Abstract
Long-term exposure to low doses of environmental carcinogens contributes to sporadic human breast cancers. Epidemiologic and experimental studies indicate that green tea catechins (GTCs) may intervene with breast cancer development. We have been developing a chronically induced breast cell carcinogenesis model wherein we repeatedly expose non-cancerous, human breast epithelial MCF10A cells to bioachievable picomolar concentrations of environmental carcinogens, such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and benzo[a]pyrene (B[a]P), to progressively induce cellular acquisition of cancer-associated properties, as measurable end points. The model is then used as a target to identify non-cytotoxic preventive agents effective in suppression of cellular carcinogenesis. Here, we demonstrate, for the first time, a two-step strategy that initially used end points that were transiently induced by short-term exposure to NNK and B[a]P as targets to detect GTCs capable of blocking the acquisition of cancer-associated properties and subsequently used end points constantly induced by long-term exposure to carcinogens as targets to verify GTCs capable of suppressing carcinogenesis. We detected that short-term exposure to NNK and B[a]P resulted in elevation of reactive oxygen species (ROS), leading to Raf-independent extracellular signal-regulated kinase (ERK) pathway activation and subsequent induction of cell proliferation and DNA damage. These GTCs, at non-cytotoxic levels, were able to suppress chronically induced cellular carcinogenesis by blocking carcinogen-induced ROS elevation, ERK activation, cell proliferation and DNA damage in each exposure cycle. Our model may help accelerate the identification of preventive agents to intervene in carcinogenesis induced by long-term exposure to environmental carcinogens, thereby safely and effectively reducing the health risk of sporadic breast cancer.
Introduction
More than 70% of sporadic breast cancers are attributable to long-term exposure to environmental factors, such as chemical carcinogens, etc.; this multiyear, multistep and multipath disease process involves cumulative genetic and epigenetic alterations to induce progressive carcinogenesis of breast cells from non-cancerous to precancerous and cancerous stages (1–4). Over 200 chemical mammary carcinogens have been experimentally detected to acutely induce cancerous cells in cultures and tumors in animals at high doses of micro- to millimolar concentrations (1,3,5). A high-dose approach may serve as a proper way to study occupational exposure; however, considering that chronic exposure of human tissues to low doses of carcinogens is responsible for most human cancers, a chronic low-dose approach might be a more proper way to study the environmental exposure most often responsible for human breast cancer development. A new approach is needed to reveal environmental mammary carcinogens, at low and bioachievable levels, capable of inducing human breast cell carcinogenesis.
We have been developing a model to mimic breast cell carcinogenesis occurring with accumulated exposures to low doses of environmental carcinogens (6–9). We used the environmental carcinogens 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and benzo[a]pyrene (B[a]P) at picomolar concentrations, like those detected in patients (10–13), to repeatedly treat immortalized, non-cancerous, human breast epithelial MCF10A cells in culture to progressively induce acquisition of cancer-associated properties (6–9). NNK is considered one of the most potent lung carcinogens in tobacco products (14); although gastric administration of NNK into rats results in DNA–adduct formation in the mammary gland and development of mammary tumors (14–17), NNK is not currently recognized as a breast carcinogen. B[a]P, a family member of polycyclic aromatic hydrocarbons, is considered an environmental, dietary and tobacco carcinogen, its metabolites forming strong DNA adducts and causing DNA lesions and it is recognized as a mammary carcinogen in rodents (3,4,12,13,18–21). Studies using human cell lines for genotoxicity tests and studies of adduct formation reveal genotoxic activity of NNK and B[a]P at concentrations as low as 25 mmol/l and 25 μmol/l, respectively (22). Our cellular model reveals the ability of NNK and B[a]P, at a bioachievable level of 100 pmol/l, to chronically and progressively induce carcinogenesis of MCF10A cells (6–9). Hence, our model system takes a new sensitive approach of validating low doses of environmental mammary carcinogens in chronic induction of human breast cell carcinogenesis.
It has been shown that a short-term exposure of MCF10A cells to the B[a]P metabolites B[a]P-quinones at 10 μmol/l for 10 min induces reactive oxygen species (ROS) elevation (23), and exposure of normal human bronchial epithelial cells to NNK at 1–5 μmol/l for 24 h induces cell proliferation (24). It has been postulated that ROS elevation and cell proliferation increase cell susceptibility to DNA damage induced by carcinogens, contributing to cellular carcinogenesis (25,26). The oxidative DNA damage caused by ROS includes strand breaks and nucleotide modifications, resulting in mutations and contributing to cellular transformation (19). Activation of the extracellular signal-regulated kinase (ERK) pathway also contributes to cell proliferation and phosphorylation of histone H2AX (27), the latter of which (on serine 139) is widely used as an indicator for DNA damage (28). In addition, B[a]P, at high doses ranging from 0.02 to 1 μmol/l, has been shown to induce cell proliferation and DNA damage in breast adenocarcinoma MCF7 cells (29,30). However, it is not clear whether picomolar levels of NNK and B[a]P are able to induce ROS elevation and cell proliferation in breast cells with short-term exposure, contributing to induction of carcinogenesis associated with long-term exposure.
Epidemiologic and experimental studies have shown that various dietary polyphenolic compounds, which are widely found in vegetables, fruits and tea, possess anticancer, antiproliferative, antioxidant and apoptotic activities (1,31). The use of green tea to increase the body’s antioxidant activity is becoming increasingly popular in the Western world (32). A typical brewed green tea contains 30–45% green tea catechins (GTCs), including epicatechin (EC), epicatechin-3-gallate (ECG), epigallocatechin (EGC) and epigallocatechin-3-gallate (EGCG) (31). GTCs have been shown to be more effective antioxidants than vitamins C and E (33), and their order of effectiveness as radical scavengers is ECG > EGCG > EGC > EC (24). Animal studies show that GTCs are able to suppress rat mammary carcinogenesis induced by 7,12-dimethylbenz[a]anthracene and N-methyl-N-nitrosourea (34,35). Laboratory studies also have shown that GTCs possess inhibitory and apoptotic activity in the growth of human breast cancer cells in cultures (36,37). Epidemiological studies have examined the benefits of tea consumption for breast cancer prevention, and some evidence has indicated that green tea consumption may help prevent breast cancer recurrence in early stage cancers; however, the results are controversial (31,38,39). In addition, studies show that EGCG and EGC exhibit higher toxicity than ECG and EC in inducing cellular DNA damage (40,41). Therefore, additional studies are needed to clarify the effectivity of individual GTCs, at non-cytotoxic levels, used in protection of breast cells from carcinogenesis in order to safely and effectively reduce the health risk of sporadic breast cancer.
Previously, we used our model system to detect the ability of a dietary GTC extract containing 60% total catechins, at non-cytotoxic concentrations (<40 μg/ml), to suppress chronically B[a]P-induced carcinogenesis of breast epithelial cells (8). In this study, we used our model system to pursue the mechanisms of NNK and B[a]P in inducing breast cell carcinogenesis and identify targeted end points transiently or constantly induced by short-term and long-term exposure to both carcinogens, respectively. Then, we used NNK- and B[a]P-induced end points as targets to identify preventive agents capable of intervening in the cellular carcinogenesis. We identified the essential role of ROS in modulating the ERK pathway leading to cell proliferation and chromosomal DNA damage in NNK- and B[a]P-induced breast cell carcinogenesis. We also revealed the preventive activity of individual EC, ECG, EGC and EGCG, at non-cytotoxic levels, in suppression of NNK- and B[a]P-induced breast cell carcinogenesis.
Materials and methods
Cell cultures, reagents and cellular carcinogenesis
Immortalized, non-cancerous human breast epithelial cell line MCF10A [American Type Culture Collection (ATCC), Rockville, MD] and derived celllines were maintained in complete MCF10A culture medium (1:1 mixture of Dulbecco's modified Eagle's medium and HAM's F12, supplemented with 100 ng/ml cholera enterotoxin, 10 μg/ml insulin, 0.5 μg/ml hydrocortisol, 20 ng/ml epidermal growth factor and 5% horse serum) (6–9). Human breast adenocarcinoma MCF7 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum. All cultures were maintained in medium supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin in 5% CO2 at 37°C. Stock solutions of NNK (Chemsyn, Lenexa, KS), B[a]P (Aldrich, Milwaukee, WI), U0126 (Cell Signaling, Beverly, MA) and chloromethyl-dichlorodihydro-fluorescein-diacetate (Invitrogen, Carlsbad, CA) were prepared in dimethyl sulfoxide and diluted with culture medium for assays. EC, ECG, EGC, EGCG (Sigma–Aldrich, St. Louis, MO) and N-acetyl-l-cysteine (NAC) (Alexis, San Diego, CA) were prepared in distilled water and diluted with culture media for assays.
Protocol for induction and suppression of cell carcinogenesis
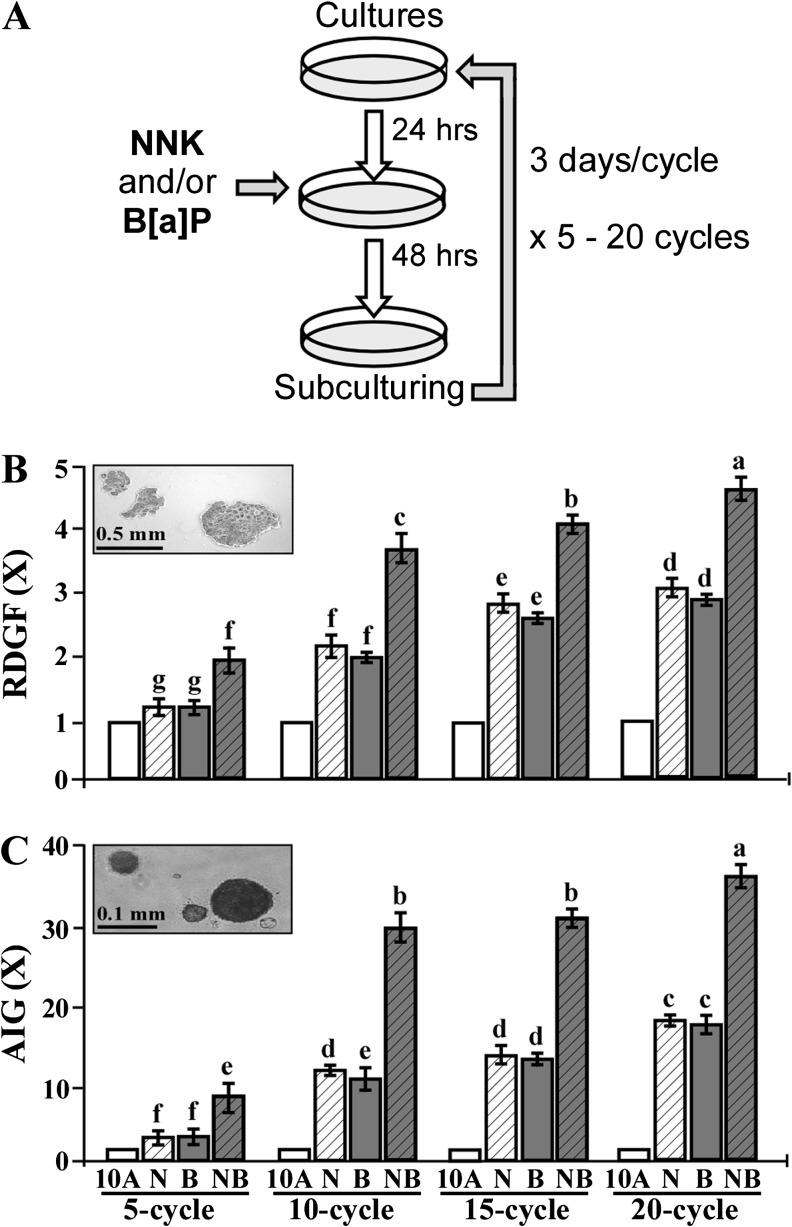
To chronically induce cell carcinogenesis for intervention, 24 h after each subculturing, cultures were exposed to combined NNK and B[a]P each at 100 pmol/l in the absence and presence of individual EC, ECG, EGC and EGCG for 48 h as one cycle of exposure for 5–20 cycles; cultures were subcultured every 3 days (3 days per cycle) (Figure 1A).
Fig. 1.
NNK- and B[a]P-induced cellular carcinogenesis. (A) MCF10A cultures were repeatedly exposed to individual (N, B) or combined NNK and B[a]P (NB) each at 100 pmol/l for 5, 10, 15, and 20 cycles. (B) To determine cellular acquisition of the cancer-associated property of reduced dependence on growth factors (RDGF), cells were maintained in LM medium for 10 days. Cell colonies (≥0.5 mm diameter) were counted. (C) To determine cellular acquisition of the cancer-associated property of anchorage-independent growth (AIG), cells were seeded in soft agar for 20 days. Cell colonies (≥0.1 mm diameter) were counted. Relative degrees of the cancer-associated properties of RDGF and AIG acquired by carcinogen-exposed cells were determined by normalizing with the colony numbers of vehicle-treated counterpart MCF10A cells, set as 1 (X, arbitrary unit), in each exposure cycle. Columns, mean of triplicates; bars, SD. All results are representative of at least three independent experiments. Mean colony numbers in each treatment group were analyzed by one-way analysis of variance at P < 0.05 to indicate significant difference in number of colonies in various groups. To further determine the significant difference between individual groups, a pairwise analysis of variables was performed using the Duncan multiple range test. Columns with different superscript letters (a, b, c, d, e, f and g) indicate significant difference at P < 0.05 between groups; no significant difference was seen between groups with the same superscript.
Assay for reduced dependence on growth factors
The low-mitogen (LM) medium contained reduced total serum and mitogenic additives to 2% of the concentration formulated in CM medium. Cells (5 × 103) were seeded in 100-mm culture dishes and maintained in LM medium. Growing cell colonies that reached 0.5 mm diameter in 10 days were stained with Coomassie brilliant blue and identified as cell clones acquiring the cancer-associated property of reduced dependence on growth factors.
Assay for anchorage-independent cell growth
The base layer consisted of 2% low-melting agarose (Sigma–Aldrich) in CM medium. Then, soft agar consisting of 0.4% low-melting agarose in a mixture (1:1) of CM medium with 3 day-conditioned medium prepared from MCF10A cultures was mixed with 1 × 104 cells and plated on top of the base layer in 60-mm diameter culture dishes. Growing colonies that reached 0.1 mm diameter by 20 days were identified as cell clones acquiring the cancer-associated property of anchorage-independent growth.
Cell mobility-healing assay
Cells were seeded in six-well plates and grown to confluence in CM medium. Cells were rinsed with phosphate-buffered saline (PBS) and starved for 15 h in Dulbecco's modified Eagle's medium/Ham’s F12 media containing 2% serum (42). The monolayer was then scratched with a 23 gauge needle (BD Sciences, Franklin Lakes, NJ) to generate wounded areas and rinsed with CM medium to remove floating cells. Cultures were maintained in CM medium, and the wounded areas were examined 6 and 24 h after scratches to detect healing of wounded areas. Wound healing area was calculated by using Total Lab TL100 software (Total Lab, Newcastle, NE).
Cell viability assay
A methyl thiazolyl tetrazolium Assay Kit (ATCC) was used to measure cell growth and viability in cultures. As described by the manufacturer, 3 × 104 cells were seeded into each well of 96-well culture plates for 24 h. After treatments, cells were incubated with methyl thiazolyl tetrazolium reagent for 4 h, followed by incubation with detergent reagent for 24 h. Quantification of reduced methyl thiazolyl tetrazolium reagent in cultures was determined with an enzyme-linked immunosorbent assay reader (Bio-Tek, Winooski, VT) at 570 nm.
Cell proliferation assay
Cell proliferation was determined using the 5-bromo-2-deoxyuridine (BrdU) cell proliferation ELISA Kit (Roche, Indianapolis, IN). Cells (5 × 104) were seeded into each well of 96-well culture plates. After the indicated treatments, the cells were labeled with BrdU for 12 h, fixed, incubated with peroxidase-conjugated BrdU-specific antibodies and stained with the peroxidase substrate. Quantification of BrdU-labeled cells was determined with an ELISA reader (Bio-Tek) at 370 nm.
Apoptotic cell death assay
An annexin-V-fluorescein isothiocyanate apoptosis detection kit with propidium iodide (BD Sciences) was used to detect apoptotic cell death by flow cytometry (43). In brief, cells were collected after trypsinization and washed with PBS. Cells were then incubated with annexin-V-fluorescein isothiocyanate and propidium iodide in a binding buffer (10 mmol/l HEPES–KOH, pH 7.4, 150 mmol/l NaCl, 1.8 mmol/l CaCl2) for 20 min at ambient temperature in the dark. Flow cytometric analysis was performed on the Coulter EPICS Elite Cytometer (Hialeah, FL) at the excitation and emission wavelengths of 488 and 550 nm, respectively, for fluorescein isothiocyanate measurements, and at 488 and 645 nm for propidium iodide measurements, respectively. The percentage of cells undergoing apoptotic death was determined using Multicycle software (Phoenix, San Diego, CA).
Measurement of ROS
To measure intracellular ROS levels, cells were incubated with 5 μmol/l chloromethyl-dichlorodihydro-fluorescein-diacetate for 1 h (44). Cells were rinsed with Ca++- and Mg++-free PBS, trypsinized from cultures and resuspended in PBS for analysis of ROS by flow cytometry, as described above, using a 15 mW air-cooled argon laser to produce 488 nm light. Dichlorodihydrofluorescein fluorescence emission was collected with a 529 nm band pass filter. The mean fluorescence intensity of 2 × 104 cells was quantified using Multicycle software (Phoneix).
DNA damage assay
DNA damage was detected with a comet assay. Cells were trypsinized and collected in PBS at a density of 2 × 104 cells/ml. Cell suspension was mixed with an equal volume of 1% low-melting agarose (Fisher, Fair Lawn, NJ) and placed on agarose-coated slides. Slides were then immersed in lysis solution (1.2 M NaCl, 100 mM Na2EDTA, 1% Triton X-100 and 0.3 nM NaOH, pH 13) at 25°C for 1 h. Slides were rinsed three times with alkaline buffer (2 mM Na2EDTA and 300 mM NaOH) for 20 min each. After electrophoresis in the same alkaline buffer at 20V for 30 min (45), slides were rinsed with distilled water, stained with 2.5 μg/ml of propidium iodide for 20 min and examined with a Zeiss fluorescence microscope (Thornwood, NY) equipped with an excitation filter of 546 nm and barrier filter of 590 nm. Fifty nuclei per slide were scored for tail moment as a parameter using CometScore software (Tritek).
Western immunoblotting
Cells were lysed in a buffer (10 mM Tris–HCl, 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 10 mM sodium pyrophosphate, 10% glycerol, 0.1% Na3VO4, 50 mM NaF, pH 7.4); cell lysates were isolated from the supernatants after centrifugation of crude lysates at 20 000g for 20 min (7,8). Protein concentration in cell lysates was measured using the BCA assay (Pierce, Rockford, IL). Equal amounts of cellular proteins were resolved by electrophoresis in 10 or 14% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose filters for western immunoblotting as described previously (9). Antibodies specific to Erk1/2 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies specific to phosphorylated Erk1/2 (p-Erk1/2), phosphorylated H2AX (p-H2AX), H2AX, phosphorylated Mek1/2 (p-Mek1/2) and Mek1/2 were purchased from Cell Signaling Technology. Antigen–antibody complexes on filters were detected by the Supersignal Chemiluminescence Kit (Pierce).
Statistical analysis
To statistically verify the cytotoxicity of EC, ECG, EGC and EGCG, the Shapiro–Wilk test (46) was used to assess normality of cell viability, cell proliferation and apoptosis. None of the variables showed evidence of lack of normality (P > 0.05). Therefore, the Student’s t-test was used to compare each of the catechins at each of the concentrations (10, 40 and 100 μg/ml) with the control. Adjustments for multiple comparisons were performed using the Simes method (47).
To statistically verify the suppression of NNK- and B[a]P-induced carcinogenesis by individual catechins, a one-way analysis of variance test was used to establish significant difference between various treatment groups; a P value of ≤0.05 was considered significant. Then, a pairwise analysis of dependent variables was performed with the Duncan multiple range test to verify the significance of differences between groups.
Statistical significance of all the other studies was analyzed by the Student’s t-test; α levels were adjusted by the Simes method (47). A P value of ≤0.05 was considered significant.
Results
NNK- and B[a]P-induced cellular carcinogenesis
Growth factors are required for normal cells to grow and survive, and cell adhesion to extracellular matrixes is important for cell survival in a multicell environment; aberrantly increased cell survivability acquired to reduce dependence on growth factors and to promote anchorage-independent growth can lead cells to tumorigenic transformation (48,49). We used these two important cancer-associated properties as long-term-targeted end points for measuring the ability of NNK and B[a]P to induce carcinogenesis of human breast cells. As shown in Figure 1A, we repeatedly exposed MCF10A cells, for 5, 10, 15 and 20 cycles, to individual or combined NNK and B[a]P each at a bioachievable concentration of 100 pmol/l, which can be detected in body fluids and tissues of cancer patients and tobacco users (11–13). We detected that accumulated exposures to individual or combined NNK and B[a]P resulted in increasing acquisition of the cancer-associated properties of reduced dependence on growth factors (Figure 1B) and anchorage-independent growth (Figure 1C) in an exposure-dependent manner, particularly for 5 and 10 cycles. Although accumulated exposures to carcinogens for 20 cycles resulted in higher levels of acquired cancer-associated properties than 10 and 15 exposure cycles, the increased levels appeared to be modest. NNK and B[a]P exhibited comparable abilities to progressively induce breast cell carcinogenesis, and the combination of NNK and B[a]P additively increased degrees of acquired cancer-associated properties. Because cumulative exposures to combined NNK and B[a]P for 10 cycles efficiently and additively induced cellular carcinogenesis versus individual carcinogens, we combined carcinogens each at 100 pmol/l in our extended studies.
Short-term-targeted end points: ROS elevation, the ERK pathway, cell proliferation and DNA damage transiently induced by short-term exposure to NNK and B[a]P
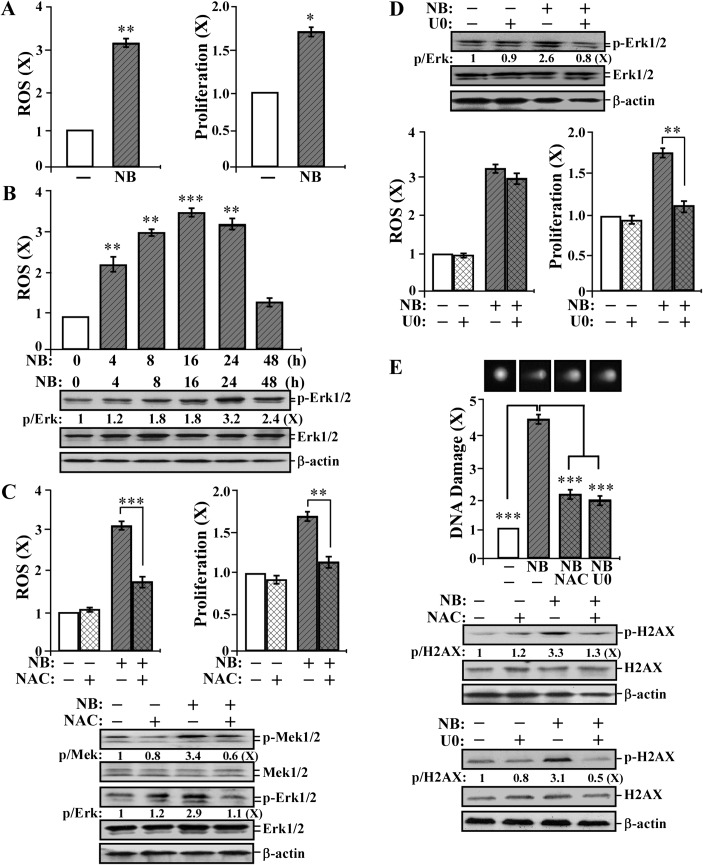
To pursue the mechanisms of NNK and B[a]P in inducing breast cell carcinogenesis, we studied the activity of combined NNK and B[a]P each at 100 pmol/l to induce ROS elevation, cell proliferation, DNA damage and the cell proliferation-related ERK pathway in each short-term exposure. As shown in Figure 2A, combined NNK and B[a]P at 100 pmol/l induced ROS elevation and cell proliferation. A transient elevation of ROS after NNK and B[a]P treatment was followed by a transient induction of the ERK pathway indexed by phosphorylation of Erk1/2 (Figure 2B); carcinogen-induced ROS reached its maximal level by 16 h, but Erk1/2 phosphorylation did not reach its maximal level until 24 h. ROS elevation appeared to be induced prior to activation of the ERK pathway during short-term exposure to NNK and B[a]P. To address whether ROS elevation cross-talked with the ERK pathway, we used the antioxidant NAC (28) to block ROS and the Mek1/2 inhibitor U0126 to block the ERK pathway (50). We detected that NAC treatment significantly reduced NNK- and B[a]P-induced ROS elevation, cell proliferation and phosphorylation of Mek1/2 and Erk1/2 (Figure 2C). However, blockage of the ERK pathway did not affect NNK- and B[a]P-induced ROS elevation but reduced cell proliferation (Figure 2D). These results indicate that NNK- and B[a]P-induced ROS elevation played a role in modulating the ERK pathway for cell proliferation.
Fig. 2.
Short-term-targeted end points: ROS elevation, the ERK pathway, cell proliferation and DNA damage transiently induced by short-term exposure to NNK and B[a]P. (A) MCF10A cells were exposed to combined NNK and B[a]P (NB) each at 100 pmol/l for 24 h. (B) MCF10A cells were exposed to NB for 0, 4, 8, 16, 24 and 48 h. (C, D and E) MCF10A cells were exposed to NB in the presence or absence of 5 mmol/l NAC or 10 μmol/l U0126 (U0) for 24 h. (A–D) ROS levels were measured with chloromethyl-dichlorodihydro-fluorescein-diacetate labeling; relative level of ROS as fold induction was normalized by the level determined in untreated counterpart cells, set as 1 (X, arbitrary unit). (A, C and D) Cell proliferation was determined; relative cell growth rate was normalized by the value of BrdU detected in untreated counterpart cells, set as 1 (X, arbitrary unit). (B–E) Cell lysates were prepared and analyzed by western immunoblotting to detect levels of phosphorylated Mek1/2 (p-Mek1/2), Mek1/2, p-Erk1/2, Erk1/2, p-H2AX and H2AX, with β-actin as a control, and these levels were quantified by densitometry. The levels of specific phosphorylation of Mek1/2 (p-Mek), Erk1/2 (p-Erk) and H2AX (p-H2AX) were calculated by normalizing the levels of p-Mek1/2, p-Erk1/2 and p-H2AX with the levels of Mek1/2, Erk1/2 and H2AX, respectively, and then further normalizing with the β-actin level and the level set in untreated cells (lane1) as 1 (X, arbitrary unit). (E) DNA damage was measured by a comet assay in 50 cells per treatment; relative DNA damage was normalized by the value determined in untreated counterpart cells, set as 1 (X, arbitrary unit). Representative images of cells treated in the comet assay are shown. Columns, mean of triplicates; bars, SD. All results are representative of at least three independent experiments. The Student’s t-test was used to analyze statistical significance, indicated by *P < 0.05, **P < 0.01, ***P < 0.001; α levels were adjusted by the Simes method.
To investigate whether NNK- and B[a]P-induced ROS and the ERK pathway were involved in DNA damage, we used a comet assay (51) to detect the extent of nuclear DNA damage by quantifying the DNA damage-produced comet tail moment in agarose gel electrophoresis. We detected that exposure of cells to NNK and B[a]P induced significant DNA damage and H2AX phosphorylation, and blockage of ROS elevation or the ERK pathway reduced carcinogen-induced DNA damage and H2AX phosphorylation (Figure 2E), indicating that ROS elevation and ERK pathway activation led to DNA damage during cell exposure to NNK and B[a]P. Thus, cell proliferation, ROS elevation, ERK pathway activation and DNA damage should be considered as short-term biological-, biochemical- and molecular-targeted end points for measuring the activity of NNK and B[a]P in inducing cellular carcinogenesis.
Cytotoxicity of EC, ECG, EGC and EGCG
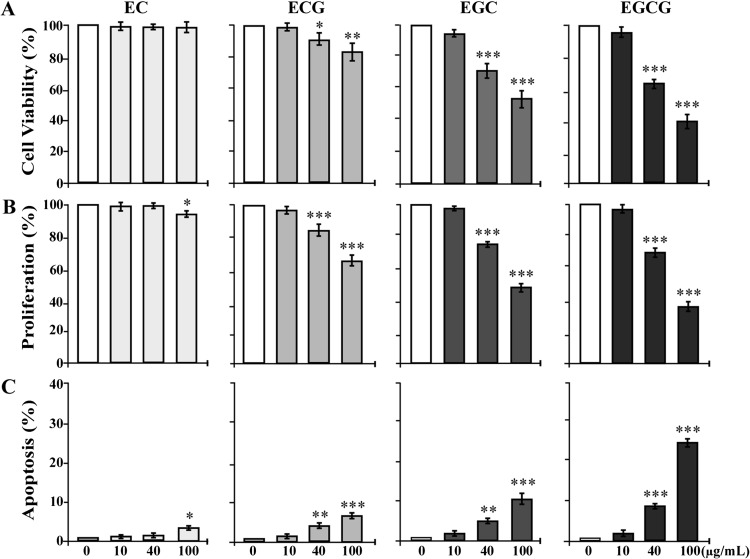
Studies showed that EGCG and EGC are more toxic than ECG and EC in inducing cellular DNA damage (40,41). Whether the cytotoxicity of EC, ECG, EGC and EGCG contributes to their preventive activity in intervention of cellular carcinogenesis needs to be clarified. To determine the cytotoxicity of individual GTCs to breast cells, we investigated the effects of EC, ECG, EGC and EGCG at various concentrations on viability, proliferation and apoptotic death of MCF10A cells. As shown in Figure 3, we detected that none of these catechins at 10 μg/ml showed any detectable effects on reducing cell viability (Figure 3A), inhibiting cell proliferation (Figure 3B) or inducing apoptosis (Figure 3C). EC at 40 μg/ml also failed to induce any detectable cytotoxic effects on MCF10A cells, but EC at 100 μg/ml induced a modest inhibition of cell proliferation and modest apoptosis. ECG, EGC and EGCG at 40 and 100 μg/ml induced reduction of cell viability (Figure 3A), inhibition of cell proliferation (Figure 3B) and apoptosis (Figure 3C), in a dose-dependent manner. Analysis of these data indicated distinct cytotoxicities of these catechins to MCF10A cells: EC < ECG < EGC < EGCG. At 10 μg/ml, these catechins were non-cytotoxic to MCF10A cells.
Fig. 3.
Cytotoxicity of EC, ECG, EGC and EGCG. MCF10A cells were treated with 0, 10, 40 and 100 μg/ml of EC, ECG, EGC and EGCG individually for 48 h. (A) Quantification of cell viability was determined with an MTT (methyl thiazolyl tetrazolium) Assay Kit, and relative cell viability was normalized by the value determined in untreated counterpart cells, set as 100%. (B) Cell proliferation was determined; relative cell growth rate was normalized by the value of BrdU detected in untreated cells, set as 100%. (C) Apoptotic cell population (%) was measured by flow cytometry with an Annexin-V-FITC Apoptosis Detection Kit. Columns, mean of triplicates; bars, SD. All results are representative of at least three independent experiments. The Student’s t-test was used to analyze statistical significance, indicated by *P < 0.05, **P < 0.01, ***P < 0.001; α levels were adjusted by the Simes method.
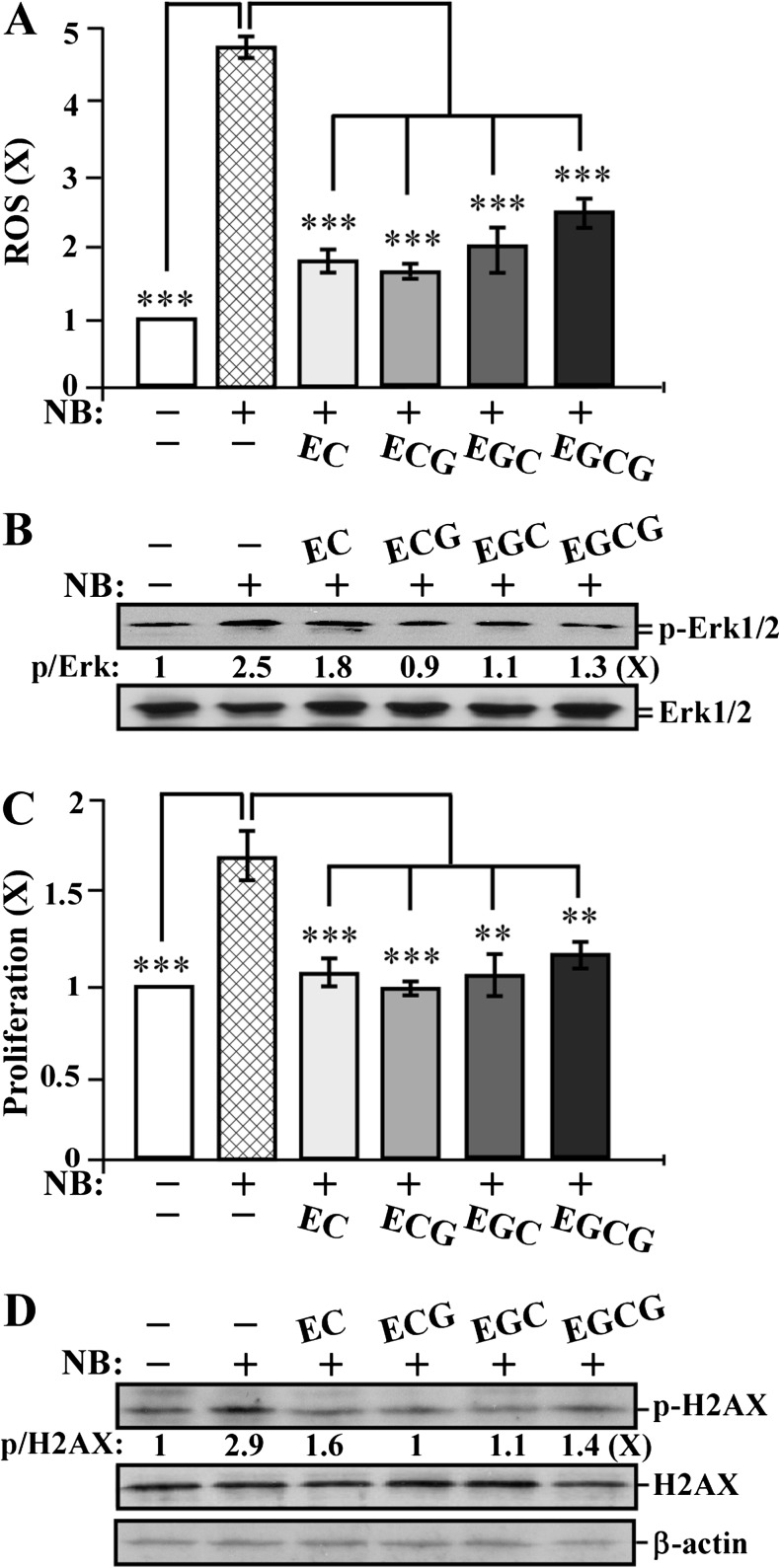
Catechin suppression of NNK- and B[a]P-induced short-term-targeted end points in MCF10A cells
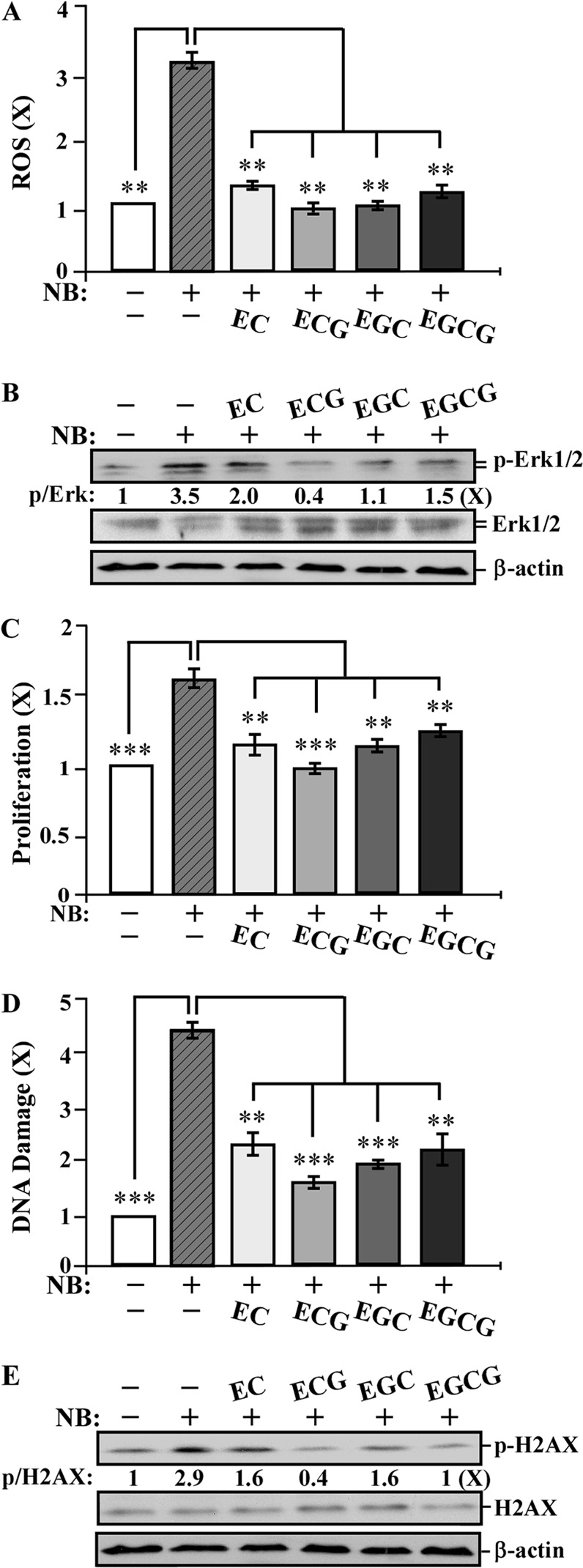
To detect whether individual catechins EC, ECG, EGC and EGCG were able to counteract against NNK and B[a]P, we studied the activity of these catechins to block NNK- and B[a]P-increased short-term-targeted end points. We exposed MCF10A cells to NNK and B[a]P in the presence and absence of individual catechins at 10 μg/ml for 24 h. As shown in Figure 4, cotreatment with EC, ECG, EGC and EGCG reduced the NNK- and B[a]P-induced ROS elevation (Figure 4A), Erk1/2 phosphorylation (Figure 4B), cell proliferation (Figure 4C) and DNA damage (Figure 4D) as well as H2AX phosphorylation (Figure 4E). Apparently, these catechins, at non-cytotoxic concentrations, were able to suppress ROS elevation, the ERK pathway, cell proliferation and DNA damage in non-cancerous breast MCF10A cells. ECG appeared to be more effective than other catechins in reducing these NNK- and B[a]P-induced short-term biological, biochemical and molecular-targeted end points.
Fig. 4.
Catechin suppression of NNK- and B[a]P-induced short-term-targeted end points in MCF10A cells. MCF10A cells were exposed to combined NNK and B[a]P (NB) each at 100 pmol/l in the absence or presence of 10 μg/ml of EC, ECG, EGC and EGCG for 24 h. (A) ROS levels were measured with chloromethyl-dichlorodihydro-fluorescein-diacetate labeling; relative level of ROS as fold induction was normalized by the level determined in untreated counterpart cells, set as 1 (X, arbitrary unit). (B and E) Cell lysates were prepared and analyzed by western immunoblotting to detect levels of p-Erk1/2, Erk1/2, p-H2AX and H2AX, with β-actin as a control, and these levels were quantified by densitometry. The levels of specific phosphorylation of Erk1/2 (p-Erk) and H2AX (p-H2AX) were calculated by normalizing the levels of p-Erk1/2 and p-H2AX with the levels of Erk1/2 and H2AX, respectively, and then further normalizing with β-actin level and the level set in untreated cells (lane1) as 1 (X, arbitrary unit). (C) Cell proliferation was determined; relative cell growth rate was normalized by the value of BrdU detected in untreated counterpart cells, set as 1 (X, arbitrary unit). (D) DNA damage was measured by comet assay; relative DNA damage was normalized by the value determined in untreated counterpart cells, set as 1 (X, arbitrary unit). Columns, mean of triplicates; bars, SD. All results are representative of at least three independent experiments. The Student’s t-test was used to analyze statistical significance, indicated by **P < 0.01, ***P < 0.001; α levels were adjusted by the Simes method.
Catechin suppression of NNK- and B[a]P-induced short-term-targeted end points in MCF7 cells
To investigate whether catechin suppression of the NNK- and B[a]P-induced short-term-targeted end points of ROS elevation, ERK pathway activation, cell proliferation and DNA damage was or was not limited to MCF10A cells, we treated human breast cancer MCF7 cells to NNK and B[a]P in the absence and presence of individual catechins for 24 h. We detected that cotreatment with EC, ECG, EGC and EGCG reduced the NNK- and B[a]P-induced ROS elevation (Figure 5A), ERK1/2 phosphorylation (Figure 5B), cell proliferation (Figure 5C) and H2AX phosphorylation (Figure 5D). These results indicated that exposure to NNK and B[a]P also induced short-term-targeted end points in breast cancer cells. Catechins, at non-cytotoxic levels, were also able to suppress NNK- and B[a]P-induced short-term-targeted end points in cancer MCF7 cells; ECG appeared to be more effective than other catechins. The results indicate that the ability of NNK and B[a]P to induce short-term-targeted end points and the activity of catechin in suppression of the NNK- and B[a]P-induced short-term-targeted end points were not limited to MCF10A cells.
Fig. 5.
Catechin suppression of NNK- and B[a]P-induced short-term-targeted end points in MCF7 cells. MCF7 cells were exposed to combined NNK and B[a]P (NB) each at 100 pmol/l in the absence or presence of 10 μg/ml of EC, ECG, EGC and EGCG for 24 h. (A) ROS levels were measured with chloromethyl-dichlorodihydro-fluorescein-diacetate labeling; relative level of ROS as fold induction was normalized by the level determined in untreated counterpart cells, set as 1 (X, arbitrary unit). (B and D) Cell lysates were prepared and analyzed by western immunoblotting to detect levels of phosphorylated Erk1/2 (p-Erk1/2), Erk1/2, p-H2AX and H2AX, with β-actin as a control, and these levels were quantified by densitometry. The levels of specific phosphorylation Erk1/2 (p-Erk) and H2AX (p-H2AX) were calculated by normalizing the levels of p-Erk1/2 and p-H2AX with the levels of Erk1/2 and H2AX, respectively, and then further normalizing with β-actin level and the level set in untreated cells (lane 1) as 1 (X, arbitrary unit). (C) Cell proliferation was determined; relative cell growth rate was normalized by the value of BrdU detected in untreated counterpart cells, set as 1 (X, arbitrary unit). Columns, mean of triplicates; bars, SD. All results are representative of at least three independent experiments. The Student’s t-test was used to analyze statistical significance, indicated by **P < 0.01, ***P < 0.001; α levels were adjusted by the Simes method.
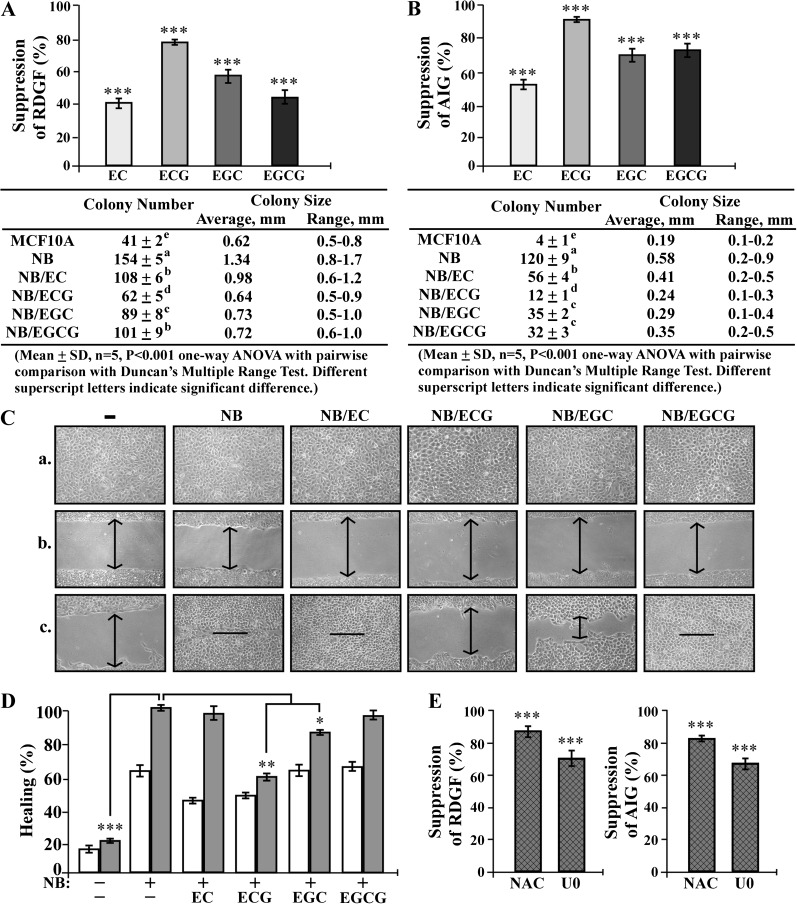
Catechin suppression of chronically NNK- and B[a]P-induced carcinogenesis
To investigate the preventive activity of individual catechins in intervention of breast cell carcinogenesis chronically induced by cumulative exposures to NNK and B[a]P, we repeatedly exposed MCF10A cells to NNK and B[a]P in the absence and presence of individual EC, ECG, EGC and EGCG at 10 μg/ml for 10 cycles, resulting in the NB, NB/EC, NB/ECG, NB/EGC and NB/EGCG cell lines, respectively. In addition to the cancer-associated properties of reduced dependence on growth factors and anchorage-independent growth, we also investigated the cancer-associated property of increased cell mobility (52) as the third long-term-targeted end point to measure carcinogen-induced and catechin-suppressed cellular carcinogenesis. We detected that EC, ECG, EGC and EGCG effectively suppressed NNK- and B[a]P-induced cellular acquisition of reduced dependence on growth factors (Figure 6A) as well as anchorage-independent growth (Figure 6B). Not only the numbers but also sizes of colonies were suppressed by catechins (Figure 6A and B, tables). The results also revealed that ECG exhibited higher activity than other catechins in suppression of carcinogen-induced cellular acquisition of these two cancer-associated properties. Using the scratch/wound assay (42), we detected that NB cells acquired higher mobility than parental MCF10A cells to heal the wounded areas, and NB/EC and NB/EGCG cells exhibited higher mobility than NB/ECG and NB/EGC cells to heal the wounded areas (Figure 6C). Analysis of healing rates revealed that NB/ECG cells exhibited a lower mobility than NB, NB/EGC, NB/EC and NB/EGCG cells (Figure 6D), indicating that ECG possessed higher activity than other catechins in suppression of carcinogen-induced cellular acquisition of increased mobility. The high effectivity of ECG in suppressing cellular carcinogenesis was correlated with its activity in blocking ROS elevation and ERK pathway activation, which were transiently induced by short-term exposure to NNK and B[a]P.
Fig. 6.
Catechin suppression of chronically NNK- and B[a]P-induced carcinogenesis. (A–D) MCF10A cultures were repeatedly exposed to combined NNK and B[a]P (NB) each at 100 pmol/l in the presence of 10 μg/ml of EC, ECG, EGC and EGCG (NB/catechin) for 10 cycles. (E) MCF10A cultures were repeatedly exposed to NB in the presence of 5 mmol/l NAC or 10 μmol/l U0126 (U0) for 10 cycles. (A and E) To detect effectivity of individual catechins, NAC and U0 on suppression of cellular acquisition of reduced dependence on growth factors (RDGF), 5 × 103 cells were seeded and maintained in LM medium for 10 days. (B and E) To detect effectivity of individual catechins, NAC or U0 on suppression of cellular acquisition of anchorage-independent growth (AIG), 1 × 104 cells were seeded in soft agar for 20 days. The value of the suppression effectivity of individual catechins on NB-induced RDGF (A and E) and AIG (B and E) was calculated by: {1 − [(# of NB/catechin-induced cell colonies) − (# of MCF10A cell colonies)] / [(# of NB-induced cell colonies) − (# of MCF10A cell colonies)]} × 100 (%). The Student’s t-test was used to analyze statistical significance, indicated by ***P < 0.001; α levels were adjusted by the Simes method. Tables show colony numbers, average colony size and range of colony size. Mean colony numbers in each treatment group were analyzed by one-way analysis of variance at P < 0.001 to indicate significant difference in number of colonies in various groups. To further determine the significant difference between individual groups, a pairwise analysis of variables was performed using the Duncan multiple range test. Means with different superscript letters (a, b, c, d and e) indicate significant difference at P < 0.001 between groups; no significant difference was seen between groups with the same superscript. (C) To detect effectivity of individual catechins in suppressing carcinogen-induced cellular acquisition of increased mobility, cells were seeded in CM medium and grown to confluence (a), a linear area of cell layer was removed from each culture with a 23-gauge needle to produce wounded cultures, and the wounded areas were examined (×100 magnification) 6 (b) and 24 h (c) after wounding. Arrows indicate width of wounded areas. Results are representative of three independent experiments. (D) To quantitatively measure cell mobility detected in (C), the area not healed by the cells was subtracted from total area of initial wound to calculate the wound healing area (%) at time intervals of 6 h (white columns) and 24 h (gray columns). Columns, mean of triplicates; bars, SD. All results are representative of three independent experiments. The Student’s t-test was used to analyze statistical significance, indicated by *P < 0.05, **P < 0.01; α levels were adjusted by the Simes method.
To validate the contributing roles of ROS elevation and the ERK pathway in cellular carcinogenesis induced by cumulative exposures to NNK and B[a]P, we repeatedly exposed MCF10A cells to NNK and B[a]P in the presence of NAC to block ROS or U0126 to block the ERK pathway for 10 cycles. We detected that blockage of ROS elevation or ERK pathway activation during each cycle of exposure resulted in significant suppression of NNK- and B[a]P-induced cellular acquisition of reduced dependence on growth factors and anchorage-independent growth (Figure 6E), verifying the contributing roles of ROS elevation and ERK pathway activation in NNK- and B[a]P-induced cellular carcinogenesis.
Discussion
Our model system addresses breast cell carcinogenesis induced by chronic exposure to carcinogens at bioachievable levels and identifies preventive agents, at non-cytotoxic levels, capable of suppressing chronically induced breast cell carcinogenesis. We demonstrated, for the first time, a two-step strategy. The first step initially uses short-term biological-, biochemical- and molecular-targeted carcinogenic end points transiently induced by short-term exposure to carcinogens for detecting preventive agents capable of blocking cellular carcinogenesis. The second step subsequently uses long-term biological, biochemical and molecular targeted carcinogenic end points induced by chronic exposure to carcinogens to verify preventive agents effective in suppression of cellular carcinogenesis.
Our studies revealed that cumulative exposures to NNK and/or B[a]P at a bioachievable concentration of 100 pmol/l resulted in progression of human breast cells to increasingly acquire cancer-associated properties in an exposure-dependent manner, without acquiring tumorigenicity. Although cellular acquisition of tumorigenicity is regarded as the gold standard for validating cell malignancy, many human cancer cells are not tumorigenic, such as MDA-MB-453 (53) and urinary bladder cancer J82 cells (54). Previously, we showed that cumulative exposures of MCF10A cells to NNK and B[a]P at 100 pmol/l for 20 cycles induce the cancer-associated property of acinar-conformational description with irregular spheroids developed on Matrigel (9). Acinar structures with a hollow lumen and apicobasally polarized cells are important characteristics found in glandular epithelia in vivo; the disruption of an intact glandular structure is a hallmark of epithelial cancer, even at its earliest premalignant stages, such as ductal carcinoma-in-situ (55–57). Clinically, breast cells involved in ductal carcinoma-in-situ are not malignant and have not acquired the ability to invade adjacent tissues through the ductal or lobular wall but often premalignant cells can either develop into malignant cells or increase the risk of becoming malignant (4,58,59). Thus, in addition to tumorigenicity, using various cancer-associated properties as measurable targeted end points should be seriously considered in studying cellular carcinogenesis and intervention of cellular carcinogenesis.
Short-term exposure to NNK and B[a]P at 100 pmol/l induced transient ROS elevation leading to ERK pathway activation, cell proliferation and chromosomal DNA damage. However, these short-term targeted end points were transient; they were not permanent in cells acquiring cancer-associated properties induced by cumulative exposures to NNK and B[a]P. In our previous studies, we detected that the ERK pathway is downregulated in two individual cell clones isolated from cultures after long-term exposure to NNK (6), and cell proliferation is not increased in cultures after long-term exposure to NNK and/or B[a]P (7–9). Aiming at these transiently induced short-term targeted end points, we detected GTCs EC, ECG, EGC and EGCG, at a non-cytotoxic concentration of 10 μg/ml, were capable of blocking NNK- and B[a]P-induced ROS elevation, ERK pathway activation, cell proliferation and DNA damage to various extents in not only non-cancerous MCF10A but also in adenocarcinoma MCF7 cells. Comparing these catechins at 10 μg/ml revealed that ECG (22.6 μmol/l) was more effective than EC (34.5 μmol/l), EGC (32.7 μmol/l) and EGCG (21.8 μmol/l) to block these short-term-targeted carcinogenic end points. Interestingly, ECG was also more effective than EC, EGC and EGCG in suppression of cellular carcinogenesis, measured by degrees of acquired cancer-associated properties in long-term-targeted carcinogenic end points, permanently induced by cumulative exposures to NNK and B[a]P; however, whether the activity of individual catechins may vary in suppression of cellular carcinogenesis induced by other carcinogens remains to be addressed. In addition, ECG was less cytotoxic to MCF10A cells than EGCG and EGC. Studies of the hepatotoxicity of GTCs in rats showed that EGCG is the most toxic of all four catechins, for example, the LD50 for EGCG (200 μM) was 10 times lower than that for ECG (2000 μM), indicating that ECG is much less toxic than EGCG (60). Thus, the cytotoxicity-independent ability of these catechins to block NNK- and B[a]P-induced, ROS elevation, ERK pathway activation, cell proliferation and DNA damage in each short-term exposure accounted for their effectiveness in suppression of breast cell carcinogenesis induced by long-term accumulated exposures to NNK and B[a]P. In addition, in our previous studies, we detected that the NNK- and B[a]P-bioactivating cytochrome P-450 enzymes CYP1A1 and CYP1B1 are elevated in cells exposed to NNK and B[a]P, and grape seed proanthocyanidin extract is able to reduce these activating enzymes (9). However, we did not detect any activity of GTCs in suppressing NNK- and B[a]P-induced CYP1A1 or CYP1B1 (data not shown).
NNK- and B[a]P-induced ROS elevation played a key role in the activation of the ERK pathway, leading to cell proliferation and DNA damage. Studies have shown that ROS is able to induce the ERK pathway via activation of membrane-associated growth factor receptors or via Raf-independent Mek1/2 activation (61, 62). In our studies, exposure of MCF10A cells to NNK and B[a]P did not induce any detectable upregulation of Ras or Raf, both of which are upstream from Mek1/2 and Erk1/2 (data not shown). Thus, NNK- and B[a]P-elevated ROS induced the ERK pathway in a Raf-independent manner. However, how ROS was induced by NNK and B[a]P in MCF10A and MCF7 cells, and how ROS was able to induce Raf-independent activation of Mek1/2 and Erk1/2 remains to be determined.
Prevention of human breast cell carcinogenesis associated with chronic exposure to low doses of environmental carcinogens is an under-investigated area. Our model system presents unique features of mimicking chronically induced carcinogenesis of human breast cells to increasingly acquire cancer-associated properties induced by chronic cumulative exposures to carcinogens at low concentrations in the picomolar range, as in environmental exposure. In contrast, many cell systems have been developed to study the activity of carcinogens at high concentrations in the micromolar range, as in occupational exposure, in acute induction of cellular carcinogenesis (2–5,18–21). Using our cellular model as a target system, we are able to verify the preventive activity of individual GTCs at non-cytotoxic levels in suppression of chronic cellular carcinogenesis and identify the mechanisms for catechins in counteracting the biological, biochemical and molecular effects of NNK and B[a]P. Use of non-cytotoxic catechin components should be seriously considered in prevention of cellular carcinogenesis induced by chronic exposure to environmental carcinogens. Using our model system will conceivably accelerate the identification of additional preventive agents that are effective in reducing the health risk of sporadic breast cancer associated with chronic exposure to carcinogens present in environmental pollution.
Funding
This work was supported by a grant from the University of Tennessee, College of Veterinary Medicine, Center of Excellence in Livestock Diseases and Human Health to H.-C.R.W.; the National Institutes of Health (CA125795 and CA129772) to H.-C.R.W.
Acknowledgments
We are grateful to Ms. M. Bailey for textual editing of the manuscript. We thank Ms. DJ Trent for technique support in flow cytometric analysis of ROS contents and cell death.
Glossary
Abbreviations
- ATCC
American Type Culture Collection
- B[a]P
benzo[a]pyrene
- BrdU
5-bromo-2′
- -deoxyuridine
CM medium
- complete MCF10A medium
EC
- epicatechin
ECG
- epicatechin-3-gallate
EGC
- epigallocatechin
EGCG
- epigallocatechin-3-gallate
ELISA
- enzyme-linked immunosorbent assay
ERK
- extracellular signal-regulated kinase
GTC
- green tea catechin
LM
- low-mitogen
NAC
- N-acetyl-L
-cysteine
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
References
- 1.Kelloff GJ, et al., editors. Cancer Chemoprevention: Strategies for Cancer Chemoprevention. Vol 2. Totowa, NJ: Human Press; (2005). [Google Scholar]
- 2.DeBruin LS, et al. Perspectives on the chemical etiology of breast cancer. Environ. Health Perspect. 2002;110(suppl. 1):119–128. doi: 10.1289/ehp.02110s1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hecht SS. Tobacco smoke carcinogens and breast cancer. Environ. Mol. Mutagen. 2002;39:119–126. doi: 10.1002/em.10071. [DOI] [PubMed] [Google Scholar]
- 4.Guengerich FP. Metabolism of chemical carcinogens. Carcinogenesis. 2000;21:345–351. doi: 10.1093/carcin/21.3.345. [DOI] [PubMed] [Google Scholar]
- 5.Mehta RG. Experimental basis for the prevention of breast cancer. Eur. J. Cancer. 2000;36:1275–1282. doi: 10.1016/s0959-8049(00)00100-3. [DOI] [PubMed] [Google Scholar]
- 6.Mei J, et al. Transformation of noncancerous human breast epithelial cell MCF10A induced by the tobacco-specific carcinogen NNK. Breast Cancer Res. Treat. 2003;79:95–105. doi: 10.1023/a:1023326121951. [DOI] [PubMed] [Google Scholar]
- 7.Siriwardhana N, et al. Precancerous carcinogenesis of human breast epithelial cells by chronic exposure to benzo[a]pyrene. Mol. Carcinog. 2008;47:338–348. doi: 10.1002/mc.20392. [DOI] [PubMed] [Google Scholar]
- 8.Siriwardhana N, et al. Precancerous model of human breast epithelial cells induced by the tobacco-specific carcinogen NNK for prevention. Breast Cancer Res. Treat. 2008;109:427–441. doi: 10.1007/s10549-007-9666-9. [DOI] [PubMed] [Google Scholar]
- 9.Song X, et al. Grape seed proanthocyanidin suppression of breast cell carcinogenesis induced by chronic exposure to combined 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo[a]pyrene. Mol. Carcinog. 2010;49:450–463. doi: 10.1002/mc.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hecht SS, et al. Quantitation of 4-oxo-4-(3-pyridyl)butanoic acid and enantiomers of 4-hydroxy-4-(3-pyridyl)butanoic acid in human urine: a substantial pathway of nicotine metabolism. Chem. Res. Toxicol. 1999;12:172–179. doi: 10.1021/tx980214i. [DOI] [PubMed] [Google Scholar]
- 11.Obana H, et al. Polycyclic aromatic hydrocarbons in human fat and liver. Bull. Environ. Contam. Toxicol. 1981;27:23–27. doi: 10.1007/BF01610981. [DOI] [PubMed] [Google Scholar]
- 12.Hecht SS, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–596. [PubMed] [Google Scholar]
- 13.Besaratinia A, et al. A molecular dosimetry approach to assess human exposure to environmental tobacco smoke in pubs. Carcinogenesis. 2002;23:1171–1176. doi: 10.1093/carcin/23.7.1171. [DOI] [PubMed] [Google Scholar]
- 14.Hecht SS. Recent studies on mechanisms of bioactivation and detoxification of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a tobacco specific lung carcinogen. Crit. Rev. Toxicol. 1996;26:163–181. doi: 10.3109/10408449609017929. [DOI] [PubMed] [Google Scholar]
- 15.Hecht SS. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 16.Chhabra SK, et al. Coexposure to ethanol with N-nitrosodimethylamine or 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone during lactation of rats: marked increase in O(6)-methylguanine-DNA adducts in maternal mammary gland and in suckling lung and kidney. Toxicol. Appl. Pharmacol. 2000;169:191–200. doi: 10.1006/taap.2000.9068. [DOI] [PubMed] [Google Scholar]
- 17.Ohnishi T, et al. Possible application of human c-Ha-ras proto-oncogene transgenic rats in a medium-term bioassay model for carcinogens. Toxicol. Pathol. 2007;35:436–443. doi: 10.1080/01926230701302541. [DOI] [PubMed] [Google Scholar]
- 18.Cavalieri E, et al. Carcinogenicity of aromatic hydrocarbons directly applied to rat mammary gland. J. Cancer Res. Clin. Oncol. 1988;114:3–9. doi: 10.1007/BF00390478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, et al. DNA adducts in normal tissue adjacent to breast cancer: a review. Cancer Detect. Prev. 1999;23:454–462. doi: 10.1046/j.1525-1500.1999.99059.x. [DOI] [PubMed] [Google Scholar]
- 20.Rundle A, et al. The relationship between genetic damage from polycyclic aromatic hydrocarbons in breast tissue and breast cancer. Carcinogenesis. 2000;21:1281–1289. [PubMed] [Google Scholar]
- 21.Rubin H. Synergistic mechanisms in carcinogenesis by polycyclic aromatic hydrocarbons and by tobacco smoke: a bio-historical perspective with updates. Carcinogenesis. 2011;22:1903–1930. doi: 10.1093/carcin/22.12.1903. [DOI] [PubMed] [Google Scholar]
- 22.Knasmüller S, et al. Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants; current state of knowledge. Toxicology. 2004;198:315–328. doi: 10.1016/j.tox.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Shi H, et al. Evaluation of spin trapping agents and trapping conditions for detection of cell-generated reactive oxygen species. Arch. Biochem. Biophys. 2005;437:59–68. doi: 10.1016/j.abb.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Ho YS, et al. Tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces cell proliferation in normal human bronchial epithelial cells through NFkappaB activation and cyclin D1 up-regulation. Toxicol. Appl. Pharmacol. 2005;205:133–148. doi: 10.1016/j.taap.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Cooke MS, et al. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;10:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 26.Preston-Martin S, et al. Epidemiologic evidence for the increased cell proliferation model of carcinogenesis. Environ. Health Perspect. 1993;5:137–138. doi: 10.1289/ehp.93101s5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu D, et al. ERK activity facilitates activation of the S-phase DNA damage checkpoint by modulating ATR function. Oncogene. 2006;8:1153–1164. doi: 10.1038/sj.onc.1209148. [DOI] [PubMed] [Google Scholar]
- 28.Rogakou EP, et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;10:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 29.Mauthe RJ, et al. Exposure of mammalian cell cultures to benzo[a]pyrene and light results in oxidative DNA damage as measured by 8-hydroxydeoxyguanosine formation. Carcinogenesis. 1995;16:133–137. doi: 10.1093/carcin/16.1.133. [DOI] [PubMed] [Google Scholar]
- 30.Plísková M, et al. Deregulation of cell proliferation by polycyclic aromatic hydrocarbons in human breast carcinoma MCF-7 cells reflects both genotoxic and nongenotoxic events. Toxicol. Sci. 2005;83:246–256. doi: 10.1093/toxsci/kfi040. [DOI] [PubMed] [Google Scholar]
- 31.Bagchi D, et al., editors. Phytopharmaceuticals in Cancer Chemoprevention. Boca Raton, FL: CRC Press; 2005. [Google Scholar]
- 32.Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Rice-Evans CA, et al. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 34.Kavanagh KT, et al. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J. Cell. Biochem. 2001;82:387–398. doi: 10.1002/jcb.1164. [DOI] [PubMed] [Google Scholar]
- 35.Roomi MW, et al. Modulation of N-methyl-N-nitrosourea induced mammary tumors in Sprague-Dawley rats by combination of lysine, proline, arginine, ascorbic acid and green tea extract. Breast Cancer Res. 2005;7:291–295. doi: 10.1186/bcr989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thangapazham RL, et al. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007;245:232–241. doi: 10.1016/j.canlet.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Sartippour MR, et al. Green tea and its catechins inhibit breast cancer xenografts. Nutr. Cancer. 2001;40:149–156. doi: 10.1207/S15327914NC402_11. [DOI] [PubMed] [Google Scholar]
- 38.Seely D, et al. The effects of green tea consumption on incidence of breast cancer and recurrence of breast cancer: a systematic review and meta-analysis. Integr. Cancer Ther. 2005;4:144–155. doi: 10.1177/1534735405276420. [DOI] [PubMed] [Google Scholar]
- 39.Boehm K, et al. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2009;((3)):CD005004. doi: 10.1002/14651858.CD005004.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yen GC, et al. Modulation of tea and tea polyphenols on benzo(a)pyrene-induced DNA damage in Chang liver cells. Free Radic. Res. 2004;38:193–200. doi: 10.1080/10715760310001638001. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto Y, et al. Cellular toxicity of catechin analogues containing gallate in opossum kidney proximal tubular (OK) cells. J. Toxicol. Sci. 2004;29:47–52. doi: 10.2131/jts.29.47. [DOI] [PubMed] [Google Scholar]
- 42.Lipton A, et al. Migration of mouse 3T3 fibroblasts in response to a serum factor. Proc. Natl Acad. Sci. U S A. 1971;11:2799–2801. doi: 10.1073/pnas.68.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choudhary S, et al. Differential induction of reactive oxygen species through Erk1/2 and Nox-1 by FK228 for selective apoptosis of oncogenic H-Ras-expressing human urinary bladder cancer J82 cells. J. Cancer Res. Clin. Oncol. 2010;137:471–480. doi: 10.1007/s00432-010-0910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trachootham D, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Olive PL, et al. The comet assay: a method to measure DNA damage in individual cells. Nat. Protoc. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro SS, et al. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 47.Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- 48.Hanahan D, et al. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 49.Reddig PJ, et al. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 50.Song P, et al. Distinct roles of the ERK pathway in modulating apoptosis of Ras-transformed and non-transformed cells induced by anticancer agent FK228. FEBS Lett. 2005;579:90–94. doi: 10.1016/j.febslet.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 51.Singh NP, et al. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 52.Madsen CD, et al. Cancer dissemination-lessons from leukocytes. Dev. Cell. 2010;1:13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Cailleau R, et al. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 54.Marshall CJ, et al. Markers of neoplastic transformation in epithelial cell lines derived from human carcinomas. J. Natl Cancer Inst. 1977;58:1743–1751. doi: 10.1093/jnci/58.6.1743. [DOI] [PubMed] [Google Scholar]
- 55.Debnath J, et al. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 56.Debnath J, et al. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 57.Nelson CM, et al. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin. Cancer Biol. 2005;15:342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Shaughnessy JA, et al. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin. Cancer Res. 2002;8:314–346. [PubMed] [Google Scholar]
- 59.Sanders ME, et al. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103:2481–2484. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 60.Galati G, et al. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic. Biol. Med. 2006;40:570–580. doi: 10.1016/j.freeradbiomed.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Martindale JL, et al. Cellular response to oxidative stress: signaling for suicide and survival. J. Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 62.Ossum CG, et al. Regulation of the mitogen-activated protein kinase p.44 ERK activity during anoxia/recovery in rainbow trout hypodermal fibroblasts. J. Exp. Biol. 2006;209:1765–1776. doi: 10.1242/jeb.02152. [DOI] [PubMed] [Google Scholar]