Abstract
Background
Chronic skin ulcers such as diabetic ulcers and venous leg ulcers are increasing and are a costly problem in health care. We have developed a novel artificial dermis, collagen/gelatin sponge (CGS), that is capable of the sustained release of basic fibroblast growth factor (bFGF) for more than 10 days. The objective of this study was to investigate the safety and efficacy of CGS impregnated with bFGF in the treatment of chronic skin ulcers. Methods/
Design
Seventeen patients (≥ 20 years of age) with chronic skin ulcers that have not healed by conventional therapy for at least 4 weeks are being recruited. Patients will be applied with CGS impregnated with bFGF of 7 μg/cm2 or 14 μg/cm2 after debridement, and the wound bed improvement will be assessed 14 days after application. “Wound bed improvement” is defined as a granulated and epithelialized area on Day 14 in proportion to the baseline wound area after debridement of 50% or higher. Patients will be followed up until 28 days after application to observe the adverse events related to the application of CGS.
Conclusion
This study has been designed to address the safety and efficacy of CGS impregnated with bFGF. If successful, this intervention may be an alternative to bioengineered skin substitutes and lead to substantial and important changes in the management of chronic skin ulcers such as diabetic ulcers and venous ulcers.
Keywords: Artificial dermis, basic fibroblast growth factor, skin ulcers, sustained release
Introduction
Non-healing or chronic skin ulcers are an increasing and costly problem in health care [1-4]. Chronic skin ulcers are caused by diabetes mellitus, venous insufficiency, pressure sores, collagen disease, trauma, or radiation. With the development of tissue engineering and advances in cell and molecular biology, novel bioengineered skin substitutes and genetically derived growth factors offer promise in the treatment of chronic skin ulcers [5-7]; however, there are still issues that remain to be solved in the treatment of those ulcers. Diabetic foot ulcers and venous leg ulcers are frequent and costly complications of their underlying diseases. The prevalence of foot ulcers ranges from 4% to 10% among persons diagnosed with diabetes mellitus [3, 4] and the annual population-based incidence of 1.0% to 5% [1, 3, 4], and the lifetime incidence may be as high as 25% [3, 4]. More than 15% of all ulcers result in some form of amputation [3, 4]. According to a previous report, venous leg ulcers recurred in 72% of cases and skin ulcers from other causes recurred in 45% of cases [2], and another report described recurrence in 48% of cases one year after skin grafting [8].
We developed a bilayered acellular artificial dermis composed of an upper silicone sheet and a lower collagen sponge [9, 10] by modifying the material described by Yannas and Burke [11, 12]. Artificial dermis has been used in the treatment of full-thickness skin defects resulting from burns, trauma injuries and tumor removal. After application of the artificial dermis to skin defects, fibroblasts and capillaries penetrate and proliferate in the collagen sponge and der-mis-like tissue is formed after degradation of the collagen sponge [9-11]. However, it is difficult to apply ordinary artificial dermis to chronic skin ulcers, because the artificial dermis has no resistance to infection and is easily infected before the infiltration of capillaries into the inner collagen sponge.
Basic fibroblast growth factor (bFGF), which was identified in 1974, promotes the proliferation of fibroblasts and capillary formation and accelerates tissue regeneration [13, 14]. In Japan, human recombinant bFGF (FIBRAST SPRAY; Kaken Pharmaceutical, Tokyo, Japan) has been used clinically for chronic skin ulcers since 2001, and its clinical effectiveness has been demonstrated [7, 15]. Recently, combination therapy involving bFGF and artificial dermis has been reported to accelerate dermis-like tissue formation in the treatment of traumatic wounds [16]. In addition, this combination therapy was reported to be effective for chronic skin ulcers such as diabetic foot ulcers, ulcers caused by collagen disease, oral steroids and arteriosclerosis obliterans [17-19]. This is because bFGF causes the proliferation of fibroblasts and strongly promotes angio-genesis in artificial dermis, leading to the early formation of dermis-like tissue and promoting wound healing; however, this combination therapy has not become the standard treatment of chronic ulcers, because once daily topical administration of bFGF is required to achieve the expected effect because the artificial dermis has no ability to retain bFGF and it rapidly diffuses away from the applied site and is also inactivated quickly after its administration in vivo; thus, significant burdens are imposed on both medical staff and patients for daily application. For these reasons, we have developed a novel artificial dermis, collagen/gelatin sponge (CGS), containing a 10wt% concentration of acidic gelatin that is capable of the sustained release of positively charged growth factors such as bFGF for more than 10 days in vivo [20]. In our previous study, CGS was used as a scaffold for dermal regeneration, the same as conventional artificial dermis, and degraded after application to the wound site, being replaced by dermis-like tissue [20].
In our previous studies to apply CGSs impregnated with 7 μg/cm2 or 14 μg/cm2 of bFGF to full-thickness skin defects of normal mice and decubitus created on diabetic mice, the time required for regeneration of dermis-like tissue in mice treated with CGSs with bFGF was half to one third of the time required in mice treated with conventional artificial dermis alone [21]. In another study using mucosal defects of dog palates, CGSs impregnated with 7 μg/cm2 bFGF accelerated the regeneration of palatal mucosa with good neovascularization and showed less contracture [22]. Alternative therapies for chronic skin ulcers have been proposed, such as tissue engineering products, growth factors, and hyperbaric oxygen therapy [23, 24]. Hyperbaric oxygen therapy is a systemic therapy; therefore, it can be combined with CGSs. The mechanism of action of tissue engineering products is considered mainly as the effects of cytokines and growth factors secreted by living cells [25]. Our CGS impregnated with bFGF can sustain and release bFGF in a controlled manner; therefore, the effectiveness of CGS will either equal or surpass and be competitive in cost to tissue engineering products. Moreover, both CGS and bFGF can be stored at room temperature and used whenever needed. Usually, growth factors must be applied once or twice a day because of their rapid inactivation after administration, and CGS with bFGF will be superior in this respect.
In view of the above, combination therapy with this novel collagen-based artificial dermis (CGS) and bFGF is anticipated to be comparably minimally invasive and effective to tissue engineering products to promote wound healing even in patients with chronic skin ulcers. Thus, we propose to investigate the safety and efficacy of this combination therapy in the treatment of chronic skin ulcers.
Materials and methods
Primary objective
The objective of this study is to evaluate the safety and efficacy of CGS impregnated with bFGF in the treatment of chronic skin ulcers that are not expected to heal with conventional treatments.
Methods and design
Open-label, randomized, multiple dose, controlled clinical trial.
Design
Two groups, a low-dose and high-dose bFGF group, have been set (Figure 1). In the initial step (Step 1), three patients will be enrolled in the low-dose group. After confirming the safety in the low-dose group, patients will be randomized to the low-dose or high-dose bFGF group in Step 2. Randomization-based comparison between dose groups can achieve significant improvements in accuracy and lack of bias. This comparison can provide useful information for designing and conducting future trials.
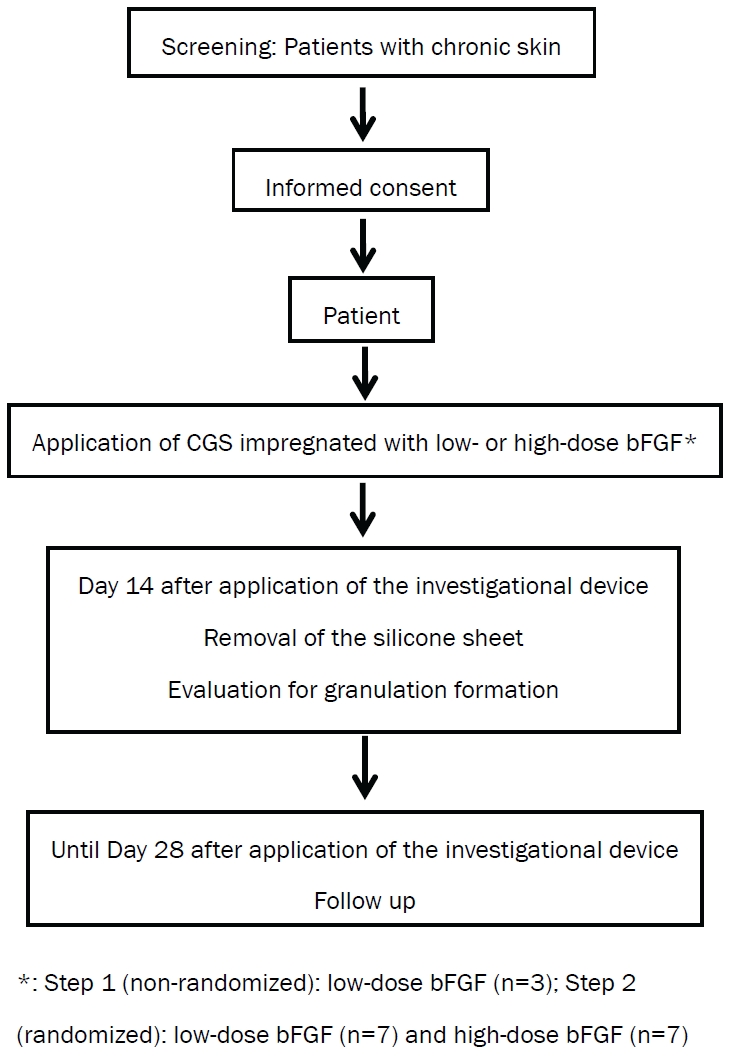
Setting and participants
This study is being conducted at Kyoto University Hospital. Patients with chronic skin ulcers are referred by physicians and also identified through a number of wound care clinics in Kyoto Prefecture and surrounding prefectures.
Inclusion criteria
1). Patients aged 20 years or older at informed consent. 2). Presence of chronic skin ulcers as below: not healing for at least 4 weeks with conventional treatments; skin graft is not expected to take; can be completely covered by a 70 mm × 100 mm device. 3). If chronic skin ulcers are present on lower extremities, the skin perfusion pressure must be 3 30 mmHg at a site proximal or distal to those ulcers. 4). Written informed consent.
Exclusion criteria
1). Have any of the following systemic diseases: uncontrolled diabetes mellitus (defined by HbA1c 3 10%) according to latest laboratory data obtained within 28 days before registration); requiring continued use of oral corticosteroid therapy (> 20 mg/day prednisolone equivalent); a history of malignant tumor with disease-free interval of 5 years or less. 2). Have a history of allergy to porcine-derived products, collagen, gelatin, bFGF, anesthetic drugs, disinfectants, etc. 3). Have participated in another clinical trial/study within the past three months. 4). Have participated in this study previously. 5). Women meeting any of the following: do not agree to avoid pregnancy during the study; currently pregnant or possibly pregnant; currently breastfeeding. 6). Other patients judged by the investigator or sub-investigator to be inappropriate as a subject of this study.
Randomization
In Step 2, patients will be randomized to either the low-dose or high-dose bFGF group at a ratio of 1:1 without stratification. Randomization will be performed using a computer-generated random sequence to ensure equal allocation to the two dose groups by a statistician of the independent data center (Department of Clinical Trial Design and Management, Translational Research Center, Kyoto University Hospital).
Interventions
Preparations of CGS impregnated with bFGF
CGS is the modification of conventional bilayered artificial dermis (Pelnac; Gunze Co., Ltd, Kyoto, Japan) and consists of an upper silicone sheet (0.1mm in thickness) and lower sponge (3 mm in thickness) [20]. In this study, the larger CGS (82 mm × 120 mm) will be used. Two different dose bFGF concentrations of 7 μg/cm2 (low dose) or 14 μg/cm2(high dose) will be used. On the day of study therapy, the investigator or sub-investigator will prepare CGS impregnated with bFGF in the operating room
Application of CGS impregnated with bFGF
This therapy will be started within 28 days of enrollment. After debridement of the chronic skin ulcers, CGS impregnated with bFGF of 7 μg/cm2 or 14 μg/cm2 and cut according to the shape of the wound will be applied and sutured to surrounding skin.
Dressing changes and silicone sheet removal
After the application of CGS, dressings will be changed as necessary. Patients will be hospitalized until Day 7 to ensure stabilization of the applied CGS and may be discharged on Day 8 after application according to the condition of the wound. On Day 14 after application, the sutures and silicone sheet of CGS will be removed. After silicone film removal, subsequent therapy may be started.
Subsequent therapy
The use of bFGF or another collagen-based artificial skin will be prohibited until Day 28 after application. The use of ointments, wound dressings and skin grafting will be allowed. After completion of the study period (Day 29 after investigational device application and onward), no particular restrictions will be imposed.
Digital photograph for healing assessment
Using a digital camera, digital images of the wounds will be taken with a calibrator (CASMATCH; BEAR Medic Corp., Tokyo, Japan) placed on the skin adjacent to the wound. The color and size of image will be adjusted using the CASMATCH and image editing software (Adobe Photoshop; Adobe Systems) to assess the wound and granulation areas. As with the primary endpoint, the granulation tissue evaluation committee members will assess the wound and granulation areas.
Primary endpoint
The primary endpoint is “wound bed improvement." Granulation tissue is wound connective tissue, which forms at the beginning of wound healing [26]. This highly fibrous tissue is usually pink because numerous small capillaries invade granulation tissue to supply oxygen and nutrients. The appearance of granulation tissue is a good sign of healing because when a wound starts granulating, it means that the healing process of the wound is starting [26-28]. The area of granulation tissue will be measured as the granulation formation area in this study. An unhealed area is defined as an area with no epithelialization and no granulation formation. In this study, the percentage of wound bed improvement is defined as the value (%) calculated from the sum of the granulated and epithelialized areas on Day 14 divided by the baseline wound area after debridement on Day 0 multiplied by 100, and the patient is diagnosed with wound bed improvement if the wound bed improvement indicator is 50% or higher. The use of 50% or more as the cutoff for the wound bed improvement indicator is based on the pressure ulcer healing assessment scale by the Japanese Society of Pressure Ulcers [28, 30, 31].
Secondary endpoints
1). Adverse events and adverse reactions. 2). Percentage of “wound bed improvement". 3). Percentage of wound reduction: The percentage of wound area reduction is defined as the value (%) calculated from the wound area of the ulcer on Day 14 divided by the baseline wound area after debridement on Day 0 multiplied by 100. 4). Percentage of granulation area: The percentage of granulation area is defined as the value (%) calculated from the granulation area divided by the wound area on Day 14 multiplied by 100.
Blinding
The baseline wound area, the wound area on Day 14 and the granulation area on Day 14 will be independently measured under blinding by central review. Patients will be unblinded, and unblinded investigators will apply CGSs and change dressings.
Sample size
This study will be conducted to determine whether CGS impregnated with bFGF is promising for the treatment of chronic skin ulcers, as evaluated based on wound bed improvement as the primary endpoint. Primary analyses will be conducted using all data treated with CGS in Step 1 and Step 2. Since debridement and conventional therapies rarely lead to wound bed improvement in this patient population, the null hypothesis tested in this study is that the proportion of patients with wound bed improvement is 10% or less. The null hypothesis is also supported by previous trials [32-34]. In consideration of the minimum clinically important difference, the expected proportion of patients with wound bed improvement in this study is set to 50% or more. When exact testing based on binomial distribution is conducted with a onesided significance level of 2.5% and a statistical power of 90% or higher, the required number of subjects is 14. Allowing for a drop-out rate of 20% or less, the total number of patients for registration is 17, specifically 3 patients in Step 1 and 14 patients in Step 2.
Study schedule
The schedule of study assessments and evaluations is shown in Table 1. The study period will be from the day of informed consent to 28 days after investigational device application. The study period will be from the day of investigational device application to 28 days after investigational device application. Data to evaluate the efficacy and safety of this study will be collected at enrollment, baseline, and Day 14 of the treatment phase and Day 28 of the observation phase.
Table 1.
Schedule of study assessments and evaluations
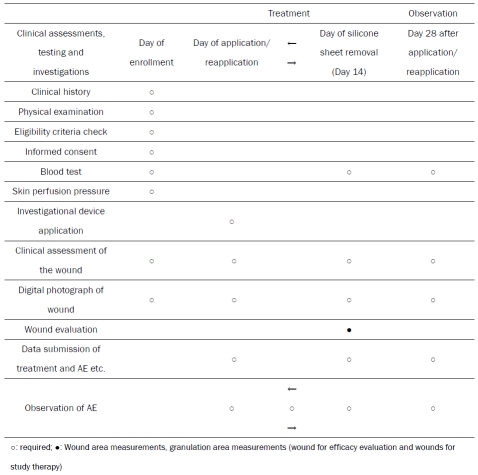 |
Statistical analysis
Patients who have been registered for the study and who have undergone investigational device application at least once will be included in the full analysis set (FAS) and the safety analysis set. From the FAS, however, patients will be excluded if they have serious protocol violations or International Conference on Harmonization Guidelines for Good Clinical Practice (ICH-GCP) violations (failure to obtain consent, major study procedure violations) or if they are found to be ineligible after registration.
Wound bed improvement
Wound bed improvement is the primary end-point of this study. The primary analysis will be conducted for the FAS using exact test based on binomial distribution with a null proportion of 10% and a one-sided significance level of 2.5%. The 95% confidence interval of the proportion of patients with wound bed improvement will be calculated using an exact method based on binomial distribution.
Percentage of wound bed improvement, would reduction and granulation area
Using the FAS, the descriptive statistics will be calculated. The interval estimation will be conducted under the assumption that this endpoint follows normal distribution.
Adverse events related to the application of the device
Using the safety analysis set, the frequency/ incidence of adverse events and adverse events that can be causally related to the investigational device in the safety analysis set will be calculated by event and severity.
Ethical considerations
This study is being conducted in compliance with the ICH-GCP and in agreement with the latest revision of the Declaration of Helsinki, Pharmaceutical Affairs Law and all applicable Japanese laws and regulations, as well as any local laws and regulations and all applicable guidelines. This protocol and any amendments have Institutional Review Board approval at Kyoto University Hospital.
Subject consent
Informed consent will be obtained from all potential study participants using the IRB-approved informed consent form. The clinical investigator informs the potential study subject of all pertinent aspects of the study. The subject must sufficiently understand the contents of the information form before providing written consent. The consent form must be dated and signed by both the investigator and the participant. Subjects are also informed that their medical care will not be affected if they do not choose to participate in this study. The consent form will be retained at Kyoto University Hospital and the information form and a copy of the consent form will be handed to the participant. Whenever the investigator obtains information that may affect the participant's willingness to continue participation in the study, the investigator or sub-investigator will immediately inform the participant and record this, and reconfirm the participant's willingness to continue participation in the study.
Adverse events
This study is being conducted according to the ICH-GCP. Adverse events and serious adverse events information will be documented according to the Medical Dictionary for Regulatory Activities (MedDRA) version 14.0.
Results and discussion
This study has been designed to address the safety and efficacy of novel treatment for chronic skin ulcers using a modified artificial dermis, CGS, that can sustain bFGF. This study will be the first randomized controlled trial to evaluate the efficacy of CGS and the appropriate concentration of bFGF impregnation for treatment of increasing non-healing ulcers. Some bioengineered skin substitutes that provide growth factors secreted by living cells have been reported to be effective for chronic skin ulcers, although they are costly and access is limited to only a few areas and countries. Both CGS and bFGF are freeze-dried and can be kept well and stored at room temperature. These are off-the-shelf products and the procedure of impregnation is simple; therefore, we can use this combination therapy anywhere when needed. If successful, this intervention may lead to substantial and important changes in the management of chronic skin ulcers, such as diabetes ulcers and venous leg ulcers
Acknowledgments
This work was supported by a grant from the Japan Science and Technology Agency.
References
- 1.Vuorisalo S, Venermo M, Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino) 2009;50:275–291. [PubMed] [Google Scholar]
- 2.Bergqvist D, Lindholm C, Nelzen O. Chronic leg ulcers: the impact of venous disease. J Vasc Surg. 1999;29:752–755. doi: 10.1016/s0741-5214(99)70330-7. [DOI] [PubMed] [Google Scholar]
- 3.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 4.Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. Vasc Health Risk Manag. 2007;3:65–76. [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrenreich M, Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12:2407–2424. doi: 10.1089/ten.2006.12.2407. [DOI] [PubMed] [Google Scholar]
- 6.Mason C, Manzotti E. Regenerative medicine cell therapies: numbers of units manufactured and patients treated between 1988 and 2010. Regen Med. 2010;5:307–313. doi: 10.2217/rme.10.37. [DOI] [PubMed] [Google Scholar]
- 7.Uchi H, Igarashi A, Urabe K, Koga T, Nakayama J, Kawamori R, Tamaki K, Hirakata H, Ohmura T, Furue M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol. 2009;19:461–468. doi: 10.1684/ejd.2009.0750. [DOI] [PubMed] [Google Scholar]
- 8.Trier WC, Peacock EE, Jr, Madden JW. Studies on the effectiveness of surgical management of chronic leg ulcers. Plast Reconstr Surg. 1970;45:20–23. doi: 10.1097/00006534-197001000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Matsuda K, Issiki N, Tamada Y, Ikada Y. Experimental study of a newly developed bilayer artificial skin. Biomaterials. 1990;11:356–360. doi: 10.1016/0142-9612(90)90114-6. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Matsuda K, Isshiki N, Tamada Y, Yoshioka K, Ikada Y. Clinical evaluation of a new bilayer ‘artificial skin’ composed of collagen sponge and silicone layer. Br J Plast Surg. 1990;43:47–54. doi: 10.1016/0007-1226(90)90044-z. [DOI] [PubMed] [Google Scholar]
- 11.Yannas IV, Orgill DP, Burke JF. Template for skin regeneration. Plast Reconstr Surg. 2011;127:60S–70S. doi: 10.1097/PRS.0b013e318200a44d. [DOI] [PubMed] [Google Scholar]
- 12.Yannas IV, Burke JF. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res. 1980;14:65–81. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- 13.Gospodarowicz D. Localization of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974;249:123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- 14.Hom DB, Unger GM, Pernell KJ, Manivel JC. Improving surgical wound healing with basic fibroblast growth factor after radiation. Laryngoscope. 2005;115:412–422. doi: 10.1097/01.mlg.0000157852.01402.12. [DOI] [PubMed] [Google Scholar]
- 15.Akita S, Akino K, Imaizumi T, Hirano A. Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen. 2008;16:635–641. doi: 10.1111/j.1524-475X.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 16.Muneuchi G, Suzuki S, Moriue T, Igawa HH. Combined treatment using artificial dermis and basic fibroblast growth factor (bFGF) for intractable fingertip ulcers caused by atypical burn injuries. Burns. 2005;31:514–517. doi: 10.1016/j.burns.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Akita S, Akino K, Tanaka K, Anraku K, Hirano A. A basic fibroblast growth factor improves lower extremity wound healing with a porcine-derived skin substitute. J Trauma. 2008;64:809–815. doi: 10.1097/TA.0b013e31802c8247. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Ito S, Sekine M, Abe M. Reconstruction of the soft tissue of a deep diabetic foot wound with artificial dermis and recombinant basic fibroblast growth factor. Plast Reconstr Surg. 2005;115:567–572. doi: 10.1097/01.prs.0000149485.60638.30. [DOI] [PubMed] [Google Scholar]
- 19.Fujioka M. Combination treatment with basic fibroblast growth factor and artificial dermis improves complex wounds in patients with a history of long-term systemic corticosteroid use. Dermatol Surg. 2009;35:1422–1425. doi: 10.1111/j.1524-4725.2009.01251.x. [DOI] [PubMed] [Google Scholar]
- 20.Takemoto S, Morimoto N, Kimura Y, Taira T, Kitagawa T, Tomihata K, Tabata Y, Suzuki S. Preparation of collagen/gelatin sponge scaffold for sustained release of bFGF. Tissue Eng Part A. 2008;14:1629–1638. doi: 10.1089/ten.tea.2007.0215. [DOI] [PubMed] [Google Scholar]
- 21.Kanda N, Morimoto N, Takemoto S, Ayvazyan AA, Kawai K, Sakamoto Y, Taira T, Suzuki S. Efficacy of Novel Collagen/Gelatin Scaffold With Sustained Release of Basic Fibroblast Growth Factor for Dermis-like Tissue Regeneration. Ann Plast Surg. 2011 doi: 10.1097/SAP.0b013e318222832f. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Ayvazyan AA, Morimoto N, Kanda N, Kawai K, Sakamoto Y, Taira T, Suzuki S. Collagen-gelatin scaffold impregnated with bFGF accelerates palatal wound healing of palatal mucosa in dogs. J Surgical Res. 2011;171:247–257. doi: 10.1016/j.jss.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 23.Langer A, Rogowski W. Systematic review of economic evaluations of human cell-derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res. 2009;9:115. doi: 10.1186/1472-6963-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Reilly D, Linden R, Fedorko L, Tarride JE, Jones WG, Bowen JM, Goeree R. A prospective, double-blind, randomized, controlled clinical trial comparing standard wound care with adjunctive hyperbaric oxygen therapy (HBOT) to standard wound care only for the treatment of chronic, non-healing ulcers of the lower limb in patients with diabetes mellitus: a study protocol. Trials. 2011;12:69. doi: 10.1186/1745-6215-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong T, McGrath JA, Navsaria H. The role of fibroblasts in tissue engineering and regeneration. Br J Dermatol. 2007;156:1149–1155. doi: 10.1111/j.1365-2133.2007.07914.x. [DOI] [PubMed] [Google Scholar]
- 26.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin P. Wound Healing-Aiming for Perfect Skin Regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 28.Sanada H, Moriguchi T, Miyachi Y, Ohura T, Nakajo T, Tokunaga K, Fukui M, Sugama J, Kitagawa A. Reliability and validity of DESIGN, a tool that classifies pressure ulcer severity and monitors healing. J Wound Care. 2004;13:13–18. doi: 10.12968/jowc.2004.13.1.26564. [DOI] [PubMed] [Google Scholar]
- 29.Lavery LA, Barnes SA, Keith MS, Seaman JW, Jr, Armstrong DG. Prediction of healing for postoperative diabetic foot wounds based on early wound area progression. Diabetes Care. 2008;31:26–29. doi: 10.2337/dc07-1300. [DOI] [PubMed] [Google Scholar]
- 30.Japanese Society of Pressure Ulcers. Guideline for Local Treatment of Pressure Ulcers. 2005.
- 31.Japanese Society of Pressure Ulcers. Guideline for Prevention and Management of Pressure Ulcers. 2009.
- 32.Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24:290–295. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- 33.Cardinal M, Eisenbud DE, Armstrong DG, Zelen C, Driver V, Attinger C, Phillips T, Harding K. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair Regen. 2009;17:306–311. doi: 10.1111/j.1524-475X.2009.00485.x. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704–1710. doi: 10.1016/S0140-6736(05)67695-7. [DOI] [PubMed] [Google Scholar]


