Abstract
Microtubule inhibitors such as vinblastine cause mitotic arrest and subsequent apoptosis through the intrinsic mitochondrial pathway. However, while Bcl-2 family proteins have been implicated as distal mediators, their precise role is largely unknown. In this study we investigated the role of Bak in vinblastine-induced apoptosis. Bak was mainly monomeric in untreated KB-3 cells, and multimers corresponding to dimer, trimer and higher oligomers were observed after vinblastine treatment. The oligomeric Bak species were strongly diminished in cells stably overexpressing Bcl-xL. Immunoprecipitation with a conformation-dependent Bak antibody revealed that vinblastine induced Bak activation. Reciprocal immunoprecipitations indicated that vinblastine induced the interaction of active Bak with active Bax. Furthermore, Bcl-xL overexpression prevented Bak and Bax interaction and strongly inhibited apoptosis, whereas Bcl-2 overexpression did not prevent Bak-Bax interaction and only weakly inhibited apoptosis. The relative contributions of Bak and Bax were investigated using fibroblasts deficient in one or both of these proteins; double knockouts were highly resistant compared to single knockouts, with vinblastine sensitivities in the order Bak+/Bax+ > Bak+/Bax− >Bak−/Bax+ > Bak−/Bax−. These results highlight Bak as a key mediator of vinblastine-induced apoptosis and show for the first time activation and oligomerization of Bak by an anti-mitotic agent. In addition, our results suggest that the interaction of the activated forms of Bak and Bax represents a key distal step in the apoptotic response to this important chemotherapeutic drug.
Keywords: apoptosis, Bak, vinblastine, oligomerization, Bak-Bax interaction
Introduction
The Bcl-2 family of proteins is a key regulator of programmed cell death or apoptosis (1–6). The prosurvival proteins include Bcl-2, Bcl-xL, Mcl-1, A1, Nr-13 and others, and the pro-apoptotic members can be further subdivided into two groups: the multidomain Bax subfamily (Bax, Bak and Bok) which contain multiple BH domains; and “the BH3-only” subfamily (Bad, Bid, Bim, Noxa, Hrk and others). The Bcl-2 proteins function in a hierarchy, with the BH3-only proteins acting as initiators of apoptosis. Under normal physiological conditions they are maintained in a latent state. However, in response to a death signal they become activated through a variety of mechanisms involving posttranslational modification or transcriptional activation. Activated BH3-only proteins play a key role in activation of multidomain Bax subfamily proteins. This leads to an increase in outer mitochondrial membrane permeability and the release of cytochrome c and other apoptogenic factors. Anti-apoptotic Bcl-2 proteins antagonize the function of the pro-apoptotic Bcl-2 proteins. BH3-only proteins appear to play a dual role, not only as direct activators of Bax or Bak, but also in neutralization of pro-survival members (1–6).
Bak and Bax were initially reported to be functionally redundant and essential for mitochondrial dysfunction and apoptosis in response to multiple death signals (7, 8). In untreated cells these proteins are distinctly localized, with Bax being largely cytosolic and translocating to the mitochondria after apoptotic stimulation, while Bak resides in the outer membrane of the mitochondria. Both proteins undergo conformational changes and homo-oligomerization in response to diverse apoptotic signals, leading to pore formation in the mitochondria and release of apoptosis promoting factors (9–12).
Fundamental to the success of cancer chemotherapy is the induction of apoptosis in tumor cells. Microtubule inhibitors have been widely used as anti-mitotic agents for cancer chemotherapeutic interventions (13). At physiological concentrations, these agents have in common the ability to suppress the dynamic instability of spindle microtubules, leading to mitotic arrest and cell death by apoptosis (14). While Bcl-2 proteins are intimately involved in regulation of apoptosis, their precise role in cell death induced by anti-mitotic drugs is largely unexplored (15–17). Bax has been implicated in apoptosis induced by vinblastine and the nontaxane microtubule inhibitor epothilone (18–20), and Bax expression is a predictor of paclitaxel sensitivity (21). We have previously shown that vinblastine induces Bax mitochondrial translocation, activation and dimerization (20). However, several studies have indicated that paclitaxel does not promote Bax mitochondrial translocation or Bax activation (22, 23), suggesting that other factors are also important. Bak also plays a key role in the regulation of mitochondrial apoptosis. A recent report indicated that Bak-deficient cells are resistant to the DNA damaging agents, VP-16 and cisplatin (24). However, whether Bak plays a role in apoptosis induced by microtubule inhibitors is not known.
In this paper we provide evidence for a key role of Bak in vinblastine-induced apoptosis. We show that vinblastine promotes the conformational activation and oligomerization of Bak, and present evidence that it is the physical interaction of Bak with Bax that is the critical step for subsequent apoptosis. Studies with cells deficient in Bak and/or Bax were also performed and double knockout cells were found to be highly vinblastine-resistant, confirming that apoptosis induced after mitotic arrest occurs most efficiently when both Bak and Bax are present.
Materials and Methods
Materials
Antibodies to caspase 3 (sc-7148), Bcl-2 (sc-509) and Mcl-1 (sc-19) were obtained from Santa Cruz; rabbit polyclonal anti-Bax antibody (catalog # 2772) was from Cell Signaling; mouse monoclonal anti-Bak antibody (catalog # AM03) was from Calbiochem; antibody to cytochrome c (catalog # 5564330) was from Pharmingen; and antibody to GAPDH (catalog # 4300) was from Ambion. 6A7 active Bax antibody was from Axxora and antibody to active Bak (NT) (catalog # 06-536) was from Upstate. DNA fragmentation apoptosis kit was obtained from Roche Applied Science and lipofectamine reagents were obtained from Invitrogen. Vinblastine and other chemicals, unless otherwise stated, were obtained from Sigma Chemical Co.
Cell Culture and Transfection
The KB-3 human carcinoma cell line was maintained in monolayer culture at 37°C and 5% CO2 in DMEM, supplemented with 10% FBS, 2 mM L-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. Cells stably overexpressing HA-Bcl-xL or HA-Bcl-2 were prepared by transfecting KB-3 cells with 10 μg of plasmid DNA (HA-Bcl-xL-pcDNA3.1 or HA-Bcl-2-pCDNA3.1) using Lipofectamine Plus reagent in serum-free DMEM. After 3 h, the transfection medium was replaced with complete medium and, after an additional 24 h, G418 was added to a final concentration of 1 mg/ml, and the cells were maintained for two weeks. Drug resistant colonies were selected, expanded, and maintained in growth medium containing 0.4 mg/ml G418. Clones were screened for Bcl-xL and HA immunoreactivity by immunoblotting using specific antibodies. MEFs that contain a homozygous disruption of the Bak or Bax alleles and wild-type MEFs were a generous gift of Stanley J. Korsmeyer’s laboratory.
Preparation of Cell Extracts and Subcellular Fractions
For whole cell extracts, cells were lysed for 30 min on ice in lysis buffer (40 mM HEPES, pH 7.4, 120 mM NaCl, 1% Triton-X-100, 1mM EDTA, 5 mM dithiothreitol, 20 mM β-glycerophosphate, 1 mM Na3VO4, 50 mM NaF, 20 μg/ml aprotinin, 50 μg/ml leupeptin, 10 μM pepstatin, 1 μM okadaic acid, and 1 mM PMSF), insoluble material was removed by centrifugation at 9,200 × g for 20 min, and protein concentration in the supernatant was determined using Bradford reagent (Biorad). Cytosolic and mitochondrial extracts were prepared using the fractionation kit from GBiosciences. Briefly, 107 cells were washed with PBS twice and then incubated with 0.5 ml of SubCell Buffer-I on ice for 10 min. The cells were lysed using a Dounce homogenizer by 20 strokes of the pestle. The lysate was transferred to a centrifuge tube, the volume adjusted to 0.7 ml, and then 0.35 ml of 3X-concentrated SubCell Buffer II was added. The sample was centrifuged at 700 × g for 10 min to pellet the nuclei and the supernatant was centrifuged at 12,000 × g for 15 min to obtain cytosol in the supernatant and a mitochondrial pellet which was resuspended in storage buffer.
To examine oligomeric forms of Bak, washed cell pellets were incubated in buffer A (10 mM HEPES, pH 7.4, 0.15 M NaCl, 1 mM EGTA, plus phosphatase and protease inhibitors, as above) containing 0.01% digitonin for 2 min at 4°C and centrifuged at 15,000 × g for 15 min. The pellet was extracted with buffer A containing 3% CHAPS for 45 min at 4°C to release membrane- and organelle-bound proteins, which were isolated in the supernatant after centrifugation at 15,000 × g for 15 min. The samples were prepared for SDS-PAGE under non-reduced conditions (without β-mercaptoethanol) with heating to 70°C for 5 min.
Immunoprecipitation
To examine the activation status of Bak, cells were lysed in 0.5 ml of lysis buffer (40 mM HEPES, pH 7.4, 120 mM NaCl, 1% CHAPS, 1 mM EDTA, supplemented with protease and phosphatase inhibitors) by incubating on ice for 30 min and centrifugation at 9,200 × g for 20 min. The extract (1 mg) was precleared with anti-rabbit or anti-mouse goat IgG agarose beads, according to the manufacturers’ directions (eBiosciences), and to the supernatant was added 5 μg of rabbit polyclonal antibody to active Bak (NT) or mouse monoclonal antibody to active Bax (6A7). After mixing for 1 h, anti-rabbit or anti-mouse IgG agarose beads were added to Bak or Bax immunoprecipitations, respectively, for an additional 3 h. The immunoprecipitates were then washed five times in the following buffers: (a) TBS containing 0.05% Tween 20; (b) 50 mM HEPES, pH 7.5, 40 mM NaCl, 2 mM EDTA, 1% CHAPS; (c) 50 mM HEPES, pH 7.5, 40 mM NaCl, 2 mM EDTA, 0.5% CHAPS, 0.5 M LiCl; (d) 50 mM HEPES, pH 7.5, 40 mM NaCl, 2 mM EDTA, 0.5 M LiCl; and (e) 50 mM HEPES, pH 7.5, 150 mM NaCl. The immunoprecipitates were incubated in SDS-PAGE sample buffer for 1 h at 37°C and resolved by 12.5% acrylamide SDS-PAGE and analyzed by immunoblotting.
Apoptosis Assays
Cells were trypsinized following drug treatment and diluted to a concentration of 5 × 104 cells/ml for measurement of apoptosis using a cell death detection enzyme-linked immunosorbent assay kit (Roche Applied Science). This is a quantitative photometric immunoassay for the determination of cytoplasmic histone-associated oligosomes generated during apoptosis. After dilution the cells were centrifuged at 200 × g for 5 min, and the cell pellet was resuspended in 0.5 ml of incubation buffer and incubated at room temperature for 30 min. After centrifugation at 16,000 × g for 10 min, 0.4 ml of the supernatant was removed and diluted 1:10 in incubation buffer for analysis. The enzyme-linked immunosorbent assay plate was prepared according to the manufacturer’s instructions, and 0.1 ml of sample was added to appropriate wells and incubated at room temperature for 90 min. After conjugation and incubation with substrate solution, the plate was shaken on an orbital shaker at 250 rpm for 15 min, and then the absorbance at 405 nm was determined using a Bio-Tek ELx800 microplate reader.
Results
Subcellular Localization of Bak in Vinblastine Induced Apoptosis
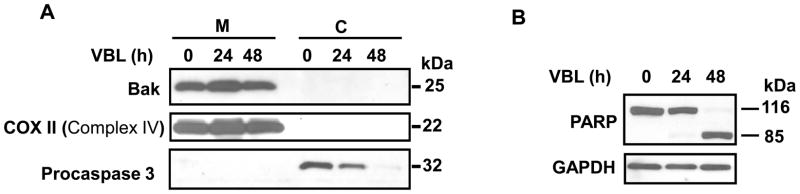
Cytosolic and mitochondrial fractions were prepared from control and vinblastine-treated KB-3 cells to determine the subcellular location of Bak and to monitor any changes with vinblastine treatment. The integrity of the fractions was demonstrated by immunoblotting for procaspase 3 (32 kDa), which was detected in the cytosolic fraction and not in the mitochondrial fraction, and for COX II (Complement IV) (22 kDa), which was detected in the mitochondrial fraction and not in the cytosolic fraction (Figure 1A). Apoptosis occurred mainly between 24 and 48 h of drug treatment, as indicated by PARP cleavage (Figure 1B) as well as loss of procaspase 3 (Figure 1A), consistent with our earlier data that apoptosis ensues as a relatively late event following a prolonged mitotic arrest (25). Bak (25 kDa) was present in the mitochondrial fraction and not in the cytosolic fraction and its location remained unchanged after vinblastine treatment (Figure 1A).
Figure 1.
Mitochondrial Bak localization. KB-3 cells were untreated or treated with 30 nM vinblastine for the times indicated. A, Cytosolic (C) and mitochondrial (M) fractions were prepared and subjected to immunoblotting for Bak, Cox II (Complex IV), and procaspase 3, as indicated. B, Whole cell extracts were prepared and subjected to immunoblotting for PARP and GAPDH as a loading control. Uncleaved (116 kDa) and cleaved (85 kDa) PARP species are indicated.
Vinblastine Induces Bak Oligomerization
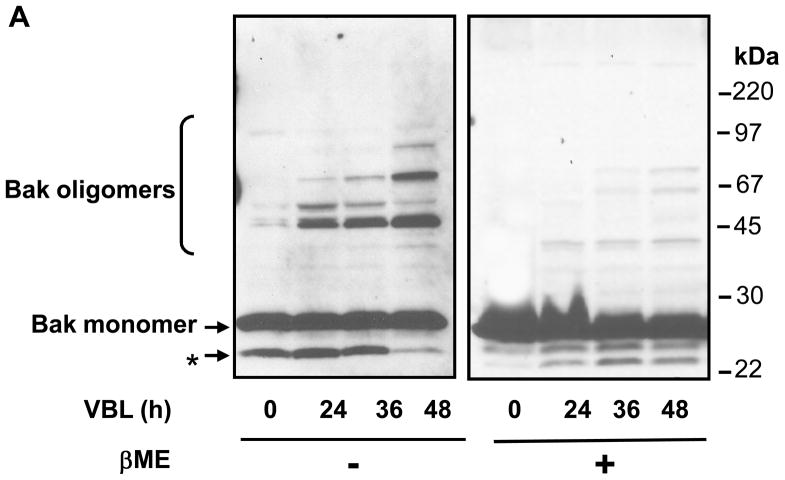
The constitutively integrated Bak has been shown to respond to multiple death stimuli by forming oligomers in the mitochondrial membrane (7, 26). To determine whether vinblastine treatment induced Bak oligomerization, KB-3 cells were untreated or treated with vinblastine and permeabilized with digitonin. The particulate fractions were extracted with CHAPS, and unreduced samples were subjected to immunoblotting for Bak. As shown in Figure 2A, Bak migrated as a monomer of 25 kDa in control cells. With vinblastine treatment, major immunoreactive bands of 50 and 75 kDa, of intensity progressive with time of treatment, were observed, consistent with Bak dimer and trimer, and other oligomeric species were also present. While vinblastine treatment consistently generated oligomeric forms of Bak, the relative abundance of the different species varied to some degree from experiment to experiment. The major oligomeric forms of Bak were eliminated by prior reduction with β–mercaptoethanol (Figure 2A), suggesting that they require disulphide bonds for their formation or maintenance. The low molecular weight immunoreactive species indicated by the asterisk in Figure 2A has previously been suggested to represent an intrachain crosslink conformer of inactive Bak (7). It was more readily apparent when samples were analyzed in the absence of β–mercaptoethanol.
Figure 2.
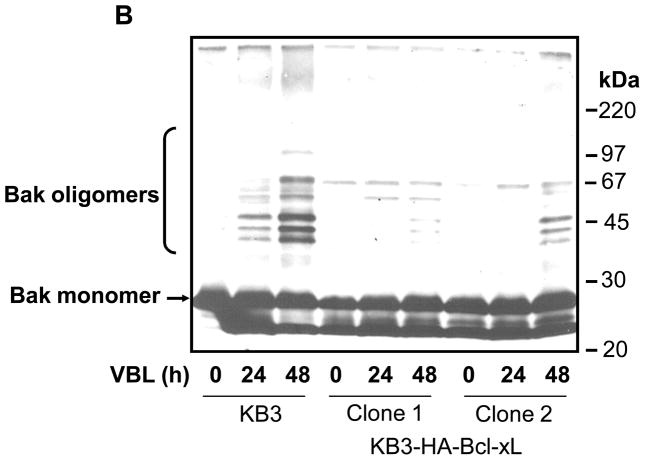
A. Vinblastine induces oligomerization of Bak. KB-3 cells were treated with vinblastine (VBL, 30 nM) for the times indicated, permeabilized with digitonin, and CHAPS solubilized particulate fractions were subjected to SDS-PAGE in the absence or presence of 1% β-mercaptoethanol (β-ME). Immunoblotting for Bak was performed with the monomeric and oligomeric forms indicated. The asterisk denotes the intrachain crosslink conformer of inactive Bak. Molecular mass standards are indicated on the right. B. Overexpression of Bcl-xL inhibits vinblastine-induced Bak oligomerization. KB-3 and two independent KB3-HA-Bcl-xL expressing stable clones were untreated or treated with 30 nM vinblastine (KB-3 cells) or 100 nM vinblastine (KB3-HA-Bcl-xL cells) as indicated, permeabilized with digitonin, and CHAPS solubilized particulate fractions were subjected to SDS-PAGE in the absence of reducing agent. Immunoblotting was performed for Bak and the monomeric and oligomeric forms are indicated. Molecular mass standards are indicated on the right.
Inhibition of Bak Oligomerization by Bcl-xL Overexpression
Anti-apoptotic proteins such as Bcl-xL and Bcl-2 have been shown to block cytochrome c release and Bak oligomerization in response to stress (27, 28). To determine whether Bcl-xL prevented vinblastine-induced Bak oligomerization, we generated stable transfectants termed KB3-HA-Bcl-xL cells, as described in “Materials and Methods.” Two representative clones were used that significantly overexpressed HA-Bcl-xL relative to control cells (20). KB-3 and KB3-HA-Bcl-xL cells were untreated or treated with vinblastine (Figure 2B). Particulate fractions were prepared and unreduced samples were analyzed by immunoblotting. Bak oligomerization in response to vinblastine treatment was much reduced in the two KB-3-HA-Bcl-xL clones in comparison to that in KB-3 cells (Figure 2B).
Vinblastine-induced Bak Activation and its Interaction with Bax
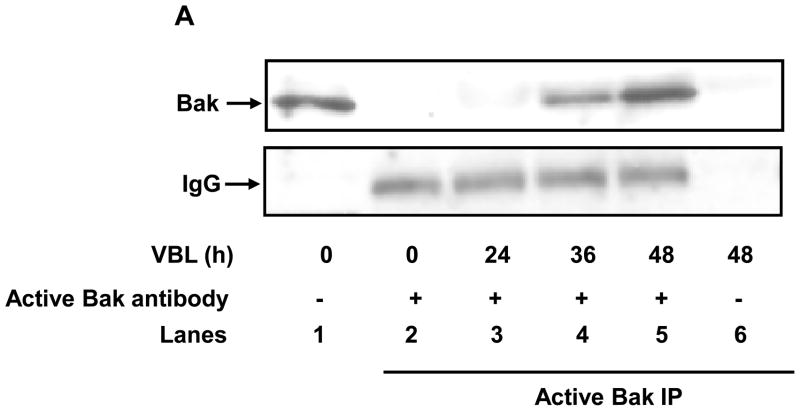
To determine whether Bak oligomerization was associated with Bak activation in KB-3 cells, immunoprecipitations were performed under native conditions using the anti-Bak (NT) rabbit polyclonal antibody, which recognizes the conformationally active form of Bak. Immunoprecipitates were analyzed by immunoblotting using a Bak antibody of mouse origin. As shown in Figure 3A (lanes 2–5), the active form of Bak was not detected in control cells and barely detected in cells treated with vinblastine for 24 h, whereas active Bak was readily detected at 36–48 h. Immunoblotting verified the presence of Bak in the original extract (lane 1) and confirmed an absence of active Bak when the precipitation was conducted in the absence of antibody (lane 6). Immunoblotting for IgG was used to show that equivalent amounts of antibody were used in the immunoprecipitations and to show equal gel loading of the immunoprecipitated material.
Figure 3.
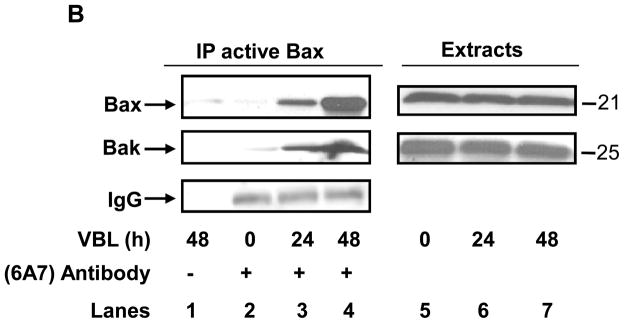
Vinblastine induces Bak activation and Bak-Bax interaction. A, KB-3 cells were treated or untreated with vinblastine (VBL, 30 nM) for the times indicated and subjected to immunoprecipitation with anti-Bak (NT) antibody followed by immunoblotting for Bak (lanes 2–5, upper panel). Sample precipitated in the absence of NT antibody (lane 6) and a whole cell extract (lane 1) were also examined as controls. Immunoblotting of IgG was used as an additional control for immunoprecipitation (lanes 2–5, lower panel). B, KB-3 cells were untreated or treated with vinblastine (VBL, 30 nM) for the times indicated and subjected to immunoprecipitation with anti-Bax 6A7 antibody (lanes 2–4), followed by immunoblotting for Bax or Bak. Precipitates prepared in the absence of 6A7 antibody (lane 1) and whole cell extracts (lanes 5–7) were also examined as controls. Immunoblotting of IgG was used as an additional control for immunoprecipitation (lanes 2–4, lower panel).
To determine whether vinblastine promoted the interaction of the two apoptotic proteins, Bak and Bax, active Bax was immunoprecipitated with 6A7 antibody which recognizes the conformationally active form of Bax. The active form of Bax was readily detected in cells treated with vinblastine for 24 and particularly at 48 h (Figure 3B, left upper panel). Bak was also detected in the immunoprecipitates that contained active Bax (Figure 3B, left middle panel), and a proportional relationship was revealed between the level of active Bak and that of active Bax. Immunoblotting for IgG in the immunoprecipitates confirmed equal loading (Figure 3B, left lower panel). Immunoblotting showed that Bax and Bak were present in equivalent amounts in the original extracts (Figure 3B, right panel), thus demonstrating the specificity of the antibodies used against the activated forms of these proteins, and confirming equal protein loading. Reciprocal immunoprecipitations confirmed the interaction of active Bak with active Bax (see Figure 4C).
Figure 4.
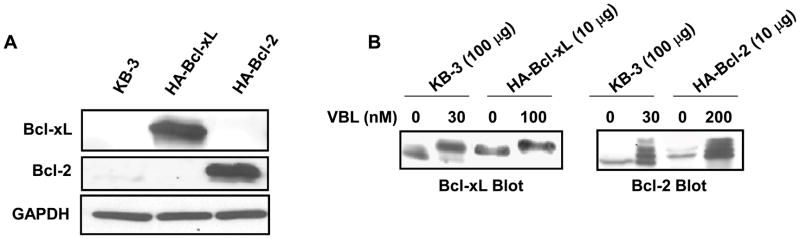
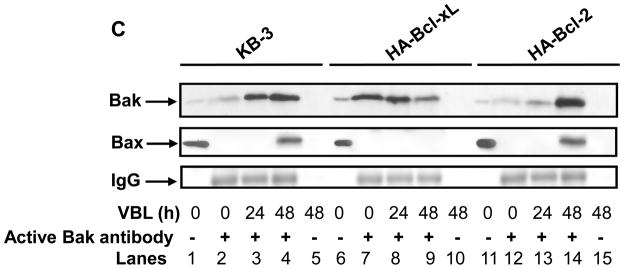
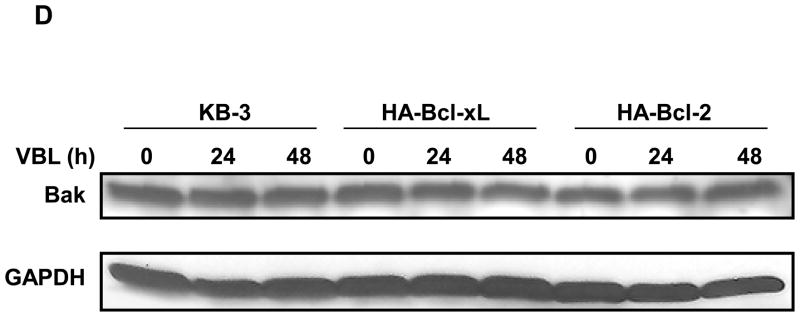
Overexpression of Bcl-xL but not Bcl-2 inhibits vinblastine–induced Bak activation and Bak–Bax interaction. A, Stable expression of HA-Bcl-2 and HA-Bcl-xL. Extracts (30 μg protein) from KB-3 cells, and stably transfected HA-Bcl-xL and HA-Bcl-2 cells, were subjected to immunoblotting for Bcl-xL or Bcl-2 antibody. GAPDH was used as the loading control. B, Phosphorylation of HA-Bcl-xL and HA-Bcl-2. KB-3 cells and cells stably expressing HA-Bcl-xL or HA-Bcl-2 were treated with the indicated concentration of vinblastine for 24 h and extracts containing the indicated amount of protein were immunoblotted for Bcl-xL or Bcl-2. C, KB-3, KB3-HA-Bcl-xL or KB3-HA-Bcl-2 cells were untreated or treated for the indicated times with vinblastine at 30, 100, and 200 nM, respectively. Extracts were subjected to immunoprecipitation with active Bak antibody as indicated (lanes 2–4, 7–9, 12–14, upper panel). The same samples were also probed for Bax (middle panel) and IgG (lower panel). Precipitates prepared in the absence of Bak antibody (lanes 5, 10, 15) and whole cell extracts (lanes 1, 6, 11) were also examined as controls. D, KB-3, KB3-HA-Bcl-xL or KB3-HA-Bcl-2 cells were treated for the indicated times with 30, 100, or 200 nM vinblastine, respectively, and extracts subjected to immunoblotting for Bak or GAPDH.
Overexpression of Bcl-xL but not Bcl-2 Blocks Bak-Bax Interaction
We next examined whether the interaction between activated Bak and Bax in response to vinblastine treatment was affected by the anti-apoptotic proteins Bcl-xL and Bcl-2. Towards this goal, we generated stable transfectants overexpressing Bcl-xL and Bcl-2, termed KB3-HA-Bcl-xL and KB3-HA-Bcl-2, respectively, as described in “Materials and Methods.” The overexpressed proteins were readily identified in the respective cell lines by immunoblotting (Figure 4A). Because Bcl-xL and Bcl-2 are both phosphorylated in response to vinblastine (25), and the phosphorylation status could affect protein:protein interaction, it was important to establish conditions where the overexpressed proteins were phosphorylated to a similar extent as the endogenous protein in KB-3 cells. Therefore, we treated KB-3, KB3-HA-Bcl-xL, and KB3-HA-Bcl-2 cells with increasing concentrations of vinblastine, and examined phosphorylation by mobility shift after immunoblotting (Figure 4B). These results indicated that while 30 nM vinblastine was sufficient to cause a characteristic phosphorylation-induced mobility shift in Bcl-xL and a characteristic “ladder’ representing multiple phosphorylated forms of Bcl-2 (25), higher concentrations, of 100 nM and 200 nM, respectively, were required to achieve the same degree of modification in KB3-HA-Bcl-xL and KB3-HA-Bcl-2 cell lines (Figure 4B). In addition, as protein overexpression obscured the mobility shift, lower amounts of protein (10 μg versus 100 μg) were used for analysis. Figure 4B also demonstrates that HA-Bcl-xL and HA-Bcl-2 are overexpressed to an extent that is approximately 10-fold greater than their respective endogenous counterparts, thus validating direct comparison of these cell lines.
The cell lines were treated with their appropriate concentration of vinblastine, and Bak immunoprecipitated with the conformation-dependent antibody and analyzed by immunoblotting (Figure 4C). In KB-3 cells, as before, vinblastine induced a time-dependent activation of Bak, and Bax was also detected in the active Bak immunoprecipitate at 48 h (Figure 4C, lanes 2–4). A very different result was found for KB3-HA-Bcl-xL cells. In this case, significant levels of conformationally altered Bak were found in untreated cells, and vinblastine treatment actually caused a decrease in “active” Bak (Figure 4C, lanes 7–9). This experimental result was highly reproducible, and essentially identical results have been observed twice in two independent Bcl-xL overexpressing cell lines. Importantly, and in contrast to KB-3 cells, Bax was not detected in the Bak immunoprecipitates from KB3-HA-Bcl-xL cells. Results with KB3-HA-Bcl-2 were highly comparable to those of KB-3 cells, with vinblastine causing time-dependent Bak activation and Bak-Bax interaction (Figure 4C, lanes 12–14). Equal protein loading was confirmed by blotting for IgG in the immunoprecipitates (Figure 4C) and for Bak and GAPDH in the original extracts (Figure 4D).
Inhibition of Vinblastine-induced Apoptosis by Bcl-xL but not Bcl-2 overexpression
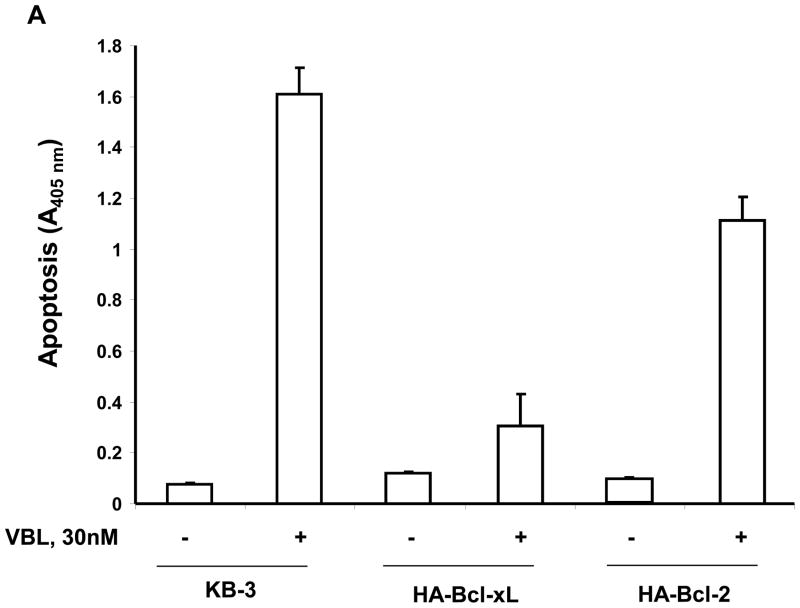
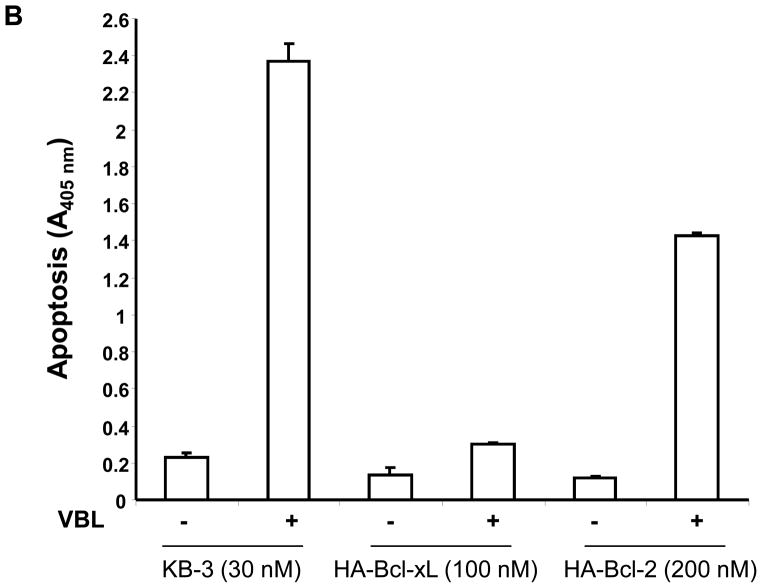
The results presented above show that overexpression of Bcl-xL induces conformational changes in Bak, independent of drug treatment, thus altering the normal process of Bak activation in response to vinblastine. In addition, Bcl-xL overexpression prevents Bak-Bax interaction. However, Bak activation and its interaction with Bax were unaffected by Bcl-2 overexpression. To determine if these observations are relevant to the induction of apoptosis, quantitative assays for apoptosis were conducted (Figure 5). In KB-3 cells, 30 nM vinblastine was used, and drug treatment strongly induced apoptosis in two independent experiments (Figure 5A, 5B). For KB3-HA-Bcl-xL cells, we used both 30 nM vinblastine, equivalent to that used for KB-3 cells, as well as 100 nM vinblastine, corresponding to conditions of maximum Bcl-xL phosphorylation. KB-3-HA-Bcl-xL cells were found to be highly vinblastine-resistant, with less than 20% the level of apoptosis observed in KB-3 cells under similar conditions (Figure 5A, 5B). In contrast, KB3-HA-Bcl-2 cells were much more susceptible to vinblastine-induced apoptosis compared to KB-3-HA-Bcl-xL cells, with levels of apoptosis of 60–70% of that found in KB-3 cells (Figure 5A, 5B).
Figure 5.
Inhibition of vinblastine-induced apoptosis by overexpressed Bcl-xL but not Bcl-2. A, KB-3, KB3-HA-Bcl-xL or KB3-HA-Bcl-2 cells were untreated or treated with vinblastine (30 nM, 48 h), and the relative extent of apoptosis was assessed quantitatively, as described under “Materials and Methods.” B, KB-3, KB3-HA-Bcl-xL or KB3-HA-Bcl-2 cells were untreated or treated with 30 nM, 100 nM or 200 nM vinblastine, respectively, for 48 h, and apoptosis was determined as in A. The results represent the mean ± S.D. (n = 6) and are representative of two independent experiments.
Studies with Bak−/− and Bax−/− MEFs
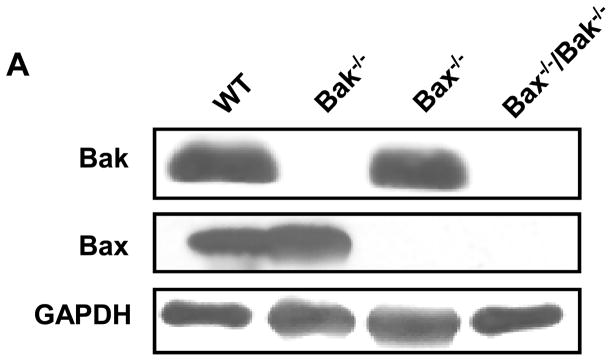
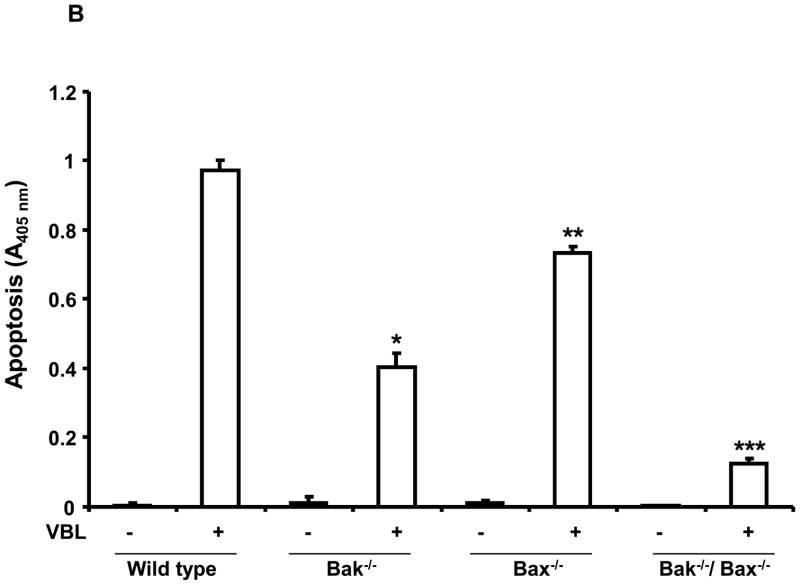
To extend these findings and further examine and compare the roles of Bak and Bax in vinblastine-induced apoptosis, we used mouse embryonic fibroblasts (MEFs) singly or doubly deficient in these pro-apoptotic regulators (7, 29). Figure 6A shows immunoblots confirming the presence or absence of Bak and Bax in wild-type, Bak−/−, Bax−/−, and Bak−/−/Bax−/− MEFs. MEFs were next treated with vinblastine and subjected to quantitative apoptotic assays (Figure 6B). Relative to wild-type cells, vinblastine-induced apoptosis was strongly impaired in Bak−/− MEFs, apoptosis was also significantly impaired in Bax−/− MEFs, and Bak−/−/Bax−/− MEFs were highly vinblastine-resistant (see P values in Figure 6B legend).
Figure 6.
Bak −/− deficient cells are resistant to vinblastine-induced apoptosis. A, Extracts were prepared from wild-type, Bak−/−, Bax−/−, and Bak−/−/Bax−/− MEFs and immunoblotted for Bak, Bax and GAPDH. B, Wild-type (WT), Bak−/−, Bax−/−, and Bak−/−/Bax−/− MEFs were untreated or treated with vinblastine (VBL, 30 nM) for the indicated times and apoptosis was quantitatively assessed, as described under “Materials and Methods.” The results are shown as the mean ± S.D. (n = 6) and are representative of two independent experiments. * P < 0.005; ** P < 0.005; *** P < 0.0005.
Phosphorylation and Degradation of Mcl-1
The studies presented above support a key role for Bak in vinblastine-induced apoptosis, and it has been reported that the anti-apoptotic Bcl-2 member Mcl-1 plays a role in Bak sequestration (12). Therefore it was of interest to examine Mcl-1 in this system. KB-3 cells were treated with vinblastine, and Mcl-1 expression probed by immunoblotting. Interestingly, Mcl-1 underwent a mobility shift at 24 h treatment, and expression was greatly diminished after 48 h (Supplemental Fig. S1). Essentially the same pattern, presumably reflecting phosphorylation and degradation of Mcl-1, was found in cells overexpressing Bcl-xL and Bcl-2 (Fig. S1). Thus vinblastine-induced Mcl-1 processing occurred largely independently of these other anti-apoptotic Bcl-2 members, and because it occurred in Bcl-xL overexpressing cells where apoptosis was shown to be defective, loss of Mcl-1 was not a consequence of cell death.
Discussion
Microtubule inhibitors such as vinblastine can be thought to exert their lethality in several stages. Their primary mechanism of action is binding to tubulin or microtubules causing disruption of spindle dynamics which leads to mitotic arrest (14). This in turn results in sustained activation of the mitotic checkpoint and subsequent initiation of apoptotic cell death. This phase is poorly characterized but appears to be regulated by the integration of several different signaling pathways involving mitogen-activated kinases, p53, NFκB, and others (17). The transition between mitotic arrest and apoptosis initiation also correlates temporally with phosphorylation of Bcl-2 and Bcl-xL, but the role of these modifications has not been firmly established. The third most distal stage involves changes in mitochondrial permeability and the release of cytochrome c and other apoptosis-promoting factors. Apoptotic stimuli that exert their effects through the mitochondrial pathway most often cause changes in the properties and activities of the Bax subfamily of Bcl-2 proteins (1–6). While several reports have implicated Bax activation in apoptosis induced after mitotic arrest (18–20), the findings presented here are the first to report, to our knowledge, a key role for Bak in this context.
In this paper we show that vinblastine induces distinct changes in the structure and properties of Bak. While Bak was mainly monomeric in untreated cells, oligomers were observed at 24–48 h of treatment, the period when overt signs of apoptosis, including PARP cleavage, caspase-3 activation, and DNA fragmentation, begin and proceed (25, 30). The presence of major species of 50 and 75 kDa (Figure 2) is consistent with vinblastine-induced Bak homo-oligomerization. However, the presence of other molecular species suggests that Bak hetero-oligomers may also be formed. Several candidates exist for specific interaction with Bak including anti-apoptotic Bcl-2 proteins as well as the voltage-dependent anion channel 2, which acts as a Bak inhibitor (31). Bak oligomerization has been mainly characterized in cells treated with truncated Bid as the apoptotic stimulus (7, 28). Bak oligomerization has also been reported to occur in response to ATP depletion (11) and in response to heat (27). In each of these cases, however, detection of the oligomers required the presence of crosslinking agents. In our case, Bak oligomers were detected after vinblastine treatment without the aid of crosslinking agents. Indeed, the crosslinker dithio(succinimidyl propionate), which we have used previously to detect Bax oligomers (20), did not enhance Bak oligomerization (data not shown). Thus, the Bak oligomers that form in response to vinblastine appear to be relatively stable, although variation in the abundance of the different species may reflect some degree of instability. When samples were prepared in the presence of β-mercaptoethanol, the Bak oligomers were not observed, indicating a requirement for disulphide bonds in their formation or maintenance.
Previously we have shown that vinblastine induces the mitochondrial translocation as well as the oligomerization and activation of Bax in KB-3 cells (20). Using a conformation-dependent Bak antibody, we show in the present report that vinblastine induces Bak activation. Active Bak was most prominent between 24 and 48 h of vinblastine treatment, corresponding to the onset and duration of apoptosis, and corresponding to the appearance of Bak oligomers. Immunoprecipitates of active Bak revealed the presence also of Bax, and the reciprocal experiment showed that active Bax co-precipitated with Bak. These results, demonstrating a physical interaction between Bak and Bax after vinblastine treatment, are the first to show, to the best of our knowledge, an interaction between these pro-apoptotic Bcl-2 family members in the apoptotic response to microtubule inhibition. While many apoptotic stimuli have been shown to activate Bax and/or Bak, very few have been reported to induce the interaction of these two multidomain pro-apoptotic proteins. ATP depletion-induced apoptosis in rat proximal tubule cells requires the interaction between Bak and Bax homo-oligomers (11), and tumor necrosis factor-α-induced apoptosis is also associated with Bak-Bax interaction (32). However, the precise topology of these proteins and their role in the permeabilization of the mitochondrial outer membrane is still under active investigation (33).
Cells overexpressing Bcl-xL and Bcl-2 were used to establish the importance of Bak/Bax interaction in vinblastine-induced apoptosis. Interestingly, Bcl-xL overexpression caused conformational changes in Bak in the absence of drug treatment, such that the protein was now recognized by the anti-Bak NT antibody. While the presence of excessive Bcl-xL appeared to unmask the Bak epitope responsible for antibody binding, the mechanism underlying this observation is not presently clear, although it is of interest that Bcl-2 overexpression did not produce such an effect. Cells overexpressing Bcl-xL did not display any signs of basal apoptosis, so this conformational change in Bak was not sufficient of itself to induce apoptosis. Overexpression of Bcl-xL was found to disrupt vinblastine-induced Bak-Bax interaction and strongly inhibit apoptosis. In contrast, overexpression of Bcl-2 did not affect the normal kinetics of Bak activation or Bak-Bax interaction, and only weakly inhibited apoptosis. These results provide evidence that Bak-Bax interaction plays a key role in vinblastine-induced apoptosis. Furthermore, these results indicate that the pro-survival proteins Bcl-xL and Bcl-2 play selective roles in this context and differ in their ability to interact with the multidomain Bax subfamily proteins.
These findings raise the question of how Bcl-xL functions to block Bak-Bax interaction, and why Bcl-2 does not possess this property. Recent results have indicated that Bak is sequestered by Bcl-xL but not by Bcl-2, and that the Bak BH3 domain is required for this interaction (12). The sequestration of mitochondrial Bak by mitochondrial Bcl-xL in KB3-HA-Bcl-xL cells may be a key inhibitory mechanism. The inhibitory effect of Bcl-xL on vinblastine-induced apoptosis in KB-3 cells may also be due to effects on Bax, as we have previously shown that Bcl-xL overexpression blocks conformational changes and oligomerization of Bax in vinblastine treated KB-3 cells (20). In addition, we showed that Bcl-xL interacts with inactive cytosolic Bax, but not with active mitochondrial Bax following its translocation in response to vinblastine. This suggests that Bcl-xL may function in the cytosol to sequester Bax and prevent its mitochondrial translocation and activation, and Bcl-xL may function in the mitochondria to directly block Bak activation. Bak has also been reported to be sequestered by Mcl-1 (12), and we found that vinblastine causes phosphorylation and subsequent loss of Mcl-1 expression (Fig. S1). Other apoptotic stimuli have been shown to induce Mcl-1 phosphorylation, targeting the protein for proteosome-mediated degradation (34). Degradation of Mcl-1 may be an important facet of apoptotic signaling in response to vinblastine, and it is intriguing that all three major anti-apoptotic Bcl-2 proteins (Bcl-2, Bcl-xL, and Mcl-1) undergo vinblastine-induced phosphorylation. Further examination of the interaction and spatial organization of these pro-survival and pro-apoptotic proteins will be needed to decipher the precise sequence of steps involved in Bak and Bax activation, the mechanism of inhibition by Bcl-xL, and the role played by Mcl-1.
Vinblastine-induced apoptosis was strongly impaired, by 87%, in Bak/Bax double deficient cells (Figure 6B), confirming their role, and consistent with these proteins acting as essential distal mediators of mitochondrial apoptosis (7). In MEFs the presence of either Bak or Bax was sufficient for a partial response to vinblastine. This is similar to the earlier study where MEFs singly deficient in either Bak or Bax showed reduced apoptosis in response to several apoptotic stimuli including UV and etoposide compared to wild-type cells (7). In another report it was observed that Bak and Bax can independently induce cytochrome c release during TNF-α induced apoptosis in baby mouse kidney epithelial cells (35). In general, though either protein can suffice to some degree, most reports are consistent with apoptosis proceeding more completely and efficiently when both Bax and Bak are present. It is tempting to speculate, based on the data reported here, that it is the interaction of these two proteins that promotes conformational changes most favorable for mitochondrial membrane permeabilization.
Supplementary Material
Acknowledgments
We are very grateful to Dr. Stanley J. Korsmeyer’s laboratory for providing the mouse embryonic fibroblasts. This work was supported by National Institutes of Health Grants CA109821 and CA075577.
Abbreviations
- PARP
Poly (ADP-ribose) polymerase
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]1-propanesulfonate
- HA
hemaglutinin
- MEF
mouse embryonic fibroblasts
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IgG
immunoglobulin
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- HEPES
(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid )
- EDTA
ethylenediamine tetraacetic acid
- PMSF
phenylmethylsulfonyl fluoride
- PBS
phosphate buffered saline
- EGTA
ethylene glycol tetraacetic acid
- TBS
Tris-buffered saline
- VBL
vinblastine
References
- 1.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–7. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walensky LD. Bcl-2 in the crosshairs: tipping the balance of life and death. Cell Death Differ. 2006;13:1339–50. doi: 10.1038/sj.cdd.4401992. [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 4.Gross A, McDonnell JM, Korsmeyer SJ. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 5.Breckenridge DG, Xue D. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr Opin Cell Biol. 2004;16:647–52. doi: 10.1016/j.ceb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax and Bak. Science. 2007;315:856–9. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 7.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nature Rev Mol Cell Biol. 2005;6:657–63. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 9.Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–34. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- 10.Wolter KG, Hsu YT, Smith CL, Nechustan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–92. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikhailov V, Mikhailova M, Degenhardt K, Venkatachalam MA, White E, Saikumar P. Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J Biol Chem. 2003;278:5367–76. doi: 10.1074/jbc.M203392200. [DOI] [PubMed] [Google Scholar]
- 12.Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowinsky EK, Donehower RC. The Chemotherapy Source Book. 2. Williams and Wilkins; Baltimore, MD: 1998. pp. 387–423. [Google Scholar]
- 14.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 15.Blagosklonny MV, Fojo T. Molecular effects of paclitaxel: myths and reality (a critical review) Int J Cancer. 1999;83:151–6. doi: 10.1002/(sici)1097-0215(19991008)83:2<151::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Wang LG, Liu XM, Kreis W, Budman DR. The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review. Cancer Chemother Pharmacol. 1999;44:355–61. doi: 10.1007/s002800050989. [DOI] [PubMed] [Google Scholar]
- 17.Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–86. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi H, Chen J, Bhalla K, Wang HG. Regulation of Bax activation and apoptotic response to microtubule-damaging agents by p53 transcription-dependent and -independent pathways. J Biol Chem. 2004;279:39431–7. doi: 10.1074/jbc.M401530200. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi H, Paranawithana SR, Lee MW, Huang Z, Bhalla KN, Wang HG. Epothilone B analogue (BMS-247550)-mediated cytotoxicity through induction of Bax conformational change in human breast cancer cells. Cancer Res. 2002;62:466–71. [PubMed] [Google Scholar]
- 20.Upreti M, Lyle CS, Skaug B, Du L, Chambers TC. Vinblastine-induced apoptosis is mediated by discrete alterations in subcellular location, oligomeric structure, and activation status of specific Bcl-2 family members. J Biol Chem. 2006;281:15941–50. doi: 10.1074/jbc.M512586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taguchi T, Kato Y, Baba Y, Nishimura G, Tanigaki Y, Horiuchi C, et al. Protein levels of p21, p27, cyclin E and Bax predict sensitivity to cisplatin and paclitaxel in head and neck squamous cell carcinomas. Oncol Rep. 2004;11:421–426. [PubMed] [Google Scholar]
- 22.Longuet M, Serduc R, Riva C. Implication of bax in apoptosis depends on microtubule network mobility. Int J Oncol. 2004;25:309–17. [PubMed] [Google Scholar]
- 23.Salah-Eldin AE, Inoue S, Tsukamoto S, Aoi H, Tsuda M. An association of Bcl-2 phosphorylation and Bax localization with their functions after hyperthermia and paclitaxel treatment. Int J Cancer. 2003;103:53–60. doi: 10.1002/ijc.10782. [DOI] [PubMed] [Google Scholar]
- 24.Wang GQ, Gastman BR, Weickowski E, et al. A role for mitochondrial Bak in apoptotic response to anticancer drugs. J Biol Chem. 2001;276:34307–17. doi: 10.1074/jbc.M103526200. [DOI] [PubMed] [Google Scholar]
- 25.Du L, Lyle CS, Chambers TC. Characterization of vinblastine-induced Bcl-xL and Bcl-2 phosphorylation: evidence for a novel protein kinase and a coordinated phosphorylation/dephosphorylation cycle associated with apoptosis induction. Oncogene. 2005;24:107–17. doi: 10.1038/sj.onc.1208189. [DOI] [PubMed] [Google Scholar]
- 26.Wei MC, Lindsten T, Mootha VK, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–71. [PMC free article] [PubMed] [Google Scholar]
- 27.Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci U S A. 2005;102:17975–80. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruffolo SC, Shore GC. Bcl-2 selectively interacts with the BID-induced open conformer of BAK, inhibiting BAK auto-oligomerization. J Biol Chem. 2003;278:25039–45. doi: 10.1074/jbc.M302930200. [DOI] [PubMed] [Google Scholar]
- 29.Lindsten T, Ross AJ, King A, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–99. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry A, Goodwin M, Moran CL, Chambers TC. AP-1 activation and altered AP-1 composition in association with increased phosphorylation and expression of specific Jun and Fos family proteins induced by vinblastine in KB-3 cells. Biochem Pharmacol. 2001;62:581–91. doi: 10.1016/s0006-2952(01)00694-3. [DOI] [PubMed] [Google Scholar]
- 31.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–7. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 32.Sundararajan R, Cuconati A, Nelson D, White E. Tumor necrosis factor-alpha induces Bax-Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K. J Biol Chem. 2001;276:45120–7. doi: 10.1074/jbc.M106386200. [DOI] [PubMed] [Google Scholar]
- 33.Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Biol. 2006;18:685–9. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Opferman JT. Unraveling Mcl-1 degradation. Cell Death Differ. 2006;12:1260–2. doi: 10.1038/sj.cdd.4401978. [DOI] [PubMed] [Google Scholar]
- 35.Degenhardt K, Sundararajan R, Lindsten T, Thompson C, White E. Bax and Bak independently promote Cytochrome c release from mitochondria. J Biol Chem. 2002;277:14127–34. doi: 10.1074/jbc.M109939200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.