Abstract
Objective
To characterize the inflammatory and coagulopathic response after endovascular thoracoabdominal aortic aneurysm (TAAA) repair and to evaluate the effect of the response on post-operative renal function.
Methods
From 7/2005 to 6/2008, 42 patients underwent elective endovascular repair of a TAAA using custom designed multi-branched stent-grafts at a single academic institution. 4 patients were excluded from the analysis. White blood cell count (WBC), platelet count, prothrombin time (PT), and creatinine were measured in all patients. In the last 9 patients, interleukin-6 (IL-6), protein C, Factor V, d-dimers, cystatin C, and neutrophil gelatinase-associated lipocalin (NGAL) levels were also measured. Change in lab values were expressed as a percentage of baseline values.
Results
The 30-day mortality rate was 5% (2/38). All patients (n=38) had a higher WBC (mean±SD: 139 ± 80%, P<0.0001), lower platelet count (56 ± 15%, P<0.0001), and higher PT (median: 17%, IQR 12 - 22%, P<0.0001) after stent-graft insertion. Twelve of 38 patients (32%) developed postoperative acute renal insufficiency (>50% rise in creatinine). Patients with renal insufficiency had significantly larger changes in WBC (178 ± 100% vs 121 ± 64%, p=0.04) and platelet count (64 ± 17% vs 52 ± 12%, p=0.02) compared to those without renal insufficiency. All patients (n=9) had significant increases in NGAL (182 ± 115%, p=0.008) after stent-graft insertion. 6/9 patients (67%) had increased cystatin C (35 ± 43%, p = 0.04) after stent-graft insertion, with a greater rise in those with postoperative renal insufficiency (87 ± 32% vs 8 ± 13%, p = 0.02). IL-6 levels were markedly increased in all patients (n=9) after repair (9,840 ± 6,160%, p=0.008). Protein C (35 ± 10%, p=0.008) and Factor V levels (28 ± 20%, p=0.008) were uniformly decreased, while d-dimers were elevated after repair in all patients (310 ± 213%, p=0.008).
Conclusions
Leukocytosis and thrombocytopenia were uniform following endovascular TAAA repair, and the severity of the response correlated with post-operative renal dysfunction. Elevation of a sensitive marker of renal injury (NGAL) suggests that renal injury may occur in all patients after stent-graft insertion.
Introduction
Thoracoabdominal aortic aneurysm (TAAA) is a lethal disease if left untreated.1 Open surgical repair is associated with high morbidity and mortality rates. Statewide audits show 30-day mortality rates of 20% and one-year mortality rates of approximately 30%.2 Endovascular TAAA repair is an alternative approach, whereby the components of a multi-branched stent-graft are inserted entirely through the femoral and brachial arteries, and assembled within the thoracoabdominal aorta.3 Short term results following endovascular TAAA repair in high risk patients demonstrate lower morbidity and mortality rates compared to the traditional open approach.4, 5
Despite the potential benefits of endovascular treatment of aortic aneurysms,6, 7 almost all patients who undergo placement of an aortic stent-graft experience a systemic response termed the post-implantation syndrome.8 This is characterized by fever, anorexia, elevated white blood cell count (WBC), and changes in coagulation parameters.8-13 For less extensive aneurysm repair, such as endovascular abdominal aortic aneurysm (AAA), the syndrome typically resolves within two weeks without any permanent ill effects. Rarely, it results in severe complications such as pulmonary dysfunction, cardiovascular events, renal insufficiency, and multi-system organ failure.13, 14 Little is known about the pathophysiology or mechanisms underlying this response. Possible causes include injury to the vascular endothelium, manipulation of thrombus in the aneurysm, or platelet activation by the graft material.
Considering the greater extent of diseased aorta, high complexity of repair, and long length of stent-graft implanted during endovascular TAAA repair, we hypothesize that endovascular TAAA repair triggers a severe form of the post-implantation syndrome. Moreover, inflammatory cytokines, disordered coagulation, and leukocyte-endothelial interactions have been suggested to play important roles in the pathogenesis of acute kidney injury.15 The purpose of this study is to characterize the inflammatory, coagulopathic, and renal response after endovascular TAAA repair.
Methods
Patients
Between July 2005 and June 2008, 42 patients underwent endovascular TAAA repair using custom-designed modular multi-branched stent-grafts (Figure 1) at the University of California-San Francisco Medical Center. All of the branched endografts were made of Dacron and stainless steel (Cook Medical Inc., Bloomington, IN). Each cuff is then bridged by a Fluency covered stent (Polytetrafluoroethylene and nitinol, C.R. Bard Inc., Tempe, AZ) into the target vessel, and then a Wallstent (Elgiloy, Boston Scientific Corp., Natick, MA) is placed. All procedures were performed under a physician-sponsored Investigational Device Exemption from the United States Food and Drug Administration (FDA), with the approval of the local Institutional Review Board. The inclusion and exclusion criteria for endovascular TAAA repair have previously been published.5 Of the 42 patients who underwent endovascular TAAA repair,4 patients were excluded from the analysis. Two patients were excluded due to pre-existing end stage renal disease requiring dialysis. One patient was excluded because of a fatal anesthetic complication, and one patient was excluded because he underwent another significant operation at the time of aneurysm repair.
Figure 1.
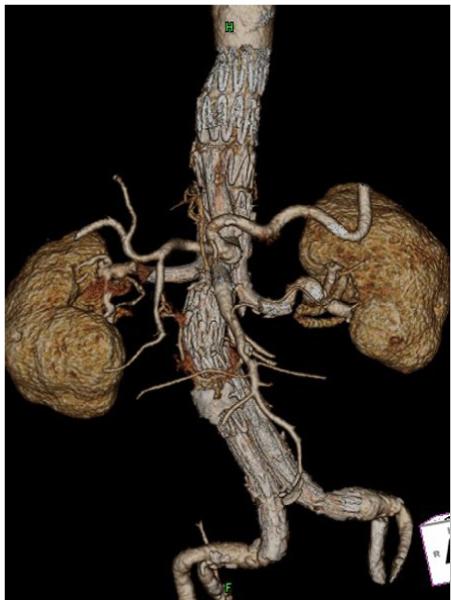
Computed tomographic angiography with 3-dimensional reconstruction after multi-branched endovascular TAAA repair.
Patient Demographics and Follow-up
Patient demographic information, including age, gender, aneurysm size, pre-operative medications, and medical co-morbidities were collected prospectively. Data on pre-existing co-morbidities were collected from patients’ self-reported history and focused on coronary artery disease (CAD), hypertension, diabetes mellitus, peripheral vascular disease, cerebrovascular disease, and prior aortic surgery (Table 1). Pre-operative use of aspirin, plavix, coumadin, hypertensive medications, and HMG-CoA reductase inhibitors (statins) were recorded at the initial clinic visit.
Tabel 1.
Patient characteristics and operative details
| n = 38 | |
| Patient Demographics | |
| Age (yrs, mean ± SD) | 75.1 ± 7.3 |
| Male (no) | 29 (76%) |
| Patient Co-morbidities | |
| Hypertension | 34 (89%) |
| Diabetes | 6 (16%) |
| Coronary artery disease | 21 (55%) |
| Cerebrovascular disease | 9 (24%) |
| Peripheral vascular disease | 11 (29%) |
| Prior aortic surgery | 18 (47%) |
| Pre-operative statin use | 28 (74%) |
| Baseline creatinine (mg/dL) | 1.25 ± 0.36 |
| Baseline GFR (mL/min/1.73 m2) | 62.3 ± 20.6 |
| Maximum Aneurysm Size(mm) | 67 ± 10 |
| Operative Details | |
| Operative time (hrs) | 6.8 ± 1.9 |
| Percent aortic coverage (%) | 69 ± 19 |
| Transfusion during surgery | 22 (58%) |
| General anesthesia | 26 (68%) |
Continuous data are presented as means ± standard deviation except where noted.
Categorical data are given as counts (percentages).
Anatomic characteristics of the TAAA (aneurysm size and extent), intra-operative procedural details, laboratory data, and post-operative outcomes were all collected prospectively. Percentage of aortic coverage was defined as the length of aorta covered by the stent-graft divided by the total length of the thoracoabdominal aorta from the origin of the left subclavian artery to the iliac bifurcation. All patients were admitted to the hospital one day prior to the procedure. If the glomerular filtration rate (GFR) calculated by the Modification of Diet in Renal Disease (MDRD) equation was less than 60 mL/min/1.73m2,16 patients were administered an intravenous bicarbonate infusion and given an oral dose of 600 mg of N-acetylcysteine. All patients received Visipaque contrast during endovascular TAAA repair.
All patients underwent post-operative computed-tomographic angiography (CTA) prior to discharge. Follow up clinical assessment, CTA, and creatinine measurement were performed at 1, 6, and 12 months, and then yearly thereafter.
Laboratory Measurements
WBC, platelet count, prothrombin time (PT), and serum creatinine were collected the day before endovascular repair, immediately post-operatively, and then daily until discharge. In the last 9 patients, additional serum markers of inflammation (interleukin-6 [IL-6]), coagulation (protein C, factor Va, d-dimer), renal injury (NGAL), and renal function (cystatin C) were collected pre-operatively, post-operatively, and for the first 3 days following surgery.
Analytical Assays
Serum Marker of Inflammation
IL-6 levels were quantified using ELISA assays (Biosource, CA, USA). Blood samples were collected, allowed to clot, and centrifuged at 1000g to isolate serum in serum separator tubes (BD Biosciences, NJ, USA). Control blood samples were collected at a single time point and processed as described above. The serum samples were stored at -20°C until ELISA assays were performed according to manufacturer’s instructions. Concentrated samples were diluted up to five-fold. Standards, samples, and controls were run in duplicates and read at 450nm (Tecan, CA, USA). IL-6 concentrations (pg/mL) were then calculated based on constructed standard curves.
Serum Markers of Coagulation
Protein C, factor Va, and d-dimer levels were measured with the Stago STA-Compact coagulation analyzer.
Serum Markers of Renal Injury and Function
Quantitative NGAL levels were measured using an ELISA sandwich immunoassay (R&D Systems, MN, USA) and performed according to manufacturer’s instructions. Concentrated samples were diluted up to five-fold with manufacturer-provided diluent. Standards, samples, and controls were run in duplicates and the resulting chromagen was read at 450nm with an additional 570nm wavelength correction (Tecan, CA, USA). NGAL concentrations (ng/mL) were then calculated based on the constructed standard curves on respective ELISA plates. Cystatin C was measured using a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring) with a nephelometer (BNII, Dade Behring).
Definitions and Measurements
Acute renal insufficiency was defined as a 50% or greater rise in serum creatinine compared to baseline values. Changes in serum creatinine, WBC, PT, platelet count, NGAL, IL-6, cystatin C, protein C, Factor V, and d-dimer levels were calculated as the differences between peak (or nadir) post-operative values and baseline (pre-operative) concentrations. Peak and nadir values were defined as the highest and lowest values, respectively, within the first 5 post-operative days. This limitation was applied in order to restrict the analysis to the post-operative systemic response and avoid changes in laboratory values resulting from later complications. Estimated GFR was determined by the MDRD study equation.16
Statistical analysis
All statistical analysis was performed using STATA 9.0 software (StataCorp, Texas, USA). Continuous variables are expressed as a mean ± standard deviation for normally distributed variables and compared using the Student’s t-test. The paired t-test was used to compare baseline and peak values for each patient. Continuous variables with a skewed distribution are expressed as a median and interquartile range and compared using the Wilcoxon rank sum test or sign rank test. The Chi-square test was used to compare categorical variables. Statistical significance was inferred at p < 0.05.
Uni-variate logistic regression analysis was used to evaluate the association between post-operative acute renal insufficiency and various predictors. Model checks included specification testing and the Hosmer-Lemeshow goodness of fit tests. In addition, uni-variate linear regression analysis was used to assess the association between absolute change in creatinine and various predictors.
Results
All patients (n=38) underwent successful endovascular TAAA repair. Patient demographics and operative details are shown in table 1. Mean patient age was 75.1 ± 7.3 years (range: 57.8 to 86.5 years) and 29/38 (76%) were men. 30 day mortality following endovascular TAAA repair was 5% (2/38 patients). 18/38 patients (47%) developed a fever (temperature ≥ 38.0°C) during the postoperative period. The mean contrast dose administered during endovascular TAAA repair was 170 ± 85 mL (range 28 to 390 mL).
Patients (n=38) had statistically significant changes in WBC, platelet count, and PT following endovascular TAAA repair (Table 2). Every patient had a higher WBC (mean±SD: 139 ± 80%; range: 10% to 390%) at a median time of 1 day after repair; a lower platelet count (mean±SD: 56 ± 15%; range 26% to 87%) at a median time of 3 days after repair; and a higher PT (median 17%; range: 4 to 175%) at a median time of 2 days after repair. Pre-operative statin use was associated with reduced peri-operative changes in WBC (118 ± 52% vs 198 ± 114%, p=0.005), but not in platelet count or PT. The magnitude of the changes in WBC, platelet count, and PT did not correlate with aneurysm size, extent of aortic coverage, contrast dose, length of operation, postoperative fever, or presence of postoperative endoleak (p>0.05 for all associations).
Table 2.
Peri-operative WBC, platelet count, PT, creatinine, and GFR
| Baseline | Peak or Nadir | Relative Change (%) | p value | |
|---|---|---|---|---|
| WBC | 6.9 ± 1.4 | 16.6 ± 6.5 | + 139 ± 80 | <0.0001a |
| Platelets | 206 ± 67 | 89 ± 41 | - 56 ± 15 | <0.0001a |
| PT | 13.7 (12.5 -14.4) | 16.6 (14.7 - 17.4) | + 22 (13 - 22) | <0.0001b |
| Creatinine | 1.25 ± 0.36 | 1.89 ± 1.08 | + 54 ± 93 | 0.0003a |
| GFR | 62 ± 21 | 46 ± 23 | - 26 ± 28 | <0.0001a |
WBC and platelets: X 109 cells/liter; PT: seconds; Creatinine: mg/dL; GFR: mL/min/1.73m2
Paired t-test
Wilcoxon signed rank test
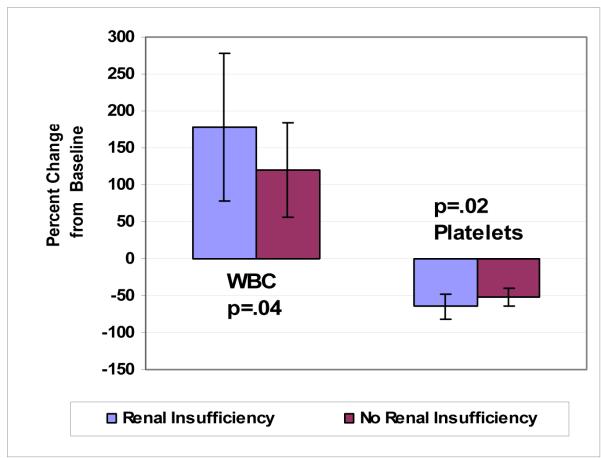
The mean baseline GFR was 62 ± 21 mL/min/1.73m2 in our cohort of patients and decreased to 46 ± 23 mL/min/1.73m2 after repair (p<0.0001). After endovascular TAAA repair, the mean baseline creatinine increased from 1.25 ± 0.36 mg/dL to 1.89 ± 1.08 mg/dL (p<0.0001) (Table 2). 12 of 38 patients (32%) developed postoperative acute renal insufficiency. Patients with renal insufficiency had larger changes in WBC (178 ± 100% vs 121 ± 64%, p=0.04) and platelet count (64 ± 17% vs 52 ± 12%, p=0.02) compared to those who did not develop renal insufficiency in the post-operative period (Figure 2). There was no significant increase in PT amongst those with postoperative renal insufficiency (median PT: 20: IQR 15 - 30%) compared to those without renal insufficiency (median PT:15: IQR 11 - 21%, p=0.09). Preoperative GFR < 60 mL/min/1.73m2 was not associated with the development of acute renal insufficiency (p=0.80). The 2 patients who died within 30 days in our series both developed acute renal insufficiency in the early post-operative period.
Figure 2.
Comparison of changes in WBC and platelet count after endovascular TAAA repair between patients with and without post-operative renal insufficiency
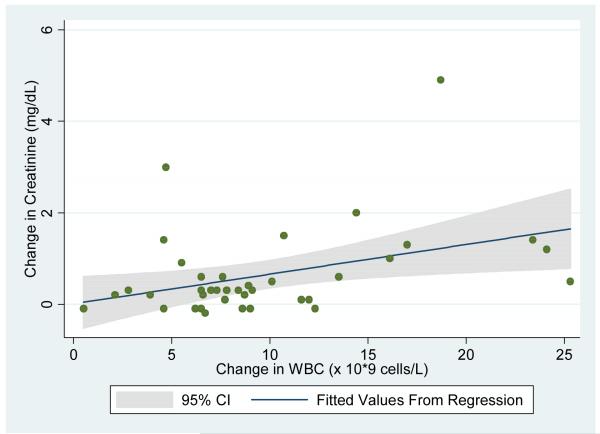
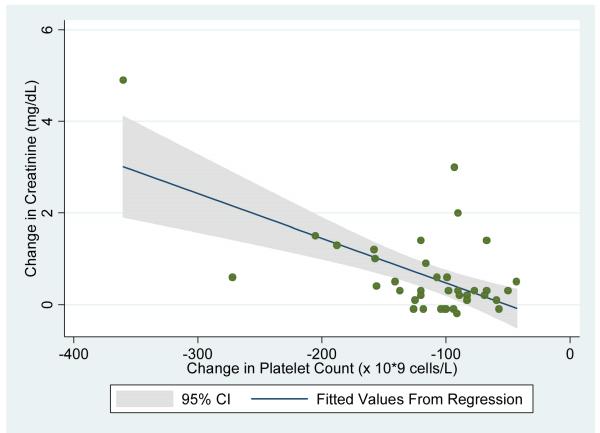
Univariate logistic regression analysis demonstrated that each 5×109 cells/liter increase in WBC in the postoperative period was associated with a 2.4 fold odds of postoperative renal insufficiency (p=0.02; 95% CI: 1.2 -4.9). Each 50×109 cells/liter decrease in platelet count was associated with a 4.0 fold odds of postoperative renal insufficiency (p=0.02; 95% CI: 1.3-12.5). Figures 3a and 3b show the relationship between postoperative change in creatinine with change in WBC and change in platelet count, respectively.
Figure 3a.
Uni-variate linear regression of post-operative change in serum creatinine using change in WBC as the predictor variable.
Figure 3b.
Uni-variate linear regression of post-operative change in serum creatinine using change in platelet count as the predictor variable.
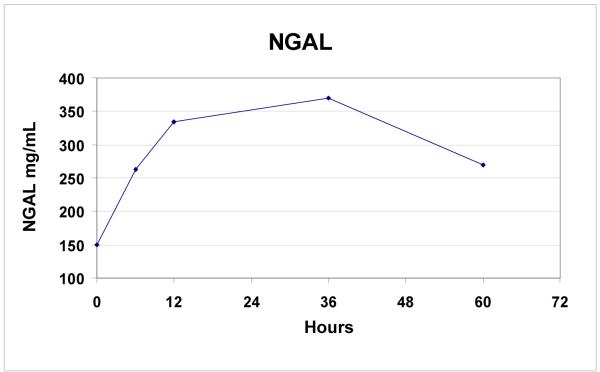
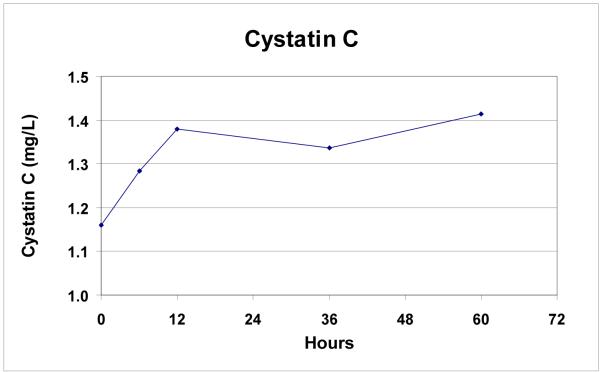
Additional markers of renal injury and function, inflammation, and coagulopathy were measured in the last 9 patients. All patients (n=9) had increases in NGAL (mean ± SD: 182 ± 115%; range: 62 to 410%, p=0.008) post-operatively (Figure 4a). Rise in NGAL levels did not correlate with the presence or absence of post-operative renal insufficiency (p = 0.66). 6/9 patients (67%) had increases in cystatin C levels after TAAA repair. Mean cystatin C levels were significantly elevated post-operatively (35 ± 43%, p = 0.04, Figure 4b), and the rise in cystatin C was greater in patients with post-operative acute renal insufficiency compared to those with no change in renal function (87 ± 32% vs 8 ± 13%, p = 0.02).
Figure 4a.
Rise in NGAL levels over time following endovascular TAAA repair (n=9).
Figure 4b.
Rise in cystatin C levels over time following endovascular TAAA repair (n=9).
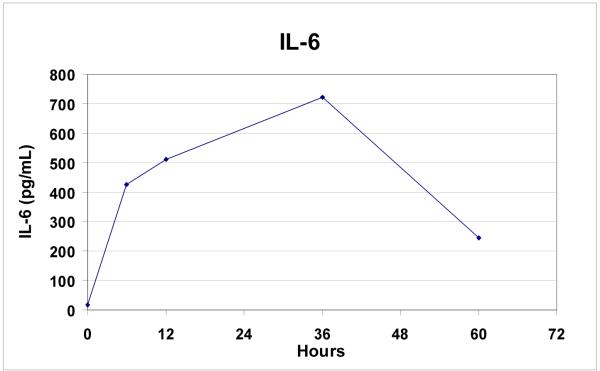
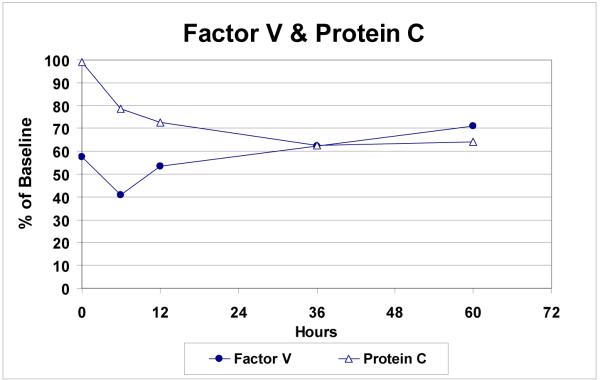
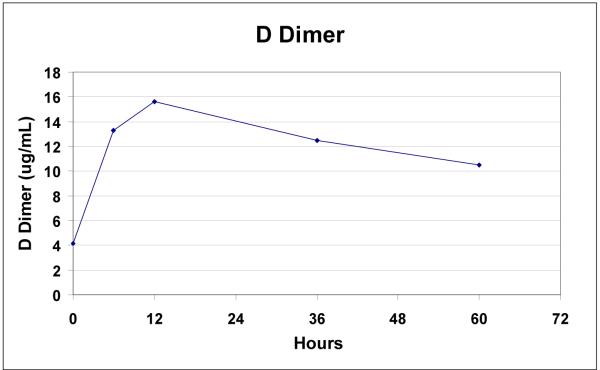
IL-6 levels were markedly elevated in all patients (mean ± SD: 9,840 ± 6,160%, p = 0.003) following endovascular repair (Figure 5). Protein C (35 ± 10%, p=0.008) and Factor V levels (28 ± 20%, p=0.008) were uniformly decreased, while d-dimers were elevated in all patients after repair (310 ± 213%, p=0.008) (Figures 6a, 6b).
Figure 5.
Rise in IL6 levels over time following endovascular TAAA repair (n=9).
Figure 6a.
Fall in factor V and protein C levels over time following endovascular TAAA repair (n=9).
Figure 6b.
Discussion
Endovascular repair of TAAA results in significant leukocytosis and thrombocytopenia, which appear to be related to postoperative acute renal insufficiency. The inflammatory sequelae may not amount to much after endovascular AAA repair, but they constitute a significant source of morbidity after endovascular TAAA repair. In our previous report on the results of multi-branched endovascular TAAA repair, the hemorrhagic complications of thrombocytopenia and coagulopathy included upper gastrointestinal bleeding, hemoptysis, and subarachnoid hemorrhage.5 Based on the findings of the current study, we can conclude that the inflammatory response to endovascular TAAA repair is also associated with renal dysfunction.
The literature on the post-implantation syndrome following AAA repair provides little indication of the underlying cause. One cannot say, based on published reports, whether the response to endovascular AAA repair is the same as the response to open surgery.10-13 Nor can one say whether the cause is surgical stress, endovascular instrumentation of the mural thrombus, endovascular instrumentation of the endothelium, the presence of a stent- graft, or thrombosis of the aneurysm. For example, some authors suggest that manipulation in the aneurysm during endovascular repair may cause white cell activation with the release of various cytokines, such as IL-1, IL-6, and TNF-α, while others suggest that injury to the vascular endothelium may prompt activation of protein C, with a subsequent coagulopathy and loss of cytoprotectivity.13-14, 17-18 Our data on endovascular TAAA repair are helpful in this regard because the inflammatory response is pronounced and consistent, occurring in every single patient treated. Moreover, there were strong correlations between inflammatory, hematologic and renal effects, suggesting that causal relationships connect them all, although we cannot yet say how. All patients in our study had markedly elevated levels of IL-6 and a two-fold increase in WBC after stent-graft implantation, suggesting a strong inflammatory response. In addition, the coagulopathic response was characterized by a decrease in protein C levels immediately after endovascular repair, followed by a significant drop in platelet count and increase in prothrombin time.
It seems likely that the inflammatory response caused the renal dysfunction, rather than the converse. Other possible explanations for a high rate of postoperative renal dysfunction in this study group include contrast nephrotoxicity and intraoperative ischemia. All of the patients in this study received Visipaque contrast. The mean contrast volume used during repair was 170 mL, with a range of 28 to 390 mL. Of note, the only patient who left the hospital on dialysis was the one who received the smallest dye load (28 mL). There is no period of obligatory renal ischemia during endovascular TAAA repair. Blood flows through the cuffs of the stent-graft into the peri-graft space, and from there into the renal and visceral arteries. In theory, the interruption of flow to a branch vessel would only be occluded for as long as it took to deploy the Fluency covered stent, or to inflate a balloon within the covered stent or the outflow artery. Based on the tapered shape of the stent-graft and the method of insertion, we believe renal ischemia occurred rarely, if ever.
We recognize that alterations in serum creatinine are not necessarily the most reliable basis for an assessment of renal function. In the latter part of this study, we started to measure novel biomarkers of renal function (cystatin C) and renal tubular injury (NGAL).19-20 Cystatin C has emerged as an important marker of acute renal dysfunction, and appears to correlate better with GFR than serum creatinine.21-24 NGAL, an indicator of acute tubular injury, may also have a role in establishing the precise timing of renal injury25, as we try to establish a chain of events in the interplay of the inflammatory, coagulopathic, and renal effects.
Most of the theories regarding the etiology of the post-implantation syndrome fall into one of two groups: those that depend on catheter-mediated injury, and those that depend on the presence of the stent graft. Since the implantation of a branched stent graft consists largely of a series of intra-arterial catheter manipulations, the duration of the operation may serve a surrogate for the extent of aortic instrumentation. Our surrogates for aneurysm and stent-graft related effects were the extent of aortic coverage and aneurysm diameter. None of these factors correlated with the inflammatory response. The only significant determinant of the extent of the inflammatory response was statin use, with a significantly less pronounced leukocytosis in those taking any form of statin therapy. If anyone needs yet another reason to promote routine statin use in vascular surgery patients, here it is.
The results of endovascular TAAA repair compare well with the results of open surgical repair, but the outcomes are marred by the effects of a severe inflammatory response.5 Not that this response is unique to endovascular repair of TAAA; some manifestations of the post-implantation syndrome occur in all patients who undergo endovascular treatment of aortic diseases (abdominal aortic aneurysms, thoracic aneurysms, and thoracic dissections).9,13,26-28 However, those undergoing endovascular TAAA repair appear to experience a more extreme response. As we have learned to overcome the technical obstacles to stent-graft insertion, the post-implantation syndrome has become the main impediment to an uncomplicated postoperative course.
Acknowledgments
This project was supported by NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130 (Dr. Hiramoto) and NIH K08 GM-085689 (Dr. Cohen). Its contents are the responsibility of the authors and do not necessarily represent the official views of the NIH.
This study was also supported in part by grants from the Hellman Award, San Francisco, CA (Dr. Hiramoto); the Research Evaluation and Allocation Committee, San Francisco, CA (Dr. Hiramoto); the Foundation of Anesthesia Research and Education, Rochester, MN (Dr. Niemann); the International Anesthesia Research Society, Cleveland, OH (Dr. Niemann); the Foundation for Accelerated Vascular Research, San Francisco, CA (Drs.Chang and Chuter); and the Established Investigator Award from the American Heart Association (Dr. Shlipak).
The Clinicaltrials.gov number is: NCT00483249.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Dr. Chuter has licensed patents to Cook Medical, Inc. and receives travel and research support from Cook Medical, Inc.
References
- 1.Crawford ES, DeNatale RW. Thoracoabdominal aortic aneurysm: observations regarding the natural course of the disease. J Vasc Surg. 1986;3(4):578–82. doi: 10.1067/mva.1986.avs0030578. [DOI] [PubMed] [Google Scholar]
- 2.Rigberg DA, McGory ML, Zingmond DS, et al. Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg. 2006;43(2):217–22. doi: 10.1016/j.jvs.2005.10.070. discussion 223. [DOI] [PubMed] [Google Scholar]
- 3.Chuter TA, Reilly LM. Endovascular treatment of thoracoabdominal aortic aneurysms. J Cardiovasc Surg (Torino) 2006;47(6):619–28. [PubMed] [Google Scholar]
- 4.Roselli EE, Greenberg RK, Pfaff K, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2007;133(6):1474–82. doi: 10.1016/j.jtcvs.2006.09.118. [DOI] [PubMed] [Google Scholar]
- 5.Chuter TA, Rapp JH, Hiramoto JS, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg. 2008;47(1):6–16. doi: 10.1016/j.jvs.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Peterson BG, Matsumura JS, Brewster DC, Makaroun MS. Five-year report of a multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysms. J Vasc Surg. 2007;45(5):885–90. doi: 10.1016/j.jvs.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Hiramoto JS, Reilly LM, Schneider DB, et al. Long-term outcome and reintervention after endovascular abdominal aortic aneurysm repair using the Zenith stent graft. J Vasc Surg. 2007;45(3):461–5. doi: 10.1016/j.jvs.2006.11.034. discussion 465-6. [DOI] [PubMed] [Google Scholar]
- 8.Blum U, Voshage G, Lammer J, et al. Endoluminal stent-grafts for infrarenal abdominal aortic aneurysms. N Engl J Med. 1997;336(1):13–20. doi: 10.1056/NEJM199701023360103. [DOI] [PubMed] [Google Scholar]
- 9.Velazquez OC, Carpenter JP, Baum RA, et al. Perigraft air, fever, and leukocytosis after endovascular repair of abdominal aortic aneurysms. Am J Surg. 1999;178(3):185–9. doi: 10.1016/s0002-9610(99)00144-0. [DOI] [PubMed] [Google Scholar]
- 10.Galle C, De Maertelaer V, Motte S, et al. Early inflammatory response after elective abdominal aortic aneurysm repair: a comparison between endovascular procedure and conventional surgery. J Vasc Surg. 2000;32(2):234–46. doi: 10.1067/mva.2000.107562. [DOI] [PubMed] [Google Scholar]
- 11.Swartbol P, Norgren L, Albrechtsson U, et al. Biological responses differ considerably between endovascular and conventional aortic aneurysm surgery. Eur J Vasc Endovasc Surg. 1996;12(1):18–25. doi: 10.1016/s1078-5884(96)80270-x. [DOI] [PubMed] [Google Scholar]
- 12.Norgren L, Albrechtsson U, Swartbol P. Side-effect of endovascular grafting to treat aortic aneurysm. Br J Surg. 1996;83(4):520–1. doi: 10.1002/bjs.1800830429. [DOI] [PubMed] [Google Scholar]
- 13.Gerasimidis T, Sfyroeras G, Trellopoulos G, et al. Impact of endograft material on the inflammatory response after elective endovascular abdominal aortic aneurysm repair. Angiology. 2005;56(6):743–53. doi: 10.1177/000331970505600612. [DOI] [PubMed] [Google Scholar]
- 14.Cross KS, Bouchier-Hayes D, Leahy AL. Consumptive coagulopathy following endovascular stent repair of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2000;19(1):94–5. doi: 10.1053/ejvs.1999.0970. [DOI] [PubMed] [Google Scholar]
- 15.Liu KD, Glidden DV, Eisner MD, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury*. Crit Care Med. 2007 [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, et al. Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Morikage N, Esato K, Zenpo N, et al. Is endovascular treatment of abdominal aortic aneurysms less invasive regarding the biological responses? Surg Today. 2000;30(2):142–6. doi: 10.1007/PL00010062. [DOI] [PubMed] [Google Scholar]
- 18.Swartbol P, Truedsson L, Norgren L. Adverse reactions during endovascular treatment of aortic aneurysms may be triggered by interleukin 6 release from the thrombotic content. J Vasc Surg. 1998;28(4):664–8. doi: 10.1016/s0741-5214(98)70092-8. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol. 2007 doi: 10.1007/s00467-007-0470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trof RJ, Di Maggio F, Leemreis J, Groeneveld AB. Biomarkers of acute renal injury and renal failure. Shock. 2006;26(3):245–53. doi: 10.1097/01.shk.0000225415.5969694.ce. [DOI] [PubMed] [Google Scholar]
- 21.Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36(1):29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 22.Chantrel F, Agin A, Offner M, et al. Comparison of cystatin C versus creatinine for detection of mild renal failure. Clin Nephrol. 2000;54(5):374–81. [PubMed] [Google Scholar]
- 23.Harmoinen AP, Kouri TT, Wirta OR, et al. Evaluation of plasma cystatin C as a marker for glomerular filtration rate in patients with type 2 diabetes. Clin Nephrol. 1999;52(6):363–70. [PubMed] [Google Scholar]
- 24.Price CP, Finney H. Developments in the assessment of glomerular filtration rate. Clin Chim Acta. 2000;297(1-2):55–66. doi: 10.1016/s0009-8981(00)00233-3. [DOI] [PubMed] [Google Scholar]
- 25.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 26.Akowuah E, Wilde P, Angelini G, Bryan AJ. Systemic inflammatory response after endoluminal stenting of the descending thoracic aorta. Interact Cardiovasc Thorac Surg. 2007;6(6):741–3. doi: 10.1510/icvts.2007.157339. [DOI] [PubMed] [Google Scholar]
- 27.Eggebrecht H, Mehta RH, Metozounve H, et al. Clinical implications of systemic inflammatory response syndrome following thoracic aortic stent-graft placement. J Endovasc Ther. 2008;15(2):135–43. doi: 10.1583/07-2284.1. [DOI] [PubMed] [Google Scholar]
- 28.Gabriel EA, Locali RF, Romano CC, et al. Analysis of the inflammatory response in endovascular treatment of aortic aneurysms. Eur J Cardiothorac Surg. 2007;31(3):406–12. doi: 10.1016/j.ejcts.2006.11.053. [DOI] [PubMed] [Google Scholar]