Abstract
The PRL-1 and PRL-2 phosphatases have been implicated as oncogenic, however the involvement of these molecules in human neoplasms is not well understood. To increase understanding of the role PRL-1 and PRL-2 play in the neoplastic process, in situ hybridization was used to examine PRL-1 and PRL-2 mRNA expression in 285 normal, benign, and malignant human tissues of diverse origin. Immunohistochemical analysis was performed on a subset of these. PRL-1 and PRL-2 mRNA expression was also assessed in a small set of samples from a variety of diseases other than cancer. Where possible, associations with clinicopathological characteristics were evaluated. Alterations in PRL-1 or -2 expression were a frequent event, but the nature of those alterations was highly tumor type specific. PRL-1 was significantly overexpressed in 100% of hepatocellular and gastric carcinomas, but significantly under-expressed in 100% of ovarian, 80% of breast, and 75% of lung tumors. PRL-2 expression was significantly increased in 100% of hepatocellular carcinomas, yet significantly downregulated in 54% of kidney carcinomas. PRL-1 expression was correlated to patient gender in the bladder and to patient age in the brain and skeletal muscle. PRL-1 expression was also associated with tumor grade in the prostate, ovary, and uterus. These results suggest a pleiotropic role for PRL-1 and PRL-2 in the neoplastic process. These molecules may associate with tumor progression and serve as clinical markers of tumor aggressiveness in some tissues, but be involved in inhibition of tumor formation or growth in others.
Keywords: Phosphatase of regenerating liver, PRL-1, PRL-2, in situ hybridization, cancer
Introduction
The PRL family of phosphatases has gained much attention in recent years as potential targets for therapeutic intervention in a variety of tumor types. The family consists of three closely related members (PRL-1, PRL-2, and PRL-3), which constitute a novel class of protein tyro-sine phosphatase (PTP). The PRLs are among the smallest of the PTPs, having molecular masses of 20-22kDa and consisting primarily of a catalytic domain. In addition, the PRL enzymes are the only PTPs known to be post-translationally isoprenylated. This post-translational modification is critical to their sub-cellular localization and biological activity [1-3].
Accumulating evidence points to a role for the PRL family in tumor formation, invasion, and metastasis. Functional studies have shown that overexpression of PRL-1, -2, or -3 in non-tumorigenic rodent cells leads to rapid cellular growth and a transformed phenotype in culture and to tumor formation in athymic, nude mice [1, 4-7]. Moreover, PRL-3 overexpression enhances the growth of human embryonic kidney fibroblasts in culture [5] and can transform a low metastatic potential melanoma cell line into a highly metastatic line both in vitro and in vivo [6]. Stable expression of PRL-1 or PRL-3 leads to enhanced cell motility and invasive ability, whereas down regulation of either of these molecules causes a significant reduction in migratory ability in vitro and suppression of metastatic tumor formation in vivo [7-14]. The most well studied PRL family member, in relation to human cancer, is PRL-3. Widespread interest in this gene was generated after Saha et al. [15] identified 144 gene transcripts with increased expression in liver metastases compared to their primary colorectal tumors and demonstrated that PRL-3 was the only gene consistently overexpressed in all 18 of the metastatic cases examined. A gradient in PRL-3 expression was also noted, with low levels of PRL-3 message in normal colorectal epithelium, intermediate levels in the primary tumors, and high expression in each of the liver metastases. Bardelli et al. [16] later showed that PRL-3 mRNA overexpression was not limited to liver metastases, but that PRL-3 was expressed more highly in all colorectal carcinoma metastases examined, regardless of the site of metastasis. PRL-3 overexpression has since been linked to such clinical parameters as disease progression, tumor aggressiveness, lymphatic invasion, venous invasion, presence and extent of metastasis, or poor patient prognosis in colon/colorectal [17-20], cervical [21], ovarian [22, 23], breast [24-26], gastric [27-35], non-small cell lung [36], esophageal [37], nasopharyngeal [12], brain [38], hepatocellular [39] and bile duct [40] cancers. These data suggest PRL-3 as a potential prognostic indicator of disease aggressiveness and clinical outcome for multiple tumor types.
In contrast to PRL-3, little data is currently available on the expression of PRL-1 or PRL-2 in human malignancies, yet it is clear from cell line and murine studies that these genes also play important roles in tumor formation, invasion, and metastasis [1, 7, 41-43]. In the current study, we provide further insight into the role that both PRL-1 and PRL-2 play in the development and progression of human disease by performing a retrospective analysis on 342 human tissue specimens from 243 individual subjects. The expression of PRL-1 and PRL-2 mRNA was assessed in a variety of normal and tumor tissues of diverse tissue origin using in situ hybridization. Where possible, correlations between PRL-1 or -2 mRNA expression and several clinicopathological features, including patient age and gender, tumor type and grade, and presence or absence of local or distant metastases were investigated. A comparison between mRNA and protein expression levels was also made in a subset of these tissues. In addition, because PRL-3 overexpression in mouse models has previously been linked to cardiovascular disease [5] and PTPs in general have been implicated in the progression of several cardiovascular, neurological, metabolic, and autoimmune diseases [44-47], we also examined the relationship between PRL-1 and PRL-2 expression and a variety of disease states other than cancer.
Materials and methods
Tissue procurement
Formalin-fixed, paraffin-embedded tissue samples were obtained from archival paraffin blocks. Tissues were acquired from the Cooperative Human Tissue Network (CHTN), National Disease Research Interchange (NDRI), or Indiana University School of Medicine, Department of Pathology, collected in accordance with the guidelines of Indiana University and with approval from the IUPUI Institutional Review Board. Tissue sections of each specimen were stained with Hematoxylin and Eosin (H&E) and were examined by a pathologist, with no prior knowledge of the available patient data, to confirm histopathologic diagnosis and tumor grading. For all cases, representative tissue sections were chosen for in situ hybridization (ISH) and/ or immunohistochemical (IHC) analysis.
In situ hybridization
Non-isotopic ISH was performed using FITC-labeled oligonucleotide probes specific for PRL-1 or PRL-2 mRNA, as previously described [48]. Briefly, 5μm thick tissue sections were deparaf-finized, rehydrated through graded alcohols to distilled water and permeabilized with 200μl of 10μg/mL proteinase K for 5-20 minutes depending on tissue type. The deproteination reaction was stopped by washing slides two times, three minutes each in Nanopure ultrapure water, followed by sequential washes in 95% and 100% ethanol for three minutes each. Slides were allowed to air dry for one hour at room temperature (RT), prior to hybridization. Tissue sections were then incubated in a humidified chamber overnight (12-14 hours) at 37°C with 50μL of PRL-1, PRL-2, or control probe diluted to a final concentration of 750 ng/mL in Perfec-tHyb™ Plus hybridization buffer (Sigma-Aldrich, St. Louis, MO, USA). Following hybridization, non-specifically bound probe was removed by washing slides two times in 2X SSC (300mM NaCI, 30mM Sodium Citrate, pH 7.0) plus 0.1% SDS for five minutes RT, one time in pre-warmed 0.5X SSC (75mM NaCI, 7.5mM Sodium Citrate, pH 7.0) + 0.1% SDS at 37°C for 20 minutes, and one time in tris-buffered saline (TBS; 50mM Tris-HCL, 150mM NaCI, pH 7.6) + 0.1% SDS at RT for 10 minutes. Detection of hybridized probe was performed by standard immunohisto-chemical techniques using a catalyzed signal amplification procedure. Non-specific background staining was blocked by incubation with DAKO Serum-Free Protein Block (DAKO Corporation, Carpenteria, CA, USA) for 30 minutes, followed by 30 minutes incubation with a mouse anti-FITC primary antibody (DAKO), diluted to 22μg/mL in DAKO Antibody Diluent. Bound primary antibody was detected using the labeled streptavidin-biotin method (LSAB2, DAKO) combined with the Renaissance® Tyramide Signal Amplification kit (TSA™ Biotin, PerkinElmer Life Sciences, Inc., Boston, MA, USA). Peroxidase bound, antibody complexes were visualized using DAB (DAB Substrate/Chromogen System, DAKO) as the chromogenic substrate. Development was allowed to proceed for 2-5 minutes and was stopped by rinsing the slides in distilled water for five minutes. Sections were counter-stained briefly with 1X Lerner's hematoxyiin, dehydrated through graded alcohols, cleared in xylene, and coverslipped with permanent mounting media (ThermoFisher Scientific, Inc., Waltham, MA, USA). All staining steps were performed on a DAKO Autostainer at room temperature and slides were rinsed for five minutes in TBS + 0.05% Tween-20 between each step of the procedure. Normal adjacent and tumor tissue sections from one organ type were always processed simultaneously.
Immunohistochemistry
Rabbit antibodies against peptides corresponding to amino acids 50-65 of human PRL-1 and 47-62 of human PRL-2 were generated by Genemed Synthesis, Inc (San Antonio, TX, USA). The antibodies were affinity purified against E. coli expressed PRL proteins. Slides containing 5μm tissue sections were deparaffinized for 9 minutes in xylene then rehydrated through a series of 100%, 80%, and 70% ethanol for 5 minutes each, followed by a 5 minute rinse in PBS. Antigen retrieval was performed by heating in a microwave for 5 minutes in 5mM Sodium Citrate. Following retrieval, slides were allowed to cool at room temperature for 15 minutes. Endogenous peroxidase activity was quenched by incubation in 3% H2O2 for 5 minutes. Sections were blocked for 15 minutes in 3% non-fat dry milk, 1% BSA, then incubated 90 minutes at 37°C with primary antibody diluted 1:200 in blocking solution. This was followed by a 30 minute incubation with biotinylated secondary, anti-rabbit antibody (Biogenex Laboratories, Inc., Fremont, CA, USA) diluted 1:20 in blocking buffer and a 30 minute incubation with streptavidin peroxidase (Biogenex). A 5 minute phosphate buffered saline (PBS) rinse was incorporated after each step in the immunostaining procedure. Colorimetric detection was carried out using AEC and allowed to proceed until color was detected in the tissues by microscopic examination at which point the reaction was quenched by rinsing the slides in distilled water. Sections were counterstained in Hematoxylin for 30 seconds and again rinsed with water.
Controls
Several positive and negative controls were used, concurrently, to confirm the specificity of the ISH or IHC signal. All controls were performed on serial sections of the same tissues as examined with the PRL-1 and PRL-2 probes or antibodies, utilizing the ISH and IHC procedures described above. For the ISH experiments, positive controls included: (a) verification of the hybridization and detection procedure by hybridization of the PRL-1 and PRL-2 antisense probes to a normal pancreas tissue (case # 032098), known to be positive for PRL-1 and PRL-2 mRNA and (b) hybridization of tissues with a fluorescein-conjugated Poly d(T) probe (Novocastra Laboratories, New Castle upon Tyne, UK) to assess the preservation and integrity of the mRNA in each sample. Negative controls consisted of: (a) omission of the oligonucleotide probes from the hybridization mixture and incubation of the tissue specimens with only PerfectHyb™ hybridization buffer, (b) substitution of the specific antisense probe with an equivalent concentration of labeled sense probe to examine the stringency of the assay, (c) hybridization using a cocktail of randomly generated, FITC-conjugated, oligonucleotide sequences (NCL-CONTROL, Novocastra), to assess binding of nonspecific sequences, and (d) Pretreatment of tissue sections with 250μg/mL RNase A (Sigma-Aldrich) for 2 hours at 37°C to demonstrate the specificity of the signal for single stranded RNA. Probe specificity was also verified by slot-blotting and shown previously [48]. As negative controls for IHC, tissue sections were incubated either in the presence of no primary antibody, no secondary antibody, or primary antibody blocked with the peptide used to generate the anti-PRL-1 or anti-PRL-2.
Staining interpretation
Evaluation of all slides was performed under bright-field microscopy. The intensity of staining and the percentage of positive normal and tumor cells for the ISH studies were evaluated with the aid of a single, experienced pathologist, in a blinded fashion. For the IHC experiments, scoring of images was performed independently by three separate individuals and the mean reading was taken for each tissue section. The appearance of a brownish-red stain over the cells was used to indicate probe hybridization or antibody binding and thus reflect the cellular levels of PRL-1 and PRL-2 mRNA or protein. Immunostaining was scored using established methods [49, 50]. Briefly, staining intensity was classified according to the following scale: (-) absent, (+/-) barely detectable, (+) weak, (++) moderate, and (+++) strong. In cases of heterogeneous staining, the average intensity across the tissue was taken as the score. Also, in a few cases where a patient sample was stained twice, the case was given a mean score, based on evaluation of the two sections. The percentage of positive cells was estimated as the number of stained cells, per total number of cells counted. The localization of staining within the cells of each tissue was also examined and noted as nuclear, cytoplasmic, membranous, or a combination of these. For semiquantitative analysis of the results, the staining intensity was assigned an arbitrary value, on a scale of 0-3, as follows: (-) = 0, (+/-) = 0.5, (+) =1, (++) = 2, (+++) = 3. An overall staining score (SS) was calculated for each sample, by multiplying the staining intensity times the percentage of positive cells. After multiplication of both values, results were graded from 0 (negative) to 300 (all cells display strong staining intensity). To confirm the reproducibility of the analysis, 25% of the slides were randomly chosen and scored twice. Duplicate readings gave similar results. Images were acquired using a SPOT digital camera and imaging software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA).
Statistical analysis
Statistical calculations were executed using Statistical Analysis System software (SAS version 8.2, SAS Institute, Inc., Cary, NC). Analyses of differences in PRL expression between cancerous and noncancerous tissues were performed using a Student's paired t-test. Results are expressed as mean ± standard error of the mean (SEM) and P < 0.05 was considered statistically significant. For most samples, the medical histories of the patients and pathological reports for each specimen were also available. These were reviewed and correlations between PRL expression and patient clinicopathological features such as patient age and gender; tumor type, subtype, and grade; and presence of local or distant metastasis were calculated using a mixed model analysis of variance. Again, P < 0.05 was deemed statistically significant.
Results
PRL-1 and PRL-2 transcripts are expressed in a broad variety of normal and tumor tissues
A total of 285 normal, benign, and malignant human tissue samples of diverse origin were obtained from archival paraffin blocks and subjected to ISH, in order to examine expression of PRL-1 and PRL-2 mRNA transcripts (Table 1). PRL-2 message was found to be expressed at moderate to high levels in almost all (279/285) of the normal and tumor tissues examined. Low levels of PRL-2 were noted only in a single case of renal cell carcinoma, one normal lymph node, one ovarian carcinoma, and three normal specimens from the spleen. PRL-1 mRNA was also expressed in the vast majority of tissues examined, however the degree and intensity of PRL-1 staining varied considerably between tissue types and between individual cases within a single tissue type. This transcript was expressed at detectable levels in 97% (133/137) of histologically normal tissues examined, as well as in 93% (14/15) of breast carcinomas, 83% (5/6) of endometrial adenocarcinomas, 78% (14/18) of ovarian tumors, 77% (10/13) of renal cell carcinomas, and in 100% of primary tumors derived from the bladder (n=9), cervix (n = 1), colon (n = 5), liver (n = 4), lung (n = 8), pancreas (n = 14), prostate (n = 28), skin (n = 1), stomach (n = 5), and testis (n = 4). PRL-1 was also expressed in all cases examined of B-cell lymphoma (n = 5, including one metastatic lesion in the testes), Hodgkin lymphoma (n = 1), chronic lymphocytic leukemia (CLL; n = 1), chronic myelogenous leukemia (CML; n = 1), colon metastases to the liver (n = 3), uterine sarcoma (n = 1), and benign lesions of the breast (n = 2). In the vast majority of cases (both normal and tumor), localization of PRL-1 and PRL-2 staining appeared to be nuclear, however in tissues of the breast, liver, pancreas, stomach, and uterus, both nuclear and cytoplasmic staining were observed.
Table 1.
Expression of PRL-1 and PRL-2 in various tumors and diseased tissues
| PRL-1 | PRL-2 | ||||||
|---|---|---|---|---|---|---|---|
| Tissue Type/Histopathology | # Samples | Weak (%) | Moderate (%) | Strong (%) | Weak (%) | Moderate (%) | Strong (%) |
| Bladder | |||||||
| Transitional Cell Carcinoma | 7 | 0 | 71 | 29 | 0 | 0 | 100 |
| Sarcomatoid Carcinoma | 1 | 100 | 0 | 0 | 0 | 0 | 100 |
| Undifferentiated Carcinoma | 1 | 100 | 0 | 0 | 0 | 0 | 100 |
| Hyperplastic Lesion | 1 | 100 | 0 | 0 | 0 | 0 | 100 |
| Brain | |||||||
| Alzheimer's | 4 | 25 | 75 | 0 | 25 | 0 | 75 |
| Multiple Sclerosis | 2 | 0 | 0 | 0 | 100 | 0 | 0 |
| Breast | |||||||
| Invasive Ductal Carcinoma | 15 | 13 | 40 | 40 | 0 | 7 | 93 |
| Benign Lesions | 2 | 0 | 100 | 0 | 0 | 0 | 100 |
| Cervix | |||||||
| Squamous Cell (LCK) | 1 | 0 | 100 | 0 | 0 | 0 | 100 |
| Colon | |||||||
| Adenocarcinoma | 5 | 20 | 40 | 40 | 0 | 0 | 100 |
| Metastatic Lesions in Liver | 3 | 0 | 33 | 67 | 0 | 0 | 100 |
| Crohn's Disease | 2 | 0 | 0 | 100 | 0 | 0 | 100 |
| Coronary Arteries | |||||||
| 30-60% Occlusion | 3 | 33 | 0 | 0 | 0 | 33 | 33 |
| 60-90% Occlusion | 2 | 0 | 0 | 0 | 0 | 50 | 0 |
| Heart | |||||||
| Heart Disease | 7 | 43 | 0 | 0 | 14 | 86 | 0 |
| Kidney | |||||||
| Renal Cell Carcinoma | 13 | 38 | 31 | 8 | 8 | 8 | 84 |
| Liver | |||||||
| Hepatocellular Carcinoma | 4 | 0 | 100 | 0 | 0 | 0 | 100 |
| Hepatitis | 2 | 100 | 0 | 0 | 0 | 50 | 50 |
| Steatosis | 2 | 50 | 50 | 0 | 0 | 50 | 50 |
| Lung | |||||||
| Non Small Cell Lung Cancer | 8 | 0 | 37 | 63 | 0 | 12 | 88 |
| Lymph Node | |||||||
| B-Cell Lymphoma | 4 | 0 | 0 | 100 | 0 | 0 | 100 |
| Hodgkin Lymphoma | 1 | 0 | 0 | 100 | 0 | 0 | 100 |
| Metastatic Lymphoma in Testes | 1 | 0 | 0 | 100 | 0 | 0 | 100 |
| Ovary | |||||||
| Epithelial Tumor | 17 | 41 | 35 | 0 | 6 | 12 | 82 |
| Dysgerminoma | 1 | 0 | 100 | 0 | 0 | 0 | 100 |
| Pancreas | |||||||
| Exocrine Tumor | 12 | 0 | 8 | 92 | 0 | 0 | 100 |
| Endocrine Tumor | 2 | 0 | 0 | 100 | 0 | 0 | 100 |
| Diabetic | 8 | 0 | 0 | 100 | 0 | 0 | 100 |
| Prostate | |||||||
| Adenocarcinoma | 28 | 25 | 25 | 50 | 0 | 0 | 100 |
| Skeletal Muscle | |||||||
| Diabetic | 4 | 0 | 50 | 0 | 0 | 25 | 75 |
| Skin | |||||||
| Basal Cell Carcinoma | 1 | 100 | 0 | 0 | 0 | 0 | 100 |
| Spleen | |||||||
| Chronic Lymphocytic Leukemia | 1 | 100 | 0 | 0 | 0 | 100 | 0 |
| Chronic Myelogenous Leukemia | 1 | 0 | 0 | 100 | 0 | 0 | 100 |
| Diabetic | 3 | 0 | 67 | 0 | 0 | 33 | 67 |
| Stomach | |||||||
| Adenocarcinoma | 4 | 0 | 0 | 100 | 0 | 0 | 100 |
| Leiomyosarcoma | 1 | 0 | 0 | 100 | 0 | 0 | 100 |
| Testis | |||||||
| Germ Cell Tumor | 4 | 0 | 50 | 50 | 0 | 0 | 100 |
| Uterus | |||||||
| Adenocarcinoma | 6 | 33 | 50 | 0 | 0 | 17 | 83 |
| Sarcoma (MMMT) | 1 | 0 | 0 | 100 | 0 | 0 | 100 |
| Vasculature (Multi Tumor Tissues) | 38 | 21 | 32 | 45 | 0 | 5 | 95 |
| Stroma (Multi Tumor Tissues) | 90 | 41 | 29 | 17 | 0 | 12 | 88 |
Abbreviations: LCK = Large Cell Keratinizing; MMMT = Malignant Mixed Mullerian Tumor
Dysregulation of PRL-1 and PRL-2 mRNA expression in tumors is highly tissue specific
To provide further insight into the role of the PRL genes in cancer development, PRL-1 and PRL-2 mRNA expression were directly compared between the tumor and normal tissues examined by ISH. In both the normal and tumor tissues, a large degree of inter-individual variability was observed, particularly in PRL-1 expression, suggesting that comparisons between normal and diseased tissue from different patients could be misleading. To account for this, only case matched tumor and normal adjacent tissue (NAT) specimens from the same patient were utilized in this analysis. Of the tissues examined, there were 94 cases where both tumor and normal samples were available (188 total tissue specimens). These included case matched specimens from the bladder (n = 5), breast (n = 15), colon (n = 5), kidney (n = 13), liver (n = 4), lung (n = 8), ovary (n = 6), pancreas (n = 10), prostate (n = 13), spleen (n = 1), stomach (n = 5), testis (n = 4), and uterus (n = 5).
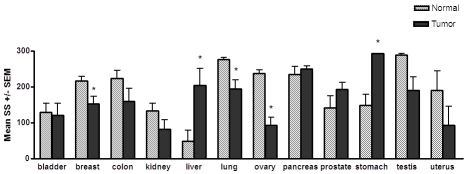
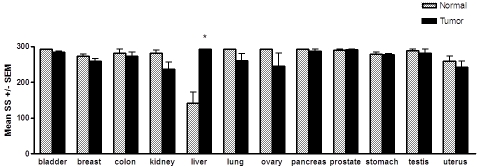
Direct comparison between normal and tumor samples revealed several significant, yet highly tissue specific differences in PRL-1 and PRL-2 mRNA expression (Figures 1 and 2). PRL-1 expression was significantly higher in 100% of the gastric carcinomas examined as compared to adjacent normal gastric tissue, with an almost 2 -fold higher mean staining score in the cancerous tissue than in the noncancerous tissue (P = 0.01, Stomach, Figures 1 and 3). PRL-1 was also significantly overexpressed in 100% of hepatocellular carcinomas (HCC) compared to the matched normal tissues examined (P = 0.0052) with 4-fold higher expression occurring in the tumor tissues in this instance. PRL-2 message was also found to be upregulated in 100% of the hepatocellular carcinomas examined (P = 0.0152, Liver, Figure 1) with levels of PRL-2 expression in the HCC tissues, on average, approximately 2-fold higher than in normal hepatocytes (P = 0.0152).
Figure 1.
Levels of PRL-1 transcript in matched tumor and adjacent normal tissue pairs. The mean staining scores (SS) ± the SEM are shown. An asterisk indicates a statistically significant difference between the matched normal versus tumor tissue, as determined by paired t-tests (P < 0.05). A statistically significant increase in PRL-1 mRNA expression was found in hepatocellular carcinomas (P = 0.0052, n = 4), and carcinomas of the stomach (P = 0.01, n = 5), compared to matched normal tissues. A significant decrease in PRL-1 mRNA expression was found in breast tumor tissue (P = 0.0058, n = 15), lung tumors (P = 0.015, n = 8), and ovarian carcinomas (P = 0.0007, n = 6) compared to matched normal tissues.
Figure 2.
Levels of PRL-2 transcript in matched tumor and adjacent normal tissue pairs. The mean staining scores (SS) ± the SEM are shown. An asterisk indicates a statistically significant difference between the matched normal versus tumor tissue, as determined by paired t-tests (P < 0.05). Only hepatocellular carcinomas exhibited a statistically significant difference, with an approximately 2-fold increase in PRL-2 mRNA expression (P = 0.015, n = 4) in the tumors compared to normal liver tissue from the same subjects.
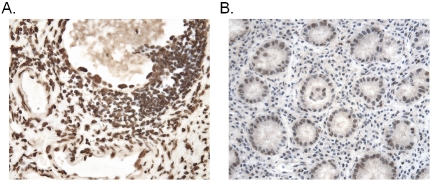
Figure 3.
Increased levels of PRL-1 mRNA in carcinomas of the stomach. A representative example shows that PRL-1 message is expressed at significantly higher levels in a gastric adenocarcinoma (A) than in the normal gastric tissue from the same individual (B). Counterstained with hematoxyiin. Magnification x400.
Given the evidence for a role of the PRL enzymes in tumor development and metastasis, it was not surprising to find PRL-1 and PRL-2 expression increased in a variety of tumor tissues. Unexpectedly however, expression of both genes was also found to be lower, relative to the normal adjacent tissues, in a number of tumor types. PRL-1 transcript levels were significantly decreased in 100% of ovarian carcinomas (P = 0.0007, Ovary, Figure 1), twelve (80%) of 15 benign and malignant breast tumors (P = 0.0058, Figures 1 and 4), and 6 (75%) of 8 lung carcinomas (P = 0.0148) with respect to the paired normal tissues for each. A similar downward trend appeared to occur for 80% of the colon carcinomas, 69% of the renal cell carcinomas, 80% of the testicular carcinomas, and 80% of the uterine carcinomas examined, however the differences in these tissues did not reach statistical significance. For PRL-2, seven (54%) out of 13 renal cell carcinomas showed a slight decrease in PRL-2 expression compared to the matched normal tissues (P = 0.049). Likewise, 38% of lung carcinomas, 40% of ovarian and 60% of uterine carcinomas showed a small decrease in PRL-2 mRNA levels compared to corresponding normal tissues, but these changes were not found to be statistically significant. Both PRL-1 and PRL-2 were also down-regulated almost 2-fold in a single case of chronic lymphocytic leukemia compared to normal splenic B cells from the same patient, but, with only one tumor and matched normal sample in this case, statistical comparisons for this tumor type could not be made. No significant changes or trends in either PRL-1 or PRL-2 were observed in the overall mRNA expression between bladder, pancreatic, or prostate tumors and their respective matched normal adjacent tissues.
Figure 4.
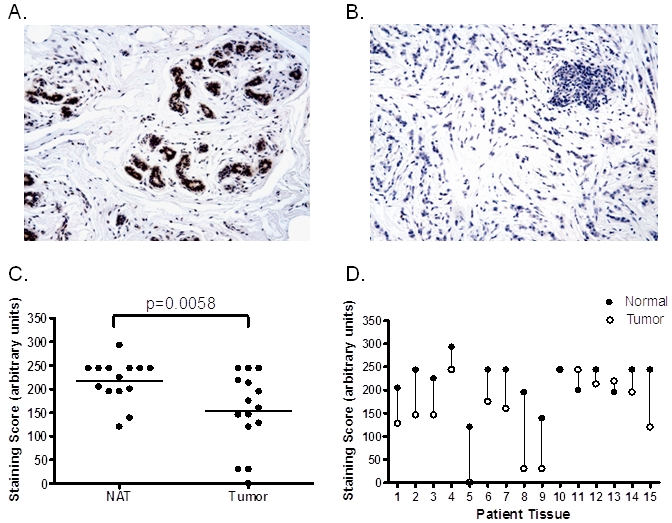
Decreased levels of PRL-1 mRNA in benign and malignant tumors of the breast. PRL-1 mRNA expression in (A) normal breast tissue and (B) a case matched, invasive ductal carcinoma of the breast. Counterstained with hema-toxylin. Magnification x200. (C) Semi-quantitative analysis of PRL-1 mRNA expression in breast tumors (n = 15) and their matched normal adjacent tissues (n = 15). Horizontal lines represent the mean values for each group. Differences between the groups were found to be statistically significant by paired t-test (P = 0.0058). (D) PRL-1 mRNA expression in individual cases of matched tumor and adjacent normal tissue of the breast, showing a decrease in PRL-1 mRNA transcripts in 12/15 (80%) tumor tissues compared to their matched normal counterparts. Vertical lines connect matched tissue pairs from the same patient.
PRL-1 and PRL-2 mRNA expression in tumor stroma and vasculature
In addition to analyzing PRL-1 and PRL-2 expression patterns in multiple tumors and their normal cellular counterparts, expression of these transcripts was also compared between the stroma and vasculature of each subject's tumor (Table 1) and normal tissue. PRL-1 message was expressed in the vasculature of 100% of the normal tissues and 98% of the tumor tissues examined, with highly variable degree and intensity of staining. Only one specimen, a uterine adenocarcinoma, did not display PRL-1 staining in the tumor vascuiature, although very low levels of PRL-1 message were detectable in the glandular tissue of this tumor. In the stroma, PRL-1 was expressed in 95% of both the normal and tumor tissue sections examined, again with highly variable levels of expression from tissue to tissue. In most of the cases where an absence of PRL-1 staining was observed in the stroma, staining of all other structures within the tissue was also weak to absent. However, in 2/4 normal breast tissues where PRL-1 mRNA expression was not detected in the stroma, PRL-1 was expressed at moderate to high levels in the ductal epithelium. Likewise, in one squamous cell carcinoma of the lung which lacked PRL-1 expression in the stroma, PRL-1 was expressed at moderate levels in the tumor epithelium. PRL-2 was expressed at moderate to high levels in the vasculature and stroma of all 190 cases examined.
Similar to its upregulation in adenocarcinomas of the stomach, PRL-1 was also overexpressed in the stroma of each stomach tumor tissue examined (Figure 3), compared to the stroma of the corresponding normal adjacent tissues (P = 0.0382). In the bladder, although no clear differences in PRL expression existed between the normal and malignant urothelial cells, a significant decrease in PRL-1 expression was observed in both the bladder tumor vasculature (P = 0.0199) and the stroma surrounding the tumor (P = 0.0182), as compared to these structures in the normal adjacent tissue samples. PRL-2 expression was also significantly decreased in the stroma of bladder carcinomas (P < 0.0001). Unlike PRL-1, PRL-2 expression was not significantly altered in the bladder tumor vasculature and displayed high levels of expression in all normal and tumor tissues. Levels of PRL-1 and PRL-2 message in the stroma and vasculature were not significantly different between the normal and tumor tissue pairs of any other tissue type.
Expression of PRL-1 and PRL-2 transcripts in other human diseases
In addition to carcinogenesis, protein tyrosine phosphatases have also been implicated in susceptibility to, or progression of, various other diseases, such as inflammatory and autoimmune diseases, neurodegenerative diseases, and cardiovascular disease. The PRL family member PRL-3 itself has been linked to a role in heart disease [5]. Therefore, in an attempt to analyze the relationship between PRL expression and various other disease states, PRL-1 and PRL-2 mRNA expression were examined in the affected organs from patients with Alzheimer's disease (n = 4), multiple sclerosis (n = 2), crohn's disease (n = 2), heart disease (n = 7), coronary artery disease (n = 5), hepatitis (n = 2), liver steatosis (n = 2), insulin-dependent diabetes mellitus (n = 6), and noninsulin-dependent diabetes (n = 2). A student's t-test was used to compare the mean staining scores between each set of diseased tissues and a set of histologically normal samples of the same tissue type, from different subjects.
PRL-1 expression was again highly variable in all tissue types examined. PRL-2 message was expressed at moderate to high levels in all tissues examined, with the exception of the brain and heart where, like PRL-1, its expression was quite variable (Table 1). In the small sample set analyzed here, no significant correlations were found between either PRL-1 or PRL-2 gene expression and any of the disease states examined. There did appear to be a trend toward increased expression of PRL-1 in the brains of Alzheimer's disease patients, where 75% of subjects had a SS > 150 in sections from the cerebral cortex and hippocampus, while 60% of normal subjects displayed SS < 50 in the same regions. However, considering the correlations with patient age discussed below, this trend is unlikely to be significant.
Correlation between PRL-1 and PRL-2 mRNA expression and patient clinicopathological parameters
To evaluate the clinical relevance of PRL-1 and PRL-2 expression in each tissue type examined, where possible (sufficient sample size and available patient data), a mixed model analysis of variance was used to analyze the relationship between the PRL staining scores and several clinicopathological features, including patient age, patient gender, tumor type, tumor subtype, tumor grade, and evidence of tumor metastasis (Tables 1 and 2). The intensity of PRL staining was also compared to the localization of the staining (whether nuclear, cytoplasmic, membranous, or a combination of these) to examine any correlations between the two and the staining localization was also individually compared to each of the various clinicopathological parameters.
Table 2.
Patient and sample characteristics
| Total Number | Gender | Age (years) | Tumor Grade | Metastasis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue Type | Samples | Subjects | Tumors | M | F | Unk | Mean | Range | High | Intermediate | Low | Unk | Y | N | Unk | |
| Bladder | 16 | 10 | 9 | 4 | 6 | 0 | 71 | 60-81 | 6 | 3 | 0 | 0 | 1 | 8 | 0 | |
| Brain | 16 | 11 | 0 | 7 | 4 | 0 | 68 | 50-87 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Breast | 35 | 20 | 17 | 0 | 20 | 0 | 58 | 38-85 | 12 | 4 | 1 | 0 | 7 | 6 | 4 | |
| Cervix | 6 | 6 | 1 | 0 | 6 | 0 | 40 | 30-44 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| Colon | 16 | 11 | 8 | 7 | 3 | 1 | 69 | 29-94 | 2 | 4 | 2 | 0 | 7 | 1 | 0 | |
| Coronary Artery | 5 | 5 | 0 | 4 | 1 | 0 | 57 | 37-78 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Heart | 11 | 11 | 0 | 7 | 4 | 0 | 45 | 9-73 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Kidney | 26 | 13 | 13 | 8 | 5 | 0 | 64 | 8-81 | 3 | 9 | 1 | 0 | 4 | 9 | 0 | |
| Liver | 16 | 12 | 4 | 5 | 7 | 0 | 54 | 1-75 | 0 | 0 | 4 | 0 | 0 | 0 | 4 | |
| Lung | 16 | 8 | 8 | 6 | 2 | 0 | 66 | 57-76 | 6 | 2 | 0 | 0 | 5 | 3 | 0 | |
| Lymph Node | 11 | 11 | 5 | 7 | 4 | 0 | 44 | 7-82 | 3 | 0 | 2 | 0 | 1 | 0 | 4 | |
| Ovary | 29 | 24 | 18 | 0 | 24 | 0 | 47 | 17-74 | 9 | 6 | 3 | 0 | 4 | 2 | 12 | |
| Pancreas | 38 | 28 | 14 | 10 | 9 | 9 | 47 | 3-79 | 8 | 5 | 0 | 1 | 6 | 0 | 8 | |
| Prostate | 41 | 28 | 28 | 28 | 0 | 0 | 63 | 51-75 | 14 | 10 | 4 | 0 | 2 | 3 | 23 | |
| Skeletal Muscle | 9 | 9 | 0 | 5 | 3 | 1 | 55 | 16-90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Skin | 4 | 4 | 1 | 1 | 1 | 2 | 52 | 51-52 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | |
| Spleen | 12 | 11 | 2 | 8 | 3 | 0 | 41 | 13-71 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | |
| Stomach | 10 | 5 | 5 | 4 | 1 | 0 | 72 | 46-85 | 4 | 1 | 0 | 0 | 4 | 0 | 1 | |
| Testis | 10 | 5 | 4 | 5 | 0 | 0 | 45 | 28-82 | 2 | 1 | 1 | 0 | 0 | 1 | 3 | |
| Uterus | 16 | 11 | 7 | 0 | 11 | 0 | 47 | 20-68 | 2 | 5 | 0 | 0 | 1 | 1 | 5 | |
Abbreviations: M = male; F = Female; Unk = Unknown; Y = Yes; N = No
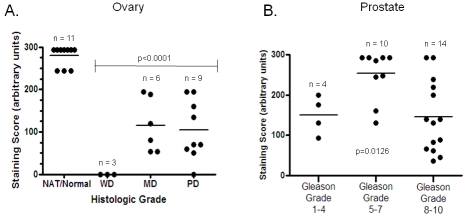
Levels of PRL-1 expression were found to be correlated with patient gender in neoplasms of the bladder (P = 0.006), where the male subjects all exhibited significantly higher PRL levels than the female subjects (Figure 5). Expression levels of PRL-1 were also correlated with age in some tissue types. PRL-1 staining scores significantly decreased with patient age in the skeletal muscle (P = 0.0031, Figure 6A) with very low expression levels attained after ages greater than 75 years. In contrast, PRL-1 strongly increased with patient age in the brain (P = 0.0252, Figure 6B) with sharp increases observed in patients over the age of 60 years. In several tumor tissues, expression of PRL-1 and -2 was significantly correlated with increasing tumor grade (increasing severity). In the ovary (Figure 7A), well-differentiated tumors expressed little to no PRL-1, while the less organized moderately-differentiated and poorly differentiated tumors tended to express higher levels of the transcript (P = <0.0001). There were no well-differentiated carcinomas of the uterus in this study, however the poorly differentiated carcinomas expressed PRL-1 to a significantly higher degree than the moderately differentiated uterine tumors (P = 0.0441). In the prostate (Figure 7B), mean PRL-1 staining scores once more increased from the low grade, more differentiated tumors (Gleason grades 1-4) to the moderate grade tumors (Grades 5-7). However, the mean staining score again decreased in the more poorly differentiated, high grade (Grades 8-10) prostate tumors (P = 0.0126). In the testes, little to no PRL-2 expression was observed in a well-differentiated germ cell tumor, but high levels of PRL-2 were noted in both the moderately and poorly differentiated tumors. In this tissue type, however, the number of samples in each category was too small to make any statistical comparisons. No clear associations were found between the intensity of PRL-1 or PRL-2 staining and the localization of the staining, nor between the localization of staining and any of the clinicopathological parameters examined. There were also no correlations found, in this data set, between PRL-1 or -2 expression and histologic subtype (e.g. clear cell vs. chromophobe cell type renal cell carcinomas of the kidney; adenocarcinoma vs. squamous cell non-small cell cancer of the lung, etc.) or with the presence or absence of local or distant metastases in any tumor type.
Figure 5.
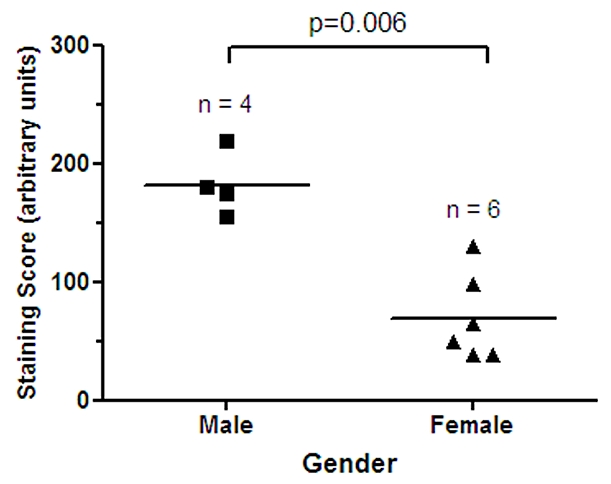
Gender specific expression of PRL-1 in the bladder. Semi-quantitative analysis of PRL-1 mRNA expression in male (n = 4) and female (n = 6) bladder tissues. Horizontal lines represent the mean values for each group. Differences between the two groups were found to be statistically significant by paired t-test (P = 0.006).
Figure 6.
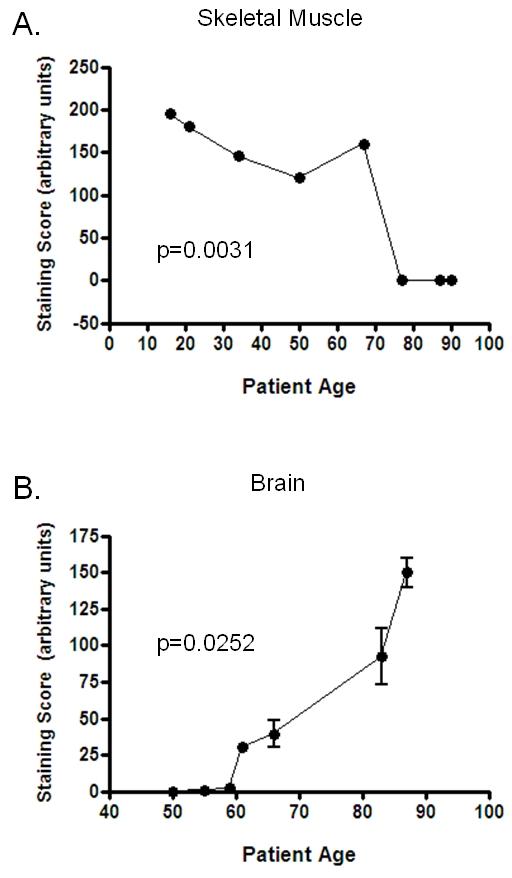
PRL-1 levels correlate with age in the skeletal muscle and brain. Semi-quantitative analysis showed that PRL-1 mRNA expression is negatively correlated with patient age in the skeletal muscle (A) and positively correlated with patient age in the brain (B). In both cases, mixed model analysis found the results to be statistically significant (P < 0.05). Brain specimens included tissue sections from the cerebrum, hippocampus, substantia nigra, and cerebellum. Different regions of the brain within the same individual displayed similar staining scores. The averages of these are represented.
Figure 7.
Correlation of PRL-1 expression with tumor grade in the ovary and prostate. (A) Expression levels, in arbitrary units, of PRL-1 mRNA in human ovarian carcinomas (n = 18) of varying histologic grade. WD = well-differentiated; MD = moderately differentiated; PD = poorly differentiated. (B) Expression levels, in arbitrary units, of PRL-1 mRNA in human prostate carcinomas (n = 28) of varying histologic grade. Horizontal lines represent the mean values for each group. In both tissue types, mixed model analysis found the results to be statistically significant (P < 0.05).
Correlation of PRL mRNA and protein expression
To examine the relationship between PRL-1 and PRL-2 expression at the mRNA and protein levels, select cases from various tissue types and representing a wide range of expression levels via ISH (RNA) were also examined by IHC (protein). The degree of expression for each was scored, in a blinded fashion, by different individuals than those who scored the ISH results and the general levels of expression (high, medium, or low) for the mRNA and protein were then compared. Using this approach it is not possible to compare absolute levels of RNA and protein, however, the relative changes seen in comparing various tissues and in comparing tumor and normal adjacent tissue can be useful. In the majority of the 30 individual cases examined, PRL-1 mRNA and protein were expressed at similar relative levels (Figure 8A). In cases where the relative levels differed, staining was always more intense for PRL-1 mRNA than for the protein. In half of the same 30 tissue sections, PRL-2 mRNA staining intensity was also higher than that for PRL-2 protein (Figure 8B). In the remaining half of cases, PRL-2 mRNA and protein appeared to be expressed at similar levels (37%) or the staining intensity for the protein was higher than that for the mRNA (13%).
Figure 8.
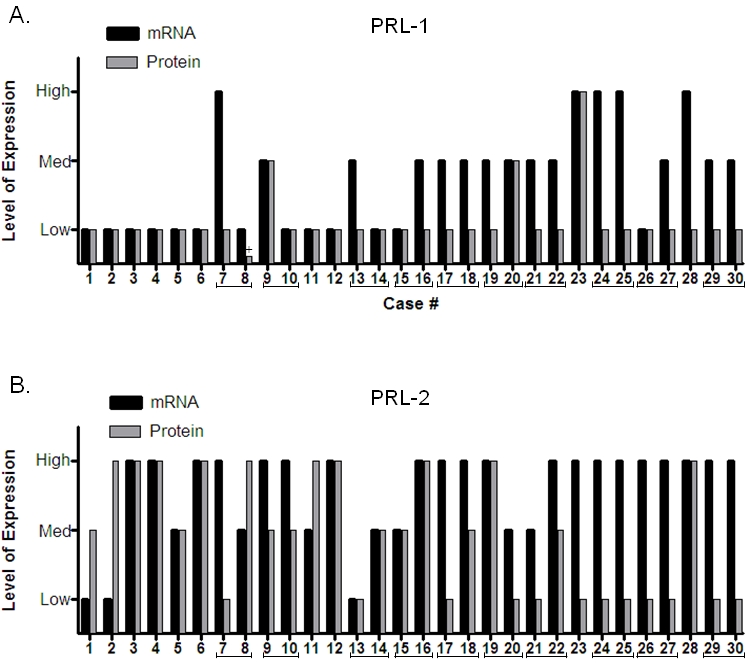
Comparison between mRNA and protein expression levels. PRL-1 (A) and PRL-2 (B) mRNA and protein expression levels were compared in a selection of 30 normal and tumor tissues from a variety of tissue types. Brackets indicate matched normal adjacent and tumor tissue pairs from the same individual. The plus sign in (A) denotes a sample for which no expression of PRL-1 protein was detected; however a small arbitrary value was assigned so that this sample would appear on the graph.
Within the 30 tissues probed by both ISH and IHC, there were 10 cases of paired tumor and normal adjacent tissue. Examination of these tissue pairs revealed that 6 of 10 cases for PRL-1 (concordance = 60%) and 8 of 10 cases for PRL-2 (concordance = 80%) displayed the same trends of expression between the RNA and protein (e.g. both increased from the normal adjacent to the tumor tissue; both decreased from the normal adjacent to the tumor tissue; or neither changed). This suggested that there was good overall concordance between the RNA and protein results and that, in general, changes occurring at the mRNA level here are reflective of those occurring at the protein level.
Discussion
Accumulating evidence has implicated the PRL family of phosphatases as having an oncogenic role in human cancers [1, 4-7]. For example, it is now well known that PRL-3 expression is generally absent from normal adult human tissues, but frequently elevated in a variety of benign and malignant human neoplasms, where it may serve as a marker for tumor aggressiveness, increased tumor angiogenesis, and/or poor prognosis [9, 10, 12, 18, 21-27, 31, 35-37, 51]. Here, we used in situ hybridization to examine the expression of PRL-1 and PRL-2 in human malignancies with the aim of providing further insight into the role these two PRL family members play in disease pathogenesis.
PRL-1 and PRL-2 transcript levels were evaluated across 285 normal, benign, and malignant tumor tissues, where both transcripts were found to be ubiquitously expressed. While PRL-2 transcripts were consistently abundant across almost all specimens, PRL-1 expression was highly variable, not only between tissue types, but also from individual to individual within a given tissue type. Since such a high degree of patient-to-patient variability in PRL-1 expression could confound results when making comparisons between groups of unmatched normal and tumor tissues from different subjects, only matched tumor and normal adjacent tissue (NAT) samples taken from the same individuals were used to evaluate changes in PRL gene expression that might occur as a result of neo-plastic transformation. Given current knowledge of the role that the PRL enzymes play in promoting tumor development and progression, we hypothesized that PRL-1 and PRL-2 gene expression would each be upregulated in a number of tumor types relative to their matched normal tissue specimens. In accordance with this theory, PRL-1 and PRL-2 transcripts were each found to be significantly overexpressed in 100% of hepatocellular carcinomas (n = 4; p = 0.0052 and 0.0152 respectively) and PRL-1 message was also significantly overexpressed in both the tumor (p = 0.01) and stroma (p = 0.0382) of 100% of carcinomas from the stomach (n = 5). Increased levels of PRL-3 expression have previously been associated with the progression and metastasis of gastric and liver carcinomas [27, 29-31, 34, 35, 39]. The current report is the first to suggest that PRL-1 and PRL-2 may also play an important role in the development and/ or progression of these tumor types.
Surprisingly however, in other tissue types, a very different result was seen. In 100% of ovarian (n=6; p = 0.0007), 80% of breast (n = 15; p = 0.0058), and 75% of lung (n = 8; p = 0.0148) tumors, PRL-1 levels were found to be significantly lower in the neoplastic cells than in their matched, unaffected counterparts. Likewise, PRL-2 levels were significantly decreased in 54% of carcinomas from the kidney (n = 13; p = 0.049) relative to the matched normal controls. These results suggest that dysregulation of PRL-1 and PRL-2 is a highly tissue specific event. This is consistent with observations of normal tissues, which have suggested that the PRL enzymes may be pleiotropic signaling molecules with a diversity of roles in different tissues and cell types [52, 53].
In addition to a role in cancer, PTPs have been implicated in a growing number of human pathologies, including cardiovascular, immunological, infectious, neurological, and metabolic diseases [44-47]. Therefore, we also sought to examine PRL-1 and PRL-2 mRNA expression in a small cohort of available samples from patients with various pathological conditions. In the panel of tissues examined here, PRL-1 and -2 were widely expressed, however no significant correlations were found between PRL-1 or PRL-2 expression levels and Alzheimer's disease, multiple sclerosis, Crohn's disease, coronary artery disease, heart disease, liver steatosis, hepatitis, or diabetes.
To evaluate the extent to which deregulation of PRL expression might be related to known patient characteristics and clinicopathological variables, where possible, PRL-1 and -2 mRNA expression levels in each tissue type were correlated to such features as patient age, patient gender, tumor histologic subtype, tumor grade, and presence/absence of tumor metastasis. In neoplasms of the bladder, expression levels of PRL-1 were found to be correlated to patient gender (p = 0.006), with male subjects displaying significantly higher PRL-1 transcript levels than female subjects. A similar trend toward increased expression in male subjects was also noted for PRL-2 in the lung (data not shown). Carter et al. [54] previously observed gender based differences of PRL-2 expression in rat brains, where PRL-2 mRNA was expressed at 3-fold higher levels in the anterior pituitaries of male rats than in female rats. The current data thus support these prior observations that the PRL enzymes may play a sexually dimorphic role in select tissue types. Increased PRL-1 expression also correlated positively with patient age in the brain (p = 0.0252), yet negatively with patient age in the skeletal muscle (p = 0.0031). Advancing age of both the brain and skeletal muscle is associated with a decline in function as well as with several common accompanying changes in gene expression [55-57]. Interestingly, in one transcriptional profiling study, aimed at identifying gene signatures for human aging in the frontal cortex [55], PRL-2 appeared on the list of genes which are significantly upregulated in the aging human brain. These data suggest that both PRL-1 and PRL-2 may be putative players in, or be heavily influenced by, the aging process. Taken together, these results suggest that age and gender should be taken into account when evaluating sample to sample variations in PRL abundance and further underscore the importance of using appropriately matched case controls in comparisons of PRL expression.
PRL-1 or -2 mRNA levels were found to be associated with tumor grade in some tissue types. Levels of PRL-1 in ovarian tumors increased significantly (p < 0.0001) in the moderate and poor grade tumors, relative to the low grade specimens, although this increase was to levels that remained appreciably lower than that seen in the NAT/normal specimens. A similar pattern of expression was observed for PRL-1 in the uterus and for PRL-2 in the testes. In the prostate, a wide range of PRL-1 expression levels were observed across the histologically normal tissue specimens, as well as across cases of prostatic intraepithelial neoplasia (PIN) and benign prostatic hyperplasia (BPH). However, in the prostate tumor specimens there was a significant increase in PRL-1 expression going from the lower grade to the more moderate grade tumors (p = 0.0126), followed by a subsequent decrease in the higher grade tumors. These data suggest that alterations in PRL expression are an early event of carcinogenesis in many organ systems and that PRL-1 and/or PRL-2 may serve as useful biomarkers for detection of tumorigenic lesions or for assessment of tumor aggressiveness in select tissue types. No correlations were found between PRL-1 or -2 expression and any of the clinicopathological features examined in the breast, heart, kidney, liver, pancreas, spleen, or stomach. Nor was any association seen between PRL expression and histologic subtype or tumor metastasis in any of the tumor types examined. There were also no significant correlations between PRL-1 or PRL-2 mRNA expression and clinical features related to colon cancer progression or metastasis, consistent with a previous report examining PRL-1 and PRL-2 protein in this tissue type [19].
To determine whether changes seen at the RNA level are reflective of what is occurring in these tissues at the protein level, immunostaining results from anti-peptide, affinity-purified polyclonal antibodies specific to PRL-1 and PRL-2 were directly compared to the ISH results in a subset of tissues from different tissue origin and demonstrating varied levels of PRL mRNA expression. Despite the presence of some variation between the absolute levels of PRL-1 or -2 mRNA and protein in the analysis of individual cases, there was a clear correlation between the two with respect to the changes occurring during tumorigenesis. In comparisons of matched normal and tumor samples from the same patient, the mRNA and protein both exhibited the same change (or conversely, lack of change) in expression 60% of the time for PRL-1 and 80% of the time for PRL-2. When differences in the general expression levels (high, medium, low) between mRNA and protein occurred, the mRNA was most often detected at higher levels than the protein. It is possible that, in each of these cases, changes occurring at the RNA level had not yet been reflected at the protein level. Alternatively, this could indicate post-transcriptional control of these molecules, perhaps through translational repression by PolyC-RNA-binding protein 1 (PCBP1) or a similar molecule, as was recently described for PRL-3 [58].
Only a handful of studies have yet examined PRL-1 or PRL-2 expression in human malignancies and even fewer have evaluated PRL-1 or PRL-2 in case matched normal and tumor samples. However, in general, the current results are in good agreement with previously published reports. In the present study, PRL-1 and PRL-2 levels were consistently lower in primary tumors from the ovary, compared to paired normal tissues, suggesting that higher levels of PRL -1 and -2 may be advantageous in this sample type. This is consistent with the observations of Reich et al. who showed that higher expression of PRL-1 or PRL-2 in ovarian cancer effusions correlated with better overall patient survival [59]. In contrast, the present data also show a relationship between increasing PRL-1 expression in ovarian carcinomas and advanced tumor grade. It is currently unclear how PRL-1 expression can be consistently downregulated in tumor specimens and positively correlated with improved patient outcome, yet also show positive correlation to increased tumor aggressiveness. However, with respect to outcome, Reich et al. did not observe the same beneficial effect of PRL-1 and -2 on patient survival when the molecules were expressed in solid tumors. It is possible then that, in solid ovarian tumors, an initial knockdown of PRL-1 expression is required for neoplastic transformation, following which enhanced levels of PRL-1 have no effect. Or PRL-1 could have an inhibitory effect on tumor formation in the early stages of ovarian carcinogenesis, but play a tumor-promoting role in the latter stages. A similar dual, opposing role has previously been reported for other molecules including Notch1 [60], MIC-1 [61], and for TGFβ [62], which has been shown to be an upstream regulator of PRL-3 [63]. In breast tissue, Hardy et al. [43] used real-time PCR to examine PRL-2 expression and found elevated levels of PRL-2 mRNA in primary breast tumors relative to matched normal tissue. The present data indicate a lack of change in PRL-2 expression between normal and neoplastic breast tissues, but PRL-2 levels in most breast tissues were extremely high and quite possibly at the limits of detection for the ISH system. In the pancreas, Stephens et al. [64] showed upregulation of PRL-1 and PRL-2 protein in 33% and 26% respectively of pancreatic tumors in relation to matched NAT specimens. In the current study, similar results were noted for PRL-1 mRNA with increased expression of PRL-1 seen in 46% of pancreatic tumor specimens with respect to matched normal controls. However, in 36% of samples, the opposite effect was seen, with an increase of PRL-1 expression in the NAT tissue relative to tumor. And, in 18% of samples, no differences were seen between the two. In the current data for PRL-2, staining was always heavy and no detectable differences between PRL-2 expression in the tumor and NAT samples were observed.
The results presented here show that, as with family member PRL-3, alterations in expression of PRL-1 and PRL-2 are a common event in human cancers; however, the nature of these alterations is highly tissue specific. In some tissue types, such as the stomach and liver, PRL-1 or -2 expression associates with tumor promotion, whereas in other tissue types, like the ovary and lung, expression of these molecules may normally serve a protective function. The frequent deregulation of these molecules in human neoplasms suggests that they may be useful markers for cancer diagnosis. They may also serve as valuable therapeutic targets and/or indicators of increasing tumor severity in select tissue types. The mechanisms of PRL action and regulation are currently poorly understood and the exact biological function of these molecules is unknown. Identifying the means by which their expression is regulated or the signaling pathways in which they act will be an important next step to provide insight into the pleiotropic role these molecules play in the carcinogenic process. Characterization of the PRL signaling pathways may also enhance our understanding of the observed gender and age related variations in PRL-1 expression. The present results help to expand our current understanding of the differences that exist between PRL-1 and PRL-2 levels in normal tissues and human malignancies and should facilitate larger scale retrospective or prospective studies examining the relevance of PRL-1 or PRL-2 in clinical cancer as well as in other human pathologies.
Acknowledgments
The authors would like to thank Dr. Mark Farmen for his oversight and review of the statistical analysis and Dr. Dean Wiseman and Jennifer Funke for their readings of the IHC slides. A portion of this work was supported in part by PHS, NIH grant CA72450, awarded to P.L.C.
References
- 1.Cates CA, Michael RL, Stayrook KR, Harvey KA, Burke YD, Randall SK, Crowell PL, Crowell DN. Prenylation of oncogenic human PTP(CAAX) protein tyrosine phosphatases. Cancer Lett. 1996;110:49–55. doi: 10.1016/s0304-3835(96)04459-x. [DOI] [PubMed] [Google Scholar]
- 2.Si X, Zeng Q, Ng CH, Hong W, Pallen CJ. Interaction of farnesylated PRL-2, a protein-tyrosine phosphatase, with the beta-subunit of geranylgeranyltransferase II. J Biol Chem. 2001;276:32875–32882. doi: 10.1074/jbc.M010400200. [DOI] [PubMed] [Google Scholar]
- 3.Zeng Q, Si X, Horstmann H, Xu Y, Hong W, Pallen CJ. Prenylation-dependent association of protein-tyrosine phosphatases PRL-1, -2, and -3 with the plasma membrane and the early en-dosome. J Biol Chem. 2000;275:21444–21452. doi: 10.1074/jbc.M000453200. [DOI] [PubMed] [Google Scholar]
- 4.Diamond RH, Cressman DE, Laz TM, Abrams CS, Taub R. PRL-1, a unique nuclear protein tyrosine phosphatase, affects cell growth. Mol Cell Biol. 1994;14:3752–3762. doi: 10.1128/mcb.14.6.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matter WF, Estridge T, Zhang C, Belagaje R, Stancato L, Dixon J, Johnson B, Bloem L, Pickard T, Donaghue M, Acton S, Jeyaseelan R, Kadambi V, Vlahos CJ. Role of PRL-3, a human muscle-specific tyrosine phosphatase, in angiotensin-ll signaling. Biochem Biophys Res Commun. 2001;283:1061–1068. doi: 10.1006/bbrc.2001.4881. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Zeng H, Zhang X, Zhao Y, Sha H, Ge X, Zhang M, Gao X, Xu Q. Phosphatase of regenerating liver-3 promotes motility and metastasis of mouse melanoma cells. Am J Pathol. 2004;164:2039–2054. doi: 10.1016/S0002-9440(10)63763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng Q, Dong JM, Guo K, Li J, Tan HX, Koh V, Pallen CJ, Manser E, Hong W. PRL-3 and PRL-1 promote cell migration, invasion, and metastasis. Cancer Res. 2003;63:2716–2722. [PubMed] [Google Scholar]
- 8.Guo K, Li J, Tang JP, Koh V, Gan BQ, Zeng Q. Catalytic domain of PRL-3 plays an essential role in tumor metastasis: formation of PRL-3 tumors inside the blood vessels. Cancer Biol Ther. 2004;3:945–951. doi: 10.4161/cbt.3.10.1111. [DOI] [PubMed] [Google Scholar]
- 9.Kato H, Semba S, Miskad UA, Seo Y, Kasuga M, Yokozaki H. High expression of PRL-3 promotes cancer cell motility and liver metastasis in human colorectal cancer: a predictive molecular marker of metachronous liver and lung metastases. Clin Cancer Res. 2004;10:7318–7328. doi: 10.1158/1078-0432.CCR-04-0485. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Zhan W, Wang Z, Zhu B, He Y, Peng J, Cai S, Ma J. Inhibition of PRL-3 gene expression in gastric cancer cell line SGC7901 via microRNA suppressed reduces peritoneal metastasis. Biochem Biophys Res Commun. 2006;348:229–237. doi: 10.1016/j.bbrc.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 11.Matsukawa Y, Semba S, Kato H, Koma Y, Yanagihara K, Yokozaki H. Constitutive suppression of PRL-3 inhibits invasion and proliferation of gastric cancer cell in vitro and in vivo. Pathobiology. 2OlO;77:155–162. doi: 10.1159/000292649. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Wang S, Lu J, Li J, Ding Y. Overexpression of phosphatase of regenerating liver -3 correlates with tumor progression and poor prognosis in nasopharyngeal carcinoma. Int J Cancer. 2009;124:1879–1886. doi: 10.1002/ijc.24096. [DOI] [PubMed] [Google Scholar]
- 13.Qian F, Li YP, Sheng X, Zhang ZC, Song R, Dong W, Cao SX, Hua ZC, Xu Q. PRL-3 siRNA inhibits the metastasis of B16-BL6 mouse melanoma cells in vitro and in vivo. Mol Med. 2007;13:151–159. doi: 10.2119/2006-00076.Qian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima M, Lazo JS. Phosphatase of regenerating liver-1 promotes cell migration and invasion and regulates filamentous actin dynamics. J Pharmacol Exp Ther. 2010;334:627–633. doi: 10.1124/jpet.110.167809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saha S, Bardelli A, Buckhaults P, Velculescu VE, Rago C, St Croix B, Romans KE, Choti MA, Lengauer C, Kinzler KW, Vogelstein B. A phosphatase associated with metastasis of colorectal cancer. Science. 2001;294:1343–1346. doi: 10.1126/science.1065817. [DOI] [PubMed] [Google Scholar]
- 16.Bardelli A, Saha S, Sager JA, Romans KE, Xin B, Markowitz SD, Lengauer C, Velculescu VE, Kinzler KW, Vogelstein B. PRL-3 expression in metastatic cancers. Clin Cancer Res. 2003;9:5607–5615. [PubMed] [Google Scholar]
- 17.Peng L, Ning J, Meng L, Shou C. The association of the expression level of protein tyrosine phosphatase PRL-3 protein with liver metastasis and prognosis of patients with colorectal cancer. J Cancer Res Clin Oncol. 2004;130:521–526. doi: 10.1007/s00432-004-0563-x. [DOI] [PubMed] [Google Scholar]
- 18.Mollevi DG, Aytes A, Padulles L, Martinez-Iniesta M, Baixeras N, Salazar R, Ramos E, Figueras J, Capella G, Villanueva A. PRL-3 is essentially overexpressed in primary colorectal tumours and associates with tumour aggressiveness. BrJ Cancer. 2008;99:1718–1725. doi: 10.1038/sj.bjc.6604747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Li ZF, He J, Li YL, Zhu GB, Zhang LH, Li YL. Expression of the human phosphata-ses of regenerating liver (PRLs) in colonic ade-nocarcinoma and its correlation with lymph node metastasis. Int J Colorectal Dis. 2007;22:1179–1184. doi: 10.1007/s00384-007-0303-1. [DOI] [PubMed] [Google Scholar]
- 20.Xing X, Peng L, Qu L, Ren T, Dong B, Su X, Shou C. Prognostic value of PRL-3 overexpression in early stages of colonic cancer. Histopathology. 2009;54:309–318. doi: 10.1111/j.1365-2559.2009.03226.x. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Li B. Expression of phosphatase of regenerating liver-3 in squamous cell carcinoma of the cervix. Med Oncol. 2011;28:775–780. doi: 10.1007/s12032-010-9514-3. [DOI] [PubMed] [Google Scholar]
- 22.Polato F, Codegoni A, Fruscio R, Perego P, Mangioni C, Saha S, Bardelli A, Broggini M. PRL-3 phosphatase is implicated in ovarian cancer growth. Clin Cancer Res. 2005;11:6835–6839. doi: 10.1158/1078-0432.CCR-04-2357. [DOI] [PubMed] [Google Scholar]
- 23.Ren T, Jiang B, Xing X, Dong B, Peng L, Meng L, Xu H, Shou C. Prognostic significance of phosphatase of regenerating liver-3 expression in ovarian cancer. Pathol Oncol Res. 2009;15:555–560. doi: 10.1007/s12253-009-9153-1. [DOI] [PubMed] [Google Scholar]
- 24.Radke I, Gotte M, Kersting C, Mattsson B, Kiesel L, Wulfing P. Expression and prognostic impact of the protein tyrosine phosphatases PRL-1, PRL-2, and PRL-3 in breast cancer. Br J Cancer. 2006;95:347–354. doi: 10.1038/sj.bjc.6603261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Peng L, Dong B, Kong L, Meng L, Yan L, Xie Y, Shou C. Overexpression of phosphatase of regenerating liver-3 in breast cancer: association with a poor clinical outcome. Ann Oncol. 2006;17:1517–1522. doi: 10.1093/annonc/mdl159. [DOI] [PubMed] [Google Scholar]
- 26.Hao RT, Zhang XH, Pan YF, Liu HG, Xiang YQ, Wan L, Wu XL. Prognostic and metastatic value of phosphatase of regenerating liver-3 in invasive breast cancer. J Cancer Res Clin Oncol. 2010;136:1349–1357. doi: 10.1007/s00432-010-0786-y. [DOI] [PubMed] [Google Scholar]
- 27.Miskad UA, Semba S, Kato H, Matsukawa Y, Kodama Y, Mizuuchi E, Maeda N, Yanagihara K, Yokozaki H. High PRL-3 expression in human gastric cancer is a marker of metastasis and grades of malignancies: an in situ hybridization study. Virchows Arch. 2007;450:303–310. doi: 10.1007/s00428-006-0361-8. [DOI] [PubMed] [Google Scholar]
- 28.Miskad UA, Semba S, Kato H, Yokozaki H. Expression of PRL-3 phosphatase in human gastric carcinomas: close correlation with invasion and metastasis. Pathobiology. 2004;71:176–184. doi: 10.1159/000078671. [DOI] [PubMed] [Google Scholar]
- 29.Ooki A, Yamashita K, Kikuchi S, Sakuramoto S, Katada N, Watanabe M. Phosphatase of regenerating liver-3 as a prognostic biomarker in histologically node-negative gastric cancer. Oncol Rep. 2009;21:1467–1475. doi: 10.3892/or_00000376. [DOI] [PubMed] [Google Scholar]
- 30.Pryczynicz A, Guzinska-Ustymowicz K, Chang XJ, Kisluk J, Kemona A. PTP4A3 (PRL-3) expression correlate with lymphatic metastases in gastric cancer. Folia Histochem Cytobiol. 2010;48:632–636. doi: 10.2478/v10042-010-0070-7. [DOI] [PubMed] [Google Scholar]
- 31.LiZR, Wang Z, Zhu BH, He YL, Peng JS, Cai SR, Ma JP, Zhan WH. Association of tyrosine PRL-3 phosphatase protein expression with peritoneal metastasis of gastric carcinoma and prognosis. Surg Today. 2007;37:646–651. doi: 10.1007/s00595-006-3437-9. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Cai SR, He YL, Zhan WH, Chen CQ, Cui J, Wu WH, Wu H, Song W, Zhang CH, Peng JJ, Huang XH. High expression of PRL-3 can promote growth of gastric cancer and exhibits a poor prognostic impact on patients. Ann Surg Oncol. 2009;16:208–219. doi: 10.1245/s10434-008-0214-6. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Cai SR, He YL, Zhan WH, Zhang CH, Wu H, Peng JJ, Xu JB, Zhang XH, Wang L, Song W. Elevated PRL-3 expression was more frequently detected in the large primary gastric cancer and exhibits a poor prognostic impact on the patients. J Cancer Res Clin Oncol. 2009;135:1041–1046. doi: 10.1007/s00432-008-0541-9. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, He YL, Cai SR, Zhan WH, Li ZR, Zhu BH, Chen CQ, Ma JP, Chen ZX, Li W, Zhang U. Expression and prognostic impact of PRL-3 in lymph node metastasis of gastric cancer: its molecular mechanism was investigated using artificial microRNA interference. Int J Cancer. 2008;123:1439–1447. doi: 10.1002/ijc.23643. [DOI] [PubMed] [Google Scholar]
- 35.Dai N, Lu AP, Shou CC, Li JY. Expression of phosphatase regenerating liver 3 is an independent prognostic indicator for gastric cancer. World J Gastroenterol. 2009;15:1499–1505. doi: 10.3748/wjg.15.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ming J, Liu N, Gu Y, Qiu X, Wang EH. PRL-3 facilitates angiogenesis and metastasis by increasing ERK phosphorylation and up-regulating the levels and activities of Rho-A/C in lung cancer. Pathology. 2009;41:118–126. doi: 10.1080/00313020802579268. [DOI] [PubMed] [Google Scholar]
- 37.Ooki A, Yamashita K, Kikuchi S, Sakuramoto S, Katada N, Watanabe M. Phosphatase of regenerating liver-3 as a convergent therapeutic target for lymph node metastasis in esophageal squamous cell carcinoma. Int J Cancer. 2010;127:543–554. doi: 10.1002/ijc.25082. [DOI] [PubMed] [Google Scholar]
- 38.Navis AC, van den Eijnden M, Schepens JT, Hooft van Huijsduijnen R, Wesseling P, Hendriks WJ. Protein tyrosine phosphatases in glioma biology. Acta Neuropathol. 2010;119:157–175. doi: 10.1007/s00401-009-0614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao WB, Li Y, Liu X, Zhang LY, Wang X. Evaluation of PRL-3 expression, and its correlation with angiogenesis and invasion in hepatocellular carcinoma. Int J Mol Med. 2008;22:187–192. [PubMed] [Google Scholar]
- 40.Xu Y, Zhu M, Zhang S, Liu H, Li T, Qin C. Expression and prognostic value of PRL-3 in human intrahepatic cholangiocarcinoma. Pathol Oncol Res. 2010;16:169–175. doi: 10.1007/s12253-009-9200-y. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Kirby CE, Herbst R. The tyrosine phosphatase PRL-1 localizes to the endoplasmic reticulum and the mitotic spindle and is required for normal mitosis. J Biol Chem. 2002;277:46659–46668. doi: 10.1074/jbc.M206407200. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Holmes Dl, Powell SM, Lu QL, Waxman J. Analysis of stromal-epithelial interactions in prostate cancer identifies PTPCAAX2 as a potential oncogene. Cancer Lett. 2002;175:63–69. doi: 10.1016/s0304-3835(01)00703-0. [DOI] [PubMed] [Google Scholar]
- 43.Hardy S, Wong NN, Muller WJ, Park M, Tremblay ML. Overexpression of the protein tyrosine phosphatase PRL-2 correlates with breast tumor formation and progression. Cancer Res. 2010;70:8959–8967. doi: 10.1158/0008-5472.CAN-10-2041. [DOI] [PubMed] [Google Scholar]
- 44.Tautz L, Pellecchia M, Mustelin T. Targeting the PTPome in human disease. Expert Opin Ther Targets. 2006;10:157–177. doi: 10.1517/14728222.10.1.157. [DOI] [PubMed] [Google Scholar]
- 45.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muise AM, Walters T, Wine E, Griffiths AM, Turner D, Duerr RH, Regueiro MD, Ngan BY, Xu W, Sherman PM, Silverberg MS, Rotin D. Protein-tyrosine phosphatase sigma is associated with ulcerative colitis. Curr Biol. 2007;17:1212–1218. doi: 10.1016/j.cub.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F, Lowe CE, Szeszko JS, Hafler JP, Zeitels L, Yang JH, Vella A, Nutland S, Stevens HE, Schuilenburg H, Coleman G, Maisuria M, Meadows W, Smink U, Healy B, Burren OS, Lam AA, Ovington NR, Allen J, Adlem E, Leung HT, Wallace C, Howson JM, Guja C, lonescu-Tirgoviste C, Simmonds MJ, Heward JM, Gough SC, Dunger DB, Wicker LS, Clayton DG. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dumaual CM, Sandusky GE, Crowell PL, Randall SK. Cellular localization of PRL-1 and PRL-2 gene expression in normal adult human tissues. J Histochem Cytochem. 2006;54:1401–1412. doi: 10.1369/jhc.6A7019.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nitadori J, Ishii G, Tsuta K, Yokose T, Murata Y, Kodama T, Nagai K, Kato H, Ochiai A. Immunohistochemical differential diagnosis between large cell neuroendocrine carcinoma and small cell carcinoma by tissue microarray analysis with a large antibody panel. Am J Clin Pathol. 2006;125:682–692. doi: 10.1309/DT6B-J698-LDX2-NGGX. [DOI] [PubMed] [Google Scholar]
- 50.Jackel MC, Mitteldorf C, Schweyer S, Fuzesi L. Clinical relevance of Fas (APO-1/CD95) expression in laryngeal squamous cell carcinoma. Head Neck. 2001;23:646–652. doi: 10.1002/hed.1091. [DOI] [PubMed] [Google Scholar]
- 51.Kong L, Li Q, Wang L, Liu Z, Sun T. The value and correlation between PRL-3 expression and matrix metalloproteinase activity and expression in human gliomas. Neuropathology. 2007;27:516–521. doi: 10.1111/j.1440-1789.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 52.Diamond RH, Peters C, Jung SP, Greenbaum LE, Haber BA, Silberg DG, Traber PG, Taub R. Expression of PRL-1 nuclear PTPase is associated with proliferation in liver but with differentiation in intestine. Am J Physiol. 1996;271:G121–129. doi: 10.1152/ajpgi.1996.271.1.G121. [DOI] [PubMed] [Google Scholar]
- 53.Rundle CH, Kappen C. Developmental expression of the murine Prl-1 protein tyrosine phosphatase gene. J Exp Zool. 1999;283:612–617. doi: 10.1002/(sici)1097-010x(19990501)283:6<612::aid-jez14>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 54.Carter DA. Expression of a novel rat protein tyrosine phosphatase gene. Biochim Biophys Acta. 1998;1442:405–408. doi: 10.1016/s0167-4781(98)00173-0. [DOI] [PubMed] [Google Scholar]
- 55.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 56.Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R, Davis RW, Becker KG, Owen AB, Kim SK. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2006;2:ell5. doi: 10.1371/journal.pgen.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bischoff-Ferrari HA, Borchers M, Gudat F, Durmuller U, Stahelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Vardy LA, Tan CP, Loo JM, Guo K, Li J, Lim SG, Zhou J, Chng WJ, Ng SB, Li HX, Zeng Q. PCBP1 suppresses the translation of metastasis-associated PRL-3 phosphatase. Cancer Cell. 2011;18:52–62. doi: 10.1016/j.ccr.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 59.Reich R, Hadar S, Davidson B. Expression and clinical role of protein of regenerating liver (PRL) phosphatases in ovarian carcinoma. Int J Mol Sci. 2011;12:1133–1145. doi: 10.3390/ijms12021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talora C, Sgroi DC, Crum CP, Dotto GP. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 2002;16:2252–2263. doi: 10.1101/gad.988902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mimeault M, Batra SK. Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer. J Cell Physiol. 2010;224:626–635. doi: 10.1002/jcp.22196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kretzschmar M. Transforming growth factor-beta and breast cancer: Transforming growth factor-beta/SMAD signaling defects and cancer. Breast Cancer Res. 2000;2:107–115. doi: 10.1186/bcr42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang Y, Liu XQ, Rajput A, Geng L, Ongchin M, Zeng Q, Taylor GS, Wang J. Phosphatase PRL-3 is a direct regulatory target of TGFbeta in colon cancer metastasis. Cancer Res. 2010;71:234–244. doi: 10.1158/0008-5472.CAN-10-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stephens B, Han H, Hostetter G, Demeure MJ, Von Hoff DD. Small interfering RNA-mediated knockdown of PRL phosphatases results in altered Akt phosphorylation and reduced clonogenicity of pancreatic cancer cells. Mol Cancer Ther. 2008;7:202–210. doi: 10.1158/1535-7163.MCT-07-0542. [DOI] [PubMed] [Google Scholar]