Abstract
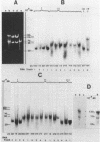
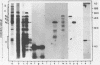
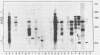
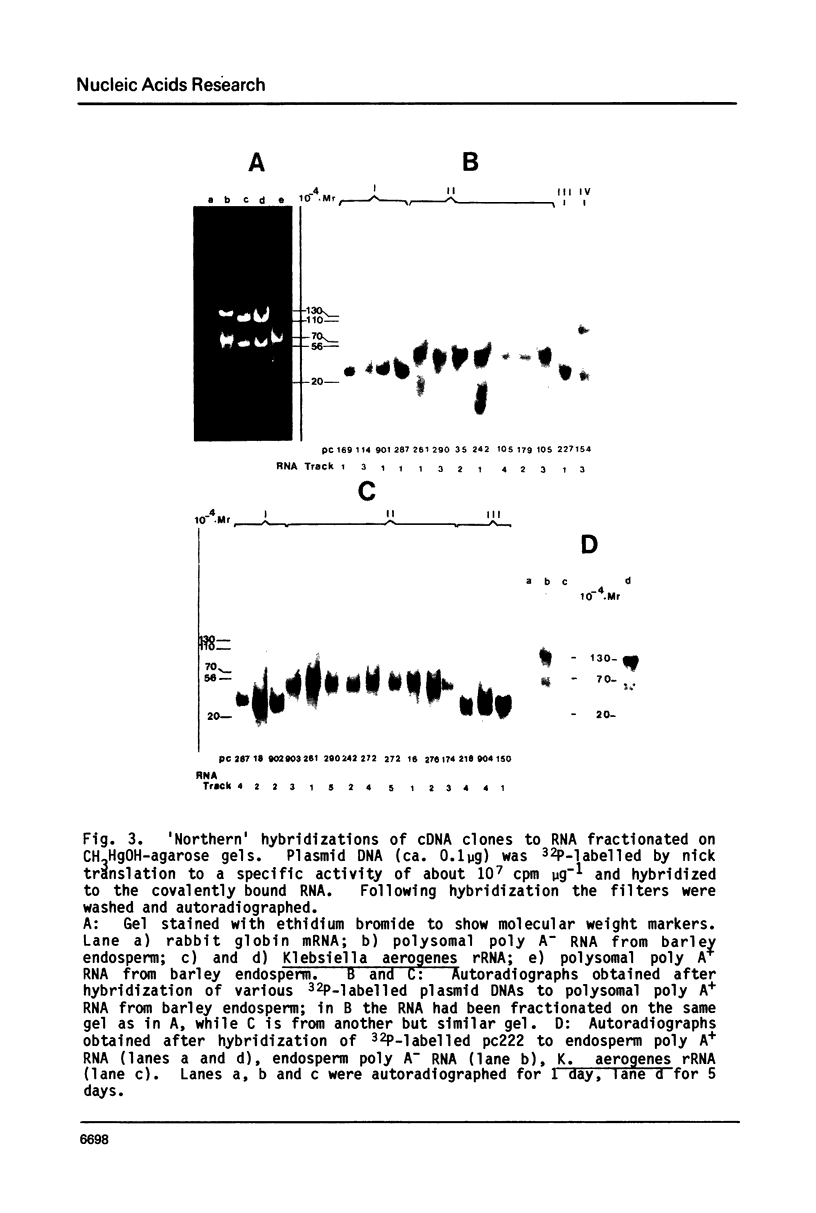
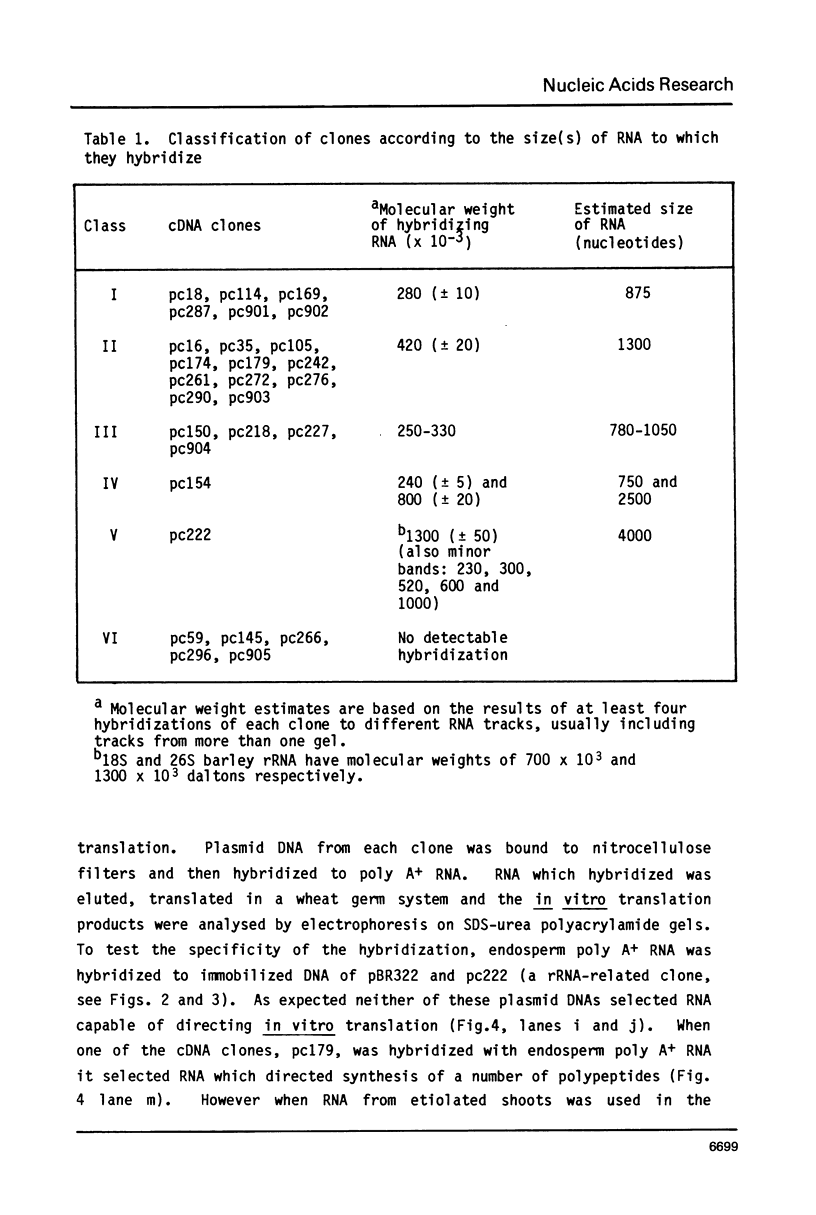
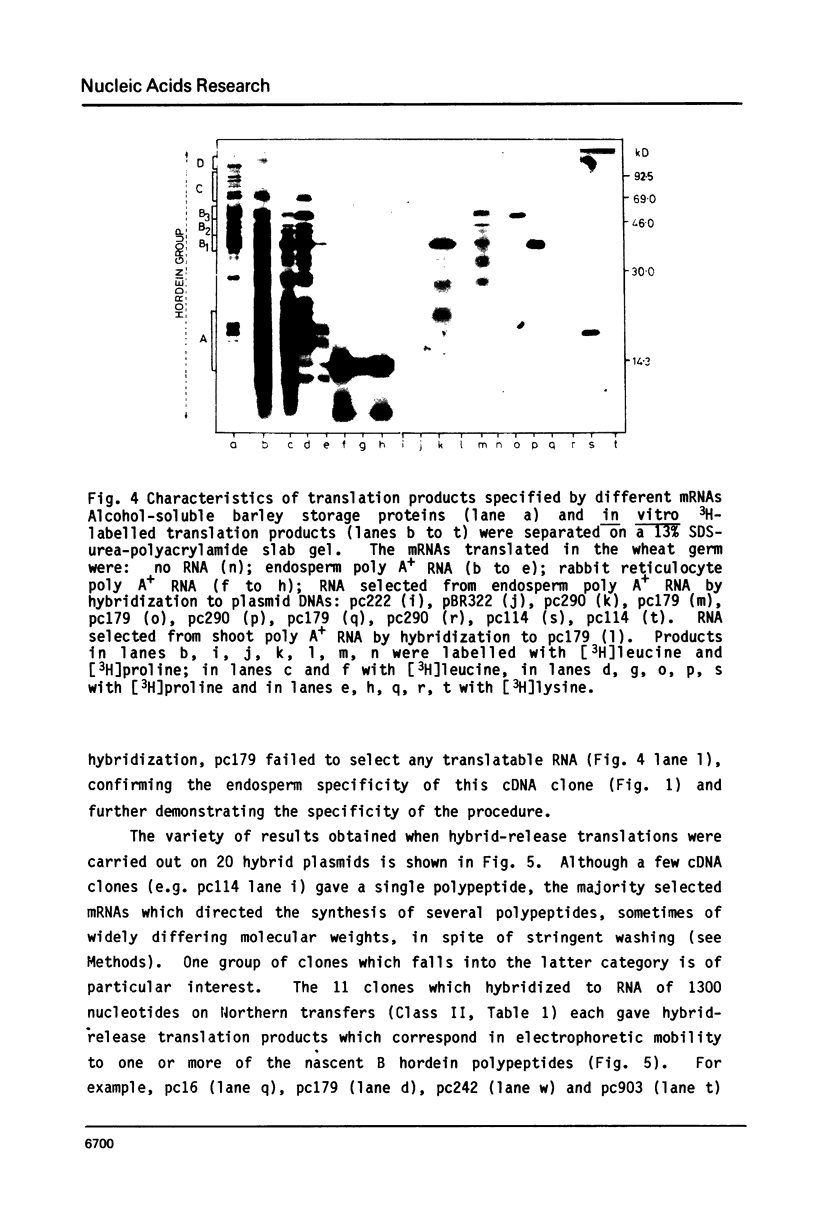
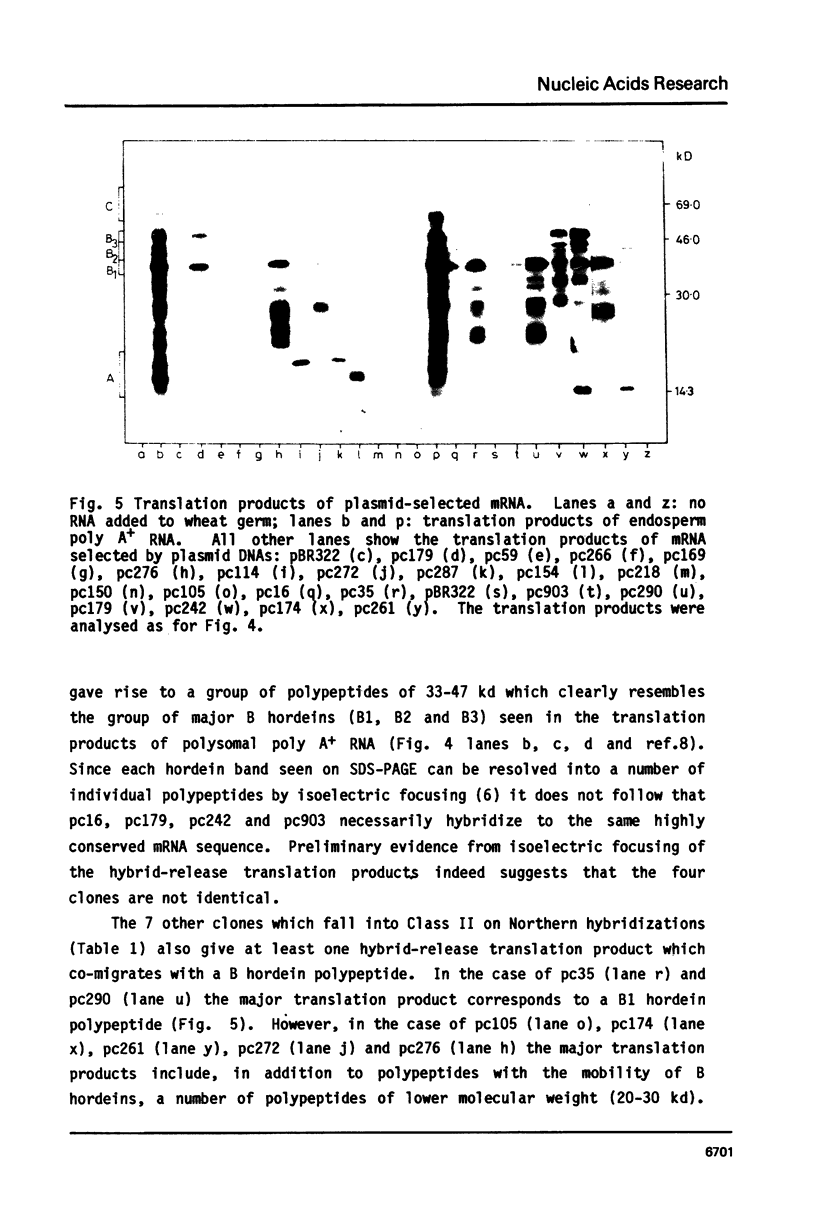
A collection of over 130 cDNA clones has been constructed in the bacterial plasmids pPH207 and pBR322 using as template the poly A+ RNA from membrane-bound polysomes of barley endosperm (cv. Sundance). Fifty four B hordein cDNA clones have been identified by cross-hybridization analysis and in vitro translation of plasmid-selected mRNAs. Hybridization of 11 of the B hordein cDNA clones to Northern blots of size-fractionated RNA indicated that the B hordein mRNA is ca. 1300 nucleotides long. One cDNA clone, pHvE-c16, has been partially sequenced and shown by comparison with C-terminal and other peptide sequences to be related to B1 hordein polypeptides. The results obtained from the analysis of the B hordein cDNA clones support the idea that the Hor 2 locus, which specifies the B hordeins, is complex and codes for a family of related mRNA species.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Davies J. A. Plasmid cloning vectors that can be nicked at a unique site. Mol Gen Genet. 1980;179(3):573–580. doi: 10.1007/BF00271747. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maxam A. M., Maniatis T. Enzymatic in vitro synthesis of globin genes. Cell. 1976 Feb;7(2):279–288. doi: 10.1016/0092-8674(76)90027-1. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Faulks A. J., Shewry P. R., Miflin B. J. The polymorphism and structural homology of storage polypeptides (hordein) coded by the Hor-2 locus in barley (Hordeum vulgare L). Biochem Genet. 1981 Oct;19(9-10):841–858. doi: 10.1007/BF00504250. [DOI] [PubMed] [Google Scholar]
- Geiser M., Döring H. P., Wöstemeyer J., Behrens U., Tillmann E., Starlinger P. A cDNA clone from Zea mays endosperm sucrose synthetase mRNA. Nucleic Acids Res. 1980 Dec 20;8(24):6175–6188. doi: 10.1093/nar/8.24.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach W. L., Bedbrook J. R. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979 Dec 11;7(7):1869–1885. doi: 10.1093/nar/7.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Cozens P. J., Cato A. C., Jost J. P. Recombinant plasmids containing avian vitellogenin structural gene sequences derived from complementary DNA. Biochim Biophys Acta. 1980;606(1):34–46. doi: 10.1016/0005-2787(80)90095-7. [DOI] [PubMed] [Google Scholar]
- Park W. D., Lewis E. D., Rubenstein I. Heterogeneity of zein mRNA and protein in maize. Plant Physiol. 1980 Jan;65(1):98–106. doi: 10.1104/pp.65.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienand U., Feix G. Electrophoretic fractionation and translation in vitro of poly(rA)-containing RNA from maize endosperm. Evidence of two mRNAs coding for zein protein. Eur J Biochem. 1978 Dec;92(2):605–611. doi: 10.1111/j.1432-1033.1978.tb12783.x. [DOI] [PubMed] [Google Scholar]