Abstract
A better understanding of the risk of local recurrence (LR) will facilitate therapeutic decision making in the management of early breast cancers. In the present study, we investigated whether telomere length in the normal breast epithelial cells surrounding the tumor is predictive of breast cancer LR; 152 women who were diagnosed with breast cancer at the Lombardi Comprehensive Cancer Center were included in this nested case–control study. Cases (patients had LR) and controls (patients had no LR) were matched on year of surgery, age at diagnosis and type of surgery. Telomere fluorescent in situ hybridization was used to determine the telomere length using formalin fixed paraffin-embedded breast tissues. Small telomere length variation (TLV), defined as the coefficient variation of telomere lengths among examined cells, in normal epithelial cells adjacent to the tumor was significantly associated with a 5-fold (95% confidence interval = 1.2–22.2) increased risk of breast cancer LR. When the subjects were categorized into quartiles, a significant inverse dose–response relationship was observed with lowest versus highest quartile odds ratio of 15.3 (Ptrend = 0.012). Patients who had large TLV had significantly better 10 year recurrence free survival rate compared with patients who had small TLV (80 versus 33%). The present study revealed that TLV in normal epithelial cells adjacent to tumor is a strong predictor of breast cancer LR. If confirmed by future studies, TLV in normal epithelial cells adjacent to tumor has the potential to become a promising biomarker for predicting breast cancer LR after breast conserving surgery.
Introduction
Breast cancer is the most common cancer in women, with estimated 209 060 new cases of breast cancer diagnosed and 40 230 deaths expected to occur in the USA in 2010 (1). With widely implemented population-based mammographic screening, breast cancers are now often detected in early stages (stages 0–II) (2). In the contemporary management of breast cancer, several options exist for local and regional treatment. Patients and their physicians must decide between various surgical options and the dose, volume and technique of radiotherapy. These decisions may have a significant impact on treatment-related morbidity and survival from breast cancer (3). A better understanding of the risk of local recurrence (LR) would facilitate therapeutic decision making. Thus, it is highly desirable to have robust biomarkers to identify breast cancer patients who are at high risk of LR.
Currently, treatment decisions for early-stage breast cancer are based on parameters relating to the tumor, including size, margin status, grade and histological features (4–9). Patient age also affects the risk of LR (10–13). Patients with large, high-grade tumors, excision margin <1 mm and diagnosed at a young age (<40 years old) are at the highest risk of developing recurrence. Moreover, gene expression profiling of tumors has been shown to separate breast cancers into distinct molecular subtypes with prognostic significance (14–16). Commercially available assays based on gene expression profiling, including Oncotype DX (Genomic Health, Redwood City, CA) and MammaPrint (Agendia, Amsterdam, The Netherlands), may provide useful prognostic information (17,18). However, their value for predicting breast cancer LR was not confirmed (19–21). Other studies have found that using a six immunohistochemical biomarker panel (ER, PR, HER2, CK5/6, EGFR and Ki-67), breast tumors can be grouped into six molecular subtypes that have significantly different risk of LR (22,23).
Although most previous studies focused on characterizing clinicopathologic features and tumor markers for the prediction of LR, relatively little attention was given to investigations on molecular changes in normal tissue surrounding the tumor as additional biomarkers for identifying patients at high risk of LR. One promising candidate biomarker in the adjacent histologically normal tissues is telomere, the nucleoprotein complexes at the end of eukaryotic chromosomes. Telomeres are specialized structures that protect chromosome ends and are essential for maintaining genome integrity and stability (24). Telomere shortening is a early and common molecular alterations in epithelial cancers (25–33). Previous studies have found that very short telomere length is a common genetic alteration in premalignant breast lesions and early breast cancer cells (34,35), and the telomere length in tumor tissues correlates with stage and prognosis in breast cancer (36). Furthermore, it was reported that significant telomere length differences in adjacent histologically normal breast tissue exist by the distance to tumor (37). In addition to telomere length, higher telomere length variation (TLV)/heterogeneity in local tissue has been associated with development of cancer in biliary tract (38) and bladder (31). High telomere heterogeneity is also an characteristic of cancer cells that use alternative lengthening of telomere (ALT) to maintain telomere length and ALT+ cancers are often aggressive and have poor clinical outcome (39). In the present study, we examined both telomere length and TLV in various types of cells surrounding breast tumor in search for potential biomarkers to predict the risk of LR.
Materials and methods
Patient population
The study was approved by the MedStar Research Institute–Georgetown University Oncology Institutional Review Board. Between 1990 and 2006, a total of 2025 breast cancer patients who had complete follow-up data were recorded in the cancer registry database of the Lombardi Comprehensive Cancer Center (LCCC), Georgetown University Medical Centers. Of these, 1654 (81.7%) had no disease recurrence at the last contact (December, 2009), 74 (3.7%) had LR, 200 (9.9%) had distant recurrence, 45 (2.2%) were never disease free and 50 (2.5%) had unknown type of recurrence. Breast cancer patients who had LR (N = 74) were considered cases in our study. Breast cancer patients who had no disease recurrence were considered controls. We randomly selected 148 controls (2 controls per case), matched to cases on year of surgery (5 year interval), type of surgery (total mastectomy, partial mastectomy/segmental mastectomy, lumpectomy) and age at diagnosis (5 year interval) to include in our study.
Tumor tissue retrieval
The list of selected patients (N = 222) was sent to the Histopathology and Tissue Shared Resources of LCCC to retrieval paraffin-embedded tissue blocks. Two blocks (a tumor and a separate benign tissue blocks) were retrieved for each of the 75 (33.8%) patients, tumor blocks were retrieved for 37 (16.7%) patients and benign blocks were retrieved for 44 (19.8%) patients. In 66 (30%) patients, no tissue blocks were found. Ten serial 5 micron sections were cut from each block and the first section was H&E stained. The second section was used for telomere fluorescent in situ hybridization (FISH), whereas the rest of the sections were reserved for other analysis. The study pathologist (BK) examined all the H&E slides and circled cell types, i.e. cancer cell, normal epithelial cell and infiltrative lymphocytes, as the guide for cell type identification. All the tissue sections were only labeled with a unique study ID number.
Clinical data
The Clinical Molecular Epidemiology Shared Resources of the LCCC provided de-identified clinical and treatment data, including age at diagnosis, date of birth, date of diagnosis, race, type of surgery, date of surgery, disease stage, tumor size, radiotherapy and type, radiotherapy date, chemotherapy and type, chemotherapy date, tumor ER/PR status, recurrence type, recurrence date, date of first contact, date of last contact, vital status and date of death. The data were downloaded from the Cancer Registry of the LCCC and a unique study ID was assigned to each patient. All patient identifiable information was removed before the data were sent out to the study team for data analysis.
Telomere quantitative fluorescent in situ hybridization
Telomere quantitative fluorescent in situ hybridization (TQ-FISH) was performed following published protocol with modifications (40). Tissue sections were deparaffined in Citrisol and hydrated through a graded ethanol series and distilled water. The slides were then incubated in 10 mM Sodium Citrate buffer (pH 6.5) at 85°C for 15 min, neutralized in PBS and dehydrated using ethanol series; 15 μl of hybridization buffer containing 1 μM Cy3-labeled telomere-specific peptide nucleic acid probe, 50% formamide, 10 mM Tris (pH 7.5), 5% blocking agent and 1× Denhardt’s solution was added to the slides. Hybridization was carried out at 30°C over night following denaturation at 75°C for 5 min. The slides were subsequently washed with occasional shaking for 10 min each in 2× SSC, 1× SSC, 0.5× SSC and 0.1× SSC at 45°C and immediately mounted with anti-fade mounting medium containing 4′,6-diamidino-2-phenylindole.
The cells were imaged using a Leica DM4000B fluorescent microscope (Leica Microsystems, Bannockburn, IL) coupled with a PixelFly charge-coupled device camera, both controlled by IPlabs software (Scanalytics). An oil lens with magnification of ×100 was used. Both Cy3 and 4′,6-diamidino-2-phenylindole images were captured. The exposure time was held constant at 150 ms for all Cy3 images to keep the signals within the linear range of the camera and enable comparison of telomere signal. Telomere length/length variations were measured in five cell types: cancer cells, adjacent normal epithelial cells, distant normal epithelial cells, carcinoma-associated fibroblast (CAF) cells and infiltrative lymphocytes. Identification of five cell types was assisted with the H&E slides cut from the same tissue block with tumor and benign areas marked out by the study pathologist. Quantification of the digitized fluorescent telomere signals was accomplished using ImageJ supplemented with a semi-automated script TeloMeter (http://bui2.win.ad.jhu.edu/telometer/). Thirty cells were randomly selected and analyzed for each cell type to estimate average telomere length and TLV. The number of cells analyzed was comparable with previous studies that reported telomere data in tissue by analyzing 20–25 cells (38,41).
TLV was defined as the coefficient of variation (CV) of telomere lengths among 30 analyzed cells. Relative telomere lengths (RTLs) were calculated as telomere fluorescent intensity units (FIUs) of cell type of interest divided by the telomere FIU of the infiltrative lymphocytes. A total number of 231 slides (112 tumor sections and 119 benign sections) were assayed in 26 batches in this study. A chromosome control slide containing metaphase spreads with known telomere length was included in each batch of TQ-FISH experiment to monitor the hybridization efficiency. The CV of the mean telomere length among the 26 chromosome control slides was 12.8%. Additionally, a tissue control slide containing the tissue section cut from the same breast tumor block was included in 19 batches of TQ-FISH experiment and the CV of the mean TLV in normal epithelial cells adjacent to tumor among the 19 tissue slides was 21.9%. The TQ-FISH assay success rate was 100% (18 samples were repeated to capture infiltrative lymphocytes that were missed in the first attempt). The laboratory personnel were blinded to the case–control status of the specimen.
Statistical analysis
Wilcoxon rank sum tests and Fisher's exact tests were used to examine the case–control differences in continuous and categorical variables, respectively. Unconditional logistic regression was used to estimate the odd ratios for the association between TLV and the risk of breast cancer LR while controlling for age at diagnosis, type of surgery, year of surgery, disease stage and estrogen receptor status. Kaplan–Meier analysis for recurrence-free survival was estimated for patients with small and large TLV in normal epithelial cells adjacent to the tumor and compared using Log-rank test. TLV was dichotomized as small/large using the median value in control patients as the cut point. Paired Student t-test was used to examine the differences in telomere length or length variation between cell types among all subjects. Spearman correlation was used to examine the correlations of TLV between cell types among all subjects. P-values were two sided and considered significant if P ≤ 0.05. All analyses were performed using SAS software, version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Characteristics of study population
The characteristics of the study subjects are presented in Table I. There were no significant case–control differences in the distributions of race, histological type and type of surgery. The mean age at diagnosis was 50.2 years for cases and 51.5 years for controls and the average tumor size was 2.49 cm for cases and 1.71 cm for controls. The median follow-up time was slightly higher in cases (78 months) than in controls (65 months). Cases had significantly higher percentage of advanced stage (III–IV) disease compared with controls (22 versus 2%, P < 0.001). The percent of patients who had ER-negative tumors was significantly higher in cases (34.8%) than in controls (11.3%, P = 0.004). Cases were significantly more likely to receive radiation therapy or chemotherapy or both, partly reflecting the factor that higher proportion of the cases had more advanced disease.
Table I.
Clinicopathologic characteristics of study population
| Variables | Cases, N = 46 | Controls, N = 106 | P valuea |
| Age at diagnosis, mean (SD) | 50.2 (11.7) | 51.5 (10.9) | 0.374 |
| Tumor size (cm), mean (SD) | 2.49 (2.70) | 1.71 (1.26) | 0.097 |
| Months of follow-up, mean (SD) | 96.5 (65.6) | 76.5 (57.0) | 0.067 |
| Race, N (%) | |||
| White | 27 (58.7) | 71 (66.4) | |
| Black | 10 (21.7) | 20 (18.7) | |
| Others | 9 (19.6) | 16 (15.0) | 0.544 |
| Histological type | |||
| Duct carcinoma | 36 (78.3) | 88 (82.2) | |
| Duct and lobular carcinoma | 1 (2.2) | 8 (7.5) | |
| Lobular carcinoma | 5 (10.9) | 6 (5.6) | |
| Others | 4 (8.1) | 5 (4.7) | 0.231 |
| Stage, N (%) | |||
| 0–I | 15 (36.6) | 47 (45.6) | |
| II | 17 (41.5) | 54 (52.4) | |
| III–IV | 9 (22.0) | 2 (1.9) | < 0.001 |
| Type of surgery, N (%) | |||
| Lumpectomy | 18 (39.1) | 42 (39.6) | |
| Partial mastectomy | 14 (30.4) | 16 (15.1) | |
| Total mastectomy | 11 (23.9) | 35 (33.0) | |
| Others | 3 (6.5) | 13 (12.3) | 0.151 |
| Year of surgery, N (%) | |||
| Before 1995 | 11 (25.6) | 13 (12.3) | |
| 1995–99 | 18 (41.9) | 39 (36.8) | |
| 1999–2004 | 13 (30.2) | 38 (35.9) | |
| After 2004 | 1 (2.3) | 16 (15.1) | 0.036 |
| Systemic therapy, N (%) | |||
| None | 8 (17.4) | 47 (44.3) | |
| Radiation therapy only | 11 (23.9) | 11 (10.4) | |
| Chemotherapy only | 13 (28.3) | 21 (19.8) | |
| Both | 14 (30.4) | 27 (25.5) | 0.006 |
| ER status, N (%) | |||
| Positive | 14 (30.4) | 47 (44.3) | |
| Negative | 16 (34.8) | 12 (11.3) | |
| Unknown | 16 (34.8) | 47 (44.3) | 0.004 |
ER, estrogen receptor.
P-values were based on Wilcoxon rank sum test (continuous variables) or Fisher's exact test (categorical variables).
Key clinicopathological factors, including age at diagnosis, tumor size, race, stage, type of surgery and ER/PR status, were compared between cases with and without tissues, and no significant differences were observed in all factors except stage. Higher percentage of cases without tissue had stage III or IV disease compared with cases with tissue (11 versus 4%, P = 0.01). The same clinicopathological factors were compared between cases with and without adjacent normal epithelial cells in tumor block and no significant differences were seen.
TLV in normal epithelial cells and breast cancer LR
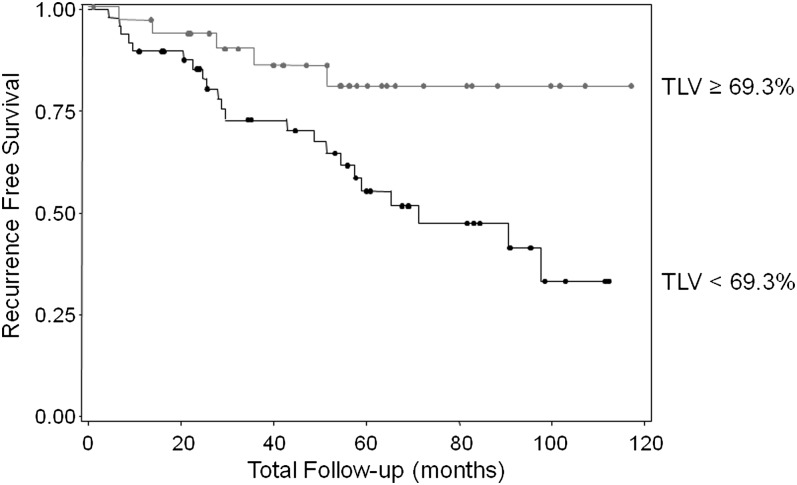
We examined if TLV, defined as the CV of the telomere lengths among 30 analyzed cells, is associated with breast cancer LR. We found that TLV in normal epithelial cells adjacent to the tumor was significantly lower in cases (mean ± SD = 60.2 ± 16.3) than in controls (72.9 ± 21.0, P = 0.008, Table II). Using the median value in controls as a cut point, patients who had smaller TLV in normal epithelial cells adjacent to the tumor had a significantly increased risk of breast cancer LR, with an adjusted odds ratio (OR) of 5.1 (95% confidence interval = 1.2–22.2, Table III). ORs were adjusted for year of surgery, type of surgery, age at diagnosis, disease stage and estrogen receptor status. When the TLV was categorized into quartiles based on the value in controls, we observed a significant dose–response relationship between TLV in normal epithelial cells adjacent to the tumor and breast cancer LR, with the lowest versus highest quartile OR of 15.3 (Ptrend = 0.012, Table III). Kaplan–Meier survival analysis indicated that patients with larger TLV (≥69.3) had 80% recurrence-free survival at the 10 year follow-up, significantly better than the 33% recurrence-free survival rate in patients who had smaller TLV (<69.3, P = 0.018, Figure 1).
Table II.
Case–control comparisons of telomere length and TLV
| Cases |
Controls |
P value | |||
| N | Mean (SD) | N | Mean (SD) | ||
| RTLa | |||||
| Tumor cells | 35 | 0.772 (0.74) | 66 | 0.726 (0.61) | 0.95 |
| CAFs | 36 | 0.887 (0.56) | 68 | 0.741 (0.35) | 0.23 |
| Adjacent epithelia cellsc | 28 | 0.699 (0.35) | 59 | 0.615 (0.31) | 0.30 |
| Distant epithelia cellsd | 23 | 0.651 (0.30) | 52 | 0.613 (0.37) | 0.40 |
| TLVb | |||||
| Tumor cells | 36 | 95.3 (52.3) | 69 | 85.6 (45.1) | 0.32 |
| CAFs | 37 | 70.6 (16.4) | 71 | 78.8 (31.8) | 0.43 |
| Adjacent epithelia cells | 29 | 60.2 (16.3) | 59 | 72.9 (21.0) | 0.008 |
| Distant epithelia cells | 30 | 67.9 (29.8) | 84 | 74.1 (21.7) | 0.09 |
| Lymphocytes | 37 | 57.1 (19.7) | 71 | 56.5 (13.8) | 0.82 |
P-values were based on Wilcoxon rank sum test.
RTL was defined as the telomere FIU of the cell type of interest divided by the telomere FIU of the infiltrative lymphocytes.
TLV is the CV% of telomere length in 30 cells.
Normal-appearing epithelial cells on the same blocks as the tumor cells.
Normal-appearing epithelial cells on the separate blocks from the tumor cells.
Table III.
Association between TLV and risk of breast cancer LR
| TLVa | Case/control (N) | ORb (95% CI) | P value |
| Adjacent normal epithelial cellsc | |||
| By median | |||
| ≥69.3 | 7/29 | Reference | |
| <69.3 | 22/30 | 5.13 (1.19–22.2) | 0.029 |
| By quartiles | |||
| ≥88.4 | 2/15 | Reference | |
| 69.3–88.4 | 5/14 | 2.68 (0.30–24.3) | |
| 55.5–69.3 | 10/15 | 6.13 (0.74–50.9) | |
| <55.5 | 12/15 | 15.31 (1.64–143) | 0.012e |
| Distant normal epithelial cellsd | |||
| By median | |||
| ≥70.4 | 12/42 | Reference | |
| <70.4 | 18/42 | 1.67 (0.59–4.71) | 0.33 |
| By quartiles | |||
| ≥91.6 | 3/22 | Reference | |
| 70.4–91.6 | 9/20 | 1.53 (0.29–8.14) | |
| 57.3–70.4 | 5/22 | 1.05 (0.17–6.40) | |
| <57.3 | 13/20 | 3.41 (0.70–16.7) | 0.13e |
CI, confidence interval. High concordance (78%) between stage and tumor size was observed; thus, only stage was included in the final logistic model.
aTLV is the CV% of telomere length in 30 cells.
ORs were adjusted for age at diagnosis, year of surgery, type of surgery, stage and estrogen receptor status (positive, negative or unknown).
Normal-appearing epithelial cells on the same block as the tumor cells.
Normal-appearing epithelial cells on the separate block from the tumor cells.
P-for-trend.
Fig. 1.
Kaplan–Meier analysis indicates that patients with large TLV (TLV ≥ 69.3%, gray line) in normal epithelial cells adjacent to the tumor had 80% 10 year recurrence-free survival rate compared with a rate of 33% in patients who had small TLV (TLV < 69.3%, black line). Log-rank P-value = 0.018.
TLV in normal epithelial cells distant to the tumor (epithelial cells were identified from the blocks without tumor cells) was non-significantly smaller in cases (mean ± SD = 67.9 ± 29.8) than in controls (74.1 ± 21.7, P = 0.09, Table II). Using the median value in controls as a cut point, patients with smaller TLV in normal epithelial cells distant to the tumor had a non-significantly increased risk of breast cancer LR, with an adjusted OR of 1.67 (95% confidence interval = 0.6–4.7, Table III). When the TLV was categorized into quartiles based on the value in controls, we observed a trend of increasing risk of LR with decreasing TLV in normal epithelial cells distant to the tumor, with the lowest versus highest quartile OR of 3.4 (Ptrend = 0.13, Table III). These results are somewhat consistent with the results from normal epithelial cells adjacent to the tumor. However, it should be noted that the exact distances between the normal epithelial cells and the tumor were unknown.
We observed no significant associations between TLV in cancer cells, in CAFs or in infiltrative lymphocytes and breast cancer LR (Table II).
TLV in normal epithelial cells and clinical factors
We also examined relationship between TLV in normal epithelial cells adjacent to tumor and selected clinical and host factors. We found that TLV in normal epithelial cells adjacent to tumor is significantly smaller in patients with large tumors (≥1.5 cM) than in patients with small tumors (<1.5 cM, Table IV). We observed no significant association between TLV and patients’ race, age at diagnosis, disease stage or tumor histological type (Table IV). We found that TLV is lower in ER-negative tumors than in ER-positive tumors, but this difference was not statistically significant (Table IV).
Table IV.
Distribution of TLV in normal epithelial cells by clinical characteristics in all subjects
| TLV in ANECa |
TLV in DNECb |
|||
| N | Mean (SD) | N | Mean (SD) | |
| Race | ||||
| Whites | 55 | 71.7 (21.6) | 70 | 76.0 (27.0) |
| Blacks | 14 | 65.1 (12.8) | 21 | 62.5 (15.2) |
| Others | 19 | 62.9 (20.2) | 23 | 70.7 (18.9) |
| P value | 0.28 | 0.13 | ||
| Age at diagnosis | ||||
| ≤40 | 19 | 61.6 (15.3) | 20 | 74.2 (30.1) |
| 41–50 | 29 | 72.3 (22.2) | 39 | 69.8 (17.3) |
| 51–60 | 25 | 66.2 (19.9) | 30 | 68.3 (19.4) |
| ≥61 | 15 | 75.0 (21.6) | 25 | 80.0 (31.6) |
| P value | 0.12 | 0.48 | ||
| Stage | ||||
| 0–I | 31 | 71.9 (22.8) | 48 | 76.6 (26.3) |
| II | 47 | 67.8 (19.7) | 55 | 70.3 (22.7) |
| III–IV | 7 | 66.9 (16.5) | 5 | 62.8 (29.1) |
| P value | 0.80 | 0.39 | ||
| Tumor size | ||||
| <1.5 cM | 21 | 81.9 (22.4) | 28 | 82.1 (31.5) |
| ≥1.5 cM | 43 | 66.2 (20.0) | 45 | 71.1 (21.3) |
| P value | 0.003 | 0.12 | ||
| ER status | ||||
| Negative | 21 | 60.5 (19.4) | 21 | 64.0 (18.6) |
| Positive | 33 | 69.5 (20.8) | 43 | 72.8 (23.8) |
| P value | 0.12 | 0.21 | ||
| Histological type | ||||
| Ductal | 72 | 69.1 (21.3) | 94 | 71.0 (21.9) |
| Lobular | 5 | 61.8 (21.0) | 6 | 88.5 (53.3) |
| Ductal and lobular | 5 | 72.0 (11.7) | 6 | 85.3 (23.2) |
| Others | 6 | 67.8 (15.1) | 8 | 67.5 (14.5) |
| P value | 0.69 | 0.49 | ||
P-values were based on Kruskal–Wallis test.
Normal-appearing epithelial cells on the same blocks as the tumor cells.
Normal-appearing epithelial cells on the separate blocks from the tumor cells.
Characteristics of TLV in cancer and stromal cells
Telomere length/length variations were measured in five cell types: cancer cells, adjacent normal epithelial cells, distant normal epithelial cells, CAF cells and infiltrative lymphocytes. The average TLV was significantly different between cell types (one-way ANOVA, P <0.001), such as average TLV in lymphocytes (56.7%) < adjacent normal epithelial cells (68.7%) < distant normal epithelial cells (72.4%) < CAFs (76.0%) < tumor cells (88.9%). The differences in TLV between lymphocytes and other four cell types and between cancer cells and other four cell types were highly significant at P < 0.001 level. The differences in mean TLV between adjacent normal epithelial cells and distant normal epithelial cells and between distant normal epithelial cells and CAFs were not statistically significant with a P-value of 0.26 and 0.15, respectively.
We also examined correlations in TLV between cell types. Significant correlation in TLV was seen between adjacent normal epithelial cells and infiltrative lymphocytes (r = 0.42, P < 0.001), between adjacent normal epithelial cells and distant normal epithelial cells (r = 0.24, P = 0.026), between adjacent normal epithelial cells and CAFs (r = 0.23, P = 0.012) and between CAFs and infiltrative lymphocytes (r = 0.24, P = 0.006). No significant correlation in TLV was seen between cancer cells and any other four cell types, between CAFs and distant normal epithelial cells (r = −0.01, P = 0.99) and between infiltrative lymphocytes and distant normal epithelial cells (r = 0.08, P = 0.40).
Characteristics of telomere length in cancer and stromal cells
The mean telomere length, expressed as FIU, in infiltrative lymphocytes (mean FIU = 208 935) was significantly longer than in tumor cells (mean FIU = 143 422, P < 0.001), in adjacent normal epithelial cells (mean FIU = 145 401, P < 0.001), in distant normal epithelial cells (mean FIU = 140 423, P < 0.001) and in CAF cells (mean FIU = 152 348, P <0.001). In 17 cases (15.3%), however, we found longer telomeres in tumor cells than in either infiltrative lymphocytes or adjacent normal epithelial cells. RTL, defined as telomere FIU in cells of interest divided by telomere FIU in infiltrative lymphocytes, was significantly shorter in adjacent normal epithelial cells (mean = 0.670) than in CAFs (mean = 0.871, P = 0.013, Table II). There are no significant differences in RTL between adjacent normal epithelial cells and distance epithelial cells (P = 0.522), between adjacent normal epithelial cells and tumor cells (P = 0.230) and between CAFs and tumor cells (P = 0.324). Our observed telomere length differences between different cell types were consistent with previous reports (35,42).
Telomere length and breast cancer LR
We observed no significant case–control differences in RTL in tumor cells, in adjacent normal epithelial cells, in distant epithelial cells or in CAFs (Table II). Logistic regression analysis indicated that RTL in any of the four cell types were not associated with risk of breast cancer LR.
Discussion
In this report, we demonstrated that, after adjustment for known breast cancer recurrence risk factors, TLV among normal-appearing epithelial cells adjacent to the tumor was significantly associated with breast cancer LR. Patient who had large TLV had significantly better 10 year recurrence-free survival. The results support the hypothesis that early molecular changes in the morphologically normal epithelial cells adjacent to the tumor are predictive of breast cancer LR. To the best of our knowledge, this study is the first to report that TLV in adjacent normal epithelial cells could be a potential molecular marker to predict breast cancer LR.
One of the most important predictors of LR is the pathological margin status after breast conserving surgery (4–7). Surgically excised tumors are routinely subject to margin examination by histology to make sure all tumor tissues has been removed; otherwise, additional surgery will be performed (43). However, histological examination based on morphology may not identify “cancerous” cells that carry early malignant molecular changes but show “normal” morphology. Thus, a negative margin classification based on current standard may not be truly negative. After breast-conserving surgery, microscopically normal-appearing but genetically altered epithelium may remain in situ. These epithelial cells can acquire additional mutations or epigenetic alterations that initiate the development of a second tumor of the same or a different histological type, representing an LR. This is probably why the vast majority of LRs occur at the site of excision (10,44). This concept is consistent with the phenomenon of field cancerization. Field cancerization describes the presence of occult but clinically important preneoplastic lesions of the epithelium within an anatomic region exposed to the same carcinogens (45) and has been described for many types of cancers including breast cancer (46,47). In the present study, we found that only TLV in normal-appearing epithelial cells was significantly associated with breast cancer LR. TLVs in tumor cells, CAF cells or infiltrative lymphocytes were not associated with LR. These data are consistent with our hypothesis that LR of breast cancer after breast conserving surgery is partly due to the failure to completely remove the morphologically normal “pre-cancerous” cells in the remaining breast tissue, further highlighting the importance of better defining the surgical margin by using molecular biomarkers.
Telomeres are specialized structures that protect chromosome integrity and are essential for maintaining genome stability (24). Cell proliferation leads to telomere shortening due to the end replication problem and very short telomeres are a frequent genetic alteration in premalignant lesions, indicating that telomere dysfunction is often an early event in carcinogenesis (25–27,29–31). Previous studies have shown that telomere shortening occurs in breast cancer premalignant lesions during the transition from ductal hyperplasia to ductal carcinoma in situ (35,48), suggesting that short telomere is one of the earliest molecular changes during breast cancer development. However, we found that telomere length in normal-appearing epithelial cells adjacent to the tumor was not associated with breast cancer LR. Nor is the telomere length in tumor cells or CAFs associated with LR (Table II). In our study set, we observed that normal epithelial cells adjacent to tumor showed typical terminal duct lobular unit structure and also had shorter average telomere length compared with CAF cells or tumor cells (Table II). This observation is consistent with previous reports, suggesting that terminal duct lobular unit of normal breast tissue typically have shorter telomeres than myoepithelial cells or normal large lactiferous ducts (35,42). In a separate study, we examined 40 samples of normal breast tissue from women who underwent breast reduction surgery. We also found that telomere length in terminal duct lobular unit was significantly shorter than in large ducts (data not shown). These observations suggest that telomere lengths in breast epithelial cells are heterogeneous and probably reflect the complex biology of breast development and differentiation during puberty, menstrual cycle, pregnancy and lactation. Importantly, we found small TLV among normal epithelial cells adjacent to tumor is significantly associated with an increased risk of LR. Further, TLV in normal epithelial cells adjacent to tumor was not associated with known recurrent risk factors, such as patient age at diagnosis, race, disease stage and tumor histological type, suggesting that it is an independent risk marker. The biology underlying this observation is unknown and remains to be elucidated.
One could speculate that TLV, like telomere length, may reflect the recent history of cell proliferation in a given cell type. For example, a uniform telomere length (small TLV) among the cells may suggest a recent clonal expansion. Clonal expansion has been reported to be a common step in cancer development, in which certain mutations transform cells into super-competitors that expand at the expense of the surrounding cells without inducing histological changes (49). The normal epithelial cells adjacent to tumor, as part of the field giving rise to the primary tumor, could be the colonies of expanding super-competitors originated from the same progenitor cell. One would expect that these colonies of expanding super-competitors will have similar telomere length and high risk of developing a tumor. To shed light on this idea, we performed immunochemical staining with anti-Ki-67 antibody in the available samples (N = 87). We found that normal epithelial cells adjacent to tumor showed positive Ki-67 staining (high proliferation marker) and had significantly smaller TLV (mean ± SD = 67.1 ± 20.3%, N = 67) than in Ki-67 negative-stained cells (mean ± SD = 78.5 ± 26.1%, N = 20, P = 0.047). These preliminary observations suggest an important biological basis of breast cancer LR, warranting further investigation. Unfortunately, the small sample size precluded us to perform detailed analysis of join effects of Ki-67 and TLV on breast cancer LR.
The present study tested a novel idea of developing non-tumor cell-related molecular markers for better defining surgical margins and for understanding the risk of LR based on normal breast tissues surrounding the tumor. By using quantitative FISH, we were able to measure telomere length at the individual cell level and calculated TLV (CV%) as a novel phenotype, in addition to traditionally used average telomere length. However, there are some weaknesses in our study: (i) our sample size is relatively small and does not have sufficient power to detect small case–control difference. Thus, future larger studies are needed to confirm our findings; (ii) the exact distance between normal epithelial cells and tumor is unknown, which precluded us to define the risk association between TLV in normal epithelial cells and LR by tissue distance to examine extend of the field effect and (iii) the tissue samples were processed and stored over 15 year period, differences in sample process and aging may affect the probe access and FISH hybridization efficiency, which could result in variations in telomere length measurement between samples. The measurement variation could be substantial if the absolute values, i.e. telomere FIU, were used. In our study, we used telomere length in infiltrative lymphocytes to normalize the hybridization variation and RTL, defined as telomere length in cells of interest divided by telomere length of infiltrative lymphocytes, was used in case–control analysis. TLV was defined as the CV of the mean (SD/mean) telomere length among the analyzed cells and this definition automatically corrects for hybridization variation. In addition, cases and controls were matched on year of surgery, which minimizes the bias of paraffin block aging on the TQ-FISH. Therefore, the observed case–control difference is not likely to be the result of technique artifact.
In summary, this study revealed that TLV in normal epithelial cells adjacent to tumor is significantly associated with breast cancer LR. Patients who had large TLV had significantly better 10 year recurrence-free survival rate (80%) than patients who had small TLV (33%). If confirmed by future larger studies, TLV in normal epithelial cells adjacent to tumor has the potential to become a promising biomarker for predicting breast cancer LR after breast conserving surgery. Because effective treatment modalities exist for the locoregional control of breast cancer, robust biomarkers that can stratify patients into low/high-risk group for LR could profoundly affect the treatment of breast cancer. Additional studies are necessary to determine if small TLV in normal adjacent epithelial cells is also associated with distant recurrence and overall survival.
Funding
Susan G Komen for the Cure (BCTR0707157 to Y.Z.); the Clinical Molecular Epidemiology Shared Resource and Histopathology and Tissue Shared Resource of the Lombardi Comprehensive Cancer Center (National Institutes of Health grant P30 CA51008).
Acknowledgments
The Clinical Molecular Epidemiology Shared Resources at the LCCC provided services for obtaining clinical and pathology data. The Histopathology and Tissue Shared Resource at the LCCC provided services for paraffin block retrieving and tissue sectioning.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CAF
carcinoma-associated fibroblasts
- CV
coefficient of variation
- FISH
fluorescent in situ hybridization
- FIU
fluorescent intensity units
- LCCC
Lombardi Comprehensive Cancer Center
- LR
local recurrence
- OR
odds ratio
- RTL
relative telomere length
- TLV
telomere length variation
- TQ-FISH
telomere quantitative fluorescent in situ hybridization
References
- 1.Jemal A, et al. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hofvind S, et al. Comparing screening mammography for early breast cancer detection in Vermont and Norway. J. Natl Cancer Inst. 2008;100:1082–1091. doi: 10.1093/jnci/djn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Punglia RS, et al. Local therapy and survival in breast cancer. N. Engl. J. Med. 2007;356:2399–2405. doi: 10.1056/NEJMra065241. [DOI] [PubMed] [Google Scholar]
- 4.Solin LJ, et al. The significance of the pathology margins of the tumor excision on the outcome of patients treated with definitive irradiation for early stage breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 1991;21:279–287. doi: 10.1016/0360-3016(91)90772-v. [DOI] [PubMed] [Google Scholar]
- 5.Smitt MC, et al. The importance of the lumpectomy surgical margin status in long-term results of breast conservation. Cancer. 1995;76:259–267. doi: 10.1002/1097-0142(19950715)76:2<259::aid-cncr2820760216>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Schnitt SJ, et al. The relationship between microscopic margins of resection and the risk of local recurrence in patients with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer. 1994;74:1746–1751. doi: 10.1002/1097-0142(19940915)74:6<1746::aid-cncr2820740617>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Pittinger TP, et al. Importance of margin status in outcome of breast-conserving surgery for carcinoma. Surgery. 1994;116:605–608. [PubMed] [Google Scholar]
- 8.Neuschatz AC, et al. Margin width as a determinant of local control with and without radiation therapy for ductal carcinoma in situ (DCIS) of the breast. Int. J. Cancer. 2001;96(suppl.):97–104. doi: 10.1002/ijc.10357. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein MJ, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N. Engl. J. Med. 1999;340:1455–1461. doi: 10.1056/NEJM199905133401902. [DOI] [PubMed] [Google Scholar]
- 10.Idvall I, et al. Histopathological and cell biological characteristics of ductal carcinoma in situ (DCIS) of the breast-a comparison between the primary DCIS and subsequent ipsilateral and contralateral tumours. Breast. 2005;14:290–297. doi: 10.1016/j.breast.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Roka S, et al. High nuclear grade and negative estrogen receptor are significant risk factors for recurrence in DCIS. Eur. J. Surg. Oncol. 2004;30:243–247. doi: 10.1016/j.ejso.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Huston TL, et al. Locally recurrent breast cancer after conservation therapy. Am. J. Surg. 2005;189:229–235. doi: 10.1016/j.amjsurg.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein MJ, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77:2267–2274. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2267::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 14.Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 15.Sorlie T, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. U.S.A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van 't Veer LJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 17.Paik S, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 18.van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 19.Nuyten DS, et al. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. Breast Cancer Res. 2006;8:R62. doi: 10.1186/bcr1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreike B, et al. Gene expression profiles of primary breast carcinomas from patients at high risk for local recurrence after breast-conserving therapy. Clin. Cancer Res. 2006;12:5705–5712. doi: 10.1158/1078-0432.CCR-06-0805. [DOI] [PubMed] [Google Scholar]
- 21.Kreike B, et al. Local recurrence after breast-conserving therapy in relation to gene expression patterns in a large series of patients. Clin. Cancer Res. 2009;15:4181–4190. doi: 10.1158/1078-0432.CCR-08-2644. [DOI] [PubMed] [Google Scholar]
- 22.Voduc KD, et al. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 23.Millar EK, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J. Clin. Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 24.McEachern MJ, et al. Telomeres and their control. Annu. Rev. Genet. 2000;34:331–358. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- 25.Engelhardt M, et al. Telomerase and telomere length in the development and progression of premalignant lesions to colorectal cancer. Clin. Cancer Res. 1997;3:1931–1941. [PubMed] [Google Scholar]
- 26.Kinouchi Y, et al. Telomere shortening in the colonic mucosa of patients with ulcerative colitis. J. Gastroenterol. 1998;33:343–348. doi: 10.1007/s005350050094. [DOI] [PubMed] [Google Scholar]
- 27.O'Sullivan JN, et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat. Genet. 2002;32:280–284. doi: 10.1038/ng989. [DOI] [PubMed] [Google Scholar]
- 28.Bailey SM, et al. Telomeres, chromosome instability and cancer. Nucleic Acids Res. 2006;34:2408–2417. doi: 10.1093/nar/gkl303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Heek NT, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am. J. Pathol. 2002;161:1541–1547. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meeker AK, et al. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- 31.Meeker AK, et al. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin. Cancer Res. 2004;10:3317–3326. doi: 10.1158/1078-0432.CCR-0984-03. [DOI] [PubMed] [Google Scholar]
- 32.Meeker AK. Telomeres and telomerase in prostatic intraepithelial neoplasia and prostate cancer biology. Urol. Oncol. 2006;24:122–130. doi: 10.1016/j.urolonc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Zheng YL, et al. Telomere attrition in cancer cells and telomere length in tumor stroma cells predict chromosome instability in esophageal squamous cell carcinoma: a genome-wide analysis. Cancer Res. 2009;69:1604–1614. doi: 10.1158/0008-5472.CAN-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meeker AK, et al. Telomere shortening occurs early during breast tumorigenesis: a cause of chromosome destabilization underlying malignant transformation? J. Mammary Gland. Biol. Neoplasia. 2004;9:285–296. doi: 10.1023/B:JOMG.0000048775.04140.92. [DOI] [PubMed] [Google Scholar]
- 35.Meeker AK, et al. Telomere shortening occurs in subsets of normal breast epithelium as well as in situ and invasive carcinoma. Am. J. Pathol. 2004;164:925–935. doi: 10.1016/S0002-9440(10)63180-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fordyce CA, et al. Telomere content correlates with stage and prognosis in breast cancer. Breast Cancer Res. Treat. 2006;99:193–202. doi: 10.1007/s10549-006-9204-1. [DOI] [PubMed] [Google Scholar]
- 37.Heaphy CM, et al. Telomere DNA content and allelic imbalance demonstrate field cancerization in histologically normal tissue adjacent to breast tumors. Int. J. Cancer. 2006;119:108–116. doi: 10.1002/ijc.21815. [DOI] [PubMed] [Google Scholar]
- 38.Hansel DE, et al. Telomere length variation in biliary tract metaplasia, dysplasia, and carcinoma. Mod. Pathol. 2006;19:772–779. doi: 10.1038/modpathol.3800591. [DOI] [PubMed] [Google Scholar]
- 39.Subhawong AP, et al. The alternative lengthening of telomeres phenotype in breast carcinoma is associated with HER-2 overexpression. Mod. Pathol. 2009;22:1423–1431. doi: 10.1038/modpathol.2009.125. [DOI] [PubMed] [Google Scholar]
- 40.Meeker AK, et al. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am. J. Pathol. 2002;160:1259–1268. doi: 10.1016/S0002-9440(10)62553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morton MJ, et al. Telomere shortening and chromosomal abnormalities in intestinal metaplasia of the urinary bladder. Clin. Cancer Res. 2007;13:6232–6236. doi: 10.1158/1078-0432.CCR-07-0121. [DOI] [PubMed] [Google Scholar]
- 42.Kurabayashi R, et al. Luminal and cancer cells in the breast show more rapid telomere shortening than myoepithelial cells and fibroblasts. Hum. Pathol. 2008;39:1647–1655. doi: 10.1016/j.humpath.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Silverstein MJ, et al. Ductal carcinoma in situ (DCIS) of the breast: diagnostic and therapeutic controversies. J. Am. Coll. Surg. 2001;192:196–214. doi: 10.1016/s1072-7515(00)00791-2. [DOI] [PubMed] [Google Scholar]
- 44.Fisher ER, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol 6). I. Intraductal carcinoma (DCIS) Cancer. 1986;57:197–208. doi: 10.1002/1097-0142(19860115)57:2<197::aid-cncr2820570203>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 45.Slaugher DP, et al. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 46.Garcia SB, et al. Field cancerization, clonality, and epithelial stem cells: the spread of mutated clones in epithelial sheets. J. Pathol. 1999;187:61–81. doi: 10.1002/(SICI)1096-9896(199901)187:1<61::AID-PATH247>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 47.Braakhuis BJ, et al. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727–1730. [PubMed] [Google Scholar]
- 48.Chin K, et al. In situ analyses of genome instability in breast cancer. Nat. Genet. 2004;36:984–988. doi: 10.1038/ng1409. [DOI] [PubMed] [Google Scholar]
- 49.Rhiner C, et al. Super competition as a possible mechanism to pioneer precancerous fields. Carcinogenesis. 2009;30:723–728. doi: 10.1093/carcin/bgp003. [DOI] [PubMed] [Google Scholar]