Abstract
Ventilator associated pneumonia is a common and costly complication in critically ill and injured surgical patients. The diagnosis of pneumonia remains problematic and non-specific. Using clinical criteria, a diagnosis of pneumonia is typically not made until an infection is well established. Semi-quantitative cultures of endotracheal aspirate and broncho-alveolar lavage are employed to improve the accuracy of diagnosis but are invasive and require time for culture results to become available. We report data that show that an inexpensive, rapid and non-invasive alternative may exist. In particular we show that: 1). Bio-aerosols evolved in the breath of ventilated patients and captured in the hygroscopic condenser humidifier filter of the ventilator circuit contain pathogenic micro-organisms. 2). The number (CFU/ml) and identity (Genus, species) of the pathogens in the aerosol samples can rapidly and inexpensively be determined by PCR. 3). Data from a convenience sample of filters correlate with clinical findings from standard microbiological methods such as broncho-alveolar lavage. The evaluation of the bacterial load evolved in exhaled breath by PCR is amenable to repeated sampling. Since increasing bacterial burden is believed to correlate with the establishment of infection, the use of quantitative PCR may provide a method to rapidly, inexpensively, and effectively detect and diagnose the early onset of pneumonia and identify pathogens involved.
Keywords: Pneumonia, VAP, BAL, HME, HCH, infection
Introduction
Critically ill patients requiring mechanical ventilation are at significant risk for developing ventilator associated pneumonia (VAP). Pneumonia is the second most common nosocomial infection of critically ill patients [1] and affects 27% of all critically ill patients [2]. VAP markedly increases ventilator, ICU, and hospital days, as well as mortality. The excess health care costs of nosocomial infections are substantial and well documented [3].
Diagnosis and treatment of pneumonia
While early diagnosis and subsequent, timely, appropriate antimicrobial therapy has been shown to significantly improve the outcome of patients with VAP [4-6] establishing the diagnosis of pneumonia remains problematic [1, 7, 8].
The clinical diagnosis of VAP requires the development of visible infiltrates on chest x-ray and the presence of physiologic changes produced by the infectious process. These findings do not occur until the infection is well established. Additionally, the clinical diagnosis of VAP is very non-specific; over estimating its occurrence relative to quantitative techniques in 40-60% of cases, contributing to significant overuse of antibiotics. On the other hand, quantitative culture techniques are generally, invasive, labor intensive and slow [1, 9]. The culture delay often promotes unnecessary antibiotic exposure with an associated increased risk of both subsequent infectious complications and risk of infection with resistant pathogens [5, 10-12].
Despite ongoing debate over optimal diagnosis and treatment of VAP [8, 10, 12], accepted pathophysiology includes an increase in the lung-borne pathogenic load during the development of pneumonia with greater numbers of pathogenic bacteria producing a higher risk of an adverse outcome [13]. Ideally, monitoring techniques that detect an increase in bacterial load early in the course of VAP, prior to the development of clinical symptoms and alterations in pulmonary function, thus allowing the appropriate initiation of targeted short courses of antibiotic therapy. Some data exists to support this concept. Cultures of endotracheal aspirates performed twice a week in critically ill mechanically ventilated patients identified the pathogenic bacteria and its sensitivity pattern in 83% of cases; later established by quantitative cultures to have VAP [1, 7, 14].
These results support the ability to identify targeted therapy more effectively with monitoring of lung borne pathogens over time. However, tracheal secretions are frequently contaminated or over grown with oral-pharyngeal flora that is not necessarily involved in pneumonic processes. This has limited the ability of endotracheal aspirates to rule out the diagnosis of pneumonia and to achieve the most targeted antibiotic therapy.
Aerosolized breath
Pathogenic microorganisms causing pneumonia have previously been detected in the exhaled breath of humans and animals [15-17]. The possibility that pathogens exhaled from the lungs of ventilated patients accrue in the ventilator circuit presents an opportunity for the quantitative assessment of bacteria over time. In the majority of ventilated patients, the ventilator circuit contains a hygroscopic condenser humidifier (HCH) filter present just external to the patients endotracheal tube that is designed to limit moisture loss from the patient , minimize heat loss, and filter bacteria that may be present in the tubing outside of the HCH filter. The HCH unit is situated in the ventilator circuit between the Y-piece and the endotracheal tube, external to the patient, see Figure 1. Condensed vapor and aerosols from the breath of intubated patients collect within this filter unit and are retained there until the unit is exchanged. Similar filters have been shown to be greater than 99.9% effective at preventing microbial and viral vectors from contaminating the ventilator circuit upstream of the Y piece [18]. HCH filters have been shown to decrease the incidence of ventilator associated pneumonia relative to circuits in which heated and humidified gases are introduced proximal to the ventilator [19]. Liquid visibly collects within the HCH filters over time and they are routinely changed every 12 hrs as part of circuit maintenance.
Figure 1.
Depiction of the HCH filter in isolation and as part of the ventilator circuit. Panel a, at left, shows an example of the HCH filter unit that stands roughly two inches tall and less than an inch in diameter. The sponge can be seen at its center. Panel b, at center, shows a photograph of the HCH filter relative to the Y-valve and the endotracheal tube insertion point - blue cap. The schematic at right indicates the airflow and ancillary HEPA filters within the ventilator circuit.
We began this study with the hypothesis that the liquid that collects within the HCH filter contains droplets of aerosolized alveolar lining fluid that may in turn contain pathogenic bacteria in cases of infection [15, 16, 20]. PCR provides a rapid, quantitative and inexpensive way to profile the HCH fluid for bacterial, fungal and viral pathogens.
Methods and materials
Study design
This study is comprised of three components: 1). Demonstration that the process of bacterial recovery from HCH filters and their subsequent detection/determination using PCR is feasible and sound. 2). Application of the process of bacterial recovery and PCR-based identification on a convenience sample of 17 HCH filter samples taken from 14 ventilated patients from the SICU. 3). Retrospective comparison of the findings from part 2, with the corresponding patients data from the medical record to determine the degree of correlation with quantitative cultures and clinical suspicion of VAP.
Bacterial growth and retention with HCH filters
Culturing of control samples of bacteria
Escherichia coli, lab strain TOP10 (Invitrogen), and a hospital isolate of methicillin resistant Staphylococcus aureus (MRSA), were cultured overnight in batch at 37 °C with 180 rpm of shaking in 5 ml of Luria broth containing (per liter): 10 g soy tryptone (Remel), 5 g yeast extract (Becton, Dickenson, and Company), 10 g NaCl (Fisher Scientific).
Coulter counting
A Beckmann Multisizer 3 Coulter counter outfitted with a 30 micron aperture was used throughout. We operated the Coulter counter with 0.2 micron filtered, 150 mM NaCl as diluent. The unit was calibrated using a 2 micron bead calibration standard (Coulter CC Size Standard L2, nominal 2 micron diameter, lot assayed as 2.05 microns). After calibration the unit was operated with an aperture current of 400 uA and a gain of 8.
Preparation of HCH pre-incubated buffer (PIB)
5 mL of 150 mM NaCl at pH 5.5, was pipetted into an HCH unit and incubated at room temperature without agitation for one hour or twelve hours and then recovered by centrifugation at 1000 rcf for 5 minutes. For simplicity we refer to this buffer as PIB throughout.
HCH growth and viability
50ul of an overnight culture of E. coli or MRSA was added to 5 mL of fresh LB medium and shaken at 37 °C for 2 hours. The growth was monitored by Coulter counting. The count was used to determine the volume required to inoculate, to a density of 106 cells/mL: 12 mL of 150 mM NaCl buffer; 12 mL of 1hr PIB; 12 mL of 12hr PIB.
Initial cell densities and densities after 1, 2, 4, 8, and 12 hours of incubation at 37 °C without agitation, were determined by Coulter counter. Concurrently, 20 uL was taken from each sample, diluted 500-fold in 10 mL 150 mM NaCl, and 100 uL of these dilutions were plated in triplicate on Luria broth agar, incubated 24 hours at 37 °C, and colonies counted to determine the density of viable cells. This density was compared to total cell density (as determined by Coulter counter) in order to calculate percent viability at the time points sampled.
HCH recovery
5 mL of 150 mM NaCl, pH 5.5, inoculated to a cell density of 106 cells/mL was pipetted into an HCH filter. An equal cell-free volume was pipetted into a HCH as control. Paired units were incubated at 37 °C without shaking. At each time point a paired sample of HCH filters were centrifuged for 5 min at 1000 rcf. The particle density distributions of the recovered fluid volumes were independently determined by Coulter counter. The recovered bacterial cell density was normalized by subtracting the particle density of the time-matched cell-free HCH. Percent cell recovery was determined by dividing the cell density at a given time point by the initial cell density.
PCR detection using universal primers
Bacterial DNA present in patient samples was identified by amplification and sequencing of a characteristic region of the 16S ribosomal DNA gene that is flanked by primer-binding sequences that are highly conserved among a broad range of bacteria [21]. Forward: 5'-TCCTACGGGAGGCAGCAGT-3'. Reverse 5'-GGACTACCAGGGTATCTAATC CTGTT-3'. (Operon, HPLC purified). The universal primers flank and amplify a 466 bp fragment.
Since fungal vectors such as Candida are very commonly reported especially among immuno-compromised patients we also adopted a strategy to identify fungal pathogens based on universal primers to 26S rDNA [22]. Forward 5'-GCATATCAATAAGCGGAGGAAAAG-3'. Reverse 5'-GGTCCGTGTTTCAAGACGG-3’ (Operon, HPLC purified).
Each PCR reaction was composed of 46 uL Platinum PCR Master Mix (Invitrogen), 1 uL of a 25 uM stock of each primer (500 nM final concentration), and 2 uL of DNA purified from recovered HCH fluid. Thermocycling program: 5 minutes at 95 °C, 35 cycles of 15 seconds at 95 °C; 30 seconds at 60 °C; 30 seconds at 72 °C. Final incubation of 5 minutes at 72 °C.
PCR products were purified using QIAquick PCR Purification Kit (Qiagen) and submitted to the Vanderbilt University DNA Sequencing Facility. The forward and reverse primers were used for the sequencing reactions. Consensus sequences from the forward and reverse reads of the PCR product were used as search queries against the Genbank nr database and the top hits were used to assign the putative pathogen identity in the HCH fluid samples.
Quantitative real time PCR technique
The construction of standard curves for real time determination of bacterial load from 16s rDNA samples is thoroughly presented in [21]. We have replicated these results and modified them to incorporate the Coulter counter. A precise dilution of 107 cells/mL in 150 mM NaCl was prepared from an overnight E. coli culture using the Coulter counter. From this, a series of half-log dilutions was prepared down to 104 cells/mL. Prior to cell lysis, 10 ug/mL of salmon sperm DNA (Stratagene) was added to enhance DNA recovery at low cell numbers. DNA was extracted from 200 uL aliquots using the DNeasy Blood and Tissue Kit (Qiagen) with a final elution volume of 200 uL. PCR reactions contained 12.5 uL iQ SYBR Green Supermix (Bio -rad), 0.5 uL of a 12.5 uM stock of each primer (250 nM final concentration), 1 uL of purified DNA, and 10.5 uL of nuclease-free dH2O. Reactions were run on an iQ5 Multi Color Real time PCR Instrument (Bio-rad) using the program: 5 minutes at 95 °C 40 cycles of 15 seconds at 95 °C; 30 seconds at 65 °C with real time fluorescence measurement.
Collection and analysis of HCH filters from ventilated patients
Collection of patient HCH samples
A sample of 17 HCH filters, AirLife (part # 003003, Cardinal Health), were collected over a 6 month period beginning in December of 2009 from ventilated patients in the SICU. The sample was random with regard to patient condition, severity or identity. Filter units were removed aseptically by a respiratory technician, placed and sealed within a sterile biohazard bag and labeled with a de-identified code. Samples to be processed were stored in a 4 °C refrigerator for up to 72 hours.
Recovery of aerosol samples from patient HCH filters
HCH units were removed from a biohazard bag in a sterile cabinet using sterile technique and inserted upright into the top of a disposable 50 mL centrifuge tube, the sides sealed with parafilm, and centrifuged at 1000 rcf for 5 minutes. Typical fluid volume recovered from a filter that had been in place for 12 hours was 5 ml. The pH of the recovered fluid was 5.5.
Extraction of bacterial DNA from recovered fluid
Fluid samples recovered from HCH filters were concentrated. Total DNA was purified from 200 uL aliquots of concentrate using the DNeasy Blood and Tissue Kit (Qiagen) with the following modifications. The 200 uL sample, 20 uL proteinase K, and 200 uL AL buffer (lysis buffer) were mixed and exposed to three rounds of freezing at -80 and thawing at 37 °C, followed by one 30 minute incubation at 56 °C and one 5 minute incubation at 95 °C. After column binding and washing, total DNA was eluted using 50 uL of nuclease-free distilled water.
Retrospective data comparison and analysis
Retrospective comparison with medical record culture data
Following approval by the institutional review board, patient medical record numbers were ascertained based on the date and room number of each sample. MRN's were stored in a secure linking table along with the corresponding sample number and date. After the laboratory processing of the HCH filter data by PCR was completed, the MRN's were queried against all clinical microbiology reports for culture data corresponding to the hospital stay inclusive of the HCH filter sample collection date(s). Pathogen type, quantity, and relative time of culture relative to HCH filter sampling were extracted by manual report review and de-linked from patient identifiers prior to analysis.
Statistics
From the frequency distribution of Table S1 we calculated the probability of a matching pair of nominal categories p1=0.12; the categories corresponding to organism identity. From the distribution in Table S2 we calculated the probability of a matching pair of ordinal categories p2=0.20; the categories corresponding to a range of organism number. Assuming that the number of organisms observed is independent of it's name we estimated the probability of observing a matching pair of both type and number to be p:=p1p2=0.02. Finally, p=0.02, was used in a right sided, exact binomial test to determine the probability of observing various numbers of matches by chance alone in repeated trials.
Results
Bacterial growth characteristics in HCH filters
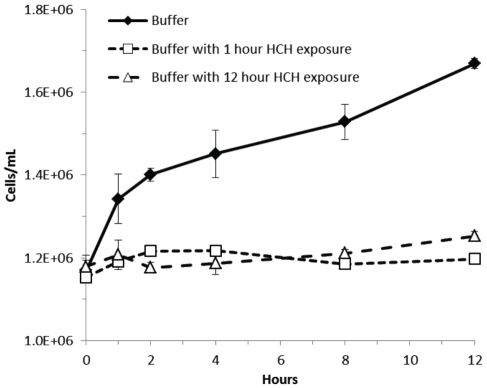
To assess the characteristics of bacterial growth within HCH filters over time we compared the growth of bacteria in buffer to growth in buffer that had been pre-incubated in the HCH filter: PIB (see materials and methods). Specifically: E. coli or MRSA were cultured as described in methods. An aliquot of an actively dividing culture was split, one half resuspended in buffer, the other resuspended in an aliquot of PIB. The number of bacteria in PIB was compared with its control over a time course of twelve hours using a commercial Coulter counter. Particu-lates arising from the HCH filter displayed a distinct distribution different from that of either bacterial species and were subtracted from PIB containing samples using matched controls. The results from triplicate experiments, shown in Figure 2, indicate that the cells placed in the HCH pre-incubated buffer did not change in number while the control culture continued to double at room temperature. Neither the gram negative E. coli nor the gram positive MRSA replicated in the HCH fluid environment.
Figure 2.
A twelve hour time course of microbial growth at room temperature. One and 12 hour PIB (see methods) innoculated with E. coli fail to show growth relative to buffer alone (dark diamond). Error bars are derived from triplicate experiments. These data indicate that the HCH environment is bacteriostatic.
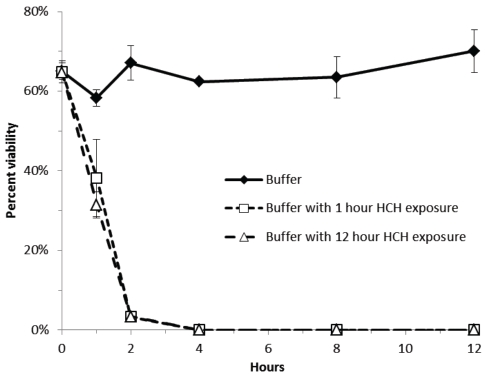
The results described above and shown in Figure 2 demonstrate that PIB is bacteriostatic. The data summarized in Figure 3, shows that PIB is not only bacteriostatic but also bacteriocidal. Aliquots of E. Coli or MRSA resuspended in PIB showed a declining number of CFU/ml when plated on LB and incubated overnight at 37 °C as compared with the control. In triplicate experiments, the data indicate that after 2 hours of exposer to buffer pre-incubated in the HCH filter fewer than 10% of the E. coli cells are viable and that after 4 hours virtually no colonies grew. We observed qualitatively the same behavior with MRSA, the difference being that the Gram positive bacteria retained 30% viability at 4 hours that declined to 0% at 8 hours (data not shown).
Figure 3.
Time course of microbial viability of E. coli innoculated into one and 12 hour PIB as compared to viability in 150 mM NaCl buffer alone. Viability was determined by plate counts done in triplicate. The result of triplicate experiments indicate that the HCH environment is bacteriocidal.
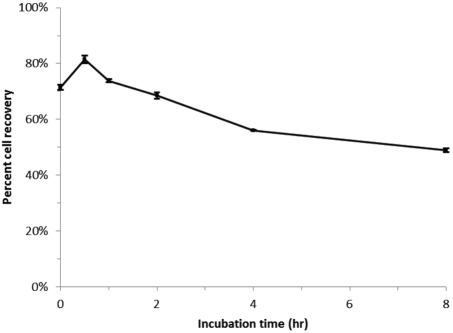
The ability to detect micro-organisms deposited in an HCH filter by PCR is contingent upon the ability to recover fragments of their DNA from the filter unit. Figure 4 demonstrates that bacteria that have been incubating in the filter can themselves be recovered with high fidelity. In this experiment a well mixed aliquot of E. coli in sodium chloride buffer were split, one half counted with a Coulter counter, the other half introduced into an HCH filter. The number of E. coli recovered from the filter after a given incubation time were recorded. The results of triplicate experiments are shown in Figure 4. The data indicate that the percentage of the cells recoverable does decline weakly with time. At least half of the organisms introduced into the filter are recoverable after 8 hours.
Figure 4.
Time course of quantitative microbial recovery from HCH filters innoculated with a prescribed initial number of E. coli. Cell numbers were determined using a Coulter counter as described in methods. The results of triplicate experiments show that percent recovery declines weakly with time but remains above 50%.
PCR is a common technique for bacterial detection and determination [21-27]. The pathogen detection from patient samples described in this work was performed using universal primers that were designed to amplify a bacteria specific 16s rDNA fragment [21, 25]. The identity of the pathogens were determined from the amplified fragment by sequence analysis.
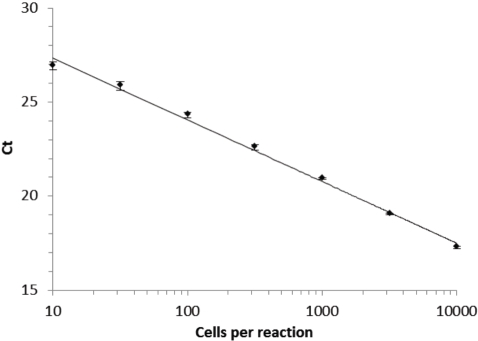
There is a vast literature on PCR sensitivity and reliability, [25, 28], far too broad to review or to comprehensively cite. In Figure 5, we illustrate our ability to sensitively and reproducibly detect small numbers of bacterial cells. Lower respiratory tract inflammatory secretions contain on the order of 105 - 106 CFU/ml [5, 7]. Since PCR can reliably detect a signal from as few as 10 bacterial cells, only a minute fraction of the total load needs to be evolved in the breath to be detectable.
Figure 5.
Standard curve relating the crossing threshold value from real time PCR with the number of E. coli cells assayed. The linear regression line has an r2 value of 0.995. Similar standard curves are reported in [21].
Application of PCR detection to clinical HCH fluid samples
Between December of 2009 and May 2010 a randomly selected convenience sample of 17 HCH filters was collected from 14 ventilated patients as described in methods. No effort was made to select patients based on their identity or condition. Only minors were excluded. Fluid samples were recovered from the patients HCH filters and were analyzed by PCR as described in methods. Table 1 provides a detailed list of the HCH-derived data in comparison with independent clinical findings. The HCH data demonstrate consistent agreement with regard to qualitative and quantitative microbiological findings in time.
Table 1.
Data comparing HCH findings with available electronic medical record culture data and clinical suspicion of VAP. Correlation of HCH fluid quantitative PCR analysis with clinical data.
| HCH | BAL(*sputum) | ||||||
|---|---|---|---|---|---|---|---|
| Patient | sample # | day | Pathogen | Quantity | collection day | Pathogen | Quantity |
| No clinical or quantitative BAL data supporting pneumonia | |||||||
| A | 1 | 33 | A baumannii | not performed | 0* | A lwoffi | NA-sputum |
| 9* | A lwoffi | NA-sputum | |||||
| B | 2 | 2 | C. albicans | not performed | 0 | No growth | NA |
| 8 | C. albicans | < 1 K | |||||
| 10 | C. albicans | < 1 K | |||||
| C | 5 | 0 | S. coag negative | Not performed | No clinical indication | NA | NA |
| D | 6 | 0 | No amplification | NA | No clinical indication | NA | NA |
| E | 7 | 0 | No amplification | NA | 6 - left | S. aureus | < 10 K |
| 6 - right | No growth | NA | |||||
| F | 9 | 0 | No amplification | NA | 9 | C. albicans | < 10 K |
| S. coag negative | < 10 K | ||||||
| 20 | C. albicans | < 1 K | |||||
| S. coag negative | < 1 K | ||||||
| G | 11 | 0 | No amplification | NA | No clinical indication | NA | NA |
| H | 12 | 0 | No amplification | NA | No clinical indication | NA | NA |
| I | 15 | 0 | No amplification | NA | 3 | S. aureus | < 1K |
| 16 | 2 | No amplification | NA | 7 | No growth | NA | |
| Clinical evidence of pneumonia and + BAL | |||||||
| J | 3 | 0 | E. faecalis | Not performed | 47 | E. faecalis | NA-sputum |
| 4 | 48 | S. aureus | 310 K | E. aerogenes | > 100 K | ||
| S. aureus | 50-100K | ||||||
| K | 8 | 0 | Enterobacter sp | ∼265 K | 0 - left | E. aerogenes | < 1K |
| 0 - right | E. aerogenes | 10-25 K | |||||
| L | 10 | 3 | S. coag negative | ∼72 K | 0 | No growth | NA |
| 6 | S. coag negative | 50-100 K | |||||
| C. albicans | 10-25 K | ||||||
| 17 | S. coag negative | < 1K | |||||
| M | 13 | 6 | A baumannii | ∼34K | 0 | No growth | NA |
| 14 | 11 | No amplification | NA | 11 | No growth | NA | |
| 12 | A baumannii | 10-25K | |||||
| N | 17 | 0 | No amplification | NA(HCH dry) | 0 | K. pneumoniae | > 100 K |
| E. sakazakii | 10-25 K | ||||||
Pathogens were detected by PCR in 8 of the 17 HCH filter samples: HCH samples 1,2,3,4,6,8,10,13 (Please refer to Table 1). In all 8 of the aforementioned samples, clinical cultures identified the same micro-organisms at some point during the patients stay. When the HCH filter data are stratified based on clinical suspicion of VAP or not, a correlation is observed. In nine HCH filter samples where PCR failed to identify pathogens, in 7 of these nine, no clinical indication for BAL existed or BAL cultures ruled out VAP.
In Filter samples HCH 13 and HCH 14, two HCH filters were collected from the same patient 5 days apart. In the first HCH sample PCR detected A. baumanii at the same level as did a BAL 3 days prior. The second filter HCH 14 was taken 8 days after the first BAL, and on the same day as a second BAL was performed, subsequent to the initiation of antibiotics for A. baumannii pneumonia. Both the PCR of HCH 14 and the BAL were performed on the same day and found no growth. We consider this latter concurrence a quantitative match and this sequence of findings strong evidence that the breath samples contain real information.
In 5 of the 6 HCH samples 2,6,10,13,14, on which we performed real time quantitation, there is order of magnitude agreement between the BAL and PCR findings. In one of the 6 matches, HCH8, where quantitative data was available the PCR finding was an order of magnitude larger than the BAL value. In 4 of 5 patients in which quantitative BAL cultures indicated pathogens greater than 104 CFU, PCR from the HCH samples both identified the pathogen and provided quantitation greater than 104 bacteria. In 2 of these 4 matches, additional bacteria were reported by BAL that were not identified by PCR. This is an artifact of our application of a specific PCR technique rather than a general limitation. In the second of the two cases, the HCH filter was completely dry and devoid of fluid at the time of analysis and no bacteria were detected by PCR despite a positive BAL.
Using pathogen frequency data from SICU encounters (Tables S1 and S2 in the Supplement) as described in the methods, we estimate that the probability of drawing two matching samples independently from these distributions at random is approximately 2%. Using this value we offer an estimate of the statistical significance of our multiple observations. The probability that 5 of 13 breath samples agree with clinical microbiology findings obtained by more invasive methods, both in the nature of the pathogen found and their number (CFU/ml) to within an order of magnitude, by chance alone is less than p = 8.873 × 10−6 or roughly 9 in one million using an exact binomial test. This calculation supports the contention that our observations are not spurious and that the HCH filters carry information that is correlated with the information contained in the more invasive assays.
Conclusions
We have demonstrated that microbial pathogens accumulate in the HCH filters of ventilated patients. We have shown that the pathogens in the HCH filters can be reliably recovered and identified. We have also shown that the pathogens in the HCH fluid match those discovered independently by standard quantitative methods such as BAL to a statistically significant degree. Alone, the finding that bacteria are recoverable from the disposable HCH filters of ventilated patients is interesting. When one factors in the agreement between the HCH and BAL results, and the observed correlation with the suspicion of VAP, the results achieve clinical significance. After taking into account the rapid, non-invasive nature of the investigational method under discussion, these results provide strongly encouraging preliminary data regarding the possible use of quantitative PCR of HCH fluid samples as an early detection method for the presence of pulmonary pathogens.
As outlined in the introduction, there are several problems encountered in the diagnosis and management of VAP in which the information from the disposable HCH filters can be utilized. Since the disposable filters are routinely replaced every 8 to 12 hours, serial analysis over time may alert the practitioner to increasing risk of pneumonia prior to the development of full clinical signs.
In conclusion, recovery and processing of aerosolized samples from standard ventilator HCH filters is straightforward, non-invasive, and well-suited for repeated sampling in time. Real time PCR detection can be performed in under an hour.
Acknowledgments
The authors wish to thank Dr Gordon Bernard for many helpful discussions and for his support. The work described was partially supported by CTSA 1 UL1 RR024975. EMB thanks Dr Sarah Gates and Professor Rick Haselton for helpful discussions and Professor Ray Mernaugh for technical and material assistance.
Supplementary Data
Table S1.
Of 192 SICU encounters reported to the CDC for Pneumonia the table describes the number and kind of different pathogens. From the results of 1638 quantitative BAL (shown in Table S2), 351 showed no growth. Accordingly we appended this proportion of “None” to the data as a fourteenth category to allow for the possibility that two samples with no growth can count as a match. The probability of drawing a matching pair of categories from this distribution is, 0.119512, approximately 12%. The probability of drawing 7 or more matches in 13 trials, at random from this distribution, is p = 0.000317092, approximately 3 in ten thousand.
| Organism(Genus) | Occurrence in CDC Data set |
|---|---|
| Enterococcus | 51 |
| Mycobacterium | 24 |
| Staphylococcus | 144 |
| Streptococcus | 18 |
| Fungi | 125 |
| Acinetobacter | 46 |
| Enterobacter | 40 |
| Escherichia | 29 |
| Haemophilus | 9 |
| Klebsiella | 43 |
| Pseudomonas | 70 |
| Serratia | 23 |
| Stenotrophomonas | 29 |
| None | 163 |
| Total | 814 |
Table S2.
Data from 1638 BAL procedures performed in the SICU prior to 11/2009, stratified according to quantitative growth observed. The five stratifications are roughly equally populated and the probability of drawing any matching pair of categories is 0.201535, as calculated from the sum of the squares of the corresponding frequencies. The probability of observing 5 or more matches in six trials is p = 0.0016 or roughly 1 in 500.
| Growth Categorization | Occurrence in SICU data set |
|---|---|
| No Growth | 351 |
| Lessthan1KCFU/ml | 353 |
| Between 1K and 10K | 294 |
| Between 10K and 100K | 349 |
| Greater than 100K | 291 |
References
- 1.Koenig S, Truwit J. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clinical Microbiology Reviews. 2006;19:637–657. doi: 10.1128/CMR.00051-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards MJ, Edwards JR, Culver DH, Gaynes R. Nosocomial infections in medical intensive care units in the united states. Critical Care Medicine. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Stone PW. Economic burden of healthcare-associated infections: an American perspective. Expert Rev Pharmacoecon Outcomes Res. 2009;9:417–422. doi: 10.1586/erp.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Thoracic Society, Guidelines for the management of adults with hospital-acquired, ventilator associated and healthcare associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 5.Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical Importance of Delays in the Initiation of Appropriate Antibiotic Treatment for Ventilator-Associated Pneumonia. Chest. 2002;122:262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 6.Rotstein C, Evans G, Born A, Grossman R, Light B, Magder S, McTaggart B, Weiss K, Zhanel GC. Clinical practice guidelines for hospital acquired pneumonia and ventilator associated pneumonia in adults. Can J Infect Dis Med Microbiol. 2008;19:19–53. doi: 10.1155/2008/593289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chastre J, Fagon J. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 8.Reo-Neto A, Youssef NCM, Tuche F, Brunk-horst F, Ranieri VM, Reinhart K, Sakr Y. Diagnosis of ventilator-associated pneumonia: a systematic review of the literature. Critical Care. 2008;12:1–14. doi: 10.1186/cc6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll KC, Glanz BD, Borek AP, Burger C, Bhally HS, Henciak S, Flayhart D. Evaluation of the BD Phoenix Automated Microbiology System for Identification and Antimicrobial Susceptibility Testing of Enterobacteriaceae. J Clin Microbiol. 2006;44:3506–3509. doi: 10.1128/JCM.00994-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez-Lerma F. Empiric broad-spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: a prospective observational study. Critical Care. 2006;10:R78. doi: 10.1186/cc4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depuyt PO, Vandijck DM, Bekaert MA, Decruyenaere JM, Blot SI, Vogelaers DP, Benoit DD. Determinants and impact of multidrug antiobiotic resistance in pathogen causing ventilator-associated pneumonia. Critical Care. 2008;12:R142. doi: 10.1186/cc7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rello J. Bench to bedside review: Therapeutic options and issues in the management of ventilator-associated bacterial pneumonia. Critical Care. 2005;9:259–265. doi: 10.1186/cc3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahar J, Nguile-Makao M, Francais A, Schwebel C, Garrouste-Orgeas M, Goldgran-Toledano D, Azoulay E, Thuong M, Jamali S, Cohen Y, de Lassence A, Timsit J. Predicting the risk of documented ventilator-associated pneumonia for benchmarking: Construction and validation of a score. Critical Care Medicine. 2009;37:2545–2551. doi: 10.1097/CCM.0b013e3181a38109. [DOI] [PubMed] [Google Scholar]
- 14.Michel F, Francheshini B, Berger P, Arnal J, Gainnier M, Sainty J, Papazian L. Early antibiotic treatment for BAL-confirmed ventillator associated pneumonia: a role for routine endotracheal aspirate cultures. Chest. 2005;127:589–597. doi: 10.1378/chest.127.2.589. [DOI] [PubMed] [Google Scholar]
- 15.Eames I, Tang J, Li Y, Wilson P. Airborne transmission of disease in hospitals. J R Soc Interface. 2009;6:S697–S702. doi: 10.1098/rsif.2009.0407.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabian P, McDevitt J, DeHaan W, Fung R, Cowling B, Chan K, Leung G, Milton D. Influenza virus in human exhaled breath: An observational study. PLoS ONE. 2008;3:e2691. doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muscatello G, Gilkerson J, Browning G. Detection of virulent Rhodococcus equi in exhaled air samples from naturally infected foals. Journal of Clinical Microbiology. 2009;47:734–737. doi: 10.1128/JCM.01395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holton J, Webb AR. An evaluation of the micro-bial retention performance of three ventilator-circuit filters. Intensive Care Med. 1994;20:233–237. doi: 10.1007/BF01704708. [DOI] [PubMed] [Google Scholar]
- 19.Kola A, Eckmanns T, Gastmeier P. Efficacy of heat and moisture exchangers in preventing ventilator associated penumonia: Meta analysis of randomized controlled trials. Intensive Care Med. 2005;31:5–11. doi: 10.1007/s00134-004-2431-1. [DOI] [PubMed] [Google Scholar]
- 20.Brodie EL, Desantis TZ, Moberg Parker JP, Zubietta IX, Piceno YM, Anderson GL. Urban aerosols harbor diverse and dynamic bacterial populations. Proc Natl Acad Sci. 2007;104:299–304. doi: 10.1073/pnas.0608255104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadkarni M, Martin F, Jacques N, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148:257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzman CP, Robnett CJ. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwen-hoek International Journal of General and Molecular Microbiology. 1998;73:331–371. doi: 10.1023/a:1001761008817. [DOI] [PubMed] [Google Scholar]
- 23.Cherkaoui A, Emonet S, Ceroni D, Candolfi B, Hibbs J, Francois P, Schrenzel J. Development and validation of a modified broad range 16s rDNA PCR for diagnostic purposes in clinical microbiology. J Microbial Methods. 2009;79:227–231. doi: 10.1016/j.mimet.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Gu F, Li Y, Zhou C, Wong D, Ho C, Qi F, Shi W. Bacterial 16s rRNA/rDNA profiling in the liquid phase of human saliva. The Open Dentistry Journal. 2009;3:80–84. doi: 10.2174/1874210600903010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horz HP, Vianna ME, Gomes BPFA, Conrads G. Evaluation of Universal Probes and Primer Sets for Assessing Total Bacterial Load in Clinical Samples: General Implications and Practical Use in Endodontic Antimicrobial Therapy. J Clin Microbiology. 2005;43:5332–5337. doi: 10.1128/JCM.43.10.5332-5337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira D, de Lencastre H. Multiplex PCR strategy for rapid identification of Structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrobial Agents and Chemotherapy. 2002;46:2155–2161. doi: 10.1128/AAC.46.7.2155-2161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siala M, Gdoura R, Fourati H, Rihl M, Jaulhac B, Younes M, Sibilia J, Baklouti S, Bargaoui N, Sellami S, Sghir A, Hammami A. Broad range PCR cloning and sequencing of the full 16s rRNA gene for the detection of bacterial DNA in synovial fluid samples of tunisian patients with reactive and undifferentiated arthritis. Arthritis Research & Therapy. 2009;11:R102. doi: 10.1186/ar2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stowers C, Haselton R, Boczko EM. An analysis of quantitative PCR reliability through replicates using the Ct method. JBiSE. 2010;5:459–469. doi: 10.4236/jbise.2010.35064. [DOI] [PMC free article] [PubMed] [Google Scholar]