradation in AITC-treated cells. However, most of the β-tubulin cysteine residues targeted by AITC have also been shown to be targeted by a number of other anticancer agents (77–81). Moreover, Chung et al. (82,83) recently reported that BITC, PEITC and SF at 10–20 μM also bind to cysteine residues of α-/β-tubulin in human lung cancer cells, including Cys347 of α-tubulin and Cys303 of β-tubulin, which triggered proteasomal degradation of the tubulins and was accompanied by disruption of tubulin polymerization. Interestingly, although BITC, PEITC and SF also inhibited tubulin polymerization (83,84), AITC showed no such effect (76).
AP-1, MEKK1 and TLR4
AP-1 is a well-known oncogene and is composed of homodimers of Jun family members or heterodimers of Jun and Fos family members and has been widely reported to play an important role in cancer development. AP-1 is a key mediator of ultraviolet radiation-induced skin carcinogenesis (85,86). We previously found that SF at 10 μM significantly inhibited ultraviolet B-induced AP-1 activation in human keratinocytes and the inhibitory effect of SF was at least partly due to direct inhibition of AP-1 DNA-binding activity (87). AP-1 binds to the 12-O-tetradecanoylphorbol-13-acetate response element (TRE), a binding site in the promoters of AP-1 target genes. It was subsequently shown that SF applied topically (1 or 2.5 μmol per mouse back, three times weekly) was effective in reducing the multiplicity and tumor burden of ultraviolet B-induced squamous cell carcinoma in a mouse model and that SF bound to Cys154 in c-Fos and Cys272 in c-Jun to inhibit AP-1 binding to TRE (88). Interestingly, in human colon cancer HT-29 cells, SF as well as AITC, BITC and PEITC stimulated AP-1 transactivation activity (89,90). Moreover, AITC, BITC and PEITC were found to stimulate the expression and phosphorylation of c-Jun and AP-1 transactivation in human bladder cancer cell lines (91). The reason for the opposite effects of ITCs on AP-1 in human skin cells and tissues versus other cell lines described above is not known.
Mitogen-activated protein kinase pathways play a key role in mediating cell growth and death. Among the members of the mitogen-activated protein kinase family, MEKK1, an upstream regulator of the SAPK/JNK signal transduction pathway, is activated in response to cytokines and various stresses. Cross et al. (92) showed that PEITC at 25 μM or high concentrations inhibited the catalytic activity of MEKK1 in LNCaP cells and other cells, which was accompanied by inhibition of its downstream target SAPK/JNK kinase, and that the inhibition required the cysteine residue at position 1238 in the ATP-binding pocket of the protein. However, the biological significance of MEKK1 inhibition by ITC requires further investigation. Hu et al. (93) have shown that PEITC causes rapid and significant elevation in the phosphorylation (activation) of all three mitogen-activated protein kinases, including ERK, JNK and p38, in human colon cancer HT-29 cells and that JNK activation was critical for the initiation of apoptosis. Chen et al. (94) reported that PEITC caused JNK activation by downregulating M3/6, a JNK-specific phosphatase.
Toll-like receptors are a family of membrane receptors that play a key role in the innate immune system. They are pattern recognition receptors that detect microorganisms and non-microbial endogenous molecules to trigger immune and inflammatory responses. TLR4 is activated by lipopolysaccharides and other molecules, resulting in its oligomerization, which in turn leads to production of proinflammatory proteins such as inducible nitric oxide synthase, cyclooxygenase-2, interleukin-1 and tumor necrosis factor-α. Youn et al. (95) showed that SF at 10 and 20 μM suppressed ligand-induced and ligand-independent TLR4 oligomerization and activation in a thiol-dependent manner in murine monocytic RAW264.7 cells and human embryonic kidney 293T cells, and that SF bound to multiple cysteine residues in the extracellular domain of this receptor, including Cys88, Cys192, Cys246, Cys281, Cys340, Cys506, Cys542 and Cys609 of human TLR4, among the 16 cysteine residues present in the extracellular domain. The results suggest that the reactivity of SF to sulfhydryl moiety contributes to its inhibitory activities, but it is not known as to which specific cysteine(s) account for the inhibitory effect of SF. SF administered orally to mice at 25 mg/kg blocked lipopolysaccharides-induced production of inflammatory cytokines, including tumor necrosis factor-α, interleukin-1β and interleukin-6 in the skin (95).
ITCs modulate other cancer-related proteins, but the underlying mechanisms remain unknown
Besides the proteins described above, ITCs have also been shown to regulate a large number of other proteins involved in inflammation, cell cycle arrest, cell death, angiogenesis and cancer invasion and metastasis. Lipopolysaccharides-induced NF-κB activation in HT-29 cells was significantly inhibited by AITC, PEITC and SF at 10–100 μM (96). In a study of human pancreatic cancer BxPC-3 cells, BITC at 20 μM also significantly decreased the level of both cytoplasmic and nuclear NF-κB/Rel-p65 (97). A subsequent study in human prostate cancer PC-3 cells showed that the inhibitory effect of the ITCs on NF-κB was mainly mediated through inhibition of IκB kinases (IKKs), particularly the inhibition of IKKβ phosphorylation (98). However, another study in BxBC-3 cells showed that BITC caused significant decrease in the expression and activity of HDAC1 and HDAC3 and suggested that this might account for BITC-induced inhibition of NF-κB (99). HDACs are a class of enzymes that remove acetyl groups from an acetyl lysine amino acid on a histone and play an important role in the regulation of gene expression. Besides BITC, both PEITC and SF as well as a number of other ITCs were also shown to inhibit HDAC activity in human colon HCT116 cells at low micromolar concentrations (100). The inhibitory effect of PEITC and SF on HDAC, inhibiting the activity and level of HDAC1 and perhaps other HDACs, was accompanied by enhanced histone acetylation and derepression of the p21 gene promoter (101–103). p21 is a cyclin-dependent kinase inhibitor; it binds and inhibits the activity of cyclin–cdk2 and cyclin–cdk4 complexes, thus functioning as a G1 phase regulator in cell cycle. The inhibitory activity of SF against HDAC was also demonstrated in human prostate cancer xenografts and spontaneous intestinal polyps in mice in vivo (103). However, little is known about how ITCs inhibit HDAC or how many HDACs may be inhibited by ITCs. In contrast to upregulation of p21 by ITCs via HDAC inhibition as described above, both AITC and SF at low micromolar concentrations caused significant downregulation of cyclin B1, cdk1, cdc25B and cdc25C in prostate cancer cell lines (17,104). Similar activities of BITC and PEITC were detected in human pancreatic and prostate cancer cell lines (97,105). Cyclin B1 binds to cdk1 and the complex is involved in the early events of mitosis, whereas cdc25B and cdc25C direct dephosphorylation/activation of cyclin B1-bound cdk1. Xiao et al. (105) previously reported that PEITC-induced downregulation of cdk1 and cdc25C in PC-3 cells was mediated via proteasome. However, Mi et al. (106) recently reported that PEITC as well as BITC at 10–20 μM significantly inhibited both the 26S and 20S proteasomes presumably through direct binding in several cell lines, including PC-3 cells.
ITCs also consistently caused downregulation of Bcl-2 and Bcl-xl in cancer cell lines, and in some cases also induction of Bax and Bak or disruption of the association between Bcl-2 and Bax or between Bcl-xl with Bak and Bax, leading to activation of multiple caspases and apoptosis (17,76,107–111). But little is known about how ITC modulates these proteins. Studies have also shown that ITCs downregulate VEGF, VEGF receptor, matrix metalloproteinase (MMP)-2 and/or MMP-9, which are known to play important roles in cancer angiogenesis, invasion and metastasis. Warin et al. (112) showed that BITC at low micromolar concentrations significantly reduced VEGF secretion and VEGF receptor 2 protein level in human breast cancer MDA-MD-231 cells in vitro and at 7.5 μmol per mouse (three times per week) significantly decreased angiogenesis and VEGF receptor 2 protein level in MDA-MD-231 xenografts in vivo. Antiangiogenic activities of AITC, PEITC and SF have also been reported (113–115). VEGF is a target gene of hypoxia-inducible factor (HIF). PEITC and SF were shown to downregulate HIF1α and/or HIF2α at low micromolar concentrations in human breast cancer cells and human tongue squamous cancer cells (116,117), suggesting that ITCs may downregulate VEGF via HIF. The ITCs appear to inhibit HIF protein synthesis, but the exact mechanism remains unknown. AITC, BITC, PEITC and SF were shown to significantly downregulate MMP-2 and/or MMP-9 at low micromolar concentrations in human hepatoma SK-Hep1 cells or HMEC-1 cells, which were associated with the inhibition of cell migration (114,118–120), but the underlying mechanism has not been revealed either. There is also evidence that ITCs inhibit STAT3. BITC at 5–10 μM significantly reduced the levels of both STAT3 and phosphorylated STAT3 in BxPC-3 cells and other pancreatic cancer cells, leading to reduced STAT3 DNA-binding and transcriptional activities (121). Moreover, the growth of BxPC-3 xenografts and STAT3 levels in the xenografts was also significantly inhibited in mice treated with BITC at 12 μmol per mouse five times a week (121). Studies of PEITC and SF in human prostate cancer cells showed that the ITCs inhibited interleukin-6-induced STAT3 phosphorylation via inhibition of its upstream kinase JAK2 (122,123).
Although the underlying mechanisms remain largely unknown for ITC modulation of the proteins described above, it is clear that different ITCs share similar activities. This suggests that the –N=C=S group of these compounds may play a key role since it is the only chemical structure that is shared by all the ITCs and is known to react with cellular nucleophiles. It is likely that reaction of the ITCs via their −N=C=S group with specific protein cysteine thiols may play a major part in at least some of the changes described above.
Concluding remarks
The evidence presented in this review shows clearly that the metabolism and cellular uptake of ITCs as well as their ability to target some of the important cancer-related cellular proteins are governed by the reaction of the –N=C=S group of ITCs with the cysteine sulfhydryl groups of GSH and proteins. These findings explain at least in part the intriguing capability of ITCs to target a diverse group of proteins and the phenomenon that different ITCs often share similar biological activities and metabolic profiles. The number of proteins that are known to be directly modulated by ITCs via reaction with cysteine residues is still relatively small at the present time, but more of such proteins will undoubtedly be discovered in the future. As described above, a large number of proteins are modulated by ITCs, and the exact mechanisms for these modulations remain unknown. Some of these modulations may eventually be attributed to direct binding of ITCs to key cysteine residues via their –N=C=S groups. However, some proteins are indirectly modulated by ITCs, e.g. induction of Phase 2 enzymes in response to modulation of Nrf2–Keap1 by ITCs. ITCs may also indirectly modify protein cysteine residues, as these compounds are known to elevate cellular levels of reactive oxygen species (Figure 3) (124), and reactive oxygen species may oxidize cysteine residues, as in the case of protein tyrosine phosphatases (125). ITCs are also capable of reacting with an amino group of proteins to generate a thiourea derivative (Figure 3). Indeed, the reaction of –N=C=S of phenyl ITC with the amino groups of proteins is the basis of the well-known Edman degradation for peptide sequencing (126). Reaction of AITC with the amino group on a lysine residue of bovine serum albumin and a synthetic peptide could occur under physiological conditions (127). PEITC was shown to bind to the N-terminal proline residue of the pleiotropic cytokine macrophage migration inhibitory factor, resulting in complete loss of its catalytic tautomerase activity and disruption of protein conformation (128). Swope et al. (129) have shown that the N-terminal proline residue of macrophage migration inhibitory factor is critical for its catalytic and cytokine activities and that its amino group has an unusually low pKa (5.6 ± 0.1), rendering it highly reactive. However, under physiological conditions, protein binding by ITCs via an amino group is probably less common than via a cysteine sulfhydryl group, as the pKa value of the former in amino acids (8.80–10.96) is much higher than that of the latter (8.18), and ITC reaction rate with NH2 groups was shown to be dramatically slower than with SH groups (130). A summary list of proteins that are known to be modulated by ITCs is provided in Figure 4.
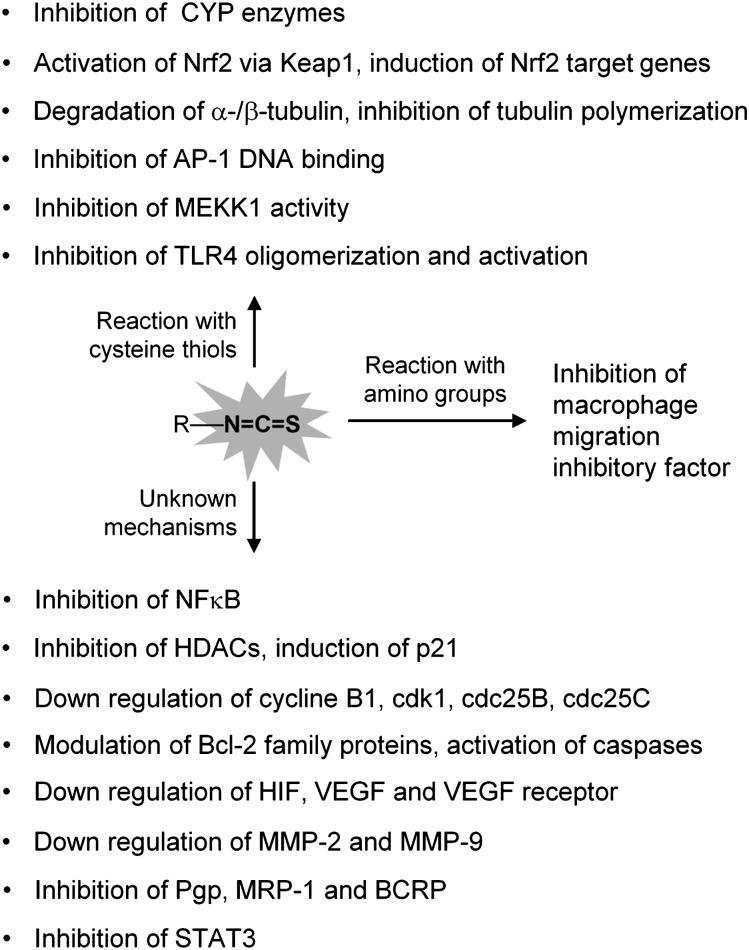
Fig. 4.
ITCs modulate a large and diverse group of proteins. The modulation of many proteins by ITCs involves direct reaction of the –N=C=S groups of ITCs with cysteine thiols of the proteins. Macrophage migration inhibitory factor is modulated via its amino group. The mechanisms for modulation of many other proteins are not yet known.
Funding
National Cancer Institute (R01CA124627).
Acknowledgments
I would like to thank Drs Rex Munday (Ruakura Research Center, Hamilton, New Zealand) and Arup Bhattacharya (Roswell Park Cancer Institute) for critical reading of this manuscript. Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AITC
allyl isothiocyanate
- AP-1
activator protein-1
- BITC
benzyl isothiocyanate
- CYP
cytochrome P450
- GSH
glutathione
- GST
glutathione S-transferase
- ITC
isothiocyanate
- HDAC
histone deacetylase
- HIF
hypoxia-inducible factor
- Keap1
Kelch-like ECH-associated protein 1
- MEKK1
mitogen-activated protein kinase kinase kinase
- MMP
matrix metalloproteinase
- NAC
N-acetylcysteine
- NF-κB
nuclear factor kappa B
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- Nrf2
NF-E2-related factor-2
- PEITC
phenethyl isothiocyanate
- STAT3
signal transducer and activator of transcription 3
- SF
sulforaphane
- TLR4
Toll-like receptor 4
- VEGF
vascular endothelial growth factor
References
- 1.Zhang Y, et al. Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer Res. 1994;54:1976s–1981s. [PubMed] [Google Scholar]
- 2.Hecht SS. Chemoprevention by isothiocyanates. J. Cell. Biochem. Suppl. 1995;22:195–209. doi: 10.1002/jcb.240590825. [DOI] [PubMed] [Google Scholar]
- 3.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab. Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 4.Conaway CC, et al. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr. Drug Metab. 2002;3:233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat. Res. 2004;555:173–190. doi: 10.1016/j.mrfmmm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, et al. Vegetable-derived isothiocyanates: anti-proliferative activity and mechanism of action. Proc. Nutr. Soc. 2006;65:68–75. doi: 10.1079/pns2005475. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y, et al. Cell death induction by isothiocyanates and their underlying molecular mechanisms. Biofactors. 2006;26:123–134. doi: 10.1002/biof.5520260203. [DOI] [PubMed] [Google Scholar]
- 8.Cheung KL, et al. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2010;54:127–135. doi: 10.1002/mnfr.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol. Sin. 2007;28:1343–1354. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 11.Mi L, et al. Proteins as binding targets of isothiocyanates in cancer prevention. Carcinogenesis. 2011;32:1405–1413. doi: 10.1093/carcin/bgr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya A, et al. Inhibition of bladder cancer development by allyl isothiocyanate. Carcinogenesis. 2010;31:281–286. doi: 10.1093/carcin/bgp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava SK, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis. 2003;24:1665–1670. doi: 10.1093/carcin/bgg123. [DOI] [PubMed] [Google Scholar]
- 14.Musk SR, et al. Allyl isothiocyanate is selectively toxic to transformed cells of the human colorectal tumour line HT29. Carcinogenesis. 1993;14:2079–2083. doi: 10.1093/carcin/14.10.2079. [DOI] [PubMed] [Google Scholar]
- 15.Gamet-Payrastre L, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 16.Gamet-Payrastre L, et al. Selective cytostatic and cytotoxic effects of glucosinolates hydrolysis products on human colon cancer cells in vitro. Anticancer Drugs. 1998;9:141–148. doi: 10.1097/00001813-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Xiao D, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 18.Uematsu Y, et al. Determination of isothiocyanates and related compounds in mustard extract and horseradish extract used as natural food additives. Shokuhin Eiseigaku Zasshi. 2002;43:10–17. doi: 10.3358/shokueishi.43.10. [DOI] [PubMed] [Google Scholar]
- 19.Gil V, et al. Benzylglucosinolate degradation in Lepidium sativum: effects of plant age and time of autolysis. Phytochemistry. 1983;19:1365–1368. [Google Scholar]
- 20.Chung FL, et al. Quantitation of human uptake of the anticarcinogen phenethyl isothiocyanate after a watercress meal. Cancer Epidemiol. Biomarkers Prev. 1992;1:383–388. [PubMed] [Google Scholar]
- 21.Zhang Y, et al. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl Acad. Sci. USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fahey JW, et al. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl Acad. Sci. USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahey JW, et al. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 24.Brusewitz G, et al. The metabolism of benzyl isothiocyanate and its cysteine conjugate. Biochem. J. 1977;162:99–107. doi: 10.1042/bj1620099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mennicke WH, et al. Studies on the metabolism and excretion of benzyl isothiocyanate in man. Xenobiotica. 1988;18:441–447. doi: 10.3109/00498258809041680. [DOI] [PubMed] [Google Scholar]
- 26.Kassahun K, et al. Biotransformation of the naturally occurring isothiocyanate sulforaphane in the rat: identification of phase I metabolites and glutathione conjugates. Chem. Res. Toxicol. 1997;10:1228–1233. doi: 10.1021/tx970080t. [DOI] [PubMed] [Google Scholar]
- 27.Gasper AV, et al. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am. J. Clin. Nutr. 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 28.Egner PA, et al. Quantification of sulforaphane mercapturic acid pathway conjugates in human urine by high-performance liquid chromatography and isotope-dilution tandem mass spectrometry. Chem. Res. Toxicol. 2008;21:1991–1996. doi: 10.1021/tx800210k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannou YM, et al. Allyl isothiocyanate: comparative disposition in rats and mice. Toxicol. Appl. Pharmacol. 1984;75:173–181. doi: 10.1016/0041-008x(84)90199-6. [DOI] [PubMed] [Google Scholar]
- 30.Bollard M, et al. The disposition of allyl isothiocyanate in the rat and mouse. Food Chem. Toxicol. 1997;35:933–943. doi: 10.1016/s0278-6915(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 31.Jiao D, et al. Identification and quantification of the N-acetylcysteine conjugate of allyl isothiocyanate in human urine after ingestion of mustard. Cancer Epidemiol. Biomarkers Prev. 1994;3:487–492. [PubMed] [Google Scholar]
- 32.Zhang Y. The 1,2-benzenedithiole-based cyclocondensation assay, a valuable tool for measurement of chemopreventive isothiocyanates. Crit. Rev. Food Sci. Nutr. 2011 doi: 10.1080/10408398.2010.503288. in press, doi: 10.1080/10408398.2010.503288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolm RH, et al. Isothiocyanates as substrates for human glutathione transferases: structure-activity studies. Biochem. J. 1995;311:453–459. doi: 10.1042/bj3110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye L, et al. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin. Chim. Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 35.Conaway CC, et al. Decomposition rates of isothiocyanate conjugates determine their activity as inhibitors of cytochrome p.450 enzymes. Chem. Res. Toxicol. 2001;14:1170–1176. doi: 10.1021/tx010029w. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro TA, et al. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol. Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 37.Eklind KI, et al. Distribution and metabolism of the natural anticarcinogen phenethyl isothiocyanate in A/J mice. Carcinogenesis. 1990;11:2033–2036. doi: 10.1093/carcin/11.11.2033. [DOI] [PubMed] [Google Scholar]
- 38.Gorler K, et al. The metabolism of benzyl isothiocyanate and its cysteine conjugate in guinea-pigs and rabbits. Xenobiotica. 1982;12:535–542. doi: 10.3109/00498258209038932. [DOI] [PubMed] [Google Scholar]
- 39.Bruggeman IM, et al. Glutathione- and cysteine-mediated cytotoxicity of allyl and benzyl isothiocyanate. Toxicol. Appl. Pharmacol. 1986;83:349–359. doi: 10.1016/0041-008x(86)90312-1. [DOI] [PubMed] [Google Scholar]
- 40.Baillie TA, et al. Glutathione: a vehicle for the transport of chemically reactive metabolites in vivo. Acc. Chem. Res. 1991;24:264–270. [Google Scholar]
- 41.Tang L, et al. The principal urinary metabolites of dietary isothiocyanates, N-acetylcysteine conjugates, elicit the same anti-proliferative response as their parent compounds in human bladder cancer cells. Anticancer Drugs. 2006;17:297–305. doi: 10.1097/00001813-200603000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Chiao JW, et al. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int. J. Oncol. 2002;20:631–636. doi: 10.3892/ijo.20.3.631. [DOI] [PubMed] [Google Scholar]
- 43.Conaway CC, et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, et al. Mechanism of differential potencies of isothiocyanates as inducers of anticarcinogenic phase 2 enzymes. Cancer Res. 1998;58:4632–4639. [PubMed] [Google Scholar]
- 45.Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis. 2000;21:1175–1182. [PubMed] [Google Scholar]
- 46.Zhang Y. Molecular mechanism of rapid cellular accumulation of anticarcinogenic isothiocyanates. Carcinogenesis. 2001;22:425–431. doi: 10.1093/carcin/22.3.425. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, et al. High cellular accumulation of sulphoraphane, a dietary anticarcinogen, is followed by rapid transporter-mediated export as a glutathione conjugate. Biochem. J. 2002;364:301–307. doi: 10.1042/bj3640301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem. Biol. Interact. 1998;111–112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 49.Mi L, et al. The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res. 2007;67:6409–6416. doi: 10.1158/0008-5472.CAN-07-0340. [DOI] [PubMed] [Google Scholar]
- 50.Mi L, et al. A cautionary note on using N-acetylcysteine as an antagonist to assess isothiocyanate-induced reactive oxygen species-mediated apoptosis. Anal. Biochem. 2010;405:269–271. doi: 10.1016/j.ab.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Callaway EC, et al. Cellular accumulation of dietary anticarcinogenic isothiocyanates is followed by transporter-mediated export as dithiocarbamates. Cancer Lett. 2004;204:23–31. doi: 10.1016/j.canlet.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 52.Tseng E, et al. Effect of organic isothiocyanates on the P-glycoprotein- and MRP1-mediated transport of daunomycin and vinblastine. Pharm. Res. 2002;19:1509–1515. doi: 10.1023/a:1020460700877. [DOI] [PubMed] [Google Scholar]
- 53.Ji Y, et al. Effect of organic isothiocyanates on breast cancer resistance protein (ABCG2)-mediated transport. Pharm. Res. 2004;21:2261–2269. doi: 10.1007/s11095-004-7679-1. [DOI] [PubMed] [Google Scholar]
- 54.Ji Y, et al. Membrane transport of dietary phenethyl isothiocyanate by ABCG2 (breast cancer resistance protein) Mol. Pharm. 2005;2:414–419. doi: 10.1021/mp050029f. [DOI] [PubMed] [Google Scholar]
- 55.Hollenberg PF, et al. Mechanism-based inactivation of human cytochromes p450s: experimental characterization, reactive intermediates, and clinical implications. Chem. Res. Toxicol. 2008;21:189–205. doi: 10.1021/tx7002504. [DOI] [PubMed] [Google Scholar]
- 56.Smith TJ, et al. Mechanisms of inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone bioactivation in mouse by dietary phenethyl isothiocyanate. Cancer Res. 1993;53:3276–3282. [PubMed] [Google Scholar]
- 57.Morse MA, et al. Effects of aromatic isothiocyanates on tumorigenicity, O6-methylguanine formation, and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Cancer Res. 1989;49:2894–2897. [PubMed] [Google Scholar]
- 58.Jiao D, et al. The essential role of the functional group in alkyl isothiocyanates for inhibition of tobacco nitrosamine-induced lung tumorigenesis. Carcinogenesis. 1996;17:755–759. doi: 10.1093/carcin/17.4.755. [DOI] [PubMed] [Google Scholar]
- 59.Conaway CC, et al. Inhibition of rat liver cytochrome P450 isozymes by isothiocyanates and their conjugates: a structure-activity relationship study. Carcinogenesis. 1996;17:2423–2427. doi: 10.1093/carcin/17.11.2423. [DOI] [PubMed] [Google Scholar]
- 60.Goosen TC, et al. Inactivation of cytochrome P450 2B1 by benzyl isothiocyanate, a chemopreventative agent from cruciferous vegetables. Chem. Res. Toxicol. 2000;13:1349–1359. doi: 10.1021/tx000133y. [DOI] [PubMed] [Google Scholar]
- 61.Fahey JW, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl Acad. Sci. USA. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iida K, et al. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 63.Sussan TE, et al. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc. Natl Acad. Sci. USA. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding Y, et al. Sulforaphane inhibits 4-aminobiphenyl-induced DNA damage in bladder cells and tissues. Carcinogenesis. 2010;31:1999–2003. doi: 10.1093/carcin/bgq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stewart D, et al. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J. Biol. Chem. 2003;278:2396–2402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi A, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 68.Dinkova-Kostova AT, et al. The role of Keap1 in cellular protective responses. Chem. Res. Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 69.Prawan A, et al. Structural influence of isothiocyanates on the antioxidant response element (ARE)-mediated heme oxygenase-1 (HO-1) expression. Pharm. Res. 2008;25:836–844. doi: 10.1007/s11095-007-9370-9. [DOI] [PubMed] [Google Scholar]
- 70.Ernst IM, et al. (2011) Allyl-, butyl- and phenylethyl-isothiocyanate activate Nrf2 in cultured fibroblasts. Pharmacol. Res. 63:233–240. doi: 10.1016/j.phrs.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Dinkova-Kostova AT, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong F, et al. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem. Res. Toxicol. 2005;18:1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 73.Zhang DD, et al. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thimmulappa RK, et al. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 75.Xu C, et al. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 76.Geng F, et al. Allyl isothiocyanate arrests cancer cells in mitosis, and mitotic arrest in turn leads to apoptosis via BCL-2 phosphorylation. J. Biol. Chem. 2011;286:32259–32267. doi: 10.1074/jbc.M111.278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu W, et al. Natural product derivative Bis(4-fluorobenzyl)trisulfide inhibits tumor growth by modification of beta-tubulin at Cys 12 and suppression of microtubule dynamics. Mol. Cancer Ther. 2009;8:3318–3330. doi: 10.1158/1535-7163.MCT-09-0548. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X, et al. Identification of arsenic-binding proteins in human breast cancer cells. Cancer Lett. 2007;255:95–106. doi: 10.1016/j.canlet.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shan B, et al. Selective, covalent modification of beta-tubulin residue Cys-239 by T138067, an antitumor agent with in vivo efficacy against multidrug-resistant tumors. Proc. Natl Acad. Sci. USA. 1999;96:5686–5691. doi: 10.1073/pnas.96.10.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bai R, et al. Identification of cysteine 354 of beta-tubulin as part of the binding site for the A ring of colchicine. J. Biol. Chem. 1996;271:12639–12645. doi: 10.1074/jbc.271.21.12639. [DOI] [PubMed] [Google Scholar]
- 81.Bai R, et al. Mapping the binding site of colchicinoids on beta-tubulin. 2-chloroacetyl-2-demethylthiocolchicine covalently reacts predominantly with cysteine 239 and secondarily with cysteine 354. J. Biol. Chem. 2000;275:40443–40452. doi: 10.1074/jbc.M005299200. [DOI] [PubMed] [Google Scholar]
- 82.Mi L, et al. Cancer preventive isothiocyanates induce selective degradation of cellular alpha- and beta-tubulins by proteasomes. J. Biol. Chem. 2009;284:17039–17051. doi: 10.1074/jbc.M901789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mi L, et al. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J. Biol. Chem. 2008;283:22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jackson SJ, et al. Sulforaphane inhibits human MCF-7 mammary cancer cell mitotic progression and tubulin polymerization. J. Nutr. 2004;134:2229–2236. doi: 10.1093/jn/134.9.2229. [DOI] [PubMed] [Google Scholar]
- 85.Barthelman M, et al. Inhibitory effects of perillyl alcohol on UVB-induced murine skin cancer and AP-1 transactivation. Cancer Res. 1998;58:711–716. [PubMed] [Google Scholar]
- 86.Huang C, et al. Inhibition of ultraviolet B-induced activator protein-1 (AP-1) activity by aspirin in AP-1-luciferase transgenic mice. J. Biol. Chem. 1997;272:26325–26331. doi: 10.1074/jbc.272.42.26325. [DOI] [PubMed] [Google Scholar]
- 87.Zhu M, et al. Phase II enzyme inducer, sulforaphane, inhibits UVB-induced AP-1 activation in human keratinocytes by a novel mechanism. Mol. Carcinog. 2004;41:179–186. doi: 10.1002/mc.20052. [DOI] [PubMed] [Google Scholar]
- 88.Dickinson SE, et al. Inhibition of activator protein-1 by sulforaphane involves interaction with cysteine in the cFos DNA-binding domain: implications for chemoprevention of UVB-induced skin cancer. Cancer Res. 2009;69:7103–7110. doi: 10.1158/0008-5472.CAN-09-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jeong WS, et al. Modulation of AP-1 by natural chemopreventive compounds in human colon HT-29 cancer cell line. Pharm. Res. 2004;21:649–660. doi: 10.1023/b:pham.0000022412.69380.d7. [DOI] [PubMed] [Google Scholar]
- 90.Patten EJ, et al. Temporal effects of the detoxification enzyme inducer, benzyl isothiocyanate: activation of c-Jun N-terminal kinase prior to the transcription factors AP-1 and NFkappaB. Biochem. Biophys. Res. Commun. 1999;257:149–155. doi: 10.1006/bbrc.1999.0422. [DOI] [PubMed] [Google Scholar]
- 91.Li J, et al. The role of c-Jun in the AP-1 activation induced by naturally occurring isothiocyanates. Food Chem. Toxicol. 2005;43:1373–1380. doi: 10.1016/j.fct.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 92.Cross JV, et al. The isothiocyanate class of bioactive nutrients covalently inhibit the MEKK1 protein kinase. BMC Cancer. 2007;7:183. doi: 10.1186/1471-2407-7-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu R, et al. The roles of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis. 2003;24:1361–1367. doi: 10.1093/carcin/bgg092. [DOI] [PubMed] [Google Scholar]
- 94.Chen YR, et al. Phenylethyl isothiocyanate induces apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J. Biol. Chem. 2002;277:39334–39342. doi: 10.1074/jbc.M202070200. [DOI] [PubMed] [Google Scholar]
- 95.Youn HS, et al. Sulforaphane suppresses oligomerization of TLR4 in a thiol-dependent manner. J. Immunol. 2010;184:411–419. doi: 10.4049/jimmunol.0803988. [DOI] [PubMed] [Google Scholar]
- 96.Jeong WS, et al. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm. Res. 2004;21:661–670. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 97.Srivastava SK, et al. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis. 2004;25:1701–1709. doi: 10.1093/carcin/bgh179. [DOI] [PubMed] [Google Scholar]
- 98.Xu C, et al. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 99.Batra S, et al. Benzyl isothiocyanate-mediated inhibition of histone deacetylase leads to NF-kappaB turnoff in human pancreatic carcinoma cells. Mol. Cancer. Ther. 2010;9:1596–1608. doi: 10.1158/1535-7163.MCT-09-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Myzak MC, et al. Dietary agents as histone deacetylase inhibitors. Mol. Carcinog. 2006;45:443–446. doi: 10.1002/mc.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang LG, et al. Dual action on promoter demethylation and chromatin by an isothiocyanate restored GSTP1 silenced in prostate cancer. Mol. Carcinog. 2007;46:24–31. doi: 10.1002/mc.20258. [DOI] [PubMed] [Google Scholar]
- 102.Wang LG, et al. De-repression of the p21 promoter in prostate cancer cells by an isothiocyanate via inhibition of HDACs and c-Myc. Int. J. Oncol. 2008;33:375–380. [PubMed] [Google Scholar]
- 103.Dashwood RH, et al. Dietary histone deacetylase inhibitors: from cells to mice to man. Semin. Cancer Biol. 2007;17:363–369. doi: 10.1016/j.semcancer.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Singh SV, et al. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J. Biol. Chem. 2004;279:25813–25822. doi: 10.1074/jbc.M313538200. [DOI] [PubMed] [Google Scholar]
- 105.Xiao D, et al. Proteasome-mediated degradation of cell division cycle 25C and cyclin-dependent kinase 1 in phenethyl isothiocyanate-induced G2-M-phase cell cycle arrest in PC-3 human prostate cancer cells. Mol. Cancer Ther. 2004;3:567–575. [PubMed] [Google Scholar]
- 106.Mi L, et al. Isothiocyanates inhibit proteasome activity and proliferation of multiple myeloma cells. Carcinogenesis. 2011;32:216–223. doi: 10.1093/carcin/bgq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiao D, et al. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol. Cancer Ther. 2006;5:2931–2945. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 108.Wu SJ, et al. Effects of antioxidants and caspase-3 inhibitor on the phenylethyl isothiocyanate-induced apoptotic signaling pathways in human PLC/PRF/5 cells. Eur. J. Pharmacol. 2005;518:96–106. doi: 10.1016/j.ejphar.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 109.Park SY, et al. Induction of apoptosis by isothiocyanate sulforaphane in human cervical carcinoma HeLa and hepatocarcinoma HepG2 cells through activation of caspase-3. Oncol. Rep. 2007;18:181–187. [PubMed] [Google Scholar]
- 110.Shankar S, et al. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin. Cancer Res. 2008;14:6855–6866. doi: 10.1158/1078-0432.CCR-08-0903. [DOI] [PubMed] [Google Scholar]
- 111.Tang L, et al. Mitochondria are the primary target in isothiocyanate-induced apoptosis in human bladder cancer cells. Mol. Cancer Ther. 2005;4:1250–1259. doi: 10.1158/1535-7163.MCT-05-0041. [DOI] [PubMed] [Google Scholar]
- 112.Warin R, et al. Inhibition of human breast cancer xenograft growth by cruciferous vegetable constituent benzyl isothiocyanate. Mol. Carcinog. 2010;49:500–507. doi: 10.1002/mc.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiao D, et al. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res. 2007;67:2239–2246. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- 114.Bertl E, et al. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol. Cancer Ther. 2006;5:575–585. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- 115.Kumar A, et al. Antiangiogenic and proapoptotic activities of allyl isothiocyanate inhibit ascites tumor growth in vivo. Integr. Cancer Ther. 2009;8:75–87. doi: 10.1177/1534735408330716. [DOI] [PubMed] [Google Scholar]
- 116.Yao H, et al. Sulforaphane inhibited expression of hypoxia-inducible factor-1alpha in human tongue squamous cancer cells and prostate cancer cells. Int. J. Cancer. 2008;123:125512–125561. doi: 10.1002/ijc.23647. [DOI] [PubMed] [Google Scholar]
- 117.Wang XH, et al. Inhibition of hypoxia inducible factor by phenethyl isothiocyanate. Biochem. Pharmacol. 2009;78:261–272. doi: 10.1016/j.bcp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 118.Hwang ES, et al. Allyl isothiocyanate and its N-acetylcysteine conjugate suppress metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in SK-Hep 1 human hepatoma cells. Exp. Biol. Med. (Maywood) 2006;231:421–430. doi: 10.1177/153537020623100408. [DOI] [PubMed] [Google Scholar]
- 119.Hwang ES, et al. Phenylethyl isothiocyanate and its N-acetylcysteine conjugate suppress the metastasis of SK-Hep1 human hepatoma cells. J. Nutr. Biochem. 2006;17:837–846. doi: 10.1016/j.jnutbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 120.Hwang ES, et al. Benzyl isothiocyanate inhibits metalloproteinase-2/-9 expression by suppressing the mitogen-activated protein kinase in SK-Hep1 human hepatoma cells. Food Chem. Toxicol. 2008;46:2358–2364. doi: 10.1016/j.fct.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 121.Sahu RP, et al. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J. Natl. Cancer Inst. 2009;101:176–193. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gong A, et al. Phenethyl isothiocyanate inhibits STAT3 activation in prostate cancer cells. Mol. Nutr. Food Res. 2009;53:878–886. doi: 10.1002/mnfr.200800253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hahm ER, et al. (2010) Sulforaphane inhibits constitutive and interleukin-6-induced activation of signal transducer and activator of transcription 3 in prostate cancer cells. Cancer Prev. Res. (Phila) 3:484–494. doi: 10.1158/1940-6207.CAPR-09-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y, et al. Cancer-preventive isothiocyanates: dichotomous modulators of oxidative stress. Free Radic. Biol. Med. 2005;38:70–77. doi: 10.1016/j.freeradbiomed.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 125.Meng T-C, et al. Cys-oxidation of protein tyrosine phosphatases: its role in regulation of signal transduction and its involvement in human cancers. J. Cancer Mol. 2006;2:9–16. [Google Scholar]
- 126.Edman P. On the mechanism of the phenyl isothiocyanate degradation of peptides. Acta Chem. Scand. 1956;10:761–765. [Google Scholar]
- 127.Nakamura T, et al. Covalent modification of lysine residues by allyl isothiocyanate in physiological conditions: plausible transformation of isothiocyanate from thiol to amine. Chem. Res. Toxicol. 2009;22:536–542. doi: 10.1021/tx8003906. [DOI] [PubMed] [Google Scholar]
- 128.Brown KK, et al. Direct modification of the proinflammatory cytokine macrophage migration inhibitory factor by dietary isothiocyanates. J. Biol. Chem. 2009;284:32425–32433. doi: 10.1074/jbc.M109.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Swope M, et al. Direct link between cytokine activity and a catalytic site for macrophage migration inhibitory factor. EMBO J. 1998;17:3534–3541. doi: 10.1093/emboj/17.13.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Podhradsky D, et al. Reactions of cysteine, its derivatives, glutathione coenzyme A, and dihydrolipoic acid with isothiocyanates. Experientia. 1979;35:154–155. doi: 10.1007/BF01920581. [DOI] [PubMed] [Google Scholar]