Abstract
Objective
To determine the outcome of adjunctive renal artery stenting for renal artery coverage at the time of endovascular abdominal aortic aneurysm repair (EVAR).
Methods
Between 8/2000 to 8/2008, 29 patients underwent elective EVAR using bifurcated Zenith stent-grafts and simultaneous renal artery stenting. Renal artery stenting during EVAR was performed with endograft “encroachment” on the renal artery ostium (n = 23) or placement of a renal stent parallel to the main body of the endograft (“snorkel”, n = 8). Follow-up included routine contrast-enhanced computed tomography (CT), multi-view abdominal x-rays, and creatinine measurement at 1, 6, and 12 months, and then yearly thereafter.
Results
31 renal arteries were stented successfully in 29 patients. All patients with planned renal artery stent placement (n=18) had a proximal neck length < 15mm. Mean proximal neck length was shorter in patients who underwent the “snorkel” technique (6.9 ± 3.1 mm) compared to those with planned endograft encroachment (9.9 ± 2.6 mm). None of the patients with unplanned endograft encroachment had neck lengths < 15mm (mean length: 26.3±10.2 mm). Mean proximal neck angulation was 42.8 ± 24.0 degrees and did not differ between the groups. One patient had a type I endoleak on completion angiography, and 2 additional patients had a type I endoleak on the first postoperative CT scan. All type I endoleaks resolved by the one-month postoperative CT scan. Primary-assisted patency of renal artery stents was 100% at a median follow-up of 12.5 months (range 2 days to 77.4 months). One patient had near occlusion of a renal artery stent noted on follow-up CT scan at 9 months; patency was restored by placement of an additional stent. One patient required dialysis following sustained hypotension from a right external iliac artery injury which resulted in prolonged post-operative bleeding. Mean creatinine at baseline was 1.1 ± 0.3 mg/dl, 1.2 ± 0.5 mg/dl at 1 month follow-up, and 1.2 ± 0.5 mg/dl at 2 years of follow-up. There were no cases of late type I endoleaks (>one month postoperatively) or stent-graft migration.
Conclusions
Adjunctive renal artery stenting during endovascular AAA repair using the “encroachment” and “snorkel” techniques is safe and effective. Short and medium term primary patency rates are excellent, but careful follow-up is needed to determine the durability of these techniques.
Introduction
Endovascular repair of infrarenal abdominal aortic aneurysms (AAA) is safe, durable, and effective only when the arterial anatomy permits sealing and fixation at the attachment sites.1-3 A sufficient length of proximal aortic neck is a critical requirement for successful endovascular aneurysm repair (EVAR) using the current FDA-approved infrarenal devices. Many patients with short infrarenal necks have been treated successfully using pararenal stent-grafts with small holes (fenestrations) to the renal arteries.4-6 However, fenestrated stent-grafts remain unavailable in the United States.
In the absence of fenestrated stent-grafts, other ways to preserve renal perfusion include pushing the upper margin of a pararenal stent-graft downwards (“encroachment” technique), or inwards, away from the renal artery orifice using a renal artery stent (“snorkel” technique). Partial encroachment on a renal orifice can be remedied after the fact by placing a stent over the top of the graft (Figure 1). More extensive coverage requires the adjunctive stent to run alongside the stent-graft, creating a “snorkel” or “chimney” to the renal artery (Figure 2). In this technique trans-brachial access to the renal artery is secured before stent-graft deployment.7-8
Figure 1.
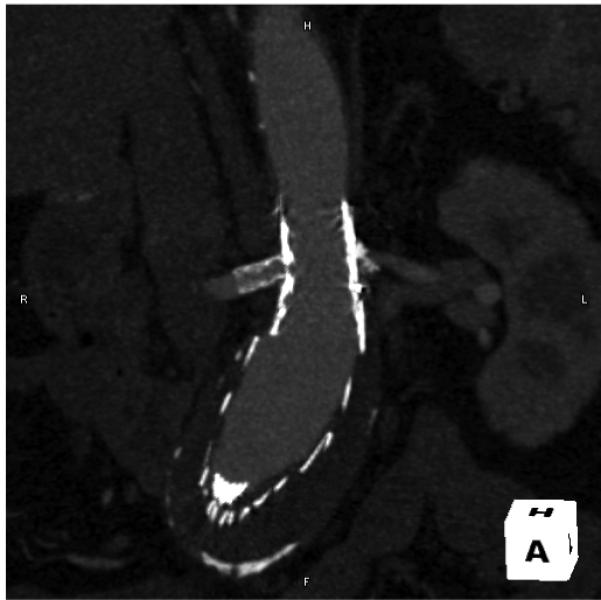
“Encroachment” of aortic stent-graft on right renal artery, with stented right renal artery.
Figure 2.
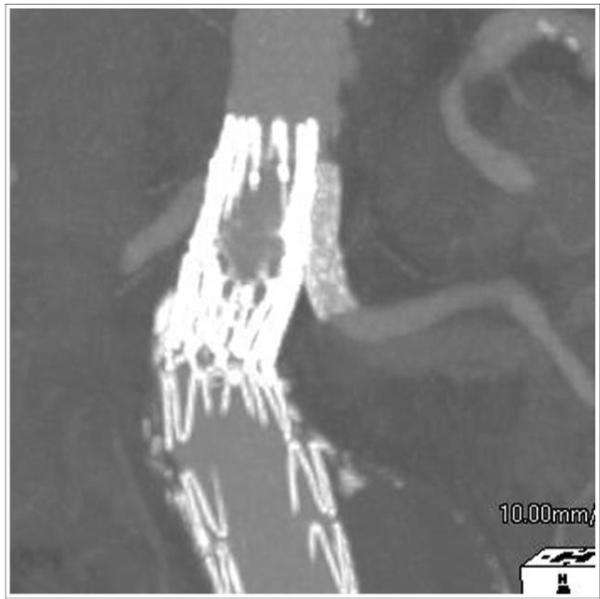
Left renal artery “snorkel” stent running parallel to main body component of aortic stent-graft.
This single-center report describes the technical feasibility and short-term outcome of two adjunctive techniques during EVAR, renal “encroachment” and renal “snorkel”. Both techniques lengthen the proximal implantation site by covering the renal arteries, while maintaining flow to the kidneys through renal stents.
Methods
Patients
Between 8/2000 and 8/2008, 29 patients underwent elective EVAR using bifurcated Zenith stent-grafts (Cook Inc., Bloomington Indiana) and simultaneous renal artery stenting at the University of California, San Francisco (UCSF) Medical Center. Only patients whose renal artery stents were placed 1) to treat planned or inadvertent encroachment of the stent-graft fabric on a renal artery orifice or 2) as part of a planned “snorkel” procedure are included in this study. Cases of renal artery stenting for stenosis in the absence of renal artery coverage were excluded. The choice of technique to lengthen the aortic neck (“encroachment” or “snorkel”) was at the discretion of the primary surgeon.
Patient demographic information, including age, gender, aneurysm size, medical co-morbidities, and creatinine levels were collected prospectively. Data on medical co-morbidities included the presence of diabetes mellitus, chronic obstructive pulmonary disease (COPD), hypertension, hypercholesterolemia, and cardiac disease. Cardiac disease was defined as any patient with a history of myocardial infarction, congestive heart failure, ischemic heart disease, or atrial fibrillation.
Techniques
Selective renal catheterization for subsequent stent placement may be performed before stent-graft deployment, or afterwards; through the proximal stent, or alongside it; from the femoral artery, or from the brachial artery. However, the net result depends mainly on the timing of renal catheterization relative to stent-graft deployment. If renal catheterization follows stent-graft deployment, the renal stent tends to run trans-axially, pushing the proximal margin of the encroaching graft down towards the aneurysm; hence, the term “renal encroachment”. If renal catheterization precedes stent-graft deployment, the renal stent tends to run axially alongside the graft as a small vertical conduit from the source of inflow to the orifice of the renal artery; hence, the term “snorkel”.
Endograft Encroachment with Adjunctive Renal Artery Stenting
The Zenith stent-graft is deployed according to the the usual sequence, with particular attention to the position of the proxmal margin of the graft relative to the most caudal renal artery. The position of the graft is shown by radio-opaque markers sutured to the fabric, approximately 1-2 mm from the proximal margin. The position of the renal ostium is identified by catheter angiography. The optimal obliquity depends on the antero-posterior position of the renal artery (especially the right) and the antero-posterior angulation of the aorta. The extent of renal artery coverage is a product of two competing goals, renal preservation and augmented aortic overlap, which depends on neck length, neck angulation, renal function, and the relative positions of the two renal arteries. We seldom cover the entire renal artery.
Catheter access to the renal artery is obtained through the base of one of the triangular interstices of the uncovered proximal stent. The failure of a 5 French catheter to follow a guidewire into the renal artery usually indicates that the wire has found its way into the renal artery through one of the narrow inter-strut apices. The only option is to remove the wire and try again.
Clearly, one cannot traverse the uncovered stent in its compressed, undeployed state, but that does not mean it has to be fully deployed. The partially deployed uncovered stent assumes a conical shape with the top cap constraining the proximal end while the distal end expands with the rest of the stent-graft. Under these circumstances, a catheter can enter the stent-graft through the contralateral gate and exit through the base of the uncovered stent on its way to the renal artery. The technique resembles a step in fenestrated stent-graft insertion.
The choice of femoral versus brachial access depends largely on the orientation of the aorta. Catheters tend to follow the outer curvature of any angulation, and, if the neck is angulated in a coronal plane, a trans-femoral catheter will track towards one renal artery and away from the other. Switching to the trans-brachial route often eliminates, or even reverses, this effect. In addition, trans-brachial access may be the preferred alternative in cases of extensive renal coverage since the trans-brachial route to the renal artery does not bend around the proximal margin of the stent-graft.
Whatever the route of access, trans-brachial or trans-femoral, the guidewire has to be robust enough to push the struts of the uncovered proximal stent and the proximal margin of the graft margin aside for unimpeded renal stent insertion. We use a stiff 0.035” guidewire. The delivery profile of the corresponding stent is larger than the delivery profile of 0.014” and 0.018” stent platforms, but not to a degree that has practical consequences.
We implant short, balloon expanded renal stents. Self-expanding stents are not robust enough. Approximately 5 mm of the renal stent is deployed into the aorta (Figures 3a, 3b). This is the functioning part of the stent. As always, accurate deployment depends on correct orientation of the imaging system. Otherwise, the inner end of the stent appears to be further into the aorta than it actually is.
Figure 3a.
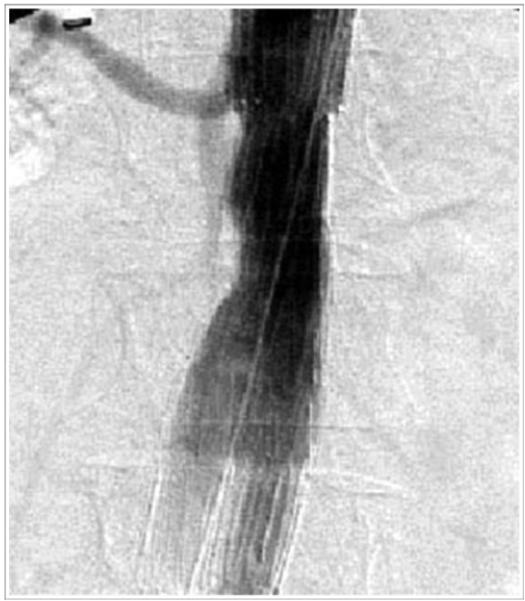
Complete “encroachment” of aortic stent-graft on orifice of left renal artery.
Figure 3b.
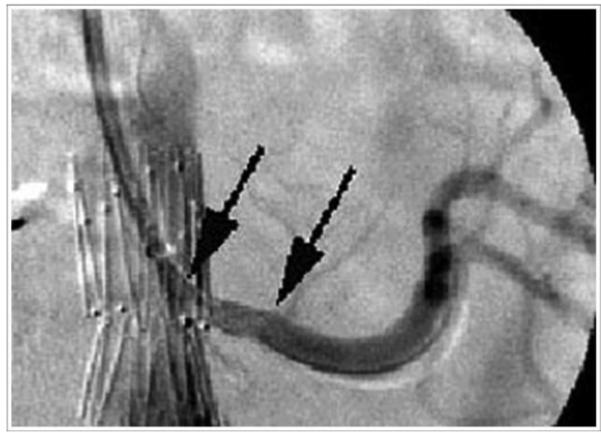
Successful stent placement into left renal artery from brachial approach. Arrows depict proximal and distal extent of renal artery stent.
Renal “Snorkel”
The “snorkel” technique is only for cases of planned renal artery coverage because access to the renal arteries must precede stent-graft deployment. As a result, the renal stent comes to lie entirely outside the stent-graft. Since the renal stent does not traverse the uncovered stent, it pushes the proximal margin of the stent-graft in, not down, and renal coverage is not limited by the maximum extent of graft deformation. In the “snorkel” technique, the only possible route of renal access is from above, through the brachial artery, because the source of arterial inflow is above the margin of the graft.
Trans-brachial catheterization of the renal artery is performed (in the absence of a stent-graft) much as it would for the treatment of renal artery stenosis. We use a stiff 0.035” guidewire in case we have to re-instrument the renal artery after stent-graft deployment, whereupon the additional stiffness would help sheaths, catheters and balloons pass obstacles such as the uncovered stent and proximal graft margin. The stent-graft is usually deployed by reference to the location of the contralateral renal artery on catheter angiograms, with the renal sheath, wire and stent already in place (Figure 4a).
Figure 4a.
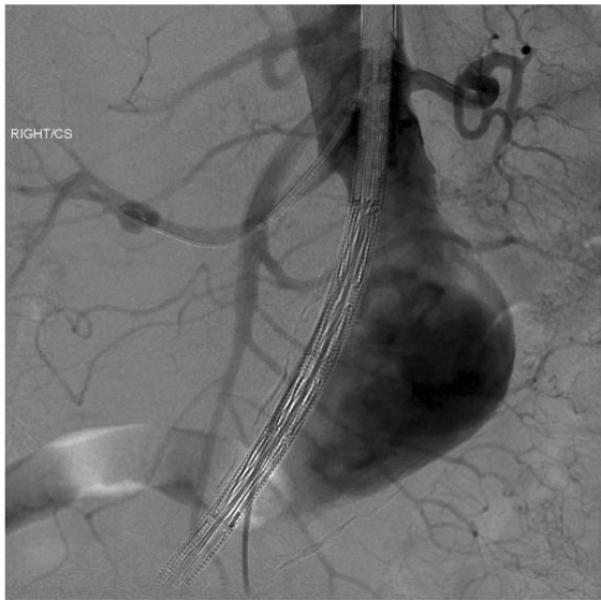
A balloon expandable stent is placed over a Rosen wire from a brachial approach, while the Zenith main body component is placed through the femoral access site.
The length of the renal stent depends on the distance between the proximal margin of the graft and the renal orifice, since the functioning part of the renal stent lies within the aorta. The upper end of the renal stent is deployed at, or above, the margin of the graft, as indicated by the positions of radio-opaque markers. We have used self-expanding stents, balloon expanded-stents, bare metal stents, and covered stents. They all require the luminal support of a balloon during intra-aortic Palmaz stent deployment (Figure 4b). Therefore, renal artery access is maintained until completion angiograms (Figure 4c) show that no further aortic interventions will be needed to treat an endoleak.
Figure 4b.
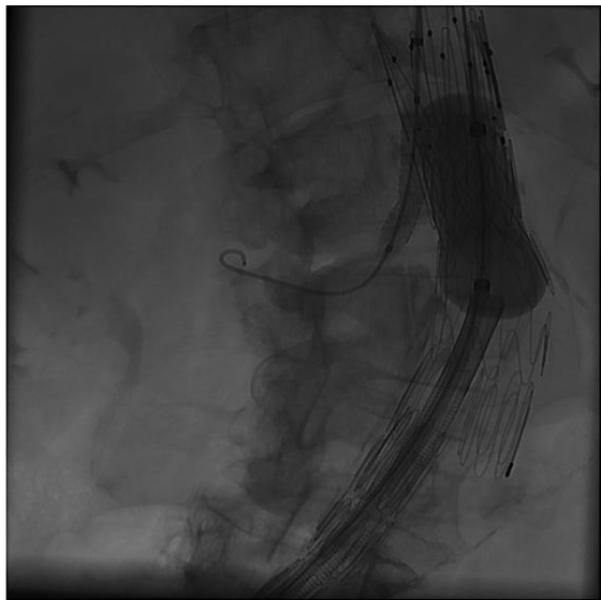
Simultaneous inflation of balloon expandable right renal artery stent and CODA balloon with Palmaz aortic stent.
Figure 4c.
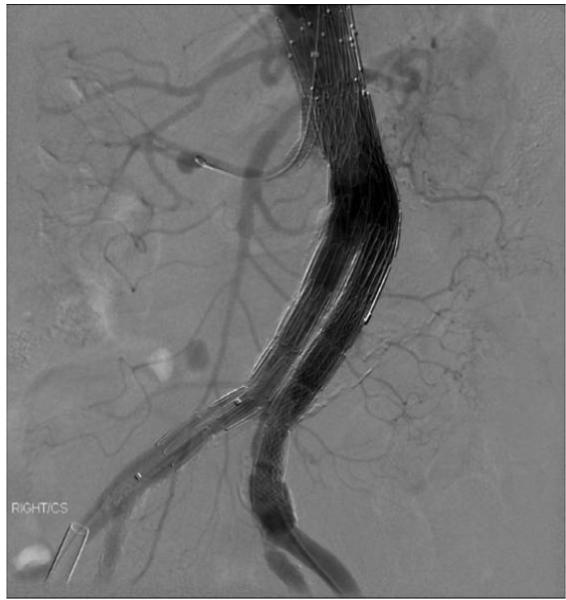
Completion angiography with right renal artery “snorkel” stent.
Follow-up
Follow-up included routine contrast-enhanced computed tomography (CT), multi-view abdominal x-rays, and serum creatinine levels postoperatively and at 1, 6, 12 months, and yearly thereafter. Two patients were followed with non-contrast CT scans in addition to renal artery ultrasound examinations to determine the patency of the renal arteries. Data on complications and interventions were collected prospectively.
Anatomic Measurements
Data on proximal neck length, diameter, and angulation were measured on pre-operative CT scans by two authors (JSH and TAMC). Neck angulation was defined as the angle between the neck of the aneurysm and the main channel of the infrarenal aortic aneurysm after three-dimensional reconstruction of the images on an Aquarius Workstation (Terarecon, San Mateo, CA).
Results
Patient demographics are summarized in Table 1. There were 23 men and 6 women, with an average age of 74.4±7.6 years (range 57.6-84.2 years). Baseline serum creatinine was 1.1 ± 0.3 mg/dL. Mean follow-up was 19.6±21.5 months (median 12.5 months, range 2 days to 6.5 years). One patient was lost to follow-up, and 6/29 (20.7%) patients died during the follow-up period.
Table 1. Patient demographic information.
| Mean Age at Operation (years±SD) | 74.4 ± 7.6 |
| Range (years) | 57.6 - 84.2 |
| Gender | |
| Male (%) | 23 (79.3%) |
| Female (%) | 6 (20.7%) |
| Pre-operative Creatinine (mg/dL ± SD) | 1.1 ± 0.3 |
| Range (mg/dL) | 0.6 - 1.7 |
| Post-operative Creatinine (mg/dL ± SD) | 1.2 ± 0.5 |
| Range (mg/dL) | 0.7 - 3.2 |
| Creatinine - 2 years(mg/dL ± SD) | 1.2 ± 0.5 |
| Range (mg/dL) | 0.7 - 2.5 |
| Mean Follow-up (days± SD) | 597 ± 655 |
| Median Follow-up (days) | 381 |
| Range (days) | 2-2354 |
| Mean Aneurysm Size (mm±SD) | 60 ± 7 |
| Range (mm) | 44-75 |
| Medical Co-Morbidities | |
| Diabetes Mellitus (%) | 5 (17.2%) |
| Cardiac Disease (%) | 20 (69.0%) |
| COPD (%) | 3 (10.3%) |
| Past Smoker (%) | 24 (82.8%) |
| Hypertension (%) | 21 (72.4%) |
| Hyperlipidemia (%) | 20 (69.0%) |
27/29 (93.1%) patients had placement of unilateral renal artery stents, and 2 patients had bilateral renal artery stents placed. 31 renal arteries were successfully stented in 29 patients. 23 renal arteries in 21 patients were stented for endograft encroachment. In 10 of these patients (12 renal arteries), renal stent insertion was part of the planned intervention, and in 11 of these patients (11 renal arteries), it was an unplanned response to inadvertent coverage. In addition, 8 renal arteries were stented in 8 patients utilizing the “snorkel” technique.
Table 2 demonstrates the details of the proximal neck, access sites, and stents used in the renal arteries. All patients with planned renal artery stent placement (n=18) had aortic neck lengths <15mm. Mean proximal neck length was shorter in patients who underwent the “snorkel” technique (6.9±3.1mm) compared to those with planned endograft encroachment (9.9±2.6mm). By using the “snorkel” technique, an additional 10.4mm (range: 4.6-25.9mm) of aortic neck length was gained. None of the cases of unplanned renal stent placement (n=11) had aortic neck lengths <15mm (mean neck length: 26.3±10.2mm). There was no significant difference in neck angulation amongst the three groups.
Table 2. Characteristics of aneurysm neck and stented renal arteries.
| Number of Renal Arteries Stented | |
| Endograft encroachment-planned | 12 |
| Endograft encroachment-inadvertent | 11 |
| Snorkel | 8 |
| Mean Neck Length (mm±SD) range mm) | 15.3 ± 10.9 |
| Range | 3.0 - 48.6 |
| Snorkel (mm±SD) | 6.9 ± 3.1 |
| Encroachment-planned (mm±SD) | 9.9 ± 2.6 |
| Encroachment-inadvertent (mm±SD) | 26.3 ± 10.2 |
| Mean Neck Diameter (mm±SD) | 24.2 ± 4.0 |
| Range (mm) | 16.1 - 32.9 |
| Mean Neck Angulation (degrees±SD) | 42.8±24.0 |
| Range (degrees) | 0-100.2 |
| Median Renal Artery Diameter (mm) | 6 |
| Range (mm) | 5 - 8 |
| Renal Artery Access Site (Number) | |
| Femoral | 17 |
| Right brachial | 3 |
| Left brachial | 9 |
| Type of Stents Placed in Renal Artery (Number) | |
| Palmaz | 9 |
| Biliary express | 13 |
| Zilver | 2 |
| Genesis | 4 |
| Cordis | 1 |
| I-cast | 1 |
| Acculink | 1 |
| Visipro | 1 |
5/29 (17.2%) patients had a type I endoleak detected intraoperatively, 4 of which occurred after endograft encroachment, and one after the “snorkel” technique. 4/5 of these type I endoleaks resolved after placement of a Palmaz stent. 3/29 (10.3%) patients had a type I endoleak on the first postoperative CT scan. Two of these had undergone a “snorkel” procedure and one had undergone renal stent placement for partial endograft encroachment on the renal artery. All of the type I endoleaks had resolved by the time of the next CT scan, one-month after stent-graft implantation. 8/29 (27.6%) patients had evidence of a type II endoleak on postoperative CT scan. There were no cases of stent-graft migration or late-appearing (> one-month after EVAR) type I endoleaks in the study cohort.
There were three complications in this cohort of patients. One patient who underwent the “snorkel” procedure had a dissection in a stented renal artery, caused by the shoulder of the balloon; this was successfully treated by placement of a self-expanding nitinol stent. One patient required dialysis following sustained hypotension from a right external iliac artery injury, which resulted in prolonged post-operative bleeding, colon ischemia, re-operation, and death. One patient (encroachment group) had CT findings suggestive of partial renal artery occlusion on a follow-up study at 9 months; subsequent angiography demonstrated a high grade stenosis in the region of the renal artery stent. This was successfully re-stented.
3/31 (9.7%) renal arteries had evidence of stenosis on pre-operative imaging, all of which underwent successfully stent placement. There were no cases of re-stenosis during the follow-up period in these patients. Primary-assisted patency was 100% at a median follow-up of 12.5 months.
Discussion
Secure hemostatic proximal stent-graft implantation is an absolute requirement for successful EVAR.1-3 Under ideal circumstances, the proximal end of the stent-graft conforms to the shape of the neck, resulting in a long zone of overlap, secure hemostatic implantation, and durable aneurysm exclusion.9 If, on the other hand, the neck is short and more than half of the proximal stent resides within the aneurysm, the two may not assume the same shape, and the overlap will be neither stable nor hemostatic. In the case of the Zenith stent-graft, the sole device in this series, the proximal stent is 15 mm long. Therefore any neck shorter than 10 mm will likely jeopardize infrarenal implantation. Other stent-grafts have shorter proximal stents and some have been used to treat short necks, but these stent-grafts have problems such as migration.10-12
The other approach is to change the aortic side of the equation by implanting the stent-graft at a more proximal level and simultaneously maintain flow through, over, or around the stent-graft using renal stents. Fenestration has the best track record of durable success, but the necessary stent-grafts are not available in the United States. 5-6 In addition, fenestrated stent-grafts take a long time to prepare and cannot be used in symptomatic patients for whom long manufacturing delays may be fatal.
The technique referred to here as “encroachment” was first used as a way to rescue inadvertent renal artery coverage. The barbs of the Zenith stent-graft permit very little caudal movement once the proximal stent has been deployed. Traction on a balloon or cross-femoral wire is ineffective and potentially dangerous. When the degree of encroachment is small, it is rarely difficult to obtain access to the renal artery, using either a transfemoral or transbrachial approach, as evidenced by our high success rate. The most difficult aspect may be making the diagnosis, since the partially occlusive graft wall may create no filling defect or apparent delay in renal perfusion.
More extensive renal coverage does impede renal catheterization. Planned coverage of the entire renal artery calls for pre-emptive renal access through a brachial approach, as in the “snorkel” technique.7 We have not performed bilateral “snorkel” procedures. We consider the proximity of the aneurysm to both renal arteries a relative contra-indication to the procedure, as we are hesitant to risk the perfusion to both kidneys. Our limited experience with this approach has yielded a high rate of success under difficult circumstances. Others have reported similar technical success with this approach, also known as the “chimney” technique, in the pararenal aorta and the aortic arch.8, 13-14 However, the long-term durability of this technique has yet to be determined. The ideal characteristics of the renal artery stent (self-expanding versus balloon-expanded, covered versus uncovered) for use in the “snorkel” procedure have also not yet been clarified. We have used all of the above in our small series of cases. We have only used one covered stent in our series because these tend to be bulkier, stiffer, and more difficult to insert than uncovered stents. We are not entirely convinced that the covered nature of the stent confers any advantage in renal patency or any protection against a type I endoleak. Our current preference is to use a balloon-expandable uncovered stent in the renal artery during “snorkel” procedures, as these stents have good trackability, deploy precisely, and provide excellent radial force.
Since our primary indication for the elective combination of renal stents and aortic stent-grafts was the presence of a short proximal aortic neck, our data on neck length warrants scrutiny (Table 2). Inadvertent renal coverage was the result of technical error. Some of the necks were short, but most were quite long, certainly long enough to meet the standard selection criteria for EVAR. The same cannot be said of planned encroachment, where the mean proximal neck length was <10mm. However, the neck lengths for the cases of planned encroachment were not as short as the necks treated using the “snorkel” technique. The need to obtain access to the already covered renal artery limited the degree of encroachment, which limited the additional neck length to be gained. In the “snorkel” technique, access to the lower renal artery was obtained before stent-graft implantation. The upper margin of the stent-graft was deployed at the inferior margin of the contralateral renal artery. The resultant increase in the length of the implantation site allowed the inclusion of shorter necks, some as short as 3 mm.
Our technical success rate and overall small number of patients in this series deprived us of the data we need to determine the minimum neck length required for each technique. In our series, there were no cases in which too much renal artery was covered for subsequent catheterization and stenting. In addition, there were very few cases of type I endoleak, and none of persistent type I endoleak.
We recognize that while adjunctive renal stenting may permit successful EVAR in the presence of a short neck, it will not work when there is no neck. Some neck is necessary to create a seal below the renal arteries, because the presence of a renal stent disrupts contact between the pararenal stent-graft and the pararenal aorta. In some cases the infrarenal seal needs a little help in the form of a Palmaz stent. This can be a problem when the inflation of a balloon within the aorta threatens the patency of an adjacent renal stent. Our current approach avoids the issue by deploying the renal stent at the same time as the routine planned deployment of an aortic Palmaz stent in cases where the “snorkel” technique is utilized.
Long-term data will be needed to assess whether a hyperplastic response to the presence of the renal stent will ultimately threaten renal perfusion. This does not appear to be a common outcome in the short to medium-term. The sole case of intraoperative renal artery injury was the result of a technical error: the balloon was inflated beyond the orifice of the stent. Only one patient in this series had a late-occurring renal artery occlusion. This observation is consistent with data from reports on stent-graft fenestration, which resemble our own series in that most stented renal arteries were free of intrinsic disease.5-6
The Zenith stent-graft has two particular advantages in cases of short proximal aortic neck. First, the two stage deployment allows precise positioning, especially when one accounts for the downward displacement of the renal arteries that sometimes accompanies sheath withdrawal in patients with iliac artery disease. Second, the uncovered proximal stent provides secure suprarenal fixation, which is especially important because the short neck provides little infrarenal fixation, and even small amounts of migration would cause type I endoleak, aneurysm pressurization and rupture. Although the uncovered proximal stent has the advantage of additional fixation, it has the potential disadvantage of complicating renal catheterization in cases of encroachment. Nevertheless, the gaps between stent apices are generally wide enough to accommodate a catheter and stent.
While the renal encroachment technique does not require any unique variation in the sizing or deployment of the aortic stent-graft, the “snorkel” procedure does. When we utilize the “snorkel” technique, we oversize the main body component by an extra 2-4mm, in order to create a gutter for the renal stent. For example, for a 28mm neck, we would use a 36mm device instead of a 32mm device. The additional oversizing allows the fabric of the main body to fold around the renal stent (Figure 5).
Figure 5.
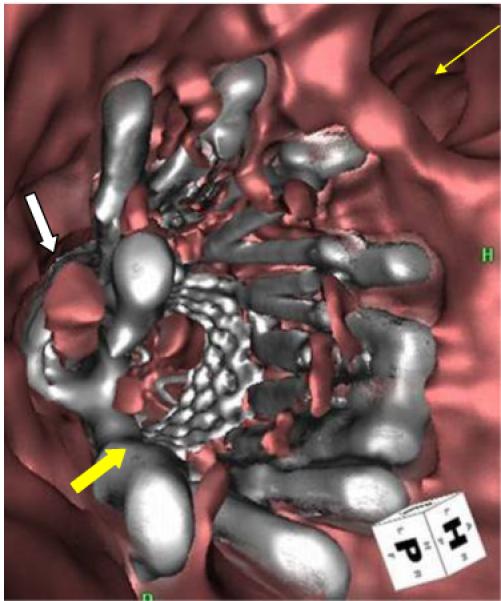
Fly-through view from postoperative CT scan in a patient who underwent placement of renal artery “snorkel.” White arrow: renal artery “snorkel” stent; block yellow arrow: aortic stent graft with Palmaz stent; thin yellow arrow: superior mesenteric artery orifice.
Conclusion
Adjunctive renal artery stents can be combined with currently available, off-the-shelf stent-grafts to increase the length of the proximal implantation site, allowing successful EVAR in cases with short proximal aortic necks. These techniques may be particularly useful for patients at high-risk for open surgical repair who do not have access to enrollment in a fenestrated or branched-graft protocol. These techniques may also be employed in the emergency setting or as part of a recovery maneuver when a renal artery is inadvertently covered. Careful follow-up is needed to determine the long-term stability of the stent-graft and the patency of the renal stent.
Acknowledgments
This publication was supported by NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competition of interest:
Dr. Chuter has licensed patents to Cook, Inc., the manufacturer of the Zenith stent-graft.
References
- 1.Abbruzzese TA, Kwolek CJ, Brewster DC, Chung TK, Kang J, Conrad MF, LaMuraglia GM, Cambria RP. Outcomes following endovascular abdominal aortic aneurysm repair (EVAR): an anatomic and device-specific analysis. J Vasc Surg. 2008;48:19–28. doi: 10.1016/j.jvs.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Leurs LJ, Kievit J, Dagnelie PC, Nelemans PJ, Buth J, EUROSTAR Collaborators Influence of infrarenal neck length on outcome of endovascular abdominal aortic aneurysm repair. J Endovasc Ther. 2006;13:640–8. doi: 10.1583/06-1882.1. [DOI] [PubMed] [Google Scholar]
- 3.Sampaio SM, Panneton JM, Mozes GI, Andrews JC, Bower TC, Karla M, Noel AA, Cherry KJ, Sullivan T, Gloviczki P. Proximal type I endoleak after endovascular abdominal aortic aneurysm repair: predictive factors. Ann Vasc Surg. 2004;18:621–8. doi: 10.1007/s10016-004-0100-z. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JL, Berce M, Hartley DE. Endoluminal aortic grafting with renal and superior mesenteric artery incorporation by graft fenestration. J Endovasc Ther. 2001;8:3–15. doi: 10.1177/152660280100800102. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill S, Greenberg RK, Haddad F, Resch T, Sereika J, Katz E. A prospective analysis of fenestrated endovascular grafting: intermediate-term outcomes. Eur J Vasc Endovasc Surg. 2006;32:115–23. doi: 10.1016/j.ejvs.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Sun Z, Mwipatayi BP, Semmens JB, Lawrence-Brown MM. Short to midterm outcomes of fenestrated endovascular grafts in the treatment of abdominal aortic aneurysms: a systematic review. J Endovasc Ther. 2006;13:747–53. doi: 10.1583/06-1919.1. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg RK, Clair D, Srivastava S, Bhandari G, Turc A, Hampton J, Popa M, Green R, Ouriel K. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003;38:990–6. doi: 10.1016/s0741-5214(03)00896-6. [DOI] [PubMed] [Google Scholar]
- 8.Ohrlander T, Sonesson B, Ivancev K, Resch T, Dias N, Malina M. The chimney graft: a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J Endovasc Ther. 2008;15:427–32. doi: 10.1583/07-2315.1. [DOI] [PubMed] [Google Scholar]
- 9.Hiramoto JS, Reilly LM, Schneider DB, Sivamurthy N, Rapp JH, Chuter TA. Long-term outcome and reintervention after endovascular abdominal aortic aneurysm repair using the Zenith stent graft. J Vasc Surg. 2007;45:461–6. doi: 10.1016/j.jvs.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 10.Coppi G, Silingardi R, Saitta G, Gennai S. Single-center experience with the Talent LPS endograft in patients with at least 5 years of follow-up. J Endovasc Ther. 2008;15:23–32. doi: 10.1583/07-2157.1. [DOI] [PubMed] [Google Scholar]
- 11.England A, Butterfield JS, Jones N, McCollum CN, Nasim A, Welch M, Ashleigh RJ. Device migration after endovascular abdominal aortic aneurysm repair: experience with a talent stent-graft. J Vasc Interv Radiol. 2004;15:1399–405. doi: 10.1097/01.RVI.0000142601.10673.00. [DOI] [PubMed] [Google Scholar]
- 12.van Marrewijk CJ, Leurs LJ, Vallabhaneni SR, Harris PL, Buth J, Laheij RJ, EUROSTAR collaborators Risk-adjusted outcome analysis of endovascular abdominal aortic aneurysm repair in a large population: how do stent-grafts compare? J Endovasc Ther. 2005;12:417–29. doi: 10.1583/05-1530R.1. [DOI] [PubMed] [Google Scholar]
- 13.Hiramoto JS, Schneider DB, Reilly LM, Chuter TA. A double-barrel stent-graft for endovascular repair of the aortic arch. J Endovasc Ther. 2006;13:72–6. doi: 10.1583/04-1711R.1. [DOI] [PubMed] [Google Scholar]
- 14.Criado FJ. A percutaneous technique for preservation of arch branch patency during thoracic endovascular aortic repair (TEVAR): retrograde catheterization and stenting. J Endovasc Ther. 2007;14:54–58. doi: 10.1583/06-2010.1. [DOI] [PubMed] [Google Scholar]


