Abstract
Exact mechanisms underlying the initiation and progression of estrogen-related cancers are not clear. Literature, evidence and our studies strongly support the role of estrogen metabolism-mediated oxidative stress in estrogen-induced breast carcinogenesis. We have recently demonstrated that antioxidants vitamin C and butylated hydroxyanisole (BHA) or estrogen metabolism inhibitor α-naphthoflavone (ANF) inhibit 17β-estradiol (E2)-induced mammary tumorigenesis in female ACI rats. The objective of the current study was to identify the mechanism of antioxidant-mediated protection against E2-induced DNA damage and mammary tumorigenesis. Female ACI rats were treated with E2 in the presence or absence of vitamin C or BHA or ANF for up to 240 days. Nuclear factor erythroid 2-related factor 2 (NRF2) and NAD(P)H-quinone oxidoreductase 1 (NQO1) were suppressed in E2-exposed mammary tissue and in mammary tumors after treatment of rats with E2 for 240 days. This suppression was overcome by co-treatment of rats with E2 and vitamin C or BHA. Time course studies indicate that NQO1 levels tend to increase after 4 months of E2 treatment but decrease on chronic exposure to E2 for 8 months. Vitamin C and BHA significantly increased NQO1 levels after 120 days. 8-Hydroxydeoxyguanosine (8-OHdG) levels were higher in E2-exposed mammary tissue and in mammary tumors compared with age-matched controls. Vitamin C or BHA treatment significantly decreased E2-mediated increase in 8-OHdG levels in the mammary tissue. In vitro studies using silencer RNA confirmed the role of NQO1 in prevention of oxidative DNA damage. Our studies further demonstrate that NQO1 upregulation by antioxidants is mediated through NRF2.
Introduction
Estrogens have been implicated in the development of breast cancer (1–3). However, the exact mechanisms by which estrogens exert their carcinogenic effects remain elusive. A growing body of clinical and epidemiological literature, our studies and those of others strongly support the role of estrogen metabolism-mediated oxidative stress in estrogen-induced breast carcinogenesis (1,2,4–8). Estrogen-mediated tumor induction is suggested to depend on formation of estrogen DNA adducts and oxidative stress resulting from redox cycling of estrogen metabolites as well as on estrogen receptor-dependent pathway of estrogen-induced cell growth (1,2,9–15). The estrogen receptor-independent pathway involves metabolism of 17β-estradiol (E2) to catechol estrogens 2-hydroxyestradiol (2-OHE2) and 4-hydroxyestradiol (4-OHE2) (14). While 2-OHE2 is known to have putative chemo-protective characteristics, 4-OHE2 is known to generate oxidant stress and possess genotoxic potential (15–18). 4-Hydroxyestradiol undergoes oxidative metabolism to form electrophilic quinones, which readily react with DNA to produce depurinating adducts (2,15,18). In addition, redox cycling of quinones and semiquinones results in the formation of free radicals and reactive oxygen species, thereby creating more opportunities for genetic damage (19,20). NAD(P)H-Quinone oxidoreductase 1 (NQO1) is a key enzyme involved in defense against reactive oxygen species and inhibition of neoplasia (21,22). NQO1 converts E2-quinones back to E2-catechols, thus making E2-quinones unavailable for reaction with DNA and oxidative stress (23–26).
The transcription of many phase II antioxidant defense genes is suggested to be regulated by nuclear transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) (27–29). The transcription factor NRF2 binds to the antioxidant response element (ARE) in the promoter regions of genes encoding these antioxidant defense enzymes to increase their transcription (30,31). NQO1 contains ARE sequence in the promoter region and is known to be regulated by NRF2 (32).
The present study was intended to examine the role of two known prototypic antioxidants vitamin C (Vit C) and butylated hydroxyanisole (BHA) on NQO1 protein expression and role of NQO1 in prevention of oxidative DNA damage during breast carcinogenesis in an ACI rat model of breast cancer. Vitamin C is one of the most prevalent antioxidative components of fruits and vegetables (33). It has also been used as a dietary supplement intended to prevent oxidative stress-mediated chronic diseases such as cancer, cardiovascular disease, hypertension, stroke, and neurodegenerative disorder (34–37). However, some studies have suggested that dietary Vit C supplementation is harmful and may cause DNA damage (38,39). BHA is one of the several widely used antioxidants food additives that has been suggested to provide protection against chemical carcinogens (40). The exact mechanisms for the anticarcinogenic activity of BHA administered subsequent to carcinogen administration remain unknown (40). The anticarcinogenic activity of BHA is often ascribed to the ability of BHA to act as a free radical scavenger and/or to induce ‘phase II’ metabolic enzymes (41,42). In our previous studies, we have demonstrated that antioxidants Vit C and BHA significantly inhibit E2-induced breast cancer (5,6). Metabolism of E2 to catechol estrogens through cytochrome P450 (Cyp) enzymes 1A1 and 1B1 is suggested to play an important role in the formation of estrogen-DNA adducts as well as in generation of oxidative stress (2,15,18). Our recent studies also demonstrate that α-naphthoflavone (ANF) a Cyp1A1/1B1 inhibitor completely abrogates E2-induced breast cancer in female ACI rats (5).
The role of NQO1 in prevention of E2-induced breast cancer by antioxidants Vit C or BHA or E2-metabolic inhibitor ANF has not been explored. In this study, we suggest that NQO1 may play an important role in the prevention of E2-induced oxidative DNA damage and breast carcinogenesis by antioxidants Vit C and BHA.
Materials and methods
Treatment of animals and histopathologic analysis
Female ACI rats (4 weeks of age; Harlan Sprague Dawley, Indianapolis, IN) were housed under controlled temperature, humidity and lighting conditions. After a 1 week acclimatization period, rats were divided into following different groups: (i) Control, (ii) E2, (iii) BHA, (iv) BHA + E2, (v) Vit C, (vi) Vit C + E2, (vii) ANF and (viii) ANF + E2. Rats were implanted subcutaneously with 3 mg E2 pellets. E2 pellets were prepared in 17 mg cholesterol as a binder as described previously (1,43). Control, Vit C, BHA and ANF groups received 17 mg cholesterol pellet only. Vitamin C (1%) was administered in drinking water. BHA (0.7%) and ANF (0.2%) were fed to animals through phytoestrogen-free AIN76A diet (Dyets, Bethlehem, PA). Water was given ad libitum to all the animals. Each of the eight treatment groups were divided into four subgroups, containing at least 10 rats in each subgroup. Each subgroup underwent treatments as described above for 7, 15, 120 or 240 days, respectively. At the end of the experimental time period, animals were anesthetized using isoflurane and euthanized. Mammary (both tumor and normal), uterus, lung, liver, spleen, heart, brain, thymus and kidney tissues were removed and snap frozen in liquid nitrogen for future analyses. Frozen tissues were stored at −70°C. Portions of the excised tissues were stored in 10% buffered formalin for histopathological evaluations. Tumor incidence and the number of tumor nodules per rat were counted at the time of dissection. Animal protocols used in the current study were approved by the Institutional Animal Care and Use Committee.
Cell culture and knockdown studies
Non-tumorigenic breast epithelial cell line MCF-10A was purchased from American Type Culture Collection. Experiments were performed in passages two to six of cells sub-cultured from a frozen stock. MCF-10A cells were grown in Dulbecco's modified Eagle's medium/F12 (50:50) media (Mediatech, Herndon, VA). Twenty-four hours prior to treatment, cells were washed twice with phosphate-buffered saline (PBS) and then grown in phenol red-free Dulbecco's modified Eagle's medium/F12 (50:50) supplemented with 5% charcoal dextran stripped horse serum (Cocalico Biologicals, Reamstown, PA). Cells were treated with E2 (10 and 50 nM), Vit C (250 μM and 1 mM), BHA (250 μM), ANF (10 μM), dicumarol (100 μM), Vit C + E2, BHA + E2, ANF + E2 for up to 48 h. Treated cells were washed with PBS and used for DNA 8-hydroxydeoxyguanosine (8-OHdG) and western blot analyses according to established methods. Small interfering RNAs against NQO1 (Ambion, Austin, TX) and NRF2 (Santa Cruz Biotechnology, Santa Cruz, CA) were used to silence the respective genes in MCF-10A cells. Cells were transfected with 20 nmol/l of siNQO1 or siNRF2. Scrambled small interfering RNA (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a negative control. MCF-10A cells transfected with siNQO1 or siNRF2 were treated with 50 nM of E2 for 48 h and used for DNA 8-OHdG analysis.
Western blot analysis
Approximately 50 mg ACI rat mammary, mammary tumor, uterus, lung, liver, brain, heart, spleen, thymus and kidney tissues were homogenized in a tissue protein extraction buffer (T-PER, Thermo Scientific, IL) using a PRO 200 rotor stator homogenizer (PRO Scientific, CT). Homogenized samples were centrifuged at 10 000g and the clear supernatant was saved and used for western blot analyses. MCF-10A cell lysates were prepared in RIPA buffer with protease inhibitor cocktail (Sigma Chemicals, MO). The Pierce BCA Protein Assay kit was used to determine protein concentrations (Pierce, Rockford IL). Eighty microgram total protein from ACI rat tissues or 30 μg protein from MCF-10A cells was size fractionated on a 12% sodium dodecyl sulfate–polyacrylamide gel, and transferred onto a polyvinylidene difluoride membrane (Millipore Corp., Billerica, MA) under standard conditions (44,45). Polyvinylidene difluoride membranes were blocked in 5% dry non-fat milk/PBS/0.05% Tween-20 at room temperature for 2 h. Affinity purified goat polyclonal antibody against NQO1 or NRF2 (Santa Cruz Biotechnology, Santa Cruz, CA) was diluted 1:1500 in PBS/0.05% Tween-20 and used for immunodetection. Chemiluminescent detection was performed using the BM Chemiluminescence Detection kit (Roche, Indianapolis, IN) and Alpha Innotech FluorChem HD2 (Alpha Innotech, San Leandro, CA) gel documentation system. Membranes probed for NQO1 were reprobed with α-tubulin monoclonal rat antibody (Santa Cruz Biotechnology, Santa Cruz, CA) using the methods described above. Intensities of the bands were quantified and normalized using AlphaEase FC StandAlone software (version 6.0.0.14; Alpha Innotech).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed with MCF-10A cells using ChIP Assay Kit (USB Corporation, Cleveland, OH) as suggested by the manufacturer. In brief, MCF-10A (∼5 × 108) cells grown in 100 mm tissue culture dishes were treated with Vit C (1 mM) or BHA (250 μM) in the presence or absence of E2 (10 nM) for 2 h and cross-linked with 1% formaldehyde and then sonicated. Soluble chromatin was collected and incubated on a rotating platform with goat polyclonal antibody against NRF2 (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. The DNA was recovered and subjected to real-time polymerase chain reaction (PCR) analysis using primers flanking ARE of the human NQO1 gene promoter. The NQO1 ARE primers used for the end point real-time PCR amplification using SYBR green method (Qiagen, Valencia) were as follows: forward primer 5′-CAGTGGCATGCACCCAGGGAA-3′ and reverse primer 5'-GCATGCCCCTTTTAGCCTTGGCA-3′. Amplification of input chromatin before immunoprecipitation at a dilution of 1:50 was used as a positive control. ChIP without any antibody served as a negative control. The assays were carried out in three replicates. Agarose gel electrophoresis and Ct (cycle threshold) values for the amplified products for ChIP DNA and input DNA samples were used to represent the results.
NQO1 activity assay
NQO1 enzymatic activity was determined using a colorimetric assay with 2,6-dichlorophenolindophenol (DCPIP) as a substrate to oxidize NADPH to NADP+ (46). Cytosolic fractions were prepared from ACI rat mammary tumor and mammary tissues as described previously (47) and used for quantification of NQO1 activity. Briefly, the mammary tumor and mammary tissues were homogenized in four volumes of 1.14% KCl solution containing 10 mM ethylenediaminetetraacetic acid, pH 7.5. The cytosolic fraction was prepared from the homogenate by successive centrifugation at 10 000g for 20 min and then 100 000g for 1 h. Protein estimation was carried out using Pierce BCA Protein Assay kit (Pierce, Rockford, IL). Ten microgram cytosolic protein from mammary or mammary tumor tissue in 200 μmol/l NADPH and Tris–HCl buffer consisting of 25 mmol/l Tris–HCl, pH 7.4, 0.7 mg/ml BSA, were read at 600 nm immediately after adding 40 μmol/l DCPIP for five time points, spanning 5 min with 1 min intervals in a microtiter plate at 27°C. Duplicate reactions with the addition of 20 μmol/l dicumarol were run to determine the rate difference from uninhibited to dicumarol-inhibited protein samples.
8-OHdG estimation
8-OHdG, a ubiquitous marker of oxidative stress-mediated DNA damage was estimated in E2-treated ACI rat mammary, mammary tumors and MCF-10A cells in the presence or absence of Vit C, BHA or ANF. siNQO1- or siNRF2-transfected MCF-10A cells were also treated with E2 and used for isolation of DNA for 8-OHdG analysis. DNA was isolated from the mammary tumors and mammary tissues from all the treatment groups treated for 240 days. MCF-10A cells treated with E2 in the presence and absence of antioxidants (Vit C and BHA), ANF and dicumarol were also used for isolation of DNA for 8-OHdG estimation. DNA was isolated using DNeasy blood and tissue kit (Qiagen, Valencia, CA) according to the supplier’s protocols with some modifications. Diethylenetriamine pentaacetic acid (0.1 mM) and ascorbic acid (2 mM) were used throughout the DNA isolation process to avoid possible spurious DNA oxidation. DNase-free RNase (Sigma-Aldrich, St. Louis, MO) was used to degrade RNA as per supplier’s recommendations. The RNA-free DNA thus obtained was used to estimate 8-OHdG levels using Oxiselect oxidative DNA damage ELISA-kit (Cell Biolabs, San Diego, CA) according to supplier’s protocols with some modifications (48). Briefly, 20 μg DNA was digested with 10 U DNase 1 (Qiagen, Valencia, CA) at 37°C for 30 min in presence of 100 mM MgCl2 and 1 M Tris-HCl (pH 7.4). After digestion of DNA with DNase 1, pH of digested DNA was adjusted to 5.2 with 3 M sodium acetate (pH 5.2) and DNA reaction mixture was subjected to 1 μl of nuclease P1 (1 U/μl) digestion for 1.5 h at 37°C. After 1.5 h of incubation, 1 M Tris-HCl (pH 8.0) was used to bring the pH back to 7.4, followed by treatment with 1 μl of alkaline phosphatase (1 U/μl stock) for 1 h. The reaction mixture was then subjected to 1 μl of each phosphodiesterase I (0.01 U/μl) and phosphodiesterase II (0.01 U/μl) digestion for 30 min at 37°C. The reaction mixture was centrifuged for 5 min at 6000g and the supernatant was used for 8-OHdG ELISA assay.
Statistical analyses
Statistical analyses were performed by using Sigma Plot 11.0 (Systat Software, San Jose, CA) or IBM SPSS Statistics 19 software (IBM, Armonk, NY). One-way analysis of variance and least significant difference post-hoc analysis was used to calculate P values for comparisons of NQO1 protein expression and 8-OHdG levels among all groups of treated animals. The unpaired t-test analysis was used to calculate P values for comparisons of NQO1 enzymatic activity between treated animals and respective age-matched controls. One-way analysis of variance with least significant difference post-hoc analysis was also used to compare 8-OHdG levels in MCF-10A cells among different treatment and control groups. Fisher’s exact test was used to compare tumor incidence between two treatment groups. A P value <0.05 was considered significant.
Results
E2 treatment decreases NQO1 protein expression
NQO1 protein expression was quantified in E2-treated female ACI rat mammary tissue and mammary tumors by western blot analyses. NQO1 protein expression was significantly decreased in mammary tissue of rats treated with E2 for 240 days and in mammary tumors (Figure 1A). Treatment of MCF-10A cells in vitro with E2 (10 nM) for 24 h also significantly decreased NQO1 protein expression compared to vehicle-treated cells (Figure 1B). Our recently published studies indicate that the morphology of the rat mammary tissue changes following estrogen treatment for 7 days (4). Therefore, we quantified NQO1 protein expression in mammary tissue during earlier time points after 7, 15 and 120 days of E2 treatment. There was no change in NQO1 messenger RNA (mRNA) and protein expression during early time points of E2 exposure (7 and 15 days) but after 120 days of E2 treatment, NQO1 mRNA and protein levels were significantly increased compared to control mammary tissue and declined after 8 months (Figures 1A and 2A and mRNA data not shown). Parallel to NQO1 mRNA expression, NRF2 mRNA expression in mammary tissues also did not change up to 15 days of E2 treatment and significantly increased after 120 days and then significantly decreased both in the mammary tissues as well as in tumors after 240 days of E2 treatment (data not shown).
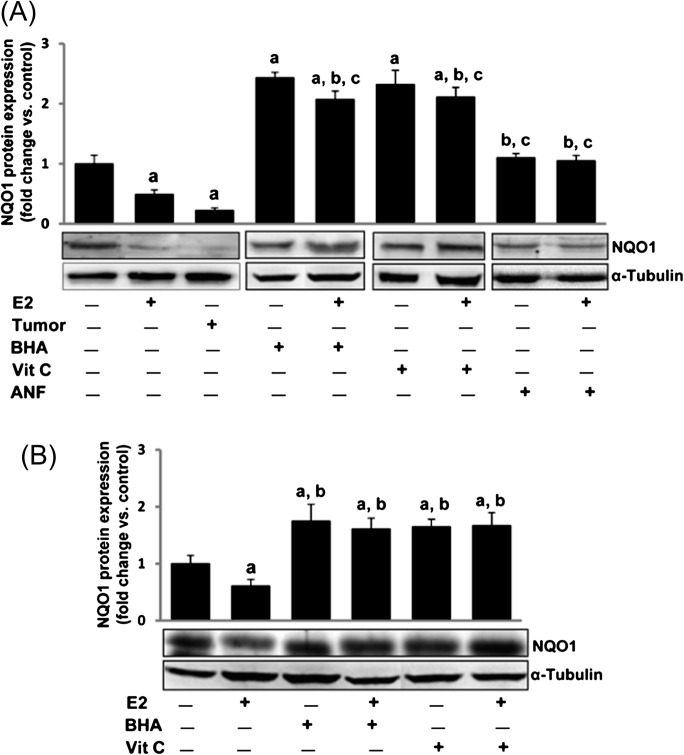
Fig. 1.
Antioxidants reverse E2-mediated decrease in NQO1 expression. Female ACI rats were treated with E2, BHA, BHA + E2, Vit C, Vit C + E2, ANF and ANF + E2 for 240 days as described in the Materials and methods section. At the end of the experiment, mammary tumors and mammary tissues were collected and used for western blot analysis for NQO1. Intensities of the bands were quantified and normalized to α-tubulin. Fold change compared to respective age-matched control mammary tissue was calculated from at least three different animals in each group. (A) NQO1 protein expression is significantly decreased in E2-treated mammary tumor and mammary tissues. Antioxidants BHA and Vit C increased NQO1 protein expression and its level remains unchanged after treatment with ANF compared to age-matched controls. The bar graph represents NQO1 protein fold change (mean ± SEM) in mammary tumors and mammary tissues from at least three different animals compared to age-matched control mammary tissues. (B) E2 (10 nM) treatment of MCF-10A cells for 24 h decreased NQO1 protein expression, whereas antioxidants BHA (250 μM) and Vit C (1 mM) significantly increased NQO1 protein levels. The bar graph represents NQO1 protein fold change (mean ± SEM) in MCF-10A from at least three different replicates compared to vehicle-treated MCF-10A. Significance was determined by analysis of variance and least significant difference post-hoc analysis. The symbols a, b and c indicate a P value <0.05 between specific treatment group and control, between specific treatment group and E2-treated mammary and between specific treatment group and mammary tumor, respectively.
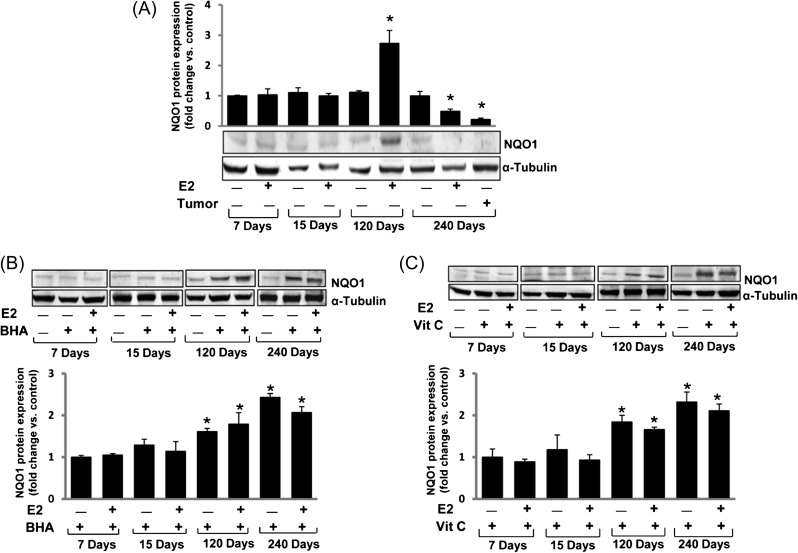
Fig. 2.
E2 inhibits, whereas BHA and Vit C increase NQO1 in time-dependent fashion. Female ACI rats were treated with E2, BHA, BHA + E2, Vit C and Vit C + E2 for 7 to 240 days as described in the Materials and methods section. At the end of the experiment, mammary tumor and mammary tissues were collected. Tissues were homogenized and approximately 80 μg protein was used for western blot analysis. Intensities of the bands were quantified and normalized to α-tubulin. (A) NQO1 protein expression in mammary tumors and E2-treated mammary tissues after 7, 15, 120 and 240 days of treatment. The bar graph represents NQO1 protein fold change (mean ± SEM) in mammary tumors and mammary tissues from at least three different animals compared to age-matched control mammary tissues. (B) NQO1 protein expression in BHA and BHA + E2-treated group mammary tissues after 7, 15, 120 and 240 days of treatment. The bar graph represents NQO1 protein fold change (mean ± SEM) in mammary tissues from at least three different animals compared to age-matched control mammary tissues. (C) NQO1 protein expression in Vit C and Vit C + E2-treated group mammary tissues after 7, 15, 120 and 240 days of treatment. The bar graph represents NQO1 protein fold change (mean ± SEM) in mammary tissues from at least three different animals compared to age-matched control mammary tissues. ‘*’ indicates P value <0.05 compared to respective control mammary tissue.
Antioxidants reverse E2-mediated decrease in NQO1 protein expression
We have recently demonstrated that treatment with Vit C or BHA or ANF significantly inhibit E2-induced breast carcinogenesis (5,6). Therefore, we were interested in finding out if these changes mirror changes in the protein expression of NQO1, a key enzyme responsible for converting E2-quinones to catechols. Western blot analyses demonstrated that treatment with antioxidants Vit C or BHA for 240 days inhibited E2-mediated decrease in NQO1 protein expression in ACI rat mammary tissue (Figure 1A). Time course studies suggest that the increase in NQO1 protein expression following BHA or Vit C treatment was apparent after 120 days of treatment (Figure 2B and C). The fold changes in NQO1 protein expression in mammary tissue of BHA, BHA + E2, Vit C and Vit C + E2 treatment groups were 1.61, 1.79, 1.84 and 1.66, respectively, after 120 days of treatment (Figure 2B and C) and 2.43, 2.07, 2.32 and 2.11, respectively, after 240 days of treatment compared to sham-operated age-matched controls (Figure 2B and C and Table I). ANF treatment did not induce NQO1 protein expression but it inhibited E2-mediated decrease in NQO1 protein expression (Figure 1A). BHA (250 μM) and Vit C (1 mM) treatment reversed E2-mediated decrease in NQO1 protein expression and significantly increased NQO1 protein expression in vitro in MCF-10A cells (Figure 1B). The fold changes in NQO1 protein expression in MCF-10A cells treated with E2, BHA, BHA + E2, Vit C and Vit C + E2 for 24 h were 0.61, 1.75, 1.61, 1.65 and 1.67, respectively, compared to vehicle-treated cells.
Table I.
NQO1 enzyme activity, protein expression, 8-OHdG levels and tumor incidence in female ACI rats after different treatments
| Treatment | NQO1 activity (nmol reduced DCPIP/min/μg protein) | NQO1 protein expression (fold change versus control) | 8-OHdG levels (fold change versus control) | Tumor incidence (%) |
| Control | 0.12 ± 0.01 | 1.00 | 1.00 | 0 |
| E2 | 0.08 ± 0.01* | 0.47* | 1.79* | 82 |
| Tumor | 0.06 ± 0.01* | 0.21* | 2.98* | –– |
| BHA | 0.26 ± 0.01* | 2.43* | 0.89 | 0 |
| BHA + E2 | 0.27 ± 0.02*,** | 2.07*,** | 1.23** | 24** |
| Vit C | 0.25 ± 0.02* | 2.32* | 0.95 | 0 |
| Vit C + E2 | 0.23 ± 0.01*,** | 2.11*,** | 1.02** | 29** |
NQO1 enzyme activity, NQO1 protein expression and DNA 8-OHdG levels were measured in mammary tumors and mammary tissues from Control, E2, BHA, BHA + E2, Vit C and Vit C + E2-treated rats after 240 days of treatment. Column one lists the different treatments each group of animals received. Column two shows the NQO1 activity as an average of values obtained for at least five different animals ± SEM. Columns three and four show fold change in NQO1 protein expression and DNA 8-OHdG levels, respectively, compared with age-matched control tissue. The percent tumor incidence in each treatment group after 240 days of treatment is listed in column five. ‘*’ indicates a P value <0.05 compared with respective control tissue and ‘**’ indicates P <0.05 compared with E2-treated group.
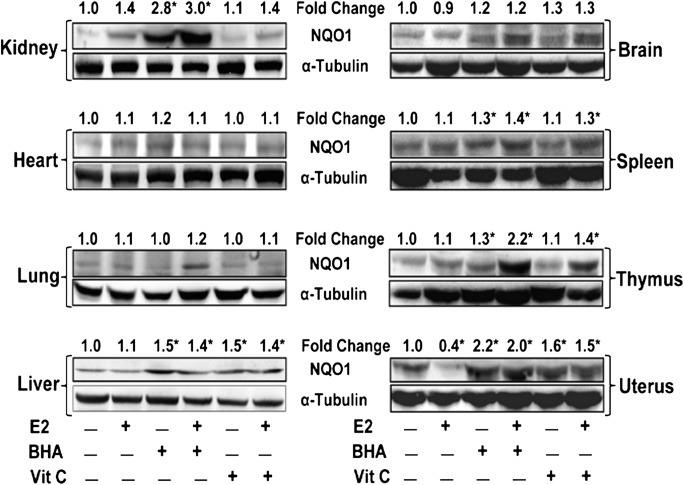
Antioxidant-mediated change in NQO1 protein expression is tissue specific
Antioxidants Vit C or BHA reversed E2-induced decrease in NQO1 protein expression in ACI rat mammary tissues after 240 days of treatment (Figure 1A). We also analyzed NQO1 protein expression in brain, heart, kidney, liver, lung, spleen, thymus and uterus of ACI rats treated with BHA or Vit C in the presence or absence of E2 for 240 days and compared with age-matched control tissues. NQO1 protein expression was detected in all organs analyzed with variable levels of expression (Figure 3). However, E2-mediated decrease in NQO1 protein was observed only in uterus, a known E2 responsive organ that behaved like mammary tissue as far as NQO1 protein expression is concerned. BHA treatment significantly increased NQO1 protein expression in kidney, liver, spleen, thymus and uterus, whereas Vit C increased NQO1 protein expression in liver and uterus only (Figure 3). While Vit C alone did not alter NQO1 protein levels in the spleen and thymus, co-treatment with E2 suggests an increase in the expression of NQO1 protein in these two organs (Figure 3).
Fig. 3.
Antioxidant-mediated change in NQO1 protein expression is tissue specific. Female ACI rats were treated with E2, BHA, BHA + E2, Vit C and Vit C + E2 for 240 days as described in the Materials and methods section. At the end of the experiment, kidney, heart, lung, liver, brain, spleen, thymus and uterus tissues were collected. Western blot analysis was carried out to investigate NQO1 protein expression in different tissues. NQO1 protein fold changes in respective organ from at least three different animals compared to respective control organ are represented at the top of each blot. ‘*’ indicates P value <0.05 compared to control mammary tissue.
Antioxidants increase NQO1 enzyme activity
NQO1 enzyme activity was evaluated in cytosolic fractions of the mammary tumors and mammary tissues of rats treated with E2, BHA, BHA + E2, Vit C and Vit C + E2 for 240 days and from age-matched controls. NQO1 activity was significantly decreased in mammary tumors and mammary tissue of rats treated with E2 compared to age-matched control mammary tissue (Table I, P < 0.05). However, significant increase in NQO1 enzyme activity was observed in mammary tissue of rats treated with BHA, BHA + E2, Vit C or Vit C + E2 for 240 days, compared to age-matched control mammary tissue (Table I, P < 0.05).
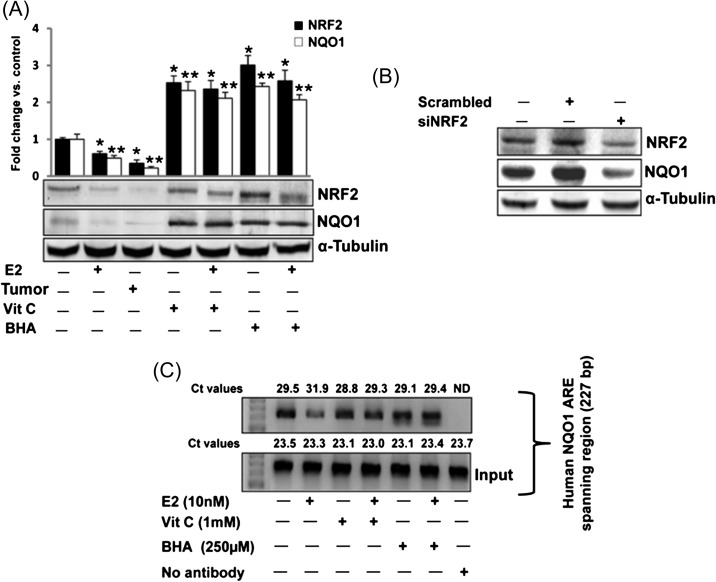
Antioxidants upregulate NQO1 via NRF2-mediated pathway
NRF2-mediated regulation of NQO1 has been suggested previously (24,27,30,31). We examined whether antioxidants BHA and Vit C upregulate NQO1 expression in rat mammary tissue in NRF2-dependent fashion. We quantified NRF2 protein expression by western blot analyses in E2-treated female ACI rat mammary tissue and mammary tumors as well as BHA and Vit C-treated mammary in the presence or absence of E2. NRF2 protein expression was significantly decreased in mammary tissue and mammary tumors of rats treated with E2 for 240 days (Figure 4A). While Vit C or BHA treatment significantly increased NRF2 protein expression (Figure 4A), a simultaneous decrease in NQO1 protein expression in E2-treated mammary tissue and mammary tumors and increase in NQO1 protein in Vit C- and BHA-treated mammary tissues suggested that NRF2-mediated regulation of NQO1 may be involved (Figure 4A). Decreased NQO1 protein expression after silencing NRF2 in MCF-10A cells was demonstrated (Figure 4B). To further confirm whether inhibition of NQO1 after E2 treatment and upregulation of NQO1 after antioxidant treatment is through differential binding of NRF2 to NQO1 promoter ARE, we carried out ChIP assay using MCF-10A cells. Following ChIP using anti-NRF2 antibody, DNA was recovered and subjected to real-time PCR analysis using PCR primers flanking the ARE of the human NQO1 gene promoter. Figure 4C shows that E2 inhibited the binding of NRF2 to the NQO1 gene promoter as shown by increase in Ct values, while antioxidants Vit C and BHA reversed E2-mediated inhibition by enhancing NRF2 binding to the NQO1 promoter relative to E2 alone as shown by decrease in Ct values compared to E2 treatment.
Fig. 4.
Antioxidants upregulate NQO1 via NRF2 -dependent pathway. (A) Female ACI rats were treated with E2, Vit C, Vit C + E2, BHA and BHA + E2 for 240 days as described in the Materials and methods section. At the end of the experiment, mammary tumor and mammary tissues were collected. Western blot analysis was carried out to investigate NRF2 and NQO1 protein expression in mammary tumor and mammary tissues. The bar graph represents NRF2 and NQO1 protein fold change (mean ± SEM) in mammary tumors and mammary tissues from at least three different animals compared to age-matched control mammary tissues. ‘*’ indicates P value <0.05 for NRF2 protein expression compared to respective control mammary. ‘**’ indicates a P value <0.05 for NQO1 protein expression compared to respective control mammary. (B) MCF-10A cells were transfected with 20 nmol/l of scrambled small interfering RNA, siNQO1 or siNRF2 for 48 h and western blot analysis was carried out using NRF2 antibody. Same membrane was reprobed with NQO1 and α-tubulin antibody. (C) MCF-10A cells were treated with Vit C (1 mM) or BHA (250 μM) in the presence or absence of E2 (10 nM) or vehicle for 2 h, fixed with formaldehyde, cross-linked and the chromatin sheared. The chromatin was immunoprecipitated with NRF2 antibody. NRF2 binding to NQO1 promoter was analyzed by real-time PCR with specific primers for the human NQO1 ARE region. The ARE region of the NQO1 promoter was also amplified from 3 μl of purified soluble chromatin before immunoprecipitation to show input DNA. Representative ChIP agarose gels and real-time PCR Ct values from three independent experiments are shown (ND, not detected).
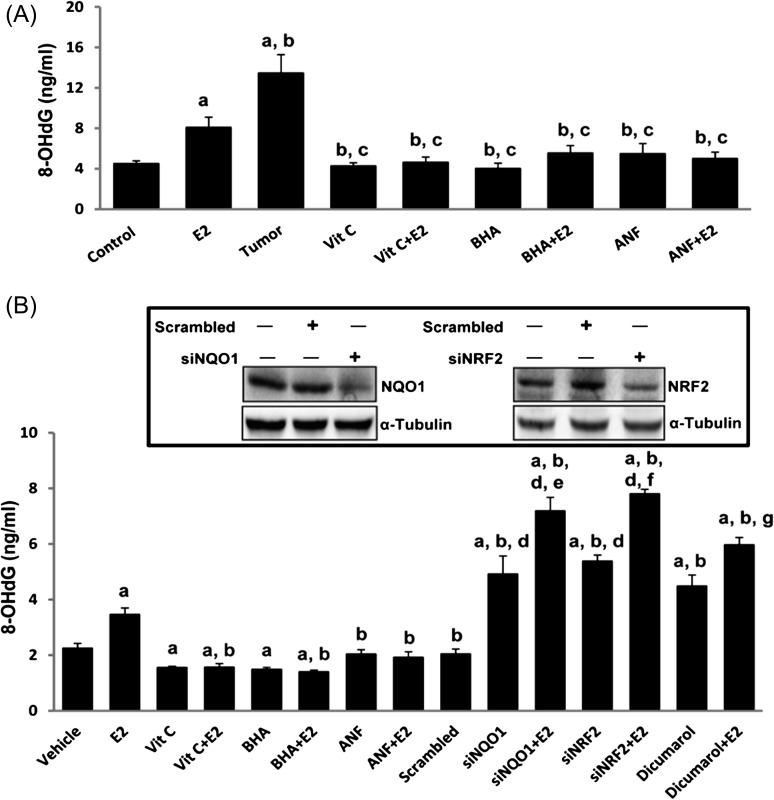
Antioxidants inhibit E2-mediated oxidative DNA damage
DNA 8-OHdG levels were quantified as a marker of oxidative DNA damage in mammary tissues as well as in human mammary epithelial cell line MCF-10A after treatment with antioxidants in the presence or absence of E2. 8-OHdG levels were also quantified in NQO1 knockdown MCF-10A cells as well as NRF2 knockdown MCF-10A cells in the presence or absence of E2. About 2- and 3-fold increases (P <0.05) in 8-OHdG levels were observed in E2-treated mammary tissue and mammary tumors, respectively, compared to control mammary tissue (Figure 5A and Table I). No significant differences in 8-OHdG levels were detected in mammary DNA samples from Vit C-, Vit C + E2-, BHA-, BHA + E2-, ANF- and ANF + E2-treated groups (Figure 5A). Treatment of MCF-10A cells with E2 for 48 h significantly increased 8-OHdG levels compared to vehicle-treated control (P < 0.05) but 8-OHdG levels were significantly decreased after treatment of MCF-10A cells with Vit C, Vit C + E2, BHA or BHA + E2 for 48 h relative to vehicle-treated MCF-10A cells (Figure 5B). No significant changes in 8-OHdG levels were detected in DNA samples from ANF or ANF + E2-treated MCF-10A cells compared to controls (Figure 5B). Dicumarol treatment of MCF-10A cells significantly increased 8-OHdG levels compared to levels in vehicle-treated MCF-10A cells (Figure 5B). Dicumarol is a known inhibitor of NQO1 and thus suggests the role of NQO1 in prevention of oxidative DNA damage. Furthermore, a significant increase in 8-OHdG levels in siNQO1 or siNRF2 -transfected MCF-10A cells compared to scrambled small interfering RNA transfected MCF-10A cells was detected (Figure 5B). Moreover, treatment with E2 further increased 8-OHdG levels in siNQO1, and siNRF2-trasfected MCF-10A cells (Figure 5B).
Fig. 5.
Antioxidants inhibit E2-mediated oxidative DNA damage. Female ACI rats were treated with E2, Vit C, Vit C + E2, BHA, BHA + E2, ANF and ANF + E2 for 240 days as described in the Materials and methods section. 8-OHdG levels were measured in mammary tumors and mammary tissues from animals in each of these groups as well as in MCF-10A cells treated with above chemicals and dicumarol. siNQO1- or siNRF2 -transfected MCF-10A cells were also treated with E2 (50 nM) for 48 h and 8-OHdG levels quantified. (A) Levels of 8-OHdG in mammary tumors and mammary tissues from different treatment groups. These data are reported as an average of values obtained for at least six different animals ± SEM. (B) Levels of 8-OHdG in MCF-10A cells treated with Vit C, BHA, ANF and dicumarol in presence or absence of E2 and in NQO1 and NRF2 knockdown MCF-10A cells. Inhibition of NQO1 and NRF2 protein expression in siNQO1 and siNRF2-transfected MCF-10A cells is shown in inset. Significance was determined by analysis of variance and least significant difference post-hoc analysis. The symbols a, b, c, d, e, f and g indicate a P value <0.05 between specific treatment group and control, between specific treatment group and E2 treatment, between specific treatment group and mammary tumor, between siNQO1 or siNRF2 and scrambled, between siNQO1 and siNQO1 + E2, between siNRF2 and siNRF2 + E2 and between dicumarol and dicumarol + E2, respectively.
Discussion
Long-term estrogen use has been associated with the development of breast cancer (4–6,49). The mechanisms of E2-induced breast carcinogenesis are, however, not clearly understood. In E2-induced breast carcinogenesis, oxidative stress produced by redox cycling between catechol estrogens and estrogen quinones is implicated to play an important role (19,20). Estrogen-quinones can also react with DNA to form depurinating adducts that are more likely to result in DNA mutations (9,19,20). Moreover, estrogen-DNA adducts are formed to a significantly higher extent in women at higher risk for breast cancer or in those diagnosed with breast cancer than in women at normal risk for breast cancer (7–9). NQO1 converts E2-quinones to E2-catechols, thus limiting the levels of E2-quinones capable of interacting with DNA and producing DNA damage (23). A role of NQO1 in chemical-induced carcinogenesis has been suggested (21,22). Although some studies suggest a role of NQO1 in cancer prevention, very little is known about the potential of NQO1 in the prevention of E2-induced breast cancer by antioxidants (21,22,24,26,50). We have recently reported for the first time that antioxidants Vit C or BHA and E2- metabolic inhibitor ANF can prevent E2-induced breast cancer in female ACI rats (5,6). In the present study, we investigated the role of above mentioned antioxidants in providing protection against oxidative DNA damage and its association with NQO1, a key enzyme responsible for converting quinones to catechols and thus capable of reducing oxidant damage and subsequent carcinogenic process. We also investigated changes in NQO1 protein expression during early phases of estrogen exposure and during pre-neoplastic and neoplastic phases of breast tumor development.
We have demonstrated that suppression of NQO1 protein expression following long-term E2 treatment can be reversed upon co-treatment with Vit C or BHA or ANF (Figure 1A). 17β-estradiol did not change NQO1 protein levels in the breast during 7–15 days of exposure (Figure 2A). However, after 4 months of E2 exposure, NQO1 protein levels increased in the breast, which then decreased after 8 months both in the breast tissue as well as in the tumor (Figures 1A and 2A). We have previously reported proliferative changes in the breast as early as 7 or 15 days of E2 exposure, a progression from normal mammary tissue to proliferative tissue such as atypical ductal hyperplasia, later progressing to tumor formation and malignancy (4). During this early neoplastic stage, most of the changes including genetic instability responsible for the initiation of carcinogenesis occur in the mammary tissue (51,52). The transient increase in NQO1 mRNA and protein expression after 120 days of estrogen treatment may be an adaptive response in an attempt to reduce E2-quinones (53,54). NRF2 mRNA expression was also unchanged up to 15 days of E2 treatment and significantly increased (∼2.8-fold) after 120 days which then significantly decreased after 240 days (8 months) both in the mammary tissues as well as in tumors (data not shown). Parallel to NQO1, increased mRNA expression of NRF2 only after 120 days of E2 treatment indicates NRF2-mediated upregulation of NQO1, suggests a possible adaptive response to reduce E2-quinones. Estrogen-mediated increase in NQO1 enzymatic activity after 6 and 12 weeks of treatment has been shown earlier in the same rat model (54). Furthermore, our time course study demonstrates that BHA or Vit C treatment increases NQO1 protein levels after 120 days of treatment and it remains high even after 240 days of co-treatment with E2 (Figure 2B and C). We also observed parallel increase in NQO1 enzymatic activity in mammary tissues following BHA or Vit C treatment for 240 days (Table I). Moreover, in E2-treated mammary tissue and in mammary tumors, both NQO1 protein expression and activity were significantly decreased compared to controls (Figure 1A and Table I). Differential expression of NQO1 protein in some organs in response to E2, BHA and Vit C indicates an organ-specific regulation of this enzyme that needs to be further investigated (Figure 3).
We did not observe either a decrease or an increase in NQO1 protein expression following treatment of rats with ANF. ANF is an E2 metabolic inhibitor that inhibits cytochrome P450-mediated conversion of E2 to 4-OHE2 (55). ANF reduces oxidative stress by minimizing the metabolism of E2 to catechols and inhibits estrogen-induced tumor development in vivo (5). No change in NQO1 protein expression in mammary tissue of ANF-treated rats is in keeping with our hypothesis that metabolic activation of E2 to catechol estrogens and subsequent redox cycling of quinone intermediates is essential for E2-induced breast carcinogenesis. ANF inhibits metabolic activation of E2 to catechol estrogens, therefore no E2-quinones will be produced. Thus, there may not be any need to increase NQO1 protein levels. We have shown that co-treatment of rats with ANF and E2 inhibits E2-induced oxidative stress and completely abrogates E2-induced breast cancer in female ACI rats (5).
NRF2-mediated regulation of NQO1 has been established previously (24,27,30,31) but we specifically examined in vivo whether the regulation of NQO1 in E2-induced breast cancer is mediated through transcription factor NRF2. Decreased protein expression of NRF2 and NQO1 in E2-treated mammary tumor and mammary tissues after 240 days of treatment and increased expression of these proteins after BHA or Vit C treatment provided a clue of NRF2-mediated regulation of NQO1 (Figure 4A). Decreased protein expression of NQO1 in NRF2 knockdown MCF-10A cells suggested NRF2-mediated regulation of NQO1 in mammary epithelial cell line MCF-10A (Figure 4B). Antioxidant resveratrol-mediated increased nuclear translocation of NRF2 and NQO1 has been shown in vitro (24). Results from ChIP assay with MCF-10A cells treated with BHA or Vit C alone or in combination of E2 further confirmed E2-mediated decreased and antioxidant-mediated increased binding of NRF2 to the ARE region of NQO1 promoter (Figure 4C). Collectively, these results confirm NRF2-mediated regulation of NQO1 in E2-induced breast carcinogenesis.
Estrogen treatment has previously been shown to induce oxidative stress and DNA damage in mammary tissue during tumorigenic process (4–6,49). To investigate the protective effects of BHA and Vit C against E2-mediated DNA damage, we examined 8-OHdG levels as a marker of oxidative DNA damage, after 240 days of E2, BHA or Vit C or ANF treatment of rats. We demonstrated significant increase in 8-OHdG levels in E2-treated mammary tissues and in mammary tumors and a pattern of decreased 8-OHdG levels after antioxidant treatments compared to age-matched control mammary tissue (Figure 5A and Table I). These studies suggest a protective role of antioxidants against oxidative DNA damage. The 8-OHdG levels in mammary tissues after 240 days of BHA or Vit C treatment were inversely correlated with expression of NQO1 protein and NQO1 activity in mammary tissues (Figures 1A, 5A and Table I). In vitro data with MCF-10A cells demonstrated significant decrease (P < 0.05) in 8-OHdG levels upon Vit C or BHA treatments compared to vehicle-treated controls (Figure 5B). In vivo, although a trend towards decrease in 8-OHdG levels was observed upon treatment with Vit C or BHA, this decrease was not significant (Figure 5A and Table I). Moreover, dicumarol treatment of MCF-10A cells resulted in further increase in 8-OHdG levels that supports the role of NQO1 in prevention of oxidative DNA damage (Figure 5B) as dicumarol is an NQO1 inhibitor (46). Significantly increased 8-OHdG levels (P < 0.05) in NQO1 knockdown MCF-10A cells compared to vehicle, scrambled or E2-treated parental MCF-10A cells further confirmed the role of NQO1 in prevention of estrogen-induced DNA damage (Figure 5B). Similarly, 8-OHdG levels were significantly increased in NRF2 knockdown MCF-10A cells compared to vehicle, scrambled or E2-treated parental MCF-10A cells. Furthermore, significant increased levels of 8-OHdG in siNQO1 or siNRF2-transfected cells after E2 treatment compared to siNQO1- or siNRF2-transfected cells confirmed that the increase in 8-OHdG levels was specific to E2-induced oxidative stress. Overall, these findings support the role of NQO1 in prevention of oxidative DNA damage during mammary carcinogenesis. NQO1 may thus have a potential in the development of therapeutic strategies for the prevention of estrogen-induced neoplasia. Our studies also indicate that NQO1 expression by antioxidants is mediated through NRF2.
Funding
National Institutes of Health Grant (CA 109551) and University of Missouri Research Board Grant (H.K.B.).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ARE
antioxidant response element
- ANF
α-naphthoflavone
- BHA
butylated hydroxyanisole
- ChIP
chromatin immunoprecipitation
- E2
estradiol
- mRNA
messenger RNA
- PCR
polymerase chain reaction
- NRF2
nuclear factor erythroid 2-related factor 2
- NQO
NAD(P)H-quinone oxidoreductase
- 8-OHdG
8-hydroxydeoxyguanosine
- OHE2
hydroxyestradiol
References
- 1.Bhat HK, et al. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:3913–3918. doi: 10.1073/pnas.0437929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavalieri EL, et al. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl. Acad. Sci. USA. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson BE, et al. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 4.Mense SM, et al. Estrogen-induced breast cancer: alterations in breast morphology and oxidative stress as a function of estrogen exposure. Toxicol. Appl. Pharmacol. 2008;232:78–85. doi: 10.1016/j.taap.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mense SM, et al. Vitamin C and alpha-naphthoflavone prevent estrogen-induced mammary tumors and decrease oxidative stress in female ACI rats. Carcinogenesis. 2009;30:1202–1208. doi: 10.1093/carcin/bgp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh B, et al. Antioxidant butylated hydroxyanisole inhibits estrogen-induced breast carcinogenesis in female ACI rats. J. Biochem. Mol. Toxicol. 2009;23:202–211. doi: 10.1002/jbt.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaikwad NW, et al. The molecular etiology of breast cancer: evidence from biomarkers of risk. Int. J. Cancer. 2008;122:1949–1957. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaikwad NW, et al. Urine biomarkers of risk in the molecular etiology of breast cancer. Breast Cancer (Auckl) 2009;3:1–8. doi: 10.4137/bcbcr.s2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalieri EL, et al. Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010;6:75–91. doi: 10.2217/fon.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemons M, et al. Estrogen and the risk of breast cancer. N. Engl. J. Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 11.Jefcoate CR, et al. Tissue-specific synthesis and oxidative metabolism of estrogens. J. Natl. Cancer Inst. Monogr. 2000;27:95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- 12.Liehr JG, et al. 32P-postlabelling in studies of hormonal carcinogenesis. IARC Sci. Publ. 1993;124:149–155. [PubMed] [Google Scholar]
- 13.Liehr JG, et al. Free radical generation by redox cycling of estrogens. Free Radic. Biol. Med. 1990;8:415–423. doi: 10.1016/0891-5849(90)90108-u. [DOI] [PubMed] [Google Scholar]
- 14.Yager JD, et al. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 15.Zahid M, et al. The greater reactivity of estradiol-3,4-quinone vs estradiol-2,3-quinone with DNA in the formation of depurinating adducts: implications for tumor-initiating activity. Chem. Res. Toxicol. 2006;19:164–172. doi: 10.1021/tx050229y. [DOI] [PubMed] [Google Scholar]
- 16.Li JJ, et al. Estrogen carcinogenesis in Syrian hamster tissues: role of metabolism. Fed. Proc. 1987;46:1858–1863. [PubMed] [Google Scholar]
- 17.Newbold RR, et al. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer Res. 2000;60:235–237. [PubMed] [Google Scholar]
- 18.Patel MM, et al. Differential oxidant potential of carcinogenic and weakly carcinogenic estrogens: involvement of metabolic activation and cytochrome P450. J. Biochem. Mol. Toxicol. 2004;18:37–42. doi: 10.1002/jbt.20005. [DOI] [PubMed] [Google Scholar]
- 19.Liehr JG, et al. Carcinogenicity of catechol estrogens in Syrian hamsters. J. Steroid Biochem. 1986;24:353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 20.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J. Natl Cancer Inst. Monogr. 2000;27:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 21.Kwak MK, et al. Chemoprevention by 1,2-dithiole-3-thiones through induction of NQO1 and other phase 2 enzymes. Methods Enzymol. 2004;382:414–423. doi: 10.1016/S0076-6879(04)82022-6. [DOI] [PubMed] [Google Scholar]
- 22.Shen J, et al. Inactivation of the quinone oxidoreductases NQO1 and NQO2 strongly elevates the incidence and multiplicity of chemically induced skin tumors. Cancer Res. 2010;70:1006–1014. doi: 10.1158/0008-5472.CAN-09-2938. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Gaikwad NW, et al. Evidence from ESI-MS for NQO1-catalyzed reduction of estrogen ortho-quinones. Free Radic. Biol. Med. 2007;43:1289–1298. doi: 10.1016/j.freeradbiomed.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu F, et al. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev. Res. (Phila. Pa) 2008;1:135–145. doi: 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross D, et al. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem. Biol. Interact. 2000;129:77–97. doi: 10.1016/s0009-2797(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 26.Zahid M, et al. Prevention of estrogen-DNA adduct formation in MCF-10F cells by resveratrol. Free Radic. Biol. Med. 2008;45:136–145. doi: 10.1016/j.freeradbiomed.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhakshinamoorthy S, et al. Small maf (MafG and MafK) proteins negatively regulate antioxidant response element-mediated expression and antioxidant induction of the NAD(P)H: quinone oxidoreductase1 gene. J. Biol. Chem. 2000;275:40134–40141. doi: 10.1074/jbc.M003531200. [DOI] [PubMed] [Google Scholar]
- 28.Kwak MK, et al. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J. Biol. Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 29.Reddy NM, et al. Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol. Genomics. 2007;32:74–81. doi: 10.1152/physiolgenomics.00126.2007. [DOI] [PubMed] [Google Scholar]
- 30.Kensler TW, et al. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kensler TW, et al. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 32.Kaspar JW, et al. Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J. Biol. Chem. 2010;285:153–162. doi: 10.1074/jbc.M109.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akindahunsi AA, et al. Antioxidant indices of some green leafy vegetables. Trop. Sci. 2005;45:33–35. [Google Scholar]
- 34.Duffy SJ, et al. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048–2049. doi: 10.1016/s0140-6736(99)04410-4. [DOI] [PubMed] [Google Scholar]
- 35.Engelhart MJ, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 36.Khaw KT, et al. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet. 2001;357:657–663. doi: 10.1016/s0140-6736(00)04128-3. [DOI] [PubMed] [Google Scholar]
- 37.Kurl S, et al. Plasma vitamin C modifies the association between hypertension and risk of stroke. Stroke. 2002;33:1568–1573. doi: 10.1161/01.str.0000017220.78722.d7. [DOI] [PubMed] [Google Scholar]
- 38.Lee SH, et al. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 39.Podmore ID, et al. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392:559. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- 40.McCormick DL, et al. Inhibition of 7,12-dimethylbenz(a)anthracene-induced rat mammary carcinogenesis by concomitant or postcarcinogen antioxidant exposure. Cancer Res. 1984;44:2858–2863. [PubMed] [Google Scholar]
- 41.Cha YN, et al. Comparative effects of dietary administration of 2(3)-tert-butyl-4-hydroxyanisole and 3,5-di-tert-butyl-4-hydroxytoluene on several hepatic enzyme activities in mice and rats. Cancer Res. 1982;42:2609–2615. [PubMed] [Google Scholar]
- 42.Iverson F. In vivo studies on butylated hydroxyanisole. Food Chem. Toxicol. 1999;37:993–997. doi: 10.1016/s0278-6915(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 43.Han X, et al. DNA single-strand breaks in kidneys of Syrian hamsters treated with steroidal estrogens: hormone-induced free radical damage preceding renal malignancy. Carcinogenesis. 1994;15:997–1000. doi: 10.1093/carcin/15.5.997. [DOI] [PubMed] [Google Scholar]
- 44.Bhat HK, et al. Suppression of calbindin D28K in estrogen-induced hamster renal tumors. J. Steroid Biochem. Mol. Biol. 2004;92:391–398. doi: 10.1016/j.jsbmb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Mense SM, et al. Preferential induction of cytochrome P450 1A1 over cytochrome P450 1B1 in human breast epithelial cells following exposure to quercetin. J. Steroid Biochem. Mol. Biol. 2008;110:157–162. doi: 10.1016/j.jsbmb.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benson AM, et al. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc. Natl Acad. Sci. USA. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan NM. Subcellular fractionation of animal tissues. Methods Mol. Biol. 1996;59:49–56. doi: 10.1385/0-89603-336-8:49. [DOI] [PubMed] [Google Scholar]
- 48.Huang X, et al. Importance of complete DNA digestion in minimizing variability of 8-oxo-dG analyses. Free Radic. Biol. Med. 2001;31:1341–1351. doi: 10.1016/s0891-5849(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 49.Montano MM, et al. Protective roles of quinone reductase and tamoxifen against estrogen-induced mammary tumorigenesis. Oncogene. 2007;26:3587–3590. doi: 10.1038/sj.onc.1210144. [DOI] [PubMed] [Google Scholar]
- 50.Patrick BA, et al. Disruption of NAD(P)H:quinone oxidoreductase 1 gene in mice leads to 20S proteasomal degradation of p63 resulting in thinning of epithelium and chemical-induced skin cancer. Oncogene. 2011;30:1098–1107. doi: 10.1038/onc.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Harvell DM, et al. Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Proc. Natl Acad. Sci. USA. 2000;97:2779–2784. doi: 10.1073/pnas.050569097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li JJ, et al. Ploidy differences between hormone- and chemical carcinogen-induced rat mammary neoplasms: comparison to invasive human ductal breast cancer. Mol. Carcinog. 2002;33:56–65. doi: 10.1002/mc.10022. [DOI] [PubMed] [Google Scholar]
- 53.Bianco NR, et al. Functional implications of antiestrogen induction of quinone reductase: inhibition of estrogen-induced deoxyribonucleic acid damage. Mol. Endocrinol. 2003;17:1344–1355. doi: 10.1210/me.2002-0382. [DOI] [PubMed] [Google Scholar]
- 54.Mesia-Vela S, et al. Dietary clofibrate inhibits induction of hepatic antioxidant enzymes by chronic estradiol in female ACI rats. Toxicology. 2004;200:103–111. doi: 10.1016/j.tox.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Peter Guengerich F, et al. Cytochrome P450 1B1: a target for inhibition in anticarcinogenesis strategies. Mutat. Res. 2003;523–524:173–182. doi: 10.1016/s0027-5107(02)00333-0. [DOI] [PubMed] [Google Scholar]