INTRODUCTION
Parathyroid carcinoma is a rare endocrine malignancy. It accounts for <1% of cases of sporadic primary hyperparathyroidism (PHPT) and is usually associated with more severe clinical manifestations than its much more common benign counterpart, parathyroid adenoma.(1–3) Its course is typically indolent but progressive. The diagnosis of malignancy is often made only when local recurrence or metastases occur, because the histology of parathyroid tumors can be equivocal or frankly misleading.(4) Most patients with recurrent disease ultimately succumb to the effects of hypercalcemia rather than to direct tumor invasion or distant metastases. A complete resection of all malignant tissue at the time of initial surgery allows for the greatest likelihood of a cure. Clinical clues to the possibility of a parathyroid cancer, therefore, should lead the surgeon to an aggressive initial operative approach. In the last decade, greater knowledge of the molecular pathogenesis of parathyroid carcinoma has led to the development of diagnostic markers that show promise, particularly when the histology is ambiguous.(2,3) Moreover, there is hope that greater understanding of the pathogenesis of parathyroid cancer will lead to the development of new therapeutic strategies for advanced, inoperable disease. This review focuses on the more recent advances in parathyroid carcinoma, particularly its molecular pathogenesis, diagnosis, and management.
EPIDEMIOLOGY
To date, >400 cases of parathyroid carcinoma have been reported.(5) It is usually a sporadic disease, but familial cases have been described. The largest series comes from the National Cancer database.(6) In most series of PHPT, parathyroid carcinoma accounts for <1% of all cases, but an incidence as high as 5% has been reported.(1,7,8) The use of varying criteria for its pathologic diagnosis is most likely the reason why later studies have shown higher incidence rates.
In contrast to benign parathyroid disease, where women predominate over men by a ratio of 3–4:1, parathyroid cancer occurs with equal frequency in both sexes. The age at diagnosis is 10 yr earlier than the typical age when the benign form of PHPT presents (mid-40s versus mid-50s).
ETIOLOGY
The etiology of parathyroid carcinoma is largely unknown. A potential role for prior neck irradiation is less clear than in the development of benign parathyroid disease.(9–13) Rarely, parathyroid carcinoma has been reported in patients with longstanding secondary hyperparathyroidism.(14,15) In the few such cases, it is unclear whether the currently accepted pathological criteria of parathyroid malignancy were met. Parathyroid carcinoma has also been reported in hereditary syndromes of hyperparathyroidism,(16–19) particularly in hyperparathyroidism-jaw tumor (HPT-JT) syndrome,(20) a rare autosomal disorder, in which as many as 15% of patients will have malignant parathyroid disease. Because cystic changes are common, this disorder has also been referred to as cystic parathyroid adenomatosis.(21) In HPT-JT, ossifying fibromas of the maxilla and mandible are seen in 30% of cases. Less commonly, kidney lesions, including cysts, polycystic disease, hamartomas, or Wilm's tumors, can be present.(22) Parathyroid carcinoma has also been reported in familial isolated hyperparathyroidism.(17,23) Recently, parathyroid carcinoma, as defined pathologically, has been reported in multiple endocrine neoplasia type 1 (MEN1) syndrome and with somatic MEN1 mutations.(24–26) However, recurrent parathyroid disease in MEN1 may mimic, but not actually be caused by, malignancy. Only one case of parathyroid carcinoma has been reported in the MEN2A syndrome.(27)
PATHOGENESIS
Oncogenes and tumor suppressor genes have been linked to parathyroid carcinomas, especially those involved in the control of the cell cycle. Examples include retinoblastoma (Rb), p53, breast carcinoma susceptibility (BRCA2), and cyclin Dl/parathyroid adenomatosis gene 1 (PRAD1) genes.(28,29) To date there is no definitive evidence for a primary role of these genes in parathyroid carcinoma, although altered expression of these gene products may participate in the process of malignant transformation.
After the report of Cryns et al.,(30) showing loss of Rb protein expression in parathyroid carcinoma, the absence of this protein in parathyroid tumors was proposed as a tool for the diagnosis of parathyroid malignancy. Since then, however, conflicting results have been reported by other investigators in terms of whether Rb gene alterations are specific for parathyroid cancer.(31–33) Moreover, Shattuck et al.(34) failed to detect microdeletions, insertions, or point mutations in the coding and promoter regions of the Rb gene in parathyroid carcinomas.
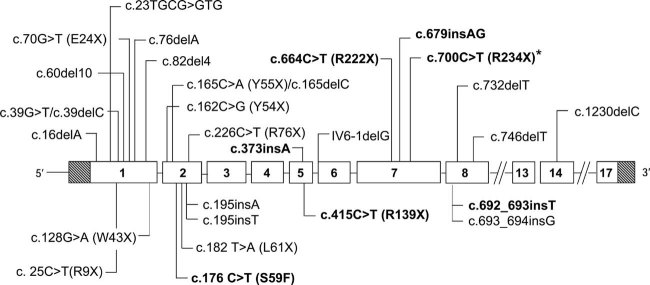
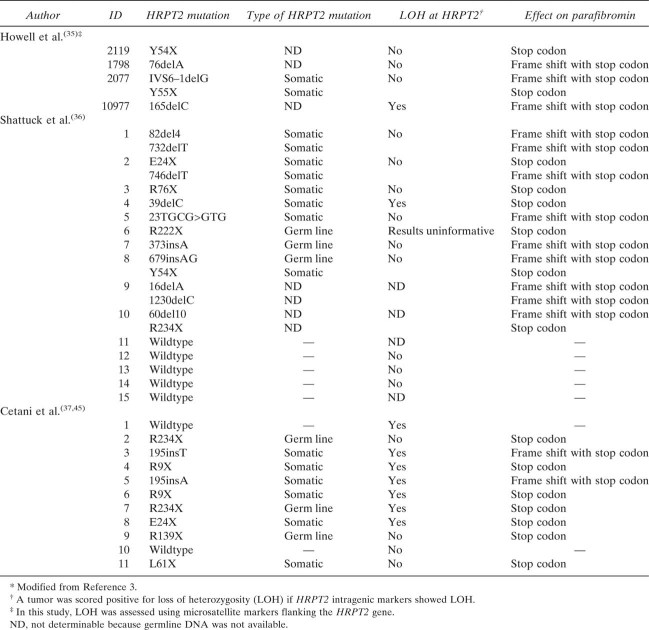
The HPT-JT syndrome has provided the best evidence for a defined gene in parathyroid cancer. The gene is known now as HRPT2 but other terms have been used, such as CDC73 and Clorf 28.(22) Evidence points to a strong association between HRPT2 mutations, the gene responsible for HPT-JT, and parathyroid carcinoma.(35–37) Parathyroid carcinoma occurs with higher frequency in HPT-JT than in sporadic PHPT (15% versus <1%). Similar germline mutations occur in a subset of kindreds with familial isolated hyperparathyroidism.(22,35,37–44) The role of the HRPT2 gene in the pathogenesis of sporadic parathyroid carcinoma was shown by Howell et al.(35) and Shattuck et al.(36) in 2003. In the former study,(35) HRPT2 mutations were detected in 4 of 4 sporadic parathyroid carcinomas and in 0 of 25 sporadic parathyroid adenomas. In the later study,(36) HRPT2 mutations were found in 10 of 15 patients with apparently sporadic parathyroid carcinoma. Cetani et al.(37,45) identified HRPT2 mutations in 9 of 11 parathyroid carcinomas but in 0 of 4 sporadic atypical adenomas. Most of the mutations are of the nonsense form and are predicted to result in lack of or reduced protein expression of the encoded parafibromin protein (see below) (Fig. 1). The prevalence of HRPT2 mutations in sporadic parathyroid carcinomas may be as high as 76.6% (Table 1). Thus, the strong association between HRPT2 mutation and parathyroid malignancy suggests that this molecular event is involved in the pathogenesis of most sporadic parathyroid carcinomas. Of particular interest is the demonstration that germline mutations were identified in about one third of subjects.(36,37,45) This finding suggests that a subset of patients with apparently sporadic parathyroid carcinomas may have the HPT-JT syndrome or a variant. Inactivating mutations of noncoding or regulatory regions could also be implicated in the pathogenesis of sporadic parathyroid carcinoma and might be present in those without alterations in the coding regions of the gene. A recent study by Haven et al.(46) found HRPT2 inactivating mutations in only 4 of 28 (15%) cases of parathyroid carcinomas. Two mutations were germinal. These tumors were classified as malignant on the basis of pathological criteria alone without the requirement for malignant behavior. In this study, the exons that harbor 85% of all known mutations (1, 2, and 7) were completely screened. The low mutation frequency could be explained in part by the fact that not all exons could be completely evaluated because of the nature of the formalin-fixed–embedded tissue. Another mechanism of HRPT2 gene inactivation, methylation of the promoter, has been reported in 2 of 11 parathyroid carcinomas.(47)
FIG. 1.
HRPT2 mutations in sporadic parathyroid carcinomas. Mutations are designed according to the format generally used in the HRPT2 literature with single-letter amino acid codes. Mutations in bold are germinal.(36,37,45,46) *This mutation has been found in three unrelated kindreds.(36,37)
Table 1.
HRPT2 Gene Analyses in Parathyroid Carcinomas*
 |
HRPT2 mutations are found, but only rarely, in sporadic benign parathyroid adenomas.(22,35,37,48) The overall prevalence among the 167 cases of benign disease in which an HRPT2 mutation has been sought is only 1.8%, or even lower 1/120 (0.8%) if the cases from Carpten et al.,(22) which were selected for cystic features, are excluded. These observations indicate that HRPT2 mutations have a very limited, if any, role in the pathogenesis of typical sporadic adenomas.
HRPT2 encodes a protein of 531 amino acids called parafibromin (parathyroid disease and fibro-osseous lesions) that is evolutionarily conserved(22) and similar in homology (54%) to a protein of Saccharomyces cerevisiae known as Cdc73. Cdc73 is a component of yeast RNA polymerase II/Paf1 complex, which is involved in the transcription elongation and RNA processing pathways. A human counterpart to the yeast Paf1 complex has been identified.(49–51) Parafibromin is mostly a nuclear protein with a functional bipartite nuclear localization signal (NLS) at residues 125–139 (nucleotides 373–417), which is evolutionary conserved and critical for its nuclear localization.(52–54) Specific HRPT2 mutations, identified in HPT-JT or sporadic parathyroid carcinoma and predicted to truncate parafibromin upstream of or within the NLS, disrupt nuclear localization.(53) Parafibromin is also localized to the nucleolus. Three nucleolar localization signals at residues 76–92, 192–194, and 393–409 have been identified.(55)
The role of parafibromin as a tumor suppressor protein comes from the observation that parathyroid tumors carrying HRPT2 mutations are frequently associated with loss of parafibromin expression or function. Transient overexpression of wildtype parafibromin in HEK-293 and NIH3T3 cells, but not its L64P mutant, which is implicated in parathyroid cancer, inhibited cell proliferation by blocking cyclin D1 expression.(56) Thus, it is conceivable that, after biallelic HRPT2 inactivation, the inhibitory effect of parafibromin on cyclin D1 activity is lost, leading to neoplastic transformation in susceptible tissue such as parathyroid glands.(56) Direct evidence of the anti-proliferative effect of parafibromin was first reported by Zhang et al.(57) in several cell lines by showing that some disease-associated HRPT2 mutant constructs abolished the ability of wildtype parafibromin to suppress colony formation. Iwata et al.(58) have recently confirmed that the transient overexpression of parafibromin inhibited the growth of HEK293 or NIH3T3 cells. Conversely, in cell lines expressing the large T (LT) antigen, such as 292T and COS-7 cells, transient overexpression of parafibromin increased cell proliferation. Thus, in LT-expressing cells, parafibromin could favor tumorigenesis.
The existence of a connection between parafibromin function and components of the transcription machinery is further supported by the fact that the Drosophila ortholog of human parafibromin, hyrax, binds to β-catenin/armadillo and is required for the nuclear transduction of the Wnt/Wingless pathway.(59) Finally, Lin et al.(53) showed that wildtype, but not NLS-mutant, parafibromin promotes apoptosis in transfected cells. Inhibition of endogenous parafibromin expression by RNA interference decreases the basal and cytotoxic-induced apoptosis.
These observations establish the central role of parafibromin in the control of the cell cycle and subsequently in determining cell fate and promoting tumorigenesis. The relationship of parafibromin with the complex network of nuclear components merits further study.
PATHOLOGY
Parathyroid carcinomas are typically large (>3 cm), irregular, grayish-white, hard tumors often adherent tenaciously to adjacent tissues.(2,4,60) The finding of gross infiltration of contiguous structures strongly suggest the diagnosis of carcinoma. The histological criteria of parathyroid carcinoma are difficult to define and identify. Schantz and Castleman(61) in 1973 established a set of criteria, including thick fibrous bands, mitotic activity, and vascular and capsular invasion. Generally, neoplastic cells (usually chief cells) are arranged in a lobular pattern and separated by dense trabeculae, with mitotic figures. Invasion of the capsule is rather common and, less frequently (10–15%), vascular invasion also occurs.(60) Capsular invasion is characterized by a “tongue-like” protrusion through the collagenous fibers and should be distinguished from pseudoinvasion, because of “trapping” of tumor cells within the capsule, which can be found in adenoma.(2,62) The criteria of vascular invasion have been differently defined according to whether capsular vessels or vessels in the surrounding tissues are involved.(2,62,63) Partial attachment of tumor cells to the wall of the vascular channel or thrombosis should also be present.(2,63)
Because metastatic behavior is rare at presentation,(64) the diagnosis of parathyroid cancer on the basis of the above morphologic criteria may be difficult at the time of the initial operation. Many of the features described above, such as adherence to surrounding tissues, fibrous bands, trabecular growth, and mitosis, are not pathognomonic of malignancy because they can also be found in parathyroid adenomas. The diagnostic value of capsular and vascular invasion is still debated. Some authors regard vascular invasion as virtually diagnostic of malignancy.(2,64) Thus, controversy and diagnostic uncertainties still exist.(2,62,63) The distinction between benign and malignant parathyroid tumors is very hard and rarely made at initial histology. Indeed, in a large series of patients with metastatic parathyroid cancers, as many as 50% were initially classified as benign tumors.(65) A full discussion of the pathology of parathyroid cancer is beyond the scope of this review.
In an attempt to improve diagnostic accuracy, other histological approaches have been studied, but none has yet proven clear diagnostic value.(33,66–72) However, the high rate of HRPT2 abnormalities in parathyroid carcinomas has paved the way for the development of new diagnostic tools (HRPT2 mutational status and/or parafibromin immunostaining) of potential utility, particularly in cases with equivocal initial histology (see below). Investigation of patients who have clinically and biochemically severe, but pathologically benign, parathyroid disease, and those with malignant pathology despite mild clinical features will help to elucidate further the utility of these diagnostic tools as markers for parathyroid carcinoma.
CLINICAL PRESENTATION
The clinical manifestations of parathyroid carcinoma are primarily caused by the effects of markedly elevated serum PTH levels and hypercalcemia rather than by the local infiltration or distant metastases.(1,2)
The typical clinical picture is characterized by signs and symptoms of severe hypercalcemia, with renal involvement (nephrocalcinosis, nephrolithiasis, impaired renal function) in up to 80% of patients, and bone involvement (osteitis fibrosa cystica, subperiosteal resorption, “salt and pepper” skull, diffuse osteopenia) in up to 90%.(1) On physical examination, up to 76% of patients with parathyroid carcinoma have a palpable neck mass.(1) Renal colic is a frequent presenting complaint. Other symptoms include muscle weakness, fatigue, depression, nausea, polydipsia and polyuria, bone pain, and fractures. Recurrent severe pancreatitis, peptic ulcer disease, and anemia can also occur. None of these features is pathognomonic of malignancy. In the majority of cases, the diagnosis of parathyroid carcinoma is made only in retrospect when hypercalcemia recurs because of local spread of tumor or distant metastases. In some patients with parathyroid cancer, a PTH moiety, different from intact PTH(1-84), is produced.(73) The clinical implications of this finding in parathyroid carcinoma await additional studies.
Rarely, parathyroid carcinomas are nonfunctional.(43,74) They can be misdiagnosed as thyroid or thymic carcinoma because of locally advanced disease (palpable neck mass, dysphagia, hoarseness caused by laryngeal nerve palsy). Immunohistochemistry for PTH, thyroglobulin, thyroid transcription factor 1, and calcitonin may help ascertain the correct diagnosis.
DIFFERENTIAL DIAGNOSIS
Clinical features
The occurrence of metastases is the only unequivocal criterion of malignancy that is generally accepted, but they usually occur late in the course of the disease.
At initial presentation, despite clinical features suggesting malignancy, it can be a challenge to differentiate between hyperparathyroidism caused by parathyroid carcinoma and that caused by its much more common benign counterpart. Because better outcomes are associated with complete resection of the tumor at the time of initial surgery, it is important to establish the correct diagnosis at the time the patient presents.
Features that might lead to suspect a parathyroid cancer in a patients with PHPT are listed here and in Table 2.
Table 2.
Clinical Features Useful in the Differential Diagnosis Between Benign and Malignant Primary Hyperparathyroidism*
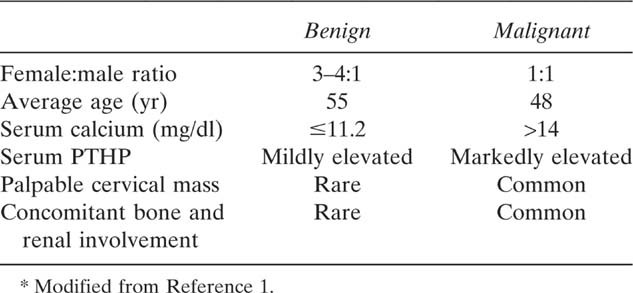 |
Male sex: there is no sex preference, whereas the female:male ratio in PHPT favors women by a ratio of 3–4:1
Relatively young age: the average age of a patient with parathyroid cancer is 50 yr, about 10 yr younger than the usual patient with benign PHPT.
Markedly elevated serum calcium and PTH: Serum calcium levels are within 1 mg/dl above the upper normal limit in most patients with parathyroid adenomas and >14–15 mg/dl in most patients with parathyroid carcinoma. PTH levels are markedly elevated in patients with parathyroid carcinoma and only slightly elevated in those with adenomas.
Bone and renal involvement: the combination of both renal and bone manifestations at the time of presentation suggests the possibility of parathyroid carcinoma. In benign PHPT, overt bone disease is unusual, and concomitant skeletal and renal involvement is uncommon.
Size and appearance of the parathyroid lesion: parathyroid carcinomas are usually >3 cm and may be palpable. The tissue is hard and gray-white and adherent to adjacent structures. Parathyroid adenomas are smaller, dark brown, and firm but not hard.
Alkaline phosphatase activity is also higher in patients with parathyroid carcinoma than in those with adenoma in whom serum levels are generally close to the upper limit of the normal range. α- and β-subunits of human chorionic gonadotropin (hCG) may be elevated in patients with parathyroid cancer but not in those with benign tumors.(75) Urinary hCG levels were found to be elevated in a small group of subjects with parathyroid carcinomas, in contrast to a control group of patients with benign PHPT.(76) In particular, the elevated hCG isotype was the hyperglycosolated form of hCG that is specifically associated with malignancy in trophoblastic and nontrophoblastic diseases. Moreover, elevations of hCG might be predictive of complications such as hip fracture and death.
When benign PHPT presents with markedly elevated serum calcium concentrations and overt target organ involvement, a clinical phenotype that was historically common but is now infrequently seen in most countries, the clinical distinction between benign and malignant disease may be difficult. It is preferable to have a high index of suspicion particularly when concomitant kidney and bone disease are present than to miss the opportunity for surgical cure by failing to consider cancer in the differential diagnosis.
Acute PHPT, sometimes called “parathyroid crisis,” shares many clinical features with parathyroid carcinoma. In view of the marked elevations of serum calcium and PTH that are common in parathyroid crisis, parathyroid cancer should be considered in any differential diagnosis of this condition. Parathyroid cancer should also be considered in any hypercalcemic patient without a history of prior neck surgery who presents with recurrent laryngeal nerve palsy.
Aids to diagnosis by pathological examination of tissue
Immunohistochemistry is used to improve the accuracy of the diagnosis of parathyroid carcinoma. One approach has involved the use of proliferation markers. Increased labeling of cell cycle–associated antigens (Ki-67, cyclin D1) has been shown in parathyroid carcinoma compared with adenoma,(66,68) but overlap among these tumor types has limited the utility of this approach. Decreased expression of p27, an inhibitor of cyclin-dependent kinase, and abnormal galectin-3 expression have been shown in carcinomas. The association between these abnormalities and high Ki-67 labeling has been suggested to increase the likelihood of malignancy.(70,72)
Evaluation of HRPT2 gene abnormalities seems to be a more promising diagnostic tool.(77) Loss of heterozygosity (LOH) or mutation at the HRPT2 gene and loss (total or focal) of parafibromin staining have been reported in the large majority of parathyroid carcinomas but very rarely in adenomas(45,78–81) (Fig. 2). To date, limited data are available in equivocal cases, where this technique would have the greatest diagnostic utility (Table 3). It is important to emphasize that the diagnostic potential of these tests hinges on their common presence in parathyroid cancer and their rarity in benign disease. Because benign parathyroid disease is so much more common than parathyroid cancer, a test that has a detectable detection rate in benign parathyroid disease (even if low) would have limited clinical utility. The positive predictive value of the test may be increased, therefore, if the HRPT2 gene/parafibromin analysis is restricted to cases that are equivocal. The combined findings of negative parafibromin staining and HRPT2 gene abnormalities increase the likelihood of a malignancy.(45,82) Based on this reasoning, it seems appropriate to evaluate all parathyroid tumors in which the diagnosis is uncertain for abnormalities of both the HRPT2 gene and its product, parafibromin.
FIG. 2.
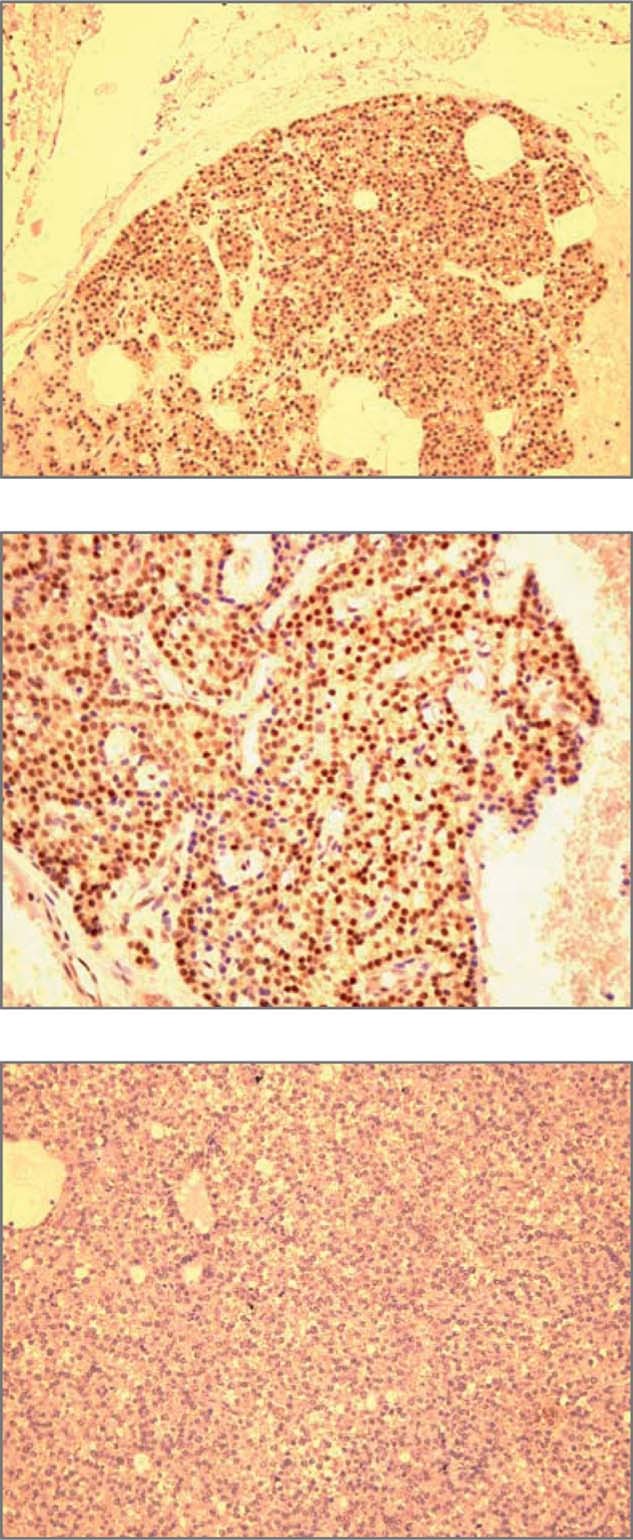
Immunohistochemical analysis of parafibromin expression. (Top) Normal parathyroid. A diffuse nuclear staining is present in most parathyroid cells (×200). (Middle) Parathyroid adenoma. The majority of cells show a positive nuclear staining (×400). (Bottom) Parathyroid carcinoma. Tumor cells show no nuclear staining (×200). (Reproduced from Eur J Endocrinol 156:547–554 with permission from the European Society of Endocrinology.)
Table 3.
Summary of Parafibromin Immunohistochemistry in Parathyroid Tumors
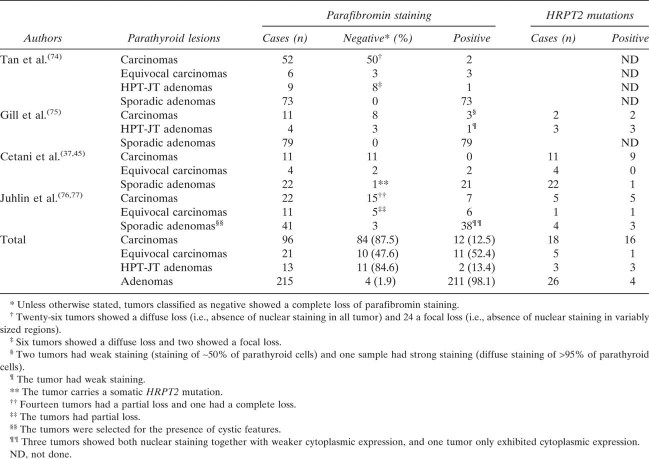 |
NATURAL HISTORY AND SURVEILLANCE
Parathyroid carcinoma typically runs an indolent, albeit progressive, course because the tumor has a rather low malignant potential. At initial presentation, very few patients show involvement of regional lymph nodes (<5%) or distant sites (<2%).(1,60) Parathyroid carcinoma recurs locally and spreads to contiguous structures in the neck. Metastases occur late in the course of the disease with spread to cervical nodes (30%) and lung (40%), followed in frequency by liver metastases (10%). Rarely, distant metastases occur in bone, pleura, pericardium, and pancreas.
The identification of HRPT2 mutations in eight patients with apparently sporadic parathyroid cancers as germline events(36,37,45,46) suggests that a subset of these patients might have HPT-JT syndrome or variant thereof. This observation has implications for the management of recurrent disease in parathyroid cancer. When a patient develops a recurrence of parathyroid cancer, in addition to the likelihood that the original carcinoma has progressed, a new tumor should be carefully sought, because additional, discrete parathyroid tumors can develop in patients with HPT-JT syndrome. Surveillance for renal and jaw lesions is also indicated. Moreover, the relatives of a patient with seemingly sporadic parathyroid carcinoma carrying a germline HRPT2 mutation are susceptible to the development of parathyroid cancer or other manifestations of HPT-JT syndrome.(43,44) In one such patient, a parathyroid cancer was imaged early by neck ultrasonography in an individual who had not yet become hypercalcemic.(43) Therefore, monitoring of family members with serum calcium determinations and neck ultrasonography is warranted. As suggested by Kelly et al.,(44) surgery should be aimed at identifying and examining all parathyroid glands and en bloc removal of any abnormal tissue. A metal-clip marking the glands left in situ may also be considered, anticipating the need for future surgery. Because of parathyroid cancer in this setting has incomplete penetrance (i.e., not all subjects harboring the HRPT2 gene abnormality will express the disease), prophylactic total parathyroidectomy is generally not recommended. In those who do undergo complete parathyroidectomy, autotransplantation of parathyroid tissue to the forearm is not recommended to avoid the introduction of potentially malignant tissue at an ectopic site.(44)
MANAGEMENT
Surgery
Surgery is the only curative treatment for parathyroid carcinoma and consists of complete resection of the primary lesion at the time of initial operation.(1,5) For this reason, both preoperative suspicion and intraoperative recognition are of great importance. Patients who present with features suggestive of parathyroid carcinoma warrant thorough exploration of all four parathyroid glands, because parathyroid carcinoma has been reported to coexist along with benign adenomas or hyperplasia.(14) The most effective surgical approach is en bloc resection.(83,84) Tracheoesophageal, paratracheal, and upper mediastinal lymph nodes should be excised, but an extensive lateral neck dissection is indicated only when there is spread to the anterior cervical nodes.
When the diagnosis of parathyroid cancer is made after parathyroid surgery on the basis of pathology, as often happens, the management plan becomes more complex. If the macroscopic characteristics of the tumor are typical of a parathyroid carcinoma, and the pathology shows extensive vascular or capsular invasion or if hypercalcemia persists, further exploration of the neck can be considered after appropriate localization studies (see below). The structures surrounding the tumor should be excised as described above. When telling histologic features are absent, the patient is normocalcemic and the diagnosis is only based on equivocal pathology, immediate reoperation is not indicated, because the simple complete resection of the tumor may turn out to be curative. However, such patients should be monitored closely with regular measurement of serum calcium and PTH levels.
Despite a potentially curative resection, parathyroid carcinoma has a recurrence rate of >50%. Most recurrences occur 2–3 yr after the initial operation, but this period is variable, and a prolonged disease-free interval of as long as 20 yr has been reported.(1,85) Imaging studies should be performed in all patients before reoperation. Fine-needle aspiration of a suspicious lesion with measurement of PTH in the eluate should be used with caution, if at all, to avoid seeding the needle track with deposits of malignant cells.(86,87) If noninvasive imaging approaches are negative, arteriography and selective venous sampling for PTH measurement may be useful. The management of recurrent or metastatic parathyroid carcinoma is primarily surgical.(1,5,83–85,88,89) Recurrences in the neck should be treated with wide resections, including the regional lymph nodes and other involved structures. Accessible distant metastases, particularly in the presence of localized metastatic disease, should also be excised, if possible.(1,90) Even a small tumor may produce a sufficient amount of PTH to cause hypercalcemia. Although resection of single metastasis or other foci of malignant tissue is rarely curative, their removal may result in periods of normocalcemia ranging from months to years.(5) Decreasing tumor mass may also render the patient's hypercalcemia more amenable to medical treatment.
Chemotherapy
Chemotherapy generally is disappointing. Several regimens have been attempted (nitrogen mustard, vincristine, cyclophosphamide, and actinomycin D, and adriamycin alone or in combination with cyclophosphamide and 5-fluorouracil), but none of them has proved to be effective.(91,92) At this time, chemotherapy has no role in the management of patients with parathyroid carcinoma.
Radiotherapy
With the exception of a single report(91) of an apparent cure (10 yr) in a patient with tumor invasion of trachea, radiation therapy has little, if any, effect in invasive parathyroid cancer.(85) Recent reports have suggested the use of irradiation as adjuvant therapy. The Mayo Clinic reported a disease-free survival at a median follow-up period of 60 mo in four patients who received postoperative adjuvant radiotherapy.(93) The MD Anderson Cancer Center experience also suggests a lower local recurrence rate if adjuvant radiation was given after surgery, independent of the type of operation and the disease stage.(94,95)
Management of hypercalcemia
When parathyroid carcinoma has became widely metastatic and surgical options are exhausted, clinical management becomes a matter of controlling the hypercalcemia.(96) Saline infusion and loop diuretics are often used, but in the majority of cases, drugs that inhibit bone resorption are needed. Potent intravenous bisphosphonates (pamidronate and zoledronate) may transiently control hypercalcemia, but patients frequently become refractory to them. Plicamicin is effective, but the response is transient, and repeated courses may be associated with toxicity. Octreotide, the long-acting somatostatin analog, has been reported to inhibit PTH secretion in two cases of metastatic parathyroid carcinoma.(97,98)
Anti-PTH immunotherapy showed promise in two recent case reports.(99–101) Dendritic cell immunotherapy may also be applicable to induce a T-cell immune response.(102)
Another approach is to target the parathyroid calcium-sensing receptor (CaSR). Calcimimetics, allosteric modulators of the CaSR, directly reduce parathyroid cell hormone secretion by binding to sites that increase the receptors' affinity for calcium.(103) Thus, sensitivity to extracellular calcium is enhanced. A first-generation calcimimetic, R-568, was used for 2 yr in a patient with metastatic parathyroid cancer with controlled hypercalcemia.(104) R-568 has been replaced by cinacalcet, a more potent second-generation agent with a longer half-life and more predictable hepatic metabolism. In benign PHPT, cinacalcet normalizes serum calcium and partially reduces PTH concentrations for up to 3 yr.(105)
Recently, the results of a multicenter study of cinacalcet in 29 patients with inoperable parathyroid carcinoma were published.(106) The primary endpoint of the study was the proportion of patients experiencing a ≥1-mg/dl reduction in serum calcium from baseline at the end of the titration phase. Secondary endpoints included changes from baseline in serum calcium, plasma PTH, bone turnover markers, and health-related quality of life variables. The dose of cinacalcet in this study was titrated from 30 mg twice daily (a dose that might be effective in benign PHPT) up to 90 mg four times daily as required to lower serum calcium levels. Duration of treatment ranged from 1 to 1051 days (mean, 328 ± 306 days). Cinacalcet effectively reduced hypercalcemia in about two thirds of patients with inoperable parathyroid carcinoma (Fig. 3). In the responders (18 of 29 patients), serum calcium levels declined from 15.0 ± 0.5 to 11.2 ± 0.3 mg/dl (p < 0.001), with the greatest responses seen in those patients with the highest levels of serum calcium at study entry. It was of interest that the marked reductions in serum calcium were not accompanied by a similar fall in circulating PTH. PTH levels reached a nadir 4 h after drug administration, but the decline was not pronounced, nor was it sustained. Although hypotheses abound, the reason for the discrepancy in calcium and PTH response to cinacalcet remains unclear at this time. Nausea and vomiting were the most common adverse events. Reported in more than one half of all patients receiving cinacalcet at these doses, these symptoms necessitated discontinuation of drug in some cases. Serious adverse events, including fracture and death, were not considered to be drug related. Instead, they were expected consequences of the patients' longstanding, often widely metastatic, underlying disease. There are no data suggesting that cinacalcet alters the course of the parathyroid cancer itself. Therefore, this agent should not be introduced to control hypercalcemia in a patient with a metastatic lesion that is accessible and amenable to surgical extirpation. However, the data do suggest that cinacalcet is useful in reducing calcium levels, and is tolerated in many patients at cumulative doses up to 360 mg/d. Furthermore, unlike other options for treatment of hypercalcemia, this agent can be used in patients with the renal impairment so common in patients with longstanding parathyroid cancer. Cinacalcet therefore represents an important new option for management of intractable hypercalcemia in patients with inoperable disease.
FIG. 3.
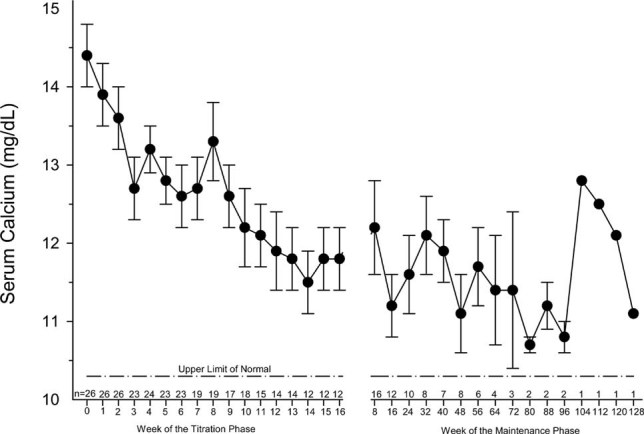
Reduction in serum calcium concentration in parathyroid cancer with cincalcet. Subjects were given cinacalcet in increasing doses, up to 90 mg four times daily, during the titration phase. The average serum calcium fell from 14.5 ± 0.4 to 12.4 ± 0.4 mg/dl (p = 0.001). (Adapted from Silverberg SJ, Rubin MR, Faiman C, Peacock M, Shoback DM, Smallridge RC, Schwanauer LE, Olson KA, Klassen P, Bilezikian JP 2007 Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab 92:3803–3808, Copyright 2007, The Endocrine Society.)
PROGNOSIS
The prognosis of parathyroid carcinoma is quite variable. No single characteristic correlates with outcome. The best prognosis depends on early recognition and complete excision of the tumor at initial surgery. The mean time to recurrence is usually 3 yr, although intervals of up to 20 yr have been reported.(1,65) Once the tumor recurs, complete cure is unlikely, although prolonged survival is still common with palliative surgery. Five-year survival rates vary from 40% to 86%. The National Cancer Database survey reported a 10-yr survival of ∼49%,(107) and the MD Andersen Cancer Center reported survival rates of 85% and 77% at 5 and 10 yr, respectively.(89) The National Surveillance, Epidemiology, and End Results database recently reported a 10-yr survival of 67.8%.(108)
SUMMARY
The best opportunity to cure parathyroid carcinoma is to diagnose it before or at the time of parathyroid surgery and for the tumor to be completely removed at the time of the initial operation. Because the diagnosis is often not clear at the time of presentation, recent attempts to distinguish between benign and malignant disease both by genetic and immunohistological analyses are promising. The disease is indolent but progressive. Attempts to remove local recurrences and distant metastases can provide short- and long-term control. Other therapeutic approaches with chemotherapy and radiotherapy are not helpful. Available medical therapy targets the consequence of the disease (hypercalcemia) rather than the disease itself.
REFERENCES
- 1.Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab. 2001;2:485–493. doi: 10.1210/jcem.86.2.7207. [DOI] [PubMed] [Google Scholar]
- 2.De Lellis RA. Parathyroid carcinoma. An overview. Adv Anat Pathol. 2005;12:53–61. doi: 10.1097/01.pap.0000151319.42376.d4. [DOI] [PubMed] [Google Scholar]
- 3.Cetani F, Pardi E, Banti C, Borsari S, Ambrogini E, Vignali E, Cianferotti L, Viccica G, Pinchera A, Marcocci C. HRPT2 gene analysis and diagnosis of parathyroid carcinoma. Expert Rev Endocrinol Metab. 2008;3:377–389. doi: 10.1586/17446651.3.3.377. [DOI] [PubMed] [Google Scholar]
- 4.Bondenson L, Grimelius L, DeLellis RA, Lloyd R, Akerstrom G, Larsson C, Arnold A, Eng C, Shane E, Bilezikian JP. Parathyroid carcinoma. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. Pathology and Genetics. Tumors of Endocrine Organs. WHO Classification of Tumours. Lyon, France: IARC Press; 2004. pp. 124–127. [Google Scholar]
- 5.Shane E. Parathyroid carcinoma. In: Bilezikian JP, Marcus R, Levine MA, editors. The Parathyroids. 2nd ed. Academic Press, San Diego, CA, USA: Basic and Clinical Concepts; 2001. pp. 515–525. [Google Scholar]
- 6.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985-1999: A National Cancer Database report. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1999;86:538–544. doi: 10.1002/(sici)1097-0142(19990801)86:3<538::aid-cncr25>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Favia G, Lumachi F, Polistina F, D'Amico DF. Parathyroid carcinoma: Sixteen new cases and suggestions for correct management. World J Surg. 1998;22:1225–1230. doi: 10.1007/s002689900549. [DOI] [PubMed] [Google Scholar]
- 8.Obara T, Fujimoto Y. Diagnosis and treatment of patients with parathyroid carcinoma: An update and review. World J Surg. 1991;15:738–744. doi: 10.1007/BF01665308. [DOI] [PubMed] [Google Scholar]
- 9.Ireland J, Fleming S, Levison D, Cattell W, Baker L. Parathyroid carcinoma associated with chronic renal failure and previous radiotherapy to the neck. J Clin Pathol. 1985;38:1114–1118. doi: 10.1136/jcp.38.10.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christmas TJ, Chapple CR, Noble JG, Milroy EJ, Cowie AG. Hyperparathyroidism after neck irradiation. Br J Surg. 1988;75:873–874. doi: 10.1002/bjs.1800750914. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J, Gierlowski TC, Schneider AB. A prospective study of hyperparathyroidism in individuals exposed to radiation in childhood. JAMA. 1990;264:581–584. [PubMed] [Google Scholar]
- 12.Schneider AB, Gierlowski TC, Shore-Freedman E, Stovall M, Ron E, Lubin J. Dose-response relationships for radiation-induced hyperparathyroidism. J Clin Endocrinol Metab. 1995;80:254–257. doi: 10.1210/jcem.80.1.7829622. [DOI] [PubMed] [Google Scholar]
- 13.Rasmuson T, Damber L, Johansson L, Johansson R, Larsson LG. Increased incidence of parathyroid adenomas following X-ray treatment of benign diseases in the cervical spine in adult patients. Clin Endocrinol (Oxf) 2002;57:731–734. doi: 10.1046/j.1365-2265.2002.01616.x. [DOI] [PubMed] [Google Scholar]
- 14.Berland Y, Olmer M, Lebreuil G, Grisoli J. Parathyroid carcinoma, adenoma and hyperplasia in a case of chronic renal insufficiency on dialysis. Clin Nephrol. 1982;18:154–158. [PubMed] [Google Scholar]
- 15.Boyle NH, Ogg CS, Hartley RB, Owen WJ. Parathyroid carcimoma secondary to prolonged hyperplasia in chronic renal failure and celiac disease. Eur J Surg Oncol. 1999;25:100–103. [PubMed] [Google Scholar]
- 16.Streeten EA, Weinstein LS, Norton JA, Mulvihill JJ, White BJ, Friedman E, Jaffe G, Brandi ML, Stewart K, Zimering MB, Spiegel AM, Aurbach GD, Marx SJ. Studies in a kindred with parathyroid carcinoma. J Clin Endocrinol Metab. 1992;75:362–366. doi: 10.1210/jcem.75.2.1639936. [DOI] [PubMed] [Google Scholar]
- 17.Wassif WS, Moniz CF, Friedman E, Wong S, Weber G, Nordenskjöld M, Peters TJ, Larsson C. Familial isolated hyperparathyroidism: A distinct genetic entity with an increased risk of parathyroid cancer. J Clin Endocrinol Metab. 1993;77:1485–1489. doi: 10.1210/jcem.77.6.7903311. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimoto K, Endo H, Tsuyuguchi M, Tanaka C, Kimura T, Iwahana H, Kato G, Sano T, Itakura M. Familial isolated primary hyperparathyroidism with parathyroid carcinomas: Clinical and molecular features. Clin Endocrinol (Oxf) 1998;48:67–72. doi: 10.1046/j.1365-2265.1998.00354.x. [DOI] [PubMed] [Google Scholar]
- 19.Marx SJ, Simonds WF, Agarwal SK, Burns AL, Weinstein LS, Cochran C, Skarulis MC, Spiegel AM, Libutti SK, Alexander HR, Jr, Chen CC, Chang R, Chandrasekharappa SC, Collins FS. Hyperparathyroidism in hereditary syndromes: Special expressions and special managements. J Bone Miner Res. 2002;17(S2):N37–N43. [PubMed] [Google Scholar]
- 20.Chen JD, Morrison C, Zhang C, Kahnoski K, Carpten JD, Teh BT. Hyperparathyroidism-jaw tumour syndrome. J Intern Med. 2003;253:634–642. doi: 10.1046/j.1365-2796.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- 21.Mallette LE, Malini S, Rappaport MP, Kirkland JL. Familial cystic parathyroid adenomatosis. Ann Intern Med. 1987;107:54–60. doi: 10.7326/0003-4819-107-1-54. [DOI] [PubMed] [Google Scholar]
- 22.Carpten JD, Robbins CM, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, Simonds WF, Gillanders EM, Kennedy AM, Chen JD, Agarwal SK, Sood R, Jones MP, Moses TY, Haven C, Petillo D, Leotlela PD, Harding B, Cameron D, Pannett AA, Hoog A, Heath H, III, James-Newton LA, Robinson B, Zarbo RJ, Cavaco BM, Wassif W, Perrier ND, Rosen IB, Kristoffersson U, Turnpenny PD, Farnebo LO, Besser GM, Jackson CE, Morreau H, Trent JM, Thakker RV, Marx SJ, The BT, Larsson C, Hobbs MR. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumors syndrome. Nat Genet. 2002;32:676–680. doi: 10.1038/ng1048. [DOI] [PubMed] [Google Scholar]
- 23.Simonds WF, James-Newton LA, Agarwal SK, Yang B, Skarulis MC, Hendy GN, Marx SJ. Familial isolated hyperparathyroidism: Clinical and genetic characteristics of 36 kindreds. Medicine (Baltimore) 2002;81:1–26. doi: 10.1097/00005792-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Dionisi S, Minisola S, Pepe J, De Geronimo S, Paglia F, Memeo L, Fitzpatrick LA. Concurrent parathyroid adenomas and carcinoma in the setting of multiple endocrine neoplasia type 1: Presentation as hypercalcemic crisis. Mayo Clin Proc. 2002;77:866–869. doi: 10.4065/77.8.866. [DOI] [PubMed] [Google Scholar]
- 25.Agha A, Carpenter R, Bhattacharya S, Edmonson SJ, Carlsen E, Monson JP. Parathyroid carcinoma in multiple endocrine neoplasia type 1 (MEN1) syndrome: Two case reports of an unrecognized entity. J Endocrinol Invest. 2007;30:145–149. doi: 10.1007/BF03347413. [DOI] [PubMed] [Google Scholar]
- 26.Haven CJ, van Puijenbroek M, Tan MH, Teh BT, Fleuren GJ, van Wezel T, Morreau H. Identification of MEN1 and HRPT2 somatic mutations in paraffin-embedded (sporadic) parathyroid carcinomas. Clin Endocrinol (Oxf) 2007;67:370–376. doi: 10.1111/j.1365-2265.2007.02894.x. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins PJ, Satta MA, Simmgen M, Drake WM, Williamson C, Lowe DG, Britton K, Chew SL, Thakker RV, Besser GM. Metastatic parathyroid carcinoma in the MEN2A syndrome. Clin Endocrinol (Oxf) 1997;47:747–751. doi: 10.1046/j.1365-2265.1997.3421147.x. [DOI] [PubMed] [Google Scholar]
- 28.Arnold A, Shattuck TM, Mallya SM, Krebs LJ, Costa J, Gallagher J, Wild Y, Saucier K. Molecular pathogenesis of primary hyperparathyroidism. J Bone Miner Res. 2002;17(S2):N30–N36. [PubMed] [Google Scholar]
- 29.Cetani F, Pardi E, Borsari S, Lemmi M, Ambrogini E, Vignali E, Cianferotti L, Pinchera M, Marcocci C. Parathyroid tumorigenesis. Clin Cases Miner Bone Metab. 2006;3:123–131. [Google Scholar]
- 30.Cryns VL, Thor A, Xu HJ, Hu SX, Wierman ME, Vickery AL, Jr, Benedict WF, Arnold A. Loss of the retinoblastoma tumor-suppressor gene in parathyroid carcinoma. N Engl J Med. 1994;330:757–761. doi: 10.1056/NEJM199403173301105. [DOI] [PubMed] [Google Scholar]
- 31.Dotzenrath C, The T, Farnebo F, Cupisti K, Svensson A, Toell A, Goretzki P, Larsson C. Allelic loss of the retinoblastoma tumor suppressor gene: A marker for aggressive parathyroid tumors? J Clin Endocrinol Metab. 1996;8:3194–3196. doi: 10.1210/jcem.81.9.8784068. [DOI] [PubMed] [Google Scholar]
- 32.Pearce SH, Trump D, Wooding W, Sheppard MN, Clayton RN, Thakker RV. Loss of heterozygosity study at the retinoblastoma and breast cancer susceptibility (BRCA2) loci in pituitary, parathyroid, pancreatic and carcinoid tumors. Clin Endocrinol (Oxf) 1996;45:195–200. doi: 10.1046/j.1365-2265.1996.d01-1561.x. [DOI] [PubMed] [Google Scholar]
- 33.Cetani F, Pardi E, Viacava P, Pollina GD, Fanelli G, Picone A, Borsari S, Gazzerro E, Miccoli P, Berti P, Pinchera A, Marcocci C. A reappraisal of the Rb1 gene abnormalities in the diagnosis of parathyroid cancer. Clin Endocrinol (Oxf) 2004;60:99–106. doi: 10.1111/j.1365-2265.2004.01954.x. [DOI] [PubMed] [Google Scholar]
- 34.Shattuck TM, Kim TS, Costa J, Yandell DW, Imanishi Y, Palanisamy N, Gaz RD, Shoback D, Clark OH, Monchik JM, Wierman ME, Hollenberg A, Tojo K, Chaganti RS, Arnold A. Mutational analyses of RB and BRCA2 as candidate tumour suppressor genes in parathyroid carcinoma. Clin Endocrinol (Oxf) 2003;59:180–189. doi: 10.1046/j.1365-2265.2003.01814.x. [DOI] [PubMed] [Google Scholar]
- 35.Howell VM, Haven CJ, Kahnoski K, Khoo SK, Petillo D, Chen J, Fleuren GJ, Robinson BG, Delbridge LW, Philips J, Nelson AE, Krause U, Hammje K, Dralle H, Hoang-Vu C, Gimm O, Marsh DJ, Morreau H, Teh BT. HRPT2 mutations are associated with malignancy in sporadic parathyroid tumours. J Med Genet. 2003;40:657–663. doi: 10.1136/jmg.40.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shattuck TM, Valimaki S, Obara T, Gaz RD, Clark OH, Shoback D, Wierman ME, Tojo K, Robbins CM, Carpten JD, Farnebo LO, Larsson C, Arnold A. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349:1722–1729. doi: 10.1056/NEJMoa031237. [DOI] [PubMed] [Google Scholar]
- 37.Cetani F, Pardi E, Borsari S, Viacava P, Dipollina G, Cianferotti L, Ambrogini E, Gazzerro E, Colussi G, Berti P, Miccoli P, Pinchera A, Marcocci C. Genetic analyses of the HRPT2 gene in primary hyperparathyroidism: Germline and somatic mutations in familial and sporadic parathyroid tumors. J Clin Endocrinol Metab. 2004;89:5583–5591. doi: 10.1210/jc.2004-0294. [DOI] [PubMed] [Google Scholar]
- 38.Simonds WF, Robbins CM, Agarwal SK, Hendy GN, Carpten JD, Marx SJ. Familial isolated hyperparathyroidism is rarely caused by germline mutation in HRPT2, the gene for the hyperparathyroidism-jaw tumor syndrome. J Clin Endocrinol Metab. 2004;89:96–102. doi: 10.1210/jc.2003-030675. [DOI] [PubMed] [Google Scholar]
- 39.Warner J, Epstein M, Sweet A, Singh D, Burgess J, Stranks S, Hill P, Perry-Keene D, Learoyd D, Robinson B, Birdsey P, Mackenzie E, Teh BT, Prins JB, Cardinal J. Genetic testing in familial isolated hyperparathyroidism: Unexpected results and their implications. J Med Genet. 2004;41:155–160. doi: 10.1136/jmg.2003.016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villablanca A, Calender A, Forsberg L, Höög A, Cheng JD, Petillo D, Bauters C, Kahnoski K, Ebeling T, Salmela P, Richardson AL, Delbridge L, Meyrier A, Proye C, Carpten JD, Teh BT, Robinson BG, Larsson C. Germline and de novo mutations in the HRPT2 tumour suppressor gene in familial isolated hyperparathyroidism (FIHP) J Med Genet. 2004;41:e32. doi: 10.1136/jmg.2003.012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradley KJ, Cavaco BM, Bowl MR, Harding B, Cranston T, Fratter C, Besser GM, Conceição Pereira M, Davie MW, Dudley N, Leite V, Sadler GP, Seller A, Thakker RV. Parafibromin mutations in hereditary hyperparathyroidism syndromes and parathyroid tumours. Clin Endocrinol (Oxf) 2006;64:299–306. doi: 10.1111/j.1365-2265.2006.02460.x. [DOI] [PubMed] [Google Scholar]
- 42.Mizusawa N, Uchino S, Iwata T, Tsuyuguchi M, Suzuki Y, Mizukoshi T, Yamashita Y, Sakurai A, Suzuki S, Beniko M, Tahara H, Fujisawa M, Kamata N, Fujisawa K, Yashiro T, Nagao D, Golam HM, Sano T, Noguchi S, Yoshimoto K. Genetic analyses in patients with familial isolated hyperparathyroidism and hyperparathyroidism-jaw tumour syndrome. Clin Endocrinol (Oxf) 2006;65:9–16. doi: 10.1111/j.1365-2265.2006.02534.x. [DOI] [PubMed] [Google Scholar]
- 43.Guarnieri V, Scillitani A, Muscarella LA, Battista C, Bonfitto N, Bisceglia M, Minisola S, Mascia ML, D'Agruma L, Cole DE. Diagnosis of parathyroid tumors in familial isolated hyperparathyroidism with HRPT2 mutation: Implications for cancer surveillance. J Clin Endocrinol Metab. 2006;91:2827–2832. doi: 10.1210/jc.2005-1239. [DOI] [PubMed] [Google Scholar]
- 44.Kelly TG, Shattuck TM, Reyes-Mugica M, Stewart AF, Simonds WF, Udelsman R, Arnold A, Carpenter TO. Surveillance for early detection of aggressive parathyroid disease: Carcinoma and atypical adenoma in familial isolated hyperparathyroidism associated with a germline HRPT2 mutation. J Bone Miner Res. 2006;21:1666–1671. doi: 10.1359/jbmr.060702. [DOI] [PubMed] [Google Scholar]
- 45.Cetani F, Ambrogini E, Viacava P, Pardi E, Fanelli G, Naccarato AG, Borsari S, Lemmi M, Berti P, Miccoli P, Pinchera A, Marcocci C. Should parafibromin staining replace HRPT2 gene analysis as an additional tool for histologic diagnosis of parathyroid carcinoma? Eur J Endocrinol. 2007;156:547–554. doi: 10.1530/EJE-06-0720. [DOI] [PubMed] [Google Scholar]
- 46.Haven CJ, van Puijenbroek M, Tan MH, Teh BT, Fleuren GJ, van Wezel T, Morreau H. Identification of MEN1 and HRPT2 somatic mutations in paraffin-embedded (sporadic) parathyroid carcinomas. Clin Endocrinol (Oxf) 2007;67:370–376. doi: 10.1111/j.1365-2265.2007.02894.x. [DOI] [PubMed] [Google Scholar]
- 47.Hewitt KM, Sharma PK, Samowitz W, Hobbs M. Aberrant methylation of the HRPT2 gene in parathyroid carcinoma. Ann Otol Rhinol Laryngol. 2007;116:928–933. doi: 10.1177/000348940711601210. [DOI] [PubMed] [Google Scholar]
- 48.Krebs LJ, Shattuck TM, Arnold A. HRPT2 mutational analysis of typical sporadic parathyroid adenomas. J Clin Endocrinol Metab. 2005;90:5015–5017. doi: 10.1210/jc.2005-0717. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhary K, Deb S, Moniaux N, Ponnusamy MP, Batra SK. Human RNA polymerase II-associated factor complex: Dysregulation in cancer. Oncogene. 2007;26:7499–7507. doi: 10.1038/sj.onc.1210582. [DOI] [PubMed] [Google Scholar]
- 50.Rozenblatt-Rosen O, Hughes CM, Nannepaga SJ, Shanmugam KS, Copeland TD, Guszczynski T, Resau JH, Meyerson M. The parafibromin tumor suppressor protein is part of a human Paf1 complex. Mol Cell Biol. 2005;25:612–620. doi: 10.1128/MCB.25.2.612-620.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yart A, Gstaiger M, Wirbelauer C, Pecnik M, Anastasiou D, Hess D, Krek W. The HRPT2 tumor suppressor gene product parafibromin associates with human PAF1 and RNA polymerase II. Mol Cell Biol. 2005;25:5052–5060. doi: 10.1128/MCB.25.12.5052-5060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hahn MA, Marsh DJ. Identification of a functional bipartite nuclear localization signal in the tumor suppressor parafibromin. Oncogene. 2005;24:6241–6248. doi: 10.1038/sj.onc.1208778. [DOI] [PubMed] [Google Scholar]
- 53.Lin L, Czapiga M, Nini L, Zhang JH, Simonds WF. Nuclear localization of the parafibromin tumor suppressor protein implicated in the hyperparathyroidism-jaw tumor syndrome enhances its proapoptotic function. Mol Cancer Res. 2007;5:183–193. doi: 10.1158/1541-7786.MCR-06-0129. [DOI] [PubMed] [Google Scholar]
- 54.Bradley KJ, Bowl MR, Williams SE, Ahmad BN, Partridge CJ, Patmanidi AL, Kennedy AM, Loh NY, Thakker RV. Parafibromin is a nuclear protein with a functional monopartite nuclear localization signal. Oncogene. 2007;26:1213–1221. doi: 10.1038/sj.onc.1209893. [DOI] [PubMed] [Google Scholar]
- 55.Hahn MA, Marsh DJ. Nucleolar localization of parafibromin is mediated by three nucleolar localization signals. FEBS Lett. 2007;581:5070–5074. doi: 10.1016/j.febslet.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 56.Woodard GE, Lin L, Zhang JH, Agarwal SK, Marx SJ, Simonds WF. Parafibromin, product of the hyperparathyroidism-jaw tumor syndrome gene HRPT2, regulates cyclin D1/PRAD1 expression. Oncogene. 2005;24:1272–1276. doi: 10.1038/sj.onc.1208274. [DOI] [PubMed] [Google Scholar]
- 57.Zhang C, Kong D, Tan MH, Pappas DL, Jr, Wang PF, Chen J, Farber L, Zhang N, Koo HM, Weinreich M, Williams BO, Teh BT. Parafibromin inhibits cancer cell growth and causes G1 phase arrest. Biochem Biophys Res Commun. 2006;350:17–24. doi: 10.1016/j.bbrc.2006.08.169. [DOI] [PubMed] [Google Scholar]
- 58.Iwata T, Mizusawa N, Taketani Y, Itakura M, Yoshimoto K. Parafibromin tumor suppressor enhances cell growth in the cells expressing SV40 large T antigen. Oncogene. 2007;26:6176–6183. doi: 10.1038/sj.onc.1210445. [DOI] [PubMed] [Google Scholar]
- 59.Mosimann C, Hausmann G, Basler K. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/Armadillo. Cell. 2006;125:327–341. doi: 10.1016/j.cell.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 60.Wang CA, Gaz RD. Natural history of parathyroid carcinoma. Diagnosis, treatment and results. Am J Surg. 1985;149:522–527. doi: 10.1016/s0002-9610(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 61.Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer. 1973;31:600–605. doi: 10.1002/1097-0142(197303)31:3<600::aid-cncr2820310316>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 62.LiVolsi VA. Pathology of the parathyroid gland. In: Barnes L, editor. Surgical Pathology of the Head and Neck. New York, NY, USA: Marcel Dekker; 1985. p. 1487. [Google Scholar]
- 63.Apel RL, Asa SL. The parathyroid glands. In: LiVolsi VA, Asa SL, editors. Endocrine Pathology. Philadelphia, PA, USA: Churchill Livingstone; 2002. pp. 103–147. [Google Scholar]
- 64.Smith JF, Coombs RRH. Histological diagnosis of carcinoma of the parathyroid gland. J Clin Pathol. 1984;37:1370–1378. doi: 10.1136/jcp.37.12.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sundelin K, Tullgren O, Farnebo LO. Clinical corse of metastatic parathyroid cancer. World J Surg. 1994;18:594–598. doi: 10.1007/BF00353773. [DOI] [PubMed] [Google Scholar]
- 66.Abbona GC, Papotti M, Gasparri G, Bussolati G. Proliferative activity in parathyroid tumors as detected by Ki-67 immunostaining. Hum Pathol. 1995;26:135–138. doi: 10.1016/0046-8177(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 67.Farnebo F, Auer G, Farnebo LO, Teh BT, Twigg S, Aspenblad U, Thompson NW, Grimelius L, Larsson C, Sandelin K. Evaluation of retinoblastoma and Ki-67 immunostaining as diagnostic markers of benign and malignant parathyroid disease. World J Surg. 1999;23:68–74. doi: 10.1007/s002689900567. [DOI] [PubMed] [Google Scholar]
- 68.Vargas MP, Vargas HI, Kleiner DE, Merino MJ. The role of prognostic markers (MiB-1, RB, and bcl-2) in the diagnosis of parathyroid tumors. Mod Pathol. 1997;10:12–17. [PubMed] [Google Scholar]
- 69.Naccarato AG, Marcocci C, Miccoli P, Bonadio AG, Cianferotti L, Vignali E, Cipollini G, Viacava P. Bcl-2, p53 and MIB-1 expression in normal and neoplastic parathyroid tissues. J Endocrinol Invest. 1998;21:136–141. doi: 10.1007/BF03347291. [DOI] [PubMed] [Google Scholar]
- 70.Erickson LA, Jin L, Wollan P, Thompson GB, van Heerden JA, Lloyd RV. Parathyroid hyperplasia, adenomas, and carcinomas: Differential expression of p27Kip1 protein. Am J Surg Pathol. 1999;23:288–295. doi: 10.1097/00000478-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 71.Haven CJ, van Puijenbroek M, Karperien M, Fleuren GJ, Morreau H. Differential expression of the calcium sensing receptor and combined loss of chromosomes 1q and 11q in parathyroid carcinoma. J Pathol. 2004;202:86–94. doi: 10.1002/path.1489. [DOI] [PubMed] [Google Scholar]
- 72.Bergero N, De Pompa R, Sacerdote C, Gasparri G, Volante M, Bussolati G, Papotti M. Galectin-3 expression in parathyroid carcinoma: Immunohistochemical study of 26 cases. Hum Pathol. 2005;36:908–914. doi: 10.1016/j.humpath.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 73.Rubin MR, Silverberg SJ, D'Amour P, Brossard JH, Rousseau L, Sliney J, Jr, Cantor T, Bilezikian JP. An N-terminal molecular form of parathyroid hormone (PTH) distinct from hPTH(1-84) is overproduced in parathyroid carcinoma. Clin Chem. 2007;53:1470–1476. doi: 10.1373/clinchem.2007.085506. [DOI] [PubMed] [Google Scholar]
- 74.Fernandez-Ranvier GG, Jensen K, Khanafshar E, Quivey JM, Glastonbury C, Kebebew E, Duh QY, Clark OH. Nonfunctioning parathyroid carcinoma: Case report and review of literature. Endocr Pract. 2007;13:750–757. doi: 10.4158/EP.13.7.750. [DOI] [PubMed] [Google Scholar]
- 75.Stock JL, Weintraub BD, Rosen SW, Aurbach GD, Spiegel AM, Marx SJ. Human chorionic gonadotropin subunit measurement in primary hyperparathyroidism. J Clin Endocrinol Metab. 1982;54:57–63. doi: 10.1210/jcem-54-1-57. [DOI] [PubMed] [Google Scholar]
- 76.Rubin MR, Bilezikian JP, Birken S, Silverberg SJ. Human chorionic gonadotropin measurements in parathyroid carcinoma. Eur J Endocrinol. 2008;159:469–474. doi: 10.1530/EJE-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rubin MR, Silverberg SJ. Editorial: HRPT2 in parathyroid cancer: A piece of the puzzle. J Clin Endocrinol Metab. 2005;90:5505–5507. doi: 10.1210/jc.2005-1578. [DOI] [PubMed] [Google Scholar]
- 78.Tan MH, Morrison C, Wang P, Yang X, Haven CJ, Zhang C, Zhao P, Tretiakova MS, Korpi-Hyovalti E, Burgess JR, Soo KC, Cheah WK, Cao B, Resau J, Morreau H, Teh BT. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clin Cancer Res. 2004;10:6629–6637. doi: 10.1158/1078-0432.CCR-04-0493. [DOI] [PubMed] [Google Scholar]
- 79.Gill AJ, Clarkson A, Gimm O, Keil J, Dralle H, Howell VM, Marsh DJ. Loss of nuclear expression of parafibromin distinguishes parathyroid carcinomas and hyperparathyroidism-jaw tumor (HPT-JT) syndrome-related adenomas from sporadic parathyroid adenomas and hyperplasias. Am J Surg Pathol. 2006;30:1140–1149. doi: 10.1097/01.pas.0000209827.39477.4f. [DOI] [PubMed] [Google Scholar]
- 80.Juhlin CC, Villablanca A, Sandelin K, Haglund F, Nordenström J, Forsberg L, Bränström R, Obara T, Arnold A, Larsson C, Höög A. Parafibromin immunoreactivity: Its use as an additional diagnostic marker for parathyroid tumor classification. Endocr Relat Cancer. 2007;14:501–512. doi: 10.1677/ERC-07-0021. [DOI] [PubMed] [Google Scholar]
- 81.Juhlin C, Larsson C, Yakoleva T, Leibiger I, Leibiger B, Alimov A, Weber G, Höög A, Villablanca A. Loss of parafibromin expression in a subset of parathyroid adenomas. Endocr Relat Cancer. 2006;13:509–523. doi: 10.1677/erc.1.01058. [DOI] [PubMed] [Google Scholar]
- 82.Cetani F, Pardi E, Ambrogini E, Banti C, Viacava P, Borsari S, Bilezikian JP, Pinchera A, Marcocci C. Hyperparathyroidism 2 gene (HRPT2, CDC73) and parafibromin studies in two patients with primary hyperparathyroidism and uncertain pathological assessment. J Endocrinol Invest. 2008 doi: 10.1007/BF03346439. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kebebew E. Parathyroid carcinoma. Curr Treat Options Oncol. 2001;2:347–354. doi: 10.1007/s11864-001-0028-2. [DOI] [PubMed] [Google Scholar]
- 84.Koea JB, Shaw JH. Parathyroid cancer: Biology and management. Surg Oncol. 1999;8:155–165. doi: 10.1016/s0960-7404(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 85.Sandelin K, Auer G, Bondeson L, Grimelius L, Farnebo LO. Prognostic factors in parathyroid cancer: A review of 95 cases. World J Surg. 1992;16:724–731. doi: 10.1007/BF02067369. [DOI] [PubMed] [Google Scholar]
- 86.Marcocci C, Mazzeo S, Bruno-Bossio G, Picone A, Vignali E, Ciampi M, Viacava P, Naccarato AG, Miccoli P, Iacconi P, Pinchera A. Preoperative localization of suspicious parathyroid adenomas by assay of parathyroid hormone in needle aspirates. Eur J Endocrinol. 1998;139:72–77. doi: 10.1530/eje.0.1390072. [DOI] [PubMed] [Google Scholar]
- 87.Spinelli C, Bonadio AG, Berti P, Materazzi G, Miccoli P. Cutaneous spreading of parathyroid carcinoma after fine needle aspiration cytology. J Endocrinol Invest. 2000;23:255–257. doi: 10.1007/BF03343718. [DOI] [PubMed] [Google Scholar]
- 88.Wang C, Gaz R. Natural history of parathyroid carcinoma: Diagnosis, treatment and results. Am J Surg. 1983;149:522–527. doi: 10.1016/s0002-9610(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 89.Anderson B, Samaan N, Vassilopoulou-Sellin R, Ordonez N, Hickey R. Parathyroid carcinoma: Features and difficulties in diagnosis and management. Surgery. 1983;94:906–915. [PubMed] [Google Scholar]
- 90.Rawat N, Khetan N, Williams DW, Baxter JN. Parathyroid carcinoma. Br J Surg. 2005;92:1345–1353. doi: 10.1002/bjs.5182. [DOI] [PubMed] [Google Scholar]
- 91.Wynne A, Heerden JV, Carney J, Fitzpatrick L. Parathyroid carcinoma: Clinical and pathological features in 43 patients. Medicine. 1992;71:197–205. [PubMed] [Google Scholar]
- 92.Rao SR, Shaha AR, Singh B, Rinaldo A, Ferito A. Management of cancer of the parathyroid. Acta Otolaryngol. 2002;122:448–452. doi: 10.1080/00016480260000184. [DOI] [PubMed] [Google Scholar]
- 93.Munson ND, Foote RL, Northcutt RC, Tiegs RD, Fitzpatrick LA, Grant CS, van Heerden JA, Thompson GB, Lloyd RV. Parathyroid carcinoma: Is there a role for adjuvant radiation therapy? Cancer. 2003;98:2378–2384. doi: 10.1002/cncr.11819. [DOI] [PubMed] [Google Scholar]
- 94.Clayman GL, Gonzalez HE, El-Naggar A, Vassilopoulou-Sellin R. Parathyroid carcinoma: Evaluation and interdisciplinary management. Cancer. 2004;100:900–905. doi: 10.1002/cncr.20089. [DOI] [PubMed] [Google Scholar]
- 95.Busaidy NL, Jimenez C, Habra MA, Schultz PN, El-Naggar AK, Clayman GL, Asper JA, Diaz EM, Jr, Evans DB, Gagel RF, Garden A, Hoff AO, Lee JE, Morrison WH, Rosenthal DI, Sherman SI, Sturgis EM, Waguespack SG, Weber RS, Wirfel K, Vassilopoulou-Sellin R. Parathyroid carcinoma: A 22-year experience. Head Neck. 2004;26:716–726. doi: 10.1002/hed.20049. [DOI] [PubMed] [Google Scholar]
- 96.Mulder JE, Bilezikian JB. Acute management of hypercalcemia. In: Bilezikian JP, Marcus R, Levine MA, editors. The Parathyroids. Basic and Clinical Concepts. 2nd ed. San Diego, CA, USA: Academic Press; 2001. pp. 729–741. [Google Scholar]
- 97.Koyano H, Shishiba Y, Shimizu T, Suzuki N, Nakazawa H, Tachibana S, Murata H, Furui S. Successful treatment by surgical removal of bone metastasis producing PTH: New approach to the management of metastatic parathyroid carcinoma. Intern Med. 1994;33:697–702. doi: 10.2169/internalmedicine.33.697. [DOI] [PubMed] [Google Scholar]
- 98.Denney AM, Watts NB. The effect of octreotide on parathyroid carcinoma. J Clin Endocrinol Metab. 2004;89:1016. doi: 10.1210/jc.2003-031825. [DOI] [PubMed] [Google Scholar]
- 99.Bradwell AR, Harvey TC. Control of hypercalcaemia of parathyroid carcinoma by immunisation. Lancet. 1999;353:370–373. doi: 10.1016/S0140-6736(98)06469-1. [DOI] [PubMed] [Google Scholar]
- 100.Shoback DM, Arends RH, Roskos L, Shetty S, Wyres M, Huang S, Raienbell GM. Treatment of parathyroid carcinoma with ABX10241, a monoclonal antibody to parathyroid hormone. J Bone Miner Res. 2004;17:SA498. [Google Scholar]
- 101.Betea D, Bradwell AR, Harvey TC, Mead GP, Schmidt-Gayk H, Ghaye B, Daly AF, Beckers A. Hormonal and biochemical normalization and tumor shrinkage induced by anti-parathyroid hormone immunotherapy in a patient with metastatic parathyroid carcinoma. J Clin Endocrinol Metab. 2004;89:3413–3420. doi: 10.1210/jc.2003-031911. [DOI] [PubMed] [Google Scholar]
- 102.Schott M, Feldkamp J, Schattenberg D, Krueger T, Dotzenrath C, Seissler J, Scherbaum WA. Induction of cellular immunity in a parathyroid carcinoma treated with tumor lysate-pulsed dendritic cells. Eur J Endocrinol. 2000;142:300–306. doi: 10.1530/eje.0.1420300. [DOI] [PubMed] [Google Scholar]
- 103.Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, Balandrin MF. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Collins MT, Skarulis MC, Bilezikian JP, Silverberg SJ, Spiegel AM, Marx SJ. Treatment of hypercalcemia secondary to parathyroid carcinoma with a novel calcimimetic agent. J Clin Endocrinol Metab. 1998;93:1083–1088. doi: 10.1210/jcem.83.4.4726. [DOI] [PubMed] [Google Scholar]
- 105.Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:135–141. doi: 10.1210/jc.2004-0842. [DOI] [PubMed] [Google Scholar]
- 106.Silverberg SJ, Rubin MR, Faiman C, Peacock M, Shoback DM, Smallridge RC, Schwanauer LE, Olson KA, Klassen P, Bilezikian JP. Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab. 2007;92:3803–3808. doi: 10.1210/jc.2007-0585. [DOI] [PubMed] [Google Scholar]
- 107.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985-1999: A National Cancer Database report. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1999;86:538–544. doi: 10.1002/(sici)1097-0142(19990801)86:3<538::aid-cncr25>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 108.Lee PK, Jarosek SL, Virnig BA, Evasovich M, Tuttle TM. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer. 2007;109:1736–1741. doi: 10.1002/cncr.22599. [DOI] [PubMed] [Google Scholar]