Abstract
The Wnt/beta-catenin (CTNNB1) and Ras-Raf-MEK-ERK signaling pathway play an important role in bladder cancer (BC) progression. Tumor-suppressive microRNAs (miRNAs) targeting these cancer pathways may provide a new therapeutic approach for BC. We initially identified miRNA-1826 potentially targeting CTNNB1, VEGFC and MEK1 using several target scan algorithms. Also 3′ untranslated region luciferase activity and protein expression of these target genes were significantly downregulated in miR-1826-transfected BC cells (J82 and T24). The expression of miR-1826 was lower in BC tissues and inverse correlation of miR-1826 with several clinical parameters (pT, grade) was observed. Also the expression of miR-1826 was much lower in three BC cell lines (J82, T24 and TCCSUP) compared with a normal bladder cell line (SV-HUC-1). We then performed analyses to look at miR-1826 function and found that miR-1826 inhibited BC cell viability, invasion and migration. We also found increased apoptosis and G1 cell cycle arrest in miR-1826-transfected BC cells. To examine whether the effect of miR-1826 was through CTNNB1 (beta-catenin) or MEK1 knockdown, we knocked down CTNNB1/MEK1 messenger RNA using a small interfering RNA (siRNA) technique. We observed that CTNNB1 or MEK1 siRNA knockdown resulted in effects similar to those with miR-1826 in BC cells. In conclusion, our data suggest that the miR-1826 plays an important role as tumor suppressor via CTNNB1/MEK1/VEGFC downregulation in BC.
Introduction
Bladder cancer (BC) is one of the most common malignancies and a leading cause of death among urological tumors. The most common histological type of BC is urothelial carcinoma (UC), which was formerly known as transitional cell carcinoma (1). Several risk factors for BC include industrial chemicals such as dyes, rubber, leather, textiles and paint products; smoking; previous treatment with the anticancer drug cyclophosphamide (Cytoxan) and pelvic radiation therapy (2). Approximately 75% of patients are ‘non-muscle-invasive UC (pTa, pTis, pT1) and have a 5 year survival rate of between 88 and 98% (3). The common treatment for these patients is endoscopic resection (1,4). Patients with muscle-invasive UC are usually treated with radical cystectomy or chemoradiotherapy (1,5). However, half of the muscle-invasive UC patients develop subsequent metastatic disease after the first aggressive treatment (1,6). Tumor grade is also an important prognostic factor for BC patients. Although tumor grade ‘G1’ is morphologically well differentiated, ‘G3’ cancers are poorly differentiated and more aggressive (1). Previous studies have identified several potential molecular biomarkers for BC (7,8). Namely inactivation of tumor suppressor genes TP53 and Rb and Ras oncogene activation have been regarded as important key players in BC carcinogenesis (7). Human Ras (H-Ras) was first identified as an oncogene in BC and mutation of the H-Ras gene occurs in ∼30 to 40% of BC patients (7,9). Activated Ras activates the protein kinase activity of Raf, which then activates MEK (MEK1 and MEK2) (10). MEK activates a mitogen-activated protein kinase (MAPK) (11), which is called ‘extracellular signal-regulated kinases’ (ERKs) and Ras-Raf-MEK-ERK is activated in BC cells (12). Several inhibitors targeting Ras-Raf-MEK-ERK have been used for cancer treatments (13,14). Wnt-beta-catenin signaling activation has also been studied and reported to be associated with cancer progression and poor prognosis (15). Ahmad et al. (16) have recently reported that Ras pathway activation cooperates with Wnt signaling to drive urothelial cell carcinoma. Growth factors such as VEGFC enhance the activation of Ras-Raf-MEK-ERK pathways and higher VEGFC expression has been reported to be associated with lymph node metastasis and poor prognosis in several cancers. Blockage of VEGF has been shown to be useful for suppression of cancer lymphangiogenesis and lymph node metastasis (17–20). VEGFC expression has also been found to be an important predictive factor for pelvic lymph node metastasis in BC (21).
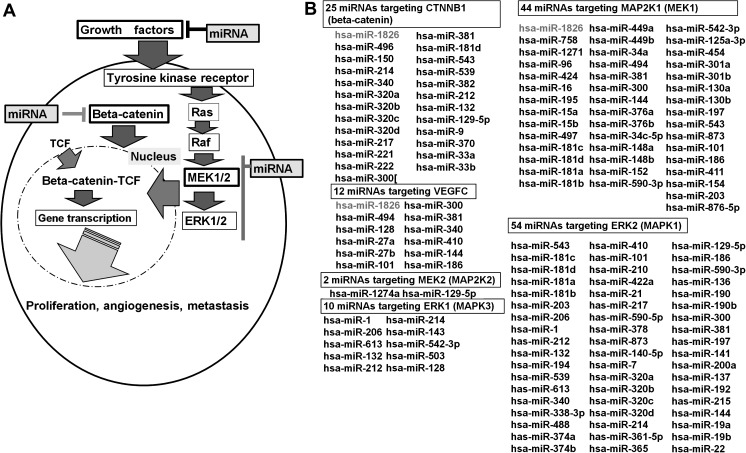
Recently, a number of microRNAs (miRNAs) have been identified and reported to be important in several cancer treatments (22). miRNAs are small non-coding RNAs, ∼22 nucleotides in length, that are capable of regulating gene expression at both the transcription and translation levels (23). miRNAs bind to the 3′ untranslated region (UTR) of target messenger RNA (mRNA) and repress translation from mRNA to protein or induce mRNA cleavage and thereby regulate the expression of target genes (24). Based on several target scan algorithms, it is possible to identify potential target genes of individual miRNAs. Initially, we searched for miRNAs targeting beta-catenin, MEK-ERK pathway genes (MEK1, MEK2, ERK1, ERK2) and several growth factors (VEGFs) using miRDB (http://mirdb.org/miRDB/), which is a miRNA target prediction and functional annotation database (25). In order to validate the miRDB results, we also used another target predicting algorithm, microRNA.org. As a result of these two algorithms, a number of miRNAs were found to target the genes of interest (75 miRNAs target VEGFA, 10 miRNAs target VEGFB, 12 miRNAs target VEGFC, 27 miRNAs target VEGFD, 25 miRNAs target beta-catenin, 44 miRNAs target MEK1, 2 miRNAs target MEK2, 10 miRNAs target ERK1 and 54 miRNAs target ERK2). Among these miRNAs, miR-1826 was identified as only targeting three genes (VEGFC, beta-catenin and MEK1). Based on these results, we hypothesized that three genes target knockdown (VEGFC, beta-catenin and MEK1) via miRNA-1826 may be a new therapeutic approach for BC. To verify this hypothesis, we performed 3′ UTR luciferase assays to confirm whether miR-1826 binds to the 3′ UTR of these target genes mRNAs and affected the function (proliferation, invasion, migration, apoptosis and cell cycle) of BC cells. We also looked at miR-1826 expression and localization in bladder tissues with real-time reverse transcription–polymerase chain reaction (RT–PCR) and in situ hybridization (ISH). Finally, we knocked down CTNNB1 and MEK1 mRNAs using a small interfering RNA (siRNA) technique to examine the mechanism of miR-1826 tumor-suppressive function.
Materials and methods
The design and schematic representation of this project
Schematic representation of the role of beta-catenin and MEK-ERK cascades in BC is shown in Figure 1A. The aim of this project was to identify new therapeutic miRNAs related to beta-catenin and MEK-ERK cascade pathways in BC cells. In order to identify miRNAs targeting these oncogenes, we initially used miRDB (25) and microRNA.org and found several miRNAs targeting them. The miRNAs identified are shown in Figure 1B.
Fig. 1.
Role of beta-catenin and the Ras-Raf-MEK-ERK cascade in BC cells and potential miRNAs targeting beta-catenin and MEK-ERK. (A) Signal transduction of beta-catenin and the MEK-ERK cascade (B) Based on miRDB and microRNA.org, several miRNAs targeting beta-catenin and the MEK-ERK cascade genes (MEK1/2, ERK1/2) were identified.
Tissue array samples
We purchased a human BC tissue array from US Biomax, Inc. (catalog#: BL801; Rockville, MD) to detect miR-1826 localization and confirm miR-1826 expression levels in BC tissues by ISH. Tissue array patient information is shown in Supplementary Table S1 (available at Carcinogenesis Online).
Clinical samples
A total of 19 patients (all male) with pathologically confirmed BC were enrolled in this study (Veterans Affairs Medical Center at San Francisco). Samples were obtained from the patients after written informed consent.
Cell culture
A normal bladder epithelial cell line (SV-HUC-1; ATCC number; CRL-9520) and three BC cell lines (J82; ATCC number: HTB-1, T24; ATCC number: HTB-4, TCCSUP; ATCC number: HTB-5) were purchased from the American Type Culture Collection (ATCC, Manassas, VA). The SV-HUC-1 cells were cultured in ATCC-formulated F-12K Medium with 10% fetal bovine serum (FBS). The J82 cell line was cultured in minimum essential medium (Eagle) in Earle’s basic salt solution supplemented with 10% FBS. The T24 cell line was cultured in McCoy’s 5A medium with 10% FBS. The TCCSUP cell line was cultured in minimum essential medium (Eagle) in Earle’s basic salt solution supplemented with non-essential amino acids, 1 mM sodium pyruvate and 10% FBS.
RNA and protein extraction
RNA (miRNA and total RNA) was extracted from formalin-fixed, paraffin-embedded human BC and non-cancerous normal bladder tissues using a miRNeasy FFPE Kit (QIAGEN) after laser microdissection based on pathologist reviews. Total RNA was also extracted from renal cancer cell lines using a miRNeasy Mini Kit (QIAGEN). Cells were lysed with RIPA buffer (Pierce, Brebieres, France) containing protease inhibitors (Sigma, St. Louis, MO). Protein quantification was done using a BCA Protein Assay Kit (Pierce).
miRNA transfection (pre-miR precursor and miR inhibitor)
Pre-miR™ miRNA precursors [negative control (miR-NC) or hsa-miR-1826 (miR-1826); Ambion] were transiently transfected into BC cells by Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Anti-miR™ miRNA inhibitor [negative control (inh-NC) or miR-1826 inhibitor (miR-1826 inhibitor); Ambion] were transiently transfected into BC cells by siPORT NeoFX Transfection Agent (Ambion) according to the manufacturer’s instructions. After transfection, cells were incubated at 37°C for 48 h until assessment.
Cell viability, cell invasion and wound healing assay
Cell viability was measured 4 days after miR-1826 transfection with MTS (CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega, Madison, WI). Data are the mean ± SD of six independent experiments. Cell invasion assay was performed with the CytoSelect 24-well cell invasion assay kit as described previously (Cell BioLab, San Diego, CA) (26). Transfected cells (miR-NC or miR-1826 transfectant—48 h) were resuspended in culture media without FBS and placed into the upper chamber in triplicate. After 48 h incubation at 37°C (5% CO2), cells migrating through the membrane were stained and counted with a microscope. The results were expressed as invaded cells quantified at OD 560 nm. Cells undergoing apoptosis generally exhibit nuclear fragmentation and the apoptotic cells cannot invade the membrane. Therefore, cells migrating through the membrane do not include apoptotic cells. The wound healing process begins with tissue matrix remodeling, migration and eventual closing of the wound area. Therefore, this assay is frequently used for assessment of cancer cell migration. Wound healing assay was performed with the CytoSelect 24-well wound healing assay kit as described previously (Cell BioLab) (26). To generate a wound field, transfected cells (miR-NC or miR-1826 transfectants—48 h) were cultured until they formed a monolayer around the insert. After removing insert, a 0.9 mm open wound field was generated and cells were allowed to migrate from either side of the gap. Wound closure was monitored and the percent closure was measured at 10 h [percent closure rate (%) = migrated cell surface area/total surface area × 100)].
Apoptosis and cell cycle analyses
Cells (48 h after transfection) were washed twice with 1× phosphate-buffered saline (PBS) and trypsinized. After inactivating trypsin in complete medium, the cells were resuspended in ice-cold 1× binding buffer (70 μl). Annexin V-FITC solution (10 μl) and 7-AAD viability dye (20 μl) were added to 70 μl of the cell suspensions. After incubation for 15 min in the dark, 400 μl of ice-cold 1× binding buffer was added. The apoptotic distribution of the cells in each sample was then determined using an FACS (Cell Lab QUANTA SC; Beckman Coulter, Fullerton, CA). The various phases of cells were determined using a DNA stain (4′,6-diamidino-2-phenylindole). Cell populations (G0/G1, S and G2/M) were measured using fluorescence and contrasted against cell volume. Data are the mean ± SD of four independent experiments.
3′ UTR luciferase assay
PmirGLO Dual-Luciferase miRNA Target Expression Vector was used for 3′ UTR luciferase assays (Promega). The primer sequences used were shown in Supplementary Table S2 (available at Carcinogenesis Online). In a total volume of 20 μl, 5 μl of 100 μM forward primer, 5 μl of 100 μM reverse primer, 2 μl of 10× annealing buffer (100 mM Tris–HCl, pH 7.5; 1 M NaCl and 10 mM ethylenediaminetetraacetic acid) and 8 μl water were added to a 200 μl PCR tube, incubated at 95°C for 5 min and then placed at room temperature at 1 h. The oligonucleotides were ligated into the PmeI–XbaI site of pmirGLO Dual-Luciferase miRNA Target Expression Vector. Colony direct PCR was performed for insert recognition using REDTaq (Sigma). The primers used were as follows: forward primer, 5′-cgtgctggaacacggtaaaa-3′; reverse primer, 5′-gcagccaactcagcttcctt-3′; PCR parameters for cycling were as follows: 94°C for 3 min, 30 cycles of PCR at 94°C for 30 s, 55°C for 30 s and 72°C for 30 s, 72°C for 10 min and 4°C for 10 min. The PCR product was digested with NotI (TaKaRa/Fisher Scientific, Pittsburgh, PA). The size of the vectors containing inserts were about 200 bp and 100 bp by electrophoresis since the NotI recognition sequence was incorporated into the primers. Vectors were sequenced directly by an outside vendor (MCLAB, South San Francisco, CA). For 3′ UTR luciferase assay, BC cells were co-transfected with miR-NC and pmirGLO or miR-1826 and pmirGLO Dual-Luciferase miRNA Target Expression Vectors using Lipofectamine 2000 (Invitrogen). Luciferase assay was performed using the Dual-Luciferase® Reporter Assay System (Promega) at 48 h after transfection.
siRNA knockdown of CTNNB1 and MEK1 mRNAs and functional analyses
BC cells were transiently transfected with CTNNB1 or MEK1 siRNA (si-CTNNB1 and si-MAP2K1, catalog#VHS50819, #VHS40795; Invitrogen) or negative control siRNA (si-NC; Invitrogen) according to the manufacturer’s instructions. Briefly, cells were grown in six-well plates and transfected individually with si-NC (negative control) or si-CTNNB1 or si-MAP2K1 at a concentration of 200 pmol per well. Transfection was performed with X-tremeGene siRNA Transfection Reagent (Roche Diagnosis, Basel, Switzerland). Then, 48 h after transfection, RNA and protein were extracted, and knockdown of CTNNB1 and MAP2K1 mRNAs and proteins were confirmed by real-time RT–PCR and western blot analysis. Cell viability (24, 48 and 72 h after transfection), invasion (48 h after transfection) and apoptosis analysis (48 h after transfection) were performed using si-NC-transfected, si-CTNNB1-transfected or si-MAP2K1-transfected BC cells.
Quantitative real-time RT–PCR
Quantitative real-time RT–PCR was performed in triplicate with an Applied Biosystems Prism 7500 Fast Sequence Detection System using TaqMan universal PCR master mix according to the manufacture’s protocol (Applied Biosystems Inc., Foster City, CA). The TaqMan probes and primers were purchased from Applied Biosystems. Human GAPDH was used as an endogenous control and RNU48 was used as internal control. Levels of RNA expression were determined using the 7500 Fast System SDS software version 1.3.1 (Applied Biosystems).
Western blot analysis
Total cell protein (15 μg) was used for western blotting. Samples were resolved in 4–20% Precise Protein Gels (Pierce) and transferred to polyvinylidene difluoride membranes (Amersham Biosciences, Fairfield, CT). The membranes were immersed in 0.3% skim milk in Tris-buffered saline containing 0.1% Tween 20 for 1 h and probed with primary polyclonal or monoclonal antibody against beta-catenin (#9562; Cell Signaling Technology, Beverly, MA), survivin (#2808; Cell Signaling Technology), MEK1 (#2352; Cell Signaling Technology), beta-Tubulin (#2128; Cell Signaling Technology) and VEGFC (#AP2042d; ABGENT, San Diego, CA) overnight at 4°C. Blots were washed in Tris-buffered saline containing 0.1% Tween 20 and labeled with horseradish peroxidase-conjugated secondary anti-mouse or anti-rabbit antibody (Cell Signaling Technology). Proteins were enhanced by chemiluminescence (Amersham ECL plus Western Blotting detection system, Fairfield, CT) for visualization. The protein expression levels are expressed relative to beta-Tubulin levels.
Immunohistochemical study
Immunostaining of beta-catenin (CTNNB1), MEK1 (MAP2K1) and VEGFC was performed on formalin-fixed paraffin-embedded specimens using an antibody against human beta-catenin (#9562; Cell Signaling Technology), MEK1 (#ab47422; Abcam, Cambridge, MA) and VEGFC (#AP2042d; ABGENT). Immunohistochemical staining was evaluated by visually assessing staining intensity (0–2) using a microscope at ×200. All specimens were scored blindly by two observers. The intensity of beta-catenin expression in cells membrane was assessed. The criteria of intensity are as follows: 0, negative expression; 1+, weakly positive expression; 2+, strongly positive expression.
In situ hybridization
We performed ISH using BC tissue array (catalog#: BL801; US Biomax, Inc) and IsHyb In Situ Hybridization Kit (BioChain, Hayward, CA) in order to detect miR-1826 in human bladder tissues (normal and cancer) following the manufacturers’ protocol. Briefly, tissue array slides were incubated at 60°C for 1 h, deparaffinized and rehydration. Slide sections were fixed with 4% paraformaldehyde in Diethyl pyrocarbonate (DEPC)-PBS at room temperature for 20 min and washed with DEPC-PBS. Then, sections were treated with proteinase K (BioChain) for 15 min at 37°C, rinsed with DEPC-PBS, fixed again with 4% paraformaldehyde for 15 min and rinsed with DEPC-water. Slides were pre-hybridized at 50°C for 3 h with pre-hybridization buffer before an overnight 45°C incubation in hybridization buffers containing either 5′-DIG-labeled miR-1826 miRCURY™ LNA detection probe (Exiqon, Woburn, MA) or scrambled negative control probe (Exiqon) at 50 nM final concentration. Probe sequence is as follows: ATTGCGTTCGAAGTGTCGATGATCAAT. Slides were washed in 2× standard saline citrate (45°C for 10 min), 1.5× standard saline citrate (45°C for 10 min) and 0.2× standard saline citrate (37°C for 20 min). Slides were then exposed to 1× blocking solution (BioChain) for 3 h at room temperature. Then, slides were exposed to AP-conjugated anti-digoxigenin antibody for 1 h at room temperature. Then, slides were washed with 1× PBS for three times at room temperature (10 min per each) and again washed with 1× alkaline phosphatase buffer twice at room temperature (5 min per each). Then, slides were incubated with BM Purple AP substrate (Roche Diagnosis) in the dark for 2 h at room temperature. After rinsing, slides were counterstained in Methyl Green (BioVision, Mountain View, CA) and rinsed with Tris-buffered saline Tween-20 before mounting. A pathologist evaluated the ISH intensity of miR-1826 in bladder and non-BC cells using the same criteria that were used for immunohistochemistry.
Statistical analysis
All statistical analyses were performed using StatView (version 5; SAS Institute Inc., NC). A P-value of <0.05 was regarded as statistically significant.
Results
3′-UTR luciferase assay and target protein expressions in miR-1826 transfectants
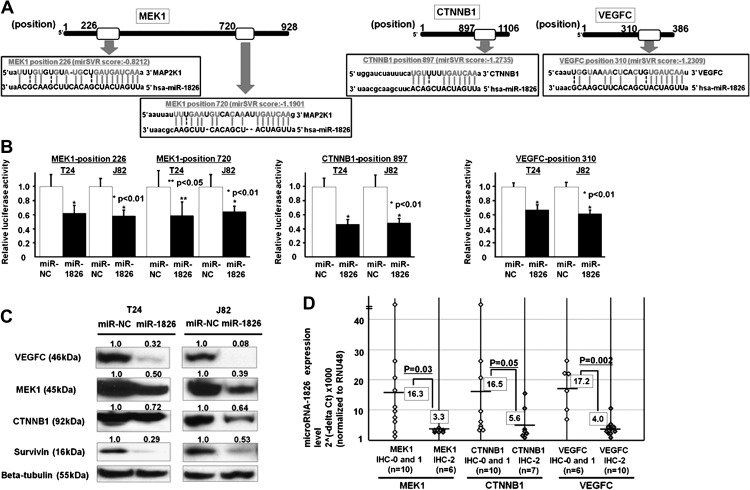
CTNNB1 mRNA has one potential complimentary binding site with miR-1826 within its 3′ UTR (mirSVR score; −1.2735) (Figure 2A). MEK1 mRNA has two potential complimentary binding sites with miR-1826 within its 3′ UTR (mirSVR score; −0.8212 and −1.1902, respectively). VEGFC mRNA has one potential complimentary binding site with miR-1826 within its 3′ UTR (mirSVR score; −1.2309) (Figure 2A). Based on these results, we performed 3′ UTR luciferase assays and found that the relative luciferase activities in these sites were significantly decreased in miR-1826-transfected BC cells (J82 and T24) (Figure 2B). These results suggest that CTNNB1, MEK1 and VEGFC mRNAs are target oncogenes of miR-1826. The CTNNB1 protein expression was also significantly decreased in miR-1826-transfected cells (Figure 2C). In order to verify CTNNB1 protein downregulation, we looked at the expression of survivin, which is a TCF/LEF down-stream effector. As expected, the protein (survivin) was significantly downregulated in miR-1826-transfected cells (Figure 2C). MEK1 and VEGFC protein expression were also significantly decreased in miR-1826-transfected BC cells (Figure 2C).
Fig. 2.
3′ UTR luciferase assay of MEK1, CTNNB1, VEGFC and correlation between miR-1826 and target protein expression in BC tissues. (A) MEK1, CTNNB1 and VEGFC 3′ UTR positions and complementary miR-1826-binding sequences. (B) 3′ UTR luciferase assay (miR-NC and miR-1826). (C) VEGFC, MEK1, CTNNB1, survivin and beta-tubulin protein expression in miR-NC-transfected or miR-1826-transfected BC cells (T24 and J82). (D) Inverse correlation between miR-1826 and MEK1/CTNNB1/VEGFC protein expression in BC tissues.
Target protein expressions in miR-1826 inhibitor transfectants
CTNNB1 and MEK1 protein expression were also significantly increased in miR-1826 inhibitor-transfected BC cells (Supplementary Figure S1, available at Carcinogenesis Online).
Inverse correlation between miR-1826 and beta-catenin/MEK1 expression levels in BC tissues
We investigated the relationship between miR-1826 expression and three target gene protein expression levels (CTNNB1, MEK1 and VEGFC) in clinical bladder samples. There was an inverse correlation between miR-1826 and the expression of these three target proteins (CTNNB1, MEK1 and VEGFC) in BC tissues (Figure 2D).
Localization of miR-1826 expression and association with clinical parameters
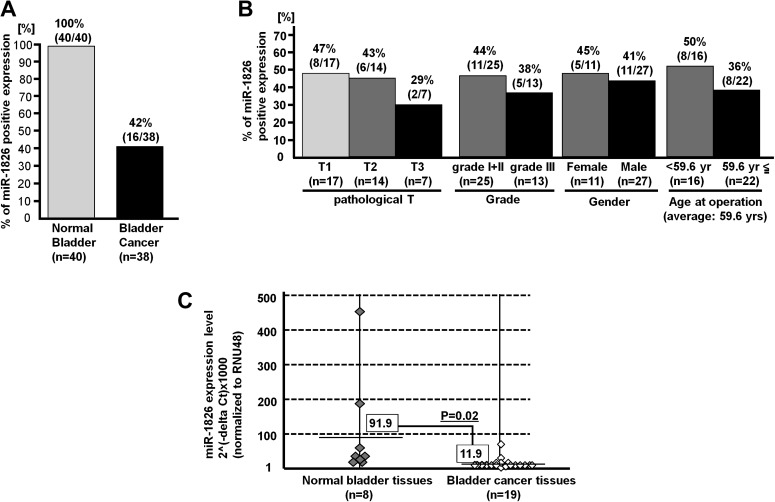
To investigate miR-1826 localization in bladder tissues, we performed ISH in bladder tissue array (normal and cancer tissues). As shown in Figure 3A, the miR-1826 expression was significantly lower in BC tissues than in normal bladder tissues. Expression of miR-1826 was identified in connective tissues, endothelial cells of blood vessels and lamina muscularis mucosa in bladder tissues (data not shown). In cancer cells, the miR-1826 expression was very much low. Moreover, we compared miR-1826 expression levels and several clinical parameters [grade, pathological (pT), gender, age at operation]. We found an inverse correlation of miR-1826 expression with tumor stage (pT) and grade in BC tissues (Figure 3B).
Fig. 3.
miR-1826 expression in bladder tissues and correlation with clinical parameters. (A) The percentage of positive miRNA-1826 expression was significantly lower in BC tissues. (B) Inverse correlation between miR-1826 expression and pathological T or tumor grade was found. (C) The miRNA-1826 expression level (normalized to RNU48) was significantly lower in BC tissues compared with normal tissues (P value = 0.02, n = 19) based on real-time PCR.
Decreased miRNA-1826 expression in BC tissues
We compared miRNA-1826 expression levels in BC tissues and normal bladder tissues by real-time PCR to validate the ISH results. The expression level was normalized to RNU48. As shown in Figure 3C, relative miR-1826 expression was significantly lower in BC tissues than in normal bladder epithelial tissues specimens (Figure 3C).
Effects of miRNA-1826 on cell viability, invasion, migration, apoptosis and cell cycle
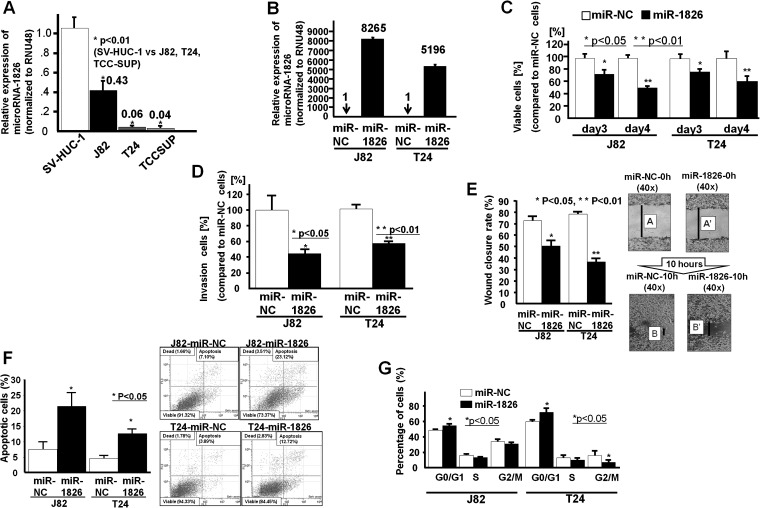
Relative miR-1826 expression was significantly lower in BC cells (J82, T24 and TCCSUP) than in normal bladder epithelial cells (SV-HUC-1) (Figure 4A). The results in BC cells were consistent with those in clinical bladder samples. We used primary BC cell lines (J82 and T24) for further functional analyses.
Fig. 4.
Effect of miR-1826 overexpression on BC cell functions (T24 and J82). (A) The miR-1826 expression level was significantly lower in BC cell lines (J82, T24 and TCCSUP) than in normal bladder cell lines (SV-HUC-1). (B) At 48 h after transfection of miR-NC or miR-1826 into BC cells (J82 and T24), the miR-1826 expression level was verified by real-time RT–PCR (fold change; 8265 and 5196, respectively). (C) Cell viability assay (miR-NC-transfected or miR-1826-transfected J82/T24 cells). (D) Invasion assay (miR-NC-transfected or miR-1826-transfected J82/T24 cells). (E) Wound healing assay (miR-NC-transfected or miR-1826-transfected J82/T24 cells). (F) Flow cytometry analysis of apoptosis in miR-NC-transfected or miR-1826-transfected J82/T24 cells. (G) Flow cytometry analysis of the cell cycle. Data are the mean ± SD of four independent experiments.
At 48 h after transfection of miR-NC or miR-1826 into BC cells (J82 and T24), the miR-1826 expression level was verified by real-time RT–PCR (fold change; 8265, 5196, respectively) (Figure 4B). Then, cell viability (MTS assay), cell migration and invasion assays were performed using transient miRNA-1826 transfectants of BC cells (Figure 4C, D and E). We observed a significant decrease in cell viability (Figure 4C), invasion (Figure 4D) and migration (Figure 4E) in miRNA-1826-transfected BC cells compared with miR-NC-transfected cells. Apoptosis and cell cycle analysis based on flow cytometry were performed with transfected BC cells (J82 and T24). A significant increase in the number of apoptotic cells in miR-1826-transfected cells (J82 and T24) was found (Figure 4F). We also observed significantly increased G1 cell cycle arrest in miR-1826-transfected BC cells (Figure 4G).
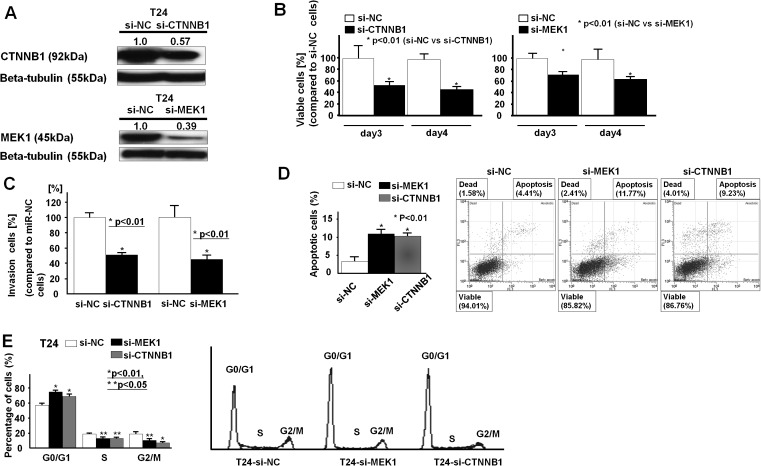
Effects of CTNNB1 and MEK1 mRNAs knockdown on BC cell functions (T24)
To look at whether miR-1826 exerts its tumor-suppressive function through CTNNB1 (beta-catenin) and MEK1 (MAP2K1), we knocked down CTNNB1 and MEK1 (MAP2K1) mRNAs using a siRNA technique. The knockdown effect was confirmed by measuring mRNA (data not shown) and protein expression levels. As shown in Figure 5A, the relative CTNNB1 and MEK1 protein expression levels were significantly decreased in siRNA-transfected cells. Then, we performed MTS and invasion assays in si-NC-transfected, si-CTNNB1-transfected or si-MEK1-transfected BC cells (T24). As shown in Figure 5B and C, cell viability (day 3, day 4) and invasion activity (48 h) were significantly inhibited in si-CTNNB1-transfected and si-MEK1-transfected T24 BC cells (48 h after transfection). As shown in Figure 5D and E, G1 cell cycle arrest was induced and apoptosis was increased in si-CTNNB1-transfected and si-MEK1-transfected T24 BC cells.
Fig. 5.
Effect of CTNNB1 and MEK1 siRNA knockdown on BC cell function (T24). (A) Protein expression of CTNNB1 and MEK1 in si-CTNNB1-transfected, si-MEK1-transfected or control (si-NC)-transfected T24 cells. (B) MTS assay (si-NC-transfected, si-CTNNB1-transfected or si-MEK1-transfected T24 cells). (C) Invasion assay. (D) Flow cytometry analysis of apoptosis in si-NC-transfected, si-CTNNB1-transfected or si-MEK1-transfected T24 cells. (E) Flow cytometry analysis of the cell cycle. Data are the mean ± SD of four independent experiments.
Discussion
UC is the most common type of BC and ∼30 to 40% of UC patients have a mutated H-Ras gene (7,9). Activating mutations in the Ras genes may affect downstream signaling pathway (Ras-Raf-MEK-ERK) (10,11). This cascade, namely the Ras-Raf-MEK-ERK pathway, leads to uncontrolled cell growth, proliferation and invasion in cancer cells. MEK1 (MAP2K1) plays an important role as an essential component of the MAPK-ERK signal transduction pathway. Several MEK1 inhibitors have been identified and have been evaluated in phases I, II and III clinical trials in several cancers including BC (14,27,28). Although other treatments targeting the Raf-MEK-ERK MAPK cascade have been verified (14), several problems remain regarding the side effect and efficacy of these treatments.
CTNNB1 (beta-catenin) is also known as a key player in the Wnt/beta-catenin signaling pathway in BC (15). Interestingly, Ahmad et al. (16) have recently reported that Ras pathway activation cooperates with Wnt signaling to drive urothelial cell carcinoma.
Recently, miRNAs have been identified as regulators of signal transduction in urological cancers (29). Thus, we focused on miRNAs as a potential new treatment strategy for both the Wnt/beta-catenin and Raf-MEK-ERK pathways in BC. We initially searched for tumor-suppressive miRNAs that inhibit the two major cancer pathways beta-catenin and Raf-MEK-ERK (MEK1, MEK2, ERK1, ERK2), with algorithms miRDB and microRNA.org. We identified miR-1826 that targets beta-catenin and MEK1. Growth factors also play an important role as triggers in Ras-Raf-MEK-ERK pathways in BC. High VEGFC expression was reported to be involved in the promotion of lymph node metastasis in bladder UC (30). We also identified that miR-1826 targets VEGFC, using the above target prediction algorithms. Thus, miR-1826 targets three oncogenes (beta-catenin, MEK1 and VEGFC) based on target prediction algorithms. To validate the target prediction algorithm results, we performed 3′ UTR luciferase assay to see whether miR-1826 binds to the 3′ UTR of these target genes. We found that relative luciferase levels for the three genes were significantly lower in miR-1826-transfected BC cells than in miR-NC-transfected controls. This was confirmed by western analysis, which shows that the protein expression of CTNNB1, MEK1 and VEGFC was significantly downregulated in miR-1826 transfectants. These results suggest that CTNNB1, MEK1 and VEGFC are direct targets of miR-1826. We also performed an immunohistochemical study of CTNNB1, MEK1 and VEGFC to examine the relationship between miR-1826 and CTNNB1 or MEK1 or VEGFC expression levels in human BC tissues. We found an inverse correlation between miR-1826 levels and CTNNB1 or MEK1 or VEGFC protein expression.
Regarding the relationship between miRNAs and target genes in the present study, Xia et al. (31) have reported that miR-200a functions as a tumor suppressor by directly regulating CTNNB1 expression in nasopharyngeal carcinoma. Saydam et al. (32) found that miR-200a plays an important role as a tumor suppressor and directly targets CTNNB1 mRNA. They also showed that miR-200a blocks Wnt/beta-catenin signaling in meningioma cells (32). MiR-34a inhibits cell proliferation by repressing MEK1 in the process of megakaryocytic differentiation (33). MiR-424-regulated cell proliferation via silencing MEK1 and cyclin E1 in senile hemangioma (34). As far as we know, there have been no reports concerning the relationship of miRNAs, BC and MEK1, CTNNB1 or VEGFC.
We also performed western analysis of survivin, a member of the TCF/LEF down-stream effectors, to assess whether miR-1826 effects down-stream Wnt/beta-catenin signaling through beta-catenin downregulation. We found that expression of survivin was also downregulated by miR-1826 in transfected cells. Horstmann et al. (35) have reported that survivin is a reliable biomarker for high-grade urothelial BC.
Based on these results, we hypothesized that knockdown of the three target genes (VEGFC, beta-catenin and MEK1) via miRNA-1826 may be a new therapeutic approach for BC. To test our hypothesis, we next looked at miR-1826 expression levels and localization in bladder tissues (normal and cancer tissues) since there have been no reports showing miR-1826 expression in BC. We found that the expression of miR-1826 was significantly downregulated in BC tissues compared with normal bladder epithelial tissues. These results are consistent with the results of miR-1826 expression in normal bladder and BC cell lines, suggesting that miR-1826 may have tumor-suppressive functions in BC. Also we observed miR-1826 expression in connective tissues and smooth muscle layers; however, the expression was lowest in BC cells. MIR-1826 localization patterns are very similar to those of another miRNA, miR-145, by ISH in bladder tissues (36). To examine the function of miR-1826, we performed transfection experiments with BC cell lines using miR-1826 precursor. As expected, we found that overexpression of miR-1826 significantly inhibited BC cell viability, migration and invasion. We also examined the cell cycle and apoptosis and found that miR-1826 induced significant G1 arrest and apoptosis in BC cells. To validate whether miR-1826 plays a tumor-suppressive role by inhibiting CTNNB1 and MEK1 expression, we performed knockdown of CTNNB1 and MEK1 in BC cells using a siRNA technique. The decreased expression of CTNNB1 and MEK1 was confirmed at the mRNA (data not shown) and protein levels. We then performed functional analyses (MTS and invasion assays) and found an effect similar to that of miR-1826 overexpression, namely that CTNNB1 or MEK1 siRNA knockdown resulted in inhibition of BC cell proliferation and invasion abilities. Taken together, this evidence suggests that miR-1826 exerts its tumor-suppressive effects through beta-catenin and MEK1 downregulation in BC cells. Since we were unable to perform an in vivo study using stable miR-1826 cells in nude mice, the mouse xenograft model will be helpful to examine the therapeutic effect of miR-1826 in BC.
In conclusion, this is the first report documenting that miR-1826 expression is significantly decreased in BC tissues where it functions as a tumor suppressor by inhibiting CTNNB1, VEGFC and MEK1 expression. These results suggest that miR-1826 may have therapeutic potential for the treatment of BC.
Supplementary material
Supplementary Tables S1 and S2 and Figure S1 can be found at http://carcin.oxfordjournals.org/
Funding
RO1CA130860, RO1CA111470, RO1CA138642, T32-DK07790 from the National Institutes of Health, VA Research Enhancement Award Program, Merit Review grants and Yamada Science Foundation.
Supplementary Material
Acknowledgments
We thank Dr Roger Erickson for his support and assistance with the preparation of the manuscript. Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BC
bladder cancer
- ERKs
extracellular signal-regulated kinases
- FBS
fetal bovine serum
- ISH
in situ hybridization
- mRNA
messenger RNA
- MAPK
mitogen-activated protein kinase
- miRNAs
microRNAs
- PBS
phosphate-buffered saline
- siRNA
small interfering RNA
- RT–PCR
reverse transcription–polymerase chain reaction
- UC
urothelial carcinoma
- UTR
untranslated region
References
- 1.Pollard C, et al. Molecular genesis of non-muscle-invasive urothelial carcinoma (NMIUC) Expert. Rev. Mol. Med. 2010;12:e10. doi: 10.1017/S1462399410001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouprêt M, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur. Urol. 2011;59:584–594. doi: 10.1016/j.eururo.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 3.Proctor I, et al. Biomarkers in bladder cancer. Histopathology. 2010;57:1–13. doi: 10.1111/j.1365-2559.2010.03592.x. [DOI] [PubMed] [Google Scholar]
- 4.Pasin E, et al. Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Rev. Urol. 2008;10:31–43. [PMC free article] [PubMed] [Google Scholar]
- 5.Stenzl A, et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur. Urol. 2011;59:1009–1018. doi: 10.1016/j.eururo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Liebert M, et al. Characteristics of invasive bladder cancers: histological and molecular markers. Semin. Urol. Oncol. 1996;14:62–72. [PubMed] [Google Scholar]
- 7.Wallerand H, et al. Molecular targeting in the treatment of either advanced or metastatic bladder cancer or both according to the signalling pathways. Curr. Opin. Urol. 2008;18:524–532. doi: 10.1097/MOU.0b013e3283097889. [DOI] [PubMed] [Google Scholar]
- 8.Netto GJ, et al. Theranostic and prognostic biomarkers: genomic applications in urological malignancies. Pathology. 2010;42:384–394. doi: 10.3109/00313021003779145. [DOI] [PubMed] [Google Scholar]
- 9.Dinney CP, et al. Focus on bladder cancer. Cancer Cell. 2004;6:111–116. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Avruch J, et al. Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog. Horm. Res. 2001;56:127–155. doi: 10.1210/rp.56.1.127. [DOI] [PubMed] [Google Scholar]
- 11.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 12.Boulalas I, et al. Activation of RAS family genes in urothelial carcinoma. J. Urol. 2009;181:2312–2319. doi: 10.1016/j.juro.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Kohno M, et al. Targeting the ERK signaling pathway in cancer therapy. Ann. Med. 2006;38:200–211. doi: 10.1080/07853890600551037. [DOI] [PubMed] [Google Scholar]
- 14.Roberts PJ, et al. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 15.Moon RT, et al. WNT and beta-catenin signalling: diseases and therapies. Nat. Rev. Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad I, et al. Ras mutation cooperates with β-catenin activation to drive bladder tumourigenesis. Cell Death Dis. 2011;2:e124. doi: 10.1038/cddis.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohta Y, et al. VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br. J. Cancer. 1999;81:54–61. doi: 10.1038/sj.bjc.6690650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto I, et al. Vascular endothelial growth factor-C expression and its relationship to pelvic lymph node status in invasive cervical cancer. Br. J. Cancer. 2001;85:93–97. doi: 10.1054/bjoc.2001.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonemura Y, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin. Cancer Res. 1999;5:1823–1829. [PubMed] [Google Scholar]
- 20.He Y, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl. Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki K, et al. Vascular endothelial growth factor-C (VEGF-C) expression predicts lymph node metastasis of transitional cell carcinoma of the bladder. Int. J. Urol. 2005;12:152–158. doi: 10.1111/j.1442-2042.2005.01010.x. [DOI] [PubMed] [Google Scholar]
- 22.Trang P, et al. MicroRNAs as potential cancer therapeutics. Oncogene. 2008;27:S52–S57. doi: 10.1038/onc.2009.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inui M, et al. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell. Biol. 2010;11:252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- 24.Fabbri M, et al. MicroRNAs. Cancer J. 2008;14:1–6. doi: 10.1097/PPO.0b013e318164145e. [DOI] [PubMed] [Google Scholar]
- 25.Wang X. miRDB: a microRNA target prediction and functional annotation database with a wiki interface. RNA. 2008;14:1012–1017. doi: 10.1261/rna.965408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirata H, et al. DICKKOPF-4 activates the noncanonical c-Jun-NH2 kinase signaling pathway while inhibiting the Wnt-canonical pathway in human renal cell carcinoma. Cancer. 2011;117:1649–1660. doi: 10.1002/cncr.25666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi T, et al. Antitumor activities of JTP-74057 (GSK1120212), a novel MEK1/2 inhibitor, on colorectal cancer cell lines in vitro and in vivo. Int. J. Oncol. 2011;39:23–31. doi: 10.3892/ijo.2011.1015. [DOI] [PubMed] [Google Scholar]
- 28.Choudhary S, et al. Differential induction of reactive oxygen species through Erk1/2 and Nox-1 by FK228 for selective apoptosis of oncogenic H-Ras-expressing human urinary bladder cancer J82 cells. J. Cancer Res. Clin. Oncol. 2011;137:471–480. doi: 10.1007/s00432-010-0910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fendler A, et al. MicroRNAs as regulators of signal transduction in urological tumors. Clin. Chem. 2011;57:954–968. doi: 10.1373/clinchem.2010.157727. [DOI] [PubMed] [Google Scholar]
- 30.Zu X, et al. Vascular endothelial growth factor-C expression in bladder transitional cell cancer and its relationship to lymph node metastasis. BJU Int. 2006;98:1090–1093. doi: 10.1111/j.1464-410X.2006.06446.x. [DOI] [PubMed] [Google Scholar]
- 31.Xia H, et al. miR-200a-mediated downregulation of ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma cell growth, migration and invasion. Biochem. Biophys. Res. Commun. 2010;391:535–541. doi: 10.1016/j.bbrc.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 32.Saydam O, et al. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway. Mol. Cell. Biol. 2009;29:5923–5940. doi: 10.1128/MCB.00332-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichimura A, et al. MicroRNA-34a inhibits cell proliferation by repressing mitogen-activated protein kinase kinase 1 during megakaryocytic differentiation of K562 cells. Mol. Pharmacol. 2010;77:1016–1024. doi: 10.1124/mol.109.063321. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima T, et al. Down-regulation of mir-424 contributes to the abnormal angiogenesis via MEK1 and cyclin E1 in senile hemangioma: its implications to therapy. PLoS One. 2010;5:e14334. doi: 10.1371/journal.pone.0014334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horstmann M, et al. Clinical experience with survivin as a biomarker for urothelial bladder cancer. World J. Urol. 2010;28:399–404. doi: 10.1007/s00345-010-0538-2. [DOI] [PubMed] [Google Scholar]
- 36.Ostenfeld MS, et al. miR-145 induces caspase-dependent and -independent cell death in urothelial cancer cell lines with targeting of an expression signature present in Ta bladder tumors. Oncogene. 2010;29:1073–1084. doi: 10.1038/onc.2009.395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.