Abstract
Here we incorporate recent advances in Drosophila neurogenetics and “optogenetics” into neuroscience laboratory exercises. We used the light-activated ion channel channelrhodopsin-2 (ChR2) and tissue-specific genetic expression techniques to study the neural basis of behavior in Drosophila larvae. We designed and implemented exercises using inexpensive, easy-to-use systems for delivering blue light pulses with fine temporal control. Students first examined the behavioral effects of activating glutamatergic neurons in Drosophila larvae and then recorded excitatory junctional potentials (EJPs) mediated by ChR2 activation at the larval neuromuscular junction (NMJ). Comparison of electrically and light-evoked EJPs demonstrates that the amplitudes and time courses of light-evoked EJPs are not significantly different from those generated by electrical nerve stimulation. These exercises introduce students to new genetic technology for remotely manipulating neural activity, and they simplify the process of recording EJPs at the Drosophila larval NMJ. Relatively little research work has been done using ChR2 in Drosophila, so students have opportunities to test novel hypotheses and make tangible contributions to the scientific record. Qualitative and quantitative assessment of student experiences suggest that these exercises help convey principles of synaptic transmission while also promoting integrative and inquiry-based studies of genetics, cellular physiology, and animal behavior.
Keywords: synaptic transmission, neurogenetics, neuromuscular junction, animal behavior
drosophila neurogeneticists have developed an impressive array of tools for studying the neural basis of animal behavior. In recent years, tissue-specific genetic expression systems, particularly the GAL4-UAS system (3), have been used to ectopically express transgenes that allow for acute, reversible manipulation of neural activity. These new techniques exploit ion channels and vesicle trafficking proteins that are gated by light and temperature (1, 9, 15, 25, 28). This allows researchers to remotely control neural activity in selected cells simply by raising the ambient temperature or shining light on behaving flies.
One powerful new tool for acutely activating neurons is the light-gated ion channel channelrhodopsin-2 (ChR2). Originally isolated from the green algae Chlamydomonas reinhardti, the channel is directly activated by blue light (24). When expressed in neurons, channel opening causes depolarization through nonspecific cation conductance (2, 23), which leads to action potential generation. This technique has been used to depolarize excitable cells in invertebrate (7, 23, 25, 28) and vertebrate (2, 5, 8, 24) preparations for research purposes.
“Optogenetic” methods for activating neurons offer attractive options for physiology educators. With the range of genetic tools available in Drosophila, teachers can design exercises that explore the neural basis of animal behavior in ways that are not possible in traditional laboratory preparations. These new tools can also be used to make technically difficult preparations more accessible to students. Our goal here is to outline one potential use of Drosophila neurogenetics and ChR2 in neuroscience education. Specifically, we show how to use ChR2 to 1) promote quantitative analysis of animal behavior, 2) teach principles of synaptic transmission, and 3) help students learn how to formulate and test their own research hypotheses.
Scientists working with the Drosophila larval neuromuscular junction (NMJ) have previously proposed using this preparation to teach synaptic physiology (16, 33). This glutamatergic synapse yields large excitatory junctional potentials (EJPs) that can be recorded with basic electrophysiology equipment (13, 14). However, to successfully record EJPs, students must precisely maneuver both an intracellular electrode and a stimulating (suction) electrode in a very small area. Here, we present inexpensive laboratory exercises that use targeted expression of ChR2 in motor neurons instead of direct electrical nerve stimulation to activate larval NMJs. Students are exposed to newly developed Drosophila neurogenetic tools and learn synaptic neurophysiology. We also report feedback on the exercises from two student cohorts across two different years in a neurophysiology laboratory course at Cornell University. Overall, this work and a companion publication (1) lay the foundation for wider use of Drosophila neurogenetics in teaching principles of neurobiology and animal behavior.
MATERIALS AND METHODS
Fly lines and animal care.
We used a GAL4 driver (OK371-GAL4), which drives expression exclusively in glutamatergic neurons (22), and a UAS construct (UAS-H134R-ChR2-mcherry) from a previous larval locomotion study (25). Virgin OK371-GAL4 females were crossed to UAS-H134R-ChR2-mcherry males. The resulting larvae were grown in darkness at 23–25°C on standard fly media containing 1 mM ATR (Toronto Research Chemicals, North York, CA). ATR is a cofactor allowing proper folding and membrane insertion of ChR2. Supplementation of fly food with ATR is essential for functional ChR2 expression. We have previously described the preparation of ATR-containing fly food in video form (10).
All fly lines are freely available from S. R. Pulver (University of Cambridge) and/or the Bloomington Drosophila Stock Center (OK371-GAL4: http://flybase.org/reports/FBst0026160.html and UAS-H134R-ChR2-mcherry: http://flybase.org/reports/FBst0028995.html). Detailed guidlelines on rearing fruit flies and making genetic crosses are available from previous publications (10, 16).
Blue light-emitting diode control system.
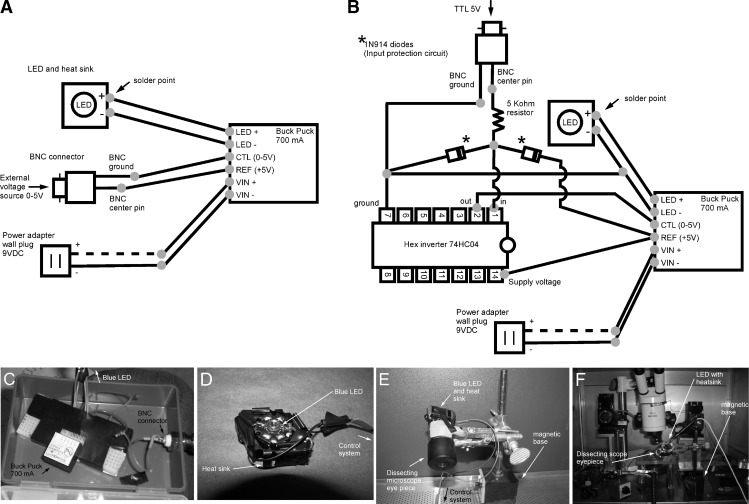
Commercially available systems for controlling blue LEDs typically cost more than US$300. This could be prohibitively expensive for many teaching laboratories, so we designed two simple, inexpensive alternatives. First, we connected an ultrabright blue LED (Luxeon V star, LED Supply, Randolph, VT) to a 700-mA “Buck Puck” power converter (LuxDrive 3021, LED Supply). When the Buck Puck is directly connected to the analog output from an A/D converter, light intensity and duration can be controlled with 0- to 5-V pulses from an external voltage source (10, 25). We attached a small heat sink to each LED (e.g., TO220, RadioShack) to dissipate heat. To ensure good heat transfer, we placed thermal paste in between the LED and heat sink and glued only the edges of the LED to the bare metal of the heat sink. The total cost of all components is under US$50. A basic wiring plan for this LED controller is shown in Fig. 1A. A typical controller is shown in Fig. 1C, and an LED mounted on a heat sink is shown in Fig. 1D. We controlled timing and light intensity with two commonly available A/D conversion systems. For demonstration here, we delivered 0- to 5-V pulses through a Powerlab 4/30 (AD Instruments, Colorado Springs, CO) with Chart5 data-acquisition software (AD Instruments). In the teaching exercises reported below, students controlled the LED through the analog output of a NIDAQ BNC-2110 A/D board (National Instruments, Austin, TX) with the free data-acquisition software “g-PRIME” (21). Both systems were able to control timing and intensity equally well.
Fig. 1.
Light-emitting diode (LED) control systems. A: diagram of the control system used in teaching exercises. Connections between the LED, “Buck Puck,” BNC connector, and power adapter are indicated. B: equivalent diagram for the control system designed for analog pulse stimulators and TTL signals with low current output. C: a typical LED control system (based on the diagram in A) showing the Buck Puck, BNC connector, and wiring. The housing was made from an empty pipette tip holder box. D: LED mounted on a heat sink. Rolls of electrical tape were placed around the LED to prevent the microscope eyepiece from crimping the wires supplying power to the LED. E: LED and heat sink mounted to an eyepiece and attached to a magnetized base. F: LED system in place on a working electrophysiology rig.
As an alternative to the above, we also designed a second simple control circuit that could be driven by analog pulse stimulators with low current output. Figure 1B shows a wiring plan for this type of control system. A 74HC04 hex inverter and a 5-kΩ resistor are used to ensure that a standard TTL signal will trigger light pulses. An input protection circuit consisting of two 1N914 diodes protects the hex inverter from a reversed connection and/or electrostatic discharge. The primary advantage of this control circuit is that it does not require A/D converters and/or data-acquisition software. The total cost of all components is under US$70.
Unfocused LEDs are not able to deliver the light intensities needed to activate ChR2 in fly neurons. To focus LEDs, we placed a Carl Zeiss ×10 dissecting scope eyepiece in front of the LED and mounted both the light source and eyepiece on magnetized bases suitable for electrophysiology “rig” tables. The make and model of eyepiece is not critical; any removable eyepiece that can cover the LED is suitable. An LED and a heat sink coupled to an eyepiece and attached to a magnetized base is shown in Fig. 1E. The complete LED setup on a working electrophysiology rig is shown in Fig. 1F. Additional views of LED system components are shown in video form in Ref. 10. It is important to note that the light emerging from the LED system outlined here is high intensity and very focused, so it is imperative that students do not look directly into active LEDs.
Larval behavior.
Animals with ChR2 in motor neurons (OK371-GAL4/UAS-H134R-ChR2) were grown in two batches: one group was raised on normal fly food and the other group on food containing 1 mM ATR. We selected third instar individuals from each group and observed behavioral responses to blue light pulses. For demonstration, larval behavior was filmed with a Leica DFC 420 C camera mounted on a Leica MZ16 F Fluorescence Stereomicroscope (Leica Camera, Solms, Germany). Blue light pulses (5 s) were delivered by manual control of shutter timing.
In classroom exercises, students placed larvae in dissection dishes and delivered light pulses using a mounted LED. The LED system typically produces a circle of light ∼5−10 mm in diameter at the eyepiece focal point. This is large enough to encompass the entire body of a third instar larva. Students observed larval responses to blue light and scored the responses manually. We did not require students to analyze larval behavior in any particular way. Instead, we encouraged students to devise their own methods for quantifying the effects of blue light stimulation on larval behavior in experimental and control animals.
Larval dissection.
For NMJ electrophysiology, third instar larvae were dissected in a clear Sylgard (Dow Corning, Midland, MI)-lined dish containing chilled “HL3.1” physiological saline solution (6). HL3.1 consisted of (in mM) 70 NaCl, 5 KCl, 0.8 CaCl2, 4 MgCl2, 10 NaHCO3, 5 trehalose, 115 sucrose, and 5 HEPES (pH 7.15). In this saline solution, preparations typically remained viable for 1–2 h at room temperature.
Each larva was positioned dorsal side up, and 0.1-mm insect pins were placed in the head and tail. Using a pair of microscissors, we made a shallow incision from the posterior pin to the anterior pin. After making the initial cut, we placed one pin into each corner of the animal's body wall and stretched each corner taut. Next, we removed fat bodies and digestive organs, exposing the anterior brain lobes, ventral ganglion, segmental nerves, and body wall muscles. In some experiments, we removed the CNS, leaving only motor axons and nerve terminals. In other preparations, we dissected away the brain lobes and cut the posterior-most nerves, leaving the ventral ganglion. In tightly pinned preparations, this reduces locomotor rhythms but leaves motor neuron cell bodies, axons, and nerve terminals intact (25). See Ref. 10 for videos describing the larval dissection.
Intracellular recordings.
Dishes with dissected preparations were first fixed to a Plexiglas stage with artist's clay and viewed through a dissecting microscope on a standard electrophysiology rig. We targeted larval muscle 6 (see Fig. 3A) for all intracellular recordings. Recordings were made with sharp glass electrodes (10–20 MΩ filled with 3 M KCl).
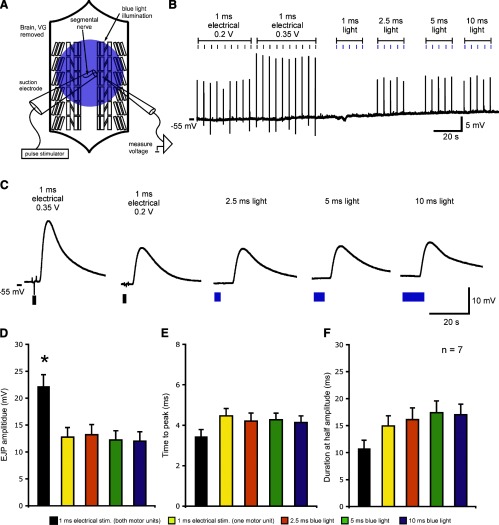
Fig. 3.
Comparison of light and electrically evoked excitatory junctional potential (LEJPs and EEJPs, respectively) in the absence of motor neuron cell bodies and ventral ganglion (VG) circuitry. A: schematic of a dissected larval preparation. The brain and ventral ganglion were removed. A single segmental nerve was stimulated via suction electrode. Larval muscle 6 was targeted for recording. B: long time-base recording showing a typical experiment. One motor unit was recruited with the lowest stimulus voltage. An additional motor unit was recruited as the electrical stimulus intensity increased. LEJPs were evoked by 2.5- to 10-ms light pulses. C: expanded time-base views of EEJPs and LEJPs shown in B. D–F: LEJPs showed amplitudes and time courses that were not statistically different from EEJPs evoked by the low-threshold motor unit (F > 0.05 by one-way ANOVA). Data from 1-ms light pulses are not shown because they did not evoke LEJPs in any preparations. In pooled data, resting membrane potentials were between −40 and −55 mV. Resting membrane potentials were not significantly different across stimulation types (F > 0.05 by one-way ANOVA; data not shown). Pooled data are presented as means ± SE. *Significant difference compared with all other conditions (P < 0.05 by one-way ANOVA with the Tukey-Kramer post hoc test).
For the demonstration electrophysiology data presented here, the electrode and headstage were maneuvered with a MP285 micromanipulator (Sutter Instruments, Novato, CA). Voltage signals were amplified with a Neuroprobe amplifier (A-M Systems, Sequim, WA). Data were digitized using a Power lab 4/30 and recorded in Chart5 (AD Instruments). Data were analyzed in Spike2 (Cambridge Electronic Design, Cambridge, UK) using custom-made analysis scripts (www.whitney.ufl.edu/BucherLab). EJPs were evoked in ChR2-expressing animals with 1-, 2.5-, 5-, and 10-ms pulses (25) to examine the effects of light pulse duration on LEJPs. We also compared LEJPs to EEJPs by attaching a suction electrode to segmental nerves and delivering 1-ms-duration electrical shocks with a model 2100 isolated pulse stimulator (A-M Systems) (see Fig. 3A).
In teaching exercises, students used MM-333 micromanipulators (Narishige, East Meadow, NY) to maneuver recording electrodes. These micromanipulators offer enough precision to record from larval NMJs and are substantially less expensive than other research-grade manipulators. Students also used Neuroprobe amplifiers to amplify voltage signals but used g-PRIME for LED control, data acquisition, and analysis (21). The quality of data recorded with teaching laboratory equipment was equivalent to the demonstration data we present here. In teaching laboratory exercises, students began by giving light pulse durations (10 ms) and intensities (5 V into the control circuit, ∼1 mW/mm2) that reliably evoked at least 1 LEJP with pulse stimulation, as demonstrated in previous work (25). Students were encouraged to design their own experiments and explore the effects of varying intensity, duration, and frequency of light pulses on synaptic transmission.
Analysis of student evaluations.
We test ran these exercises with two different student cohorts in two successive years (Spring semesters, 2009 and 2010) of an undergraduate neurophysiology course (BIONB/BME 4910) at Cornell University. The 2009 students completed the exercise in one laboratory session; they were undergraduate students from Biology (n = 11), Biological Engineering (n = 2), Psychology (n = 1), Mathematics (n =1), and Human Ecology (n = 1) majors and first-year graduate students from Neurobiology and Behavior (n = 6), Biomedical Engineering (n = 2), and Electrical/Computer Engineering (n = 2). In 2010, we spread the exercise over 2 wk; undergraduate students were from Biology (n = 11), Biology and Society (n = 1), Psychology (n = 1), Biological Engineering (n = 1), and Electrical/Computer Engineering (n = 1) majors and first-year graduate students from Neurobiology and Behavior (n = 4), Biomedical Engineering (n = 6), Electrical/Computer Engineering (n = 1), Entomology (n = 1), and Psychology (n = 1). Students worked in groups of two or three students at each physiology rig. Their background in neuroscience ranged from very little (Engineering students) to a sophomore-level class in Neuroscience (Biology students), which used the Purves et al. (26) textbook. Student experiences were evaluated qualitatively in 2009; we asked for a one-page informal opinion on the exercise from each student. In the second year, we quantified student experiences by asking them 12 questions designed to evaluate various technical and conceptual aspects of the exercise. Student responses were measured on a Likert scale (19). All students had previous electrophysiological experience earlier in the semester with exercises from the Crawdad CD (32), including recording synaptic potentials from the crayfish NMJ. N. J. Hornstein and S. R. Pulver presented background lectures on fly genetics and Drosophila NMJ electrophysiology before students started the laboratory exercises.
RESULTS
Behavioral responses to blue light.
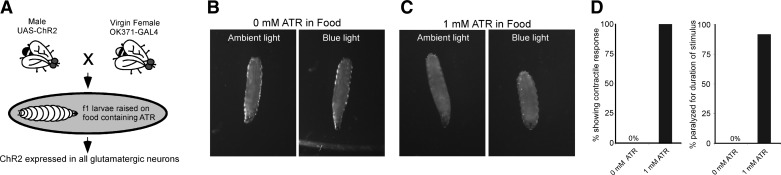
Previous work has demonstrated that simultaneous activation of all larval motor neurons with ChR2 leads to tetanic paralysis (25). To assess whether these effects are robust enough for use in teaching laboratories, we expressed ChR2 in motor neurons (Fig. 2A) and then filmed behavioral responses to blue light. OK371-GAL4 × UAS-H134R-ChR2 animals raised on normal fly food were not affected by blue light pulses (n = 10; Fig. 2, B, left and right, and D). In contrast, genetically identical animals reared on food containing ATR showed immediate, obvious responses to blue light. In ambient light or green light, these larvae usually crawled normally, showing well-coordinated posterior-to-anterior peristaltic waves of muscle contractions (Fig. 2C, left; Supplemental Material, Supplemental Movie 1).1 In blue light, all body segments contracted at once, and peristaltic waves stopped (Fig. 2C, right; Supplemental Movie 1). All animals (100%) raised on ATR food showed immediate, strong contraction of all body segments (Fig. 2D, left). Over 90% of these animals were completely paralyzed for the duration of a 5-s light pulse (n = 12; Fig. 2D, right). Paralyzed animals recovered within 5 s after a 5-s light pulse (Supplemental Movie 1). In demonstration experiments (shown here), we delivered blue light pulses through a dissecting microscope equipped for fluorescence microscopy. In classroom exercises, we obtained similar results using the LED control system described above.
Fig. 2.
Activation of glutamatergic neurons with channelrhodopsin-2 (ChR2) causes tetanic paralysis in larvae raised on food containing all-trans-retinal (ATR). A: schematic of the genetic crossing scheme and larval rearing. B: third instar larva raised on food without ATR. Locomotion and body posture under ambient light were the same as those under blue light. C: third instar larva raised on food containing 1 mM ATR. Locomotion was unimpaired under ambient light. Under blue light, all body segments contracted, and the animal stopped crawling. D: pooled data. Animals raised without dietary ATR did not respond to blue light, whereas 100% of animals expressing ChR2 showed contractile responses to blue light (left; n = 10); 92% of these animals were paralyzed for the duration of a 5-s light pulse (right; n = 12).
Each student group was encouraged to devise their own methods for measuring ChR2-mediated behavioral effects. One example of a student-conceived analysis is shown in Table 1. This student group compared crawling behavior in control and ChR2-expressing animals under ambient and blue light. They measured the frequency of forward peristaltic waves by counting the number of waves in a 30-s trial. They also estimated the total distance traveled by placing a grid of 1 × 1-cm squares beneath each larva and measuring the number of squares traveled during the same 30-s trial. Under ambient light, both genotypes showed similar crawling parameters. In the presence of rhythmic blue light pulses (1-s duration, 0.5-Hz cycle period), controls continued to crawl, whereas animals expressing ChR2 showed no forward peristalsis. Consistent with previous work (25), behavioral effects were strong at first but gradually wore off after 20–30 s under constant illumination (data not shown). Several student groups noted that high-intensity white light could also elicit behavioral responses in ChR2-expressing animals. Students were therefore encouraged to minimize the intensity of dissection scope lamps during experiments.
Table 1.
Example of student-initiated behavioral analysis
|
Group A: No ChR2 |
Group B: ChR2 Expression |
|||
|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 1 | Trial 2 | |
| Control condition: no blue light stimulation | ||||
| Number of peristaltic waves | 21 | 24 | 23 | 23 |
| Total distance traveled, no. of squares | 8 | 12 | 12 | 14 |
| Experimental condition: 1-s blue light pulses | ||||
| Number of peristaltic waves | 19 | 21 | 0 | 0 |
| Total distance traveled, no. of squares | 12 | 13 | 0 | 0 |
Students counted the number of peristaltic waves and distance traveled during 30-s trials in control [no channelrhodopsin2 (ChR2) expression, n = 2] and experimental (ChR2 expressed in glutamatergic neurons, n = 2) animals. In both groups, locomotion was measured in ambient light and in the presence of rhythmic (1-s pulses, 0.5 Hz) blue light pulses.
LEJPs at the larval NMJ.
Previous work has shown that the LED system presented here can reliably generate single LEJPs at the larval NMJ (10, 25). We asked students to first apply light pulses of varying durations to the larval preparation (Fig. 3A) and record LEJPS to ensure that they had a working preparation (demonstration examples in Fig. 3C). Next, we encouraged them to formulate and investigate their own research questions. Several groups chose to examine how these LEJPs compared with EEJPs at the larval NMJ. They easily recorded LEJPs but had difficulty successfully stimulating motor nerves to record EEJPs. For demonstration purposes, we repeated this experiment. In the preparation shown in Fig. 3A, the CNS was removed, and a suction electrode was placed on a single segmental nerve. Nerve shocks (1 ms) reliably evoked single EEJPs. Consistent with previous work, as stimulus intensity increased, a second motor unit innervating larval muscle 6 was recruited, leading to a stepwise increase in EEJP amplitude (Fig. 3B). Blue light pulses of 1 ms failed to evoked LEJPs in seven of seven preparations, but 2.5-, 5-, and 10-ms light pulses evoked LEJPs in most preparations (2.5 ms: 5 of 7 preparations; 5 ms: 7 of 7 preparations; and 10 ms: 7 of 7 preparations). LEJP and low-threshold EEJP amplitudes and time courses were not significantly different (P > 0.05 by one-way ANOVA with the Tukey-Kramer post hoc test; Fig. 3, B–F).
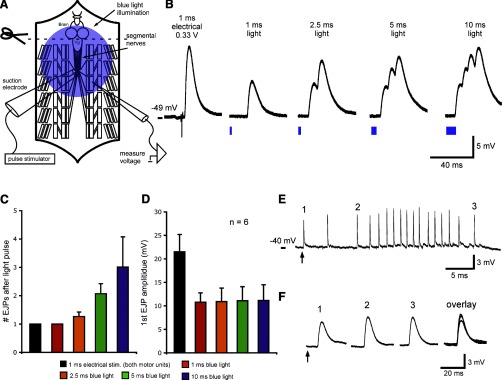
In previous work, LEJPs have been measured in preparations in which motor neuron cell bodies were present and ventral ganglion circuitry was intact (25). Several student groups chose to study LEJPs in this type of preparation (a schematic is shown in Fig. 4A). In demonstration experiments, 1-ms electrical pulses recruited both motor units with amplitudes and time courses similar to those seen in reduced nerve-muscle preparations (data not shown). With the ventral ganglion intact, we reliably evoked single low-threshold LEJPs with light pulse durations as short as 1 ms (Fig. 4B). Longer light pulses evoked summating trains of EJPs (Fig. 4, B and C). Increasing the light pulse duration did not affect the amplitudes of leading LEJPs (Fig. 4D).
Fig. 4.
Comparison of LEJPs and EEJPs with motor neuron cell bodies and ventral ganglion intact. A: schematic of a dissected larval preparation, showing the brain, ventral ganglion segmental nerves, and an intracellular electrode in larval muscle 6. The brain is removed, but the ventral ganglion is intact. B: EJPs in response to a 1-ms electrical stimulus and four different blue light pulse durations. The electrical stimulus intensity was adjusted to activate both motor units innervating larval muscle 6. Note the multiple summating LEJPs after longer light pulse durations. C: number of EJPs for each light pulse duration. D: increasing light pulse durations did not affect the amplitudes of leading LEJPs. E: short light pulses can trigger long trains of spontaneous EJPs. A 1-ms light pulse (arrow) triggered a single EJP in larval muscle 6 (1) followed by a train of endogenously generated EJPs (2 and 3). F: LEJP was similar in amplitude and duration to spontaneous EJPs. Data in B, E, and F are from two different animals. In pooled data, resting membrane potentials were between −40 and −55 mV. Leading EJP amplitudes and resting membrane potentials were not significantly different across stimulation types (F > 0.05 by one-way ANOVA). Pooled data are presented as means ± SE.
In several preparations with intact ventral ganglia, (3/7), short light pulses evoked a single LEJP followed by a long (1–5 s) train of spontaneously generated EJPs (Fig. 4E). In these experiments, LEJPs were similar in amplitude and time course to spontaneous EJPs (Fig. 4F). Trains of spontaneously generated EJPs were not seen in preparations in which the ventral ganglion had been removed. In classroom experiments, several groups noted that in preparations with intact ventral ganglia, high-intensity white light pulses from dissection lamps could trigger trains of LEJPs.
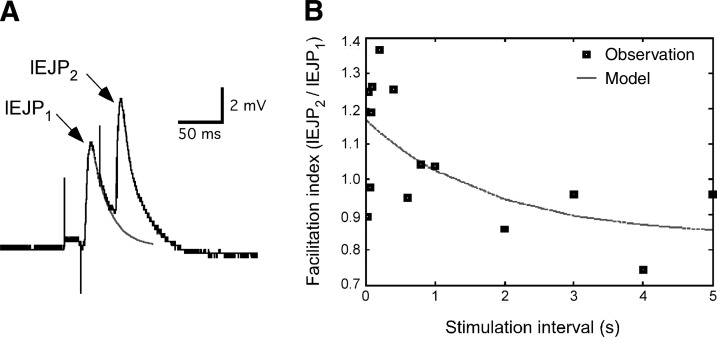
An example of the data collected during a student-initiated classroom project is shown in Fig. 5. This particular group recorded LEJPs in response to paired pulses of blue light (Fig. 5A). They then calculated facilitation ratios (EJP2 amplitude/EJP1 amplitude) at various stimulation intervals (Fig. 5B) to compare these data with previously published descriptions of short-term plasticity at the larval NMJ. Students used offline analysis tools in g-PRIME to compensate for summation at short stimulus intervals. Specifically, they fitted an exponential curve to the repolarizing phase of leading EJPs and used that as a baseline to estimate trailing EJP amplitudes. This allowed them to accurately estimate facilitation ratios even at stimulus intervals where summation dominated in the synaptic responses. The students' results suggested the presence of short-term facilitation at stimulus intervals of <1 s.
Fig. 5.
Example of a student-initiated electrophysiology experiment: analysis of short-term plasticity at the larval neuromuscular junction. A: pair of LEJPs evoked by 20-ms light pulses spaced 40 ms apart. Arrows indicate LEJPs. To compensate for additive summation at short stimulation intervals, an exponential curve (shaded line) was fit to the repolarizing phase of the first EJP. The amplitude of the LEJP2 was determined by the difference between its peak voltage and the exponential fit voltage at the time of peak voltage. B: paired pulse facilitation indexes over a range of stimulation intervals (■). Data were fit to an exponential decay equation. The calculated long-term facilitation ratio was 0.8 ± 4 (95% confidence interval). Data were from a single neuromuscular junction. All experimental design, data collection, analysis, and figure preparation were carried out by students.
Student evaluations.
In the first-year qualitative evaluation, student reviews of the exercises were generally favorable. Students were excited to be working with a novel research preparation, they enjoyed the integration of behavior and physiology, and they seemed to be inspired by the idea of using genetics to remotely control neural activity. From a practical point of view, students liked being able to see light-evoked muscle contractions in dissected preparations; it helped them target healthy muscle cells for intracellular recording. In the first year, students complained that 1) the LED control system was not 100% reliable, 2) 1 wk was too short to complete the exercise, and 3) there was not enough time allocated for exploring their own research questions.
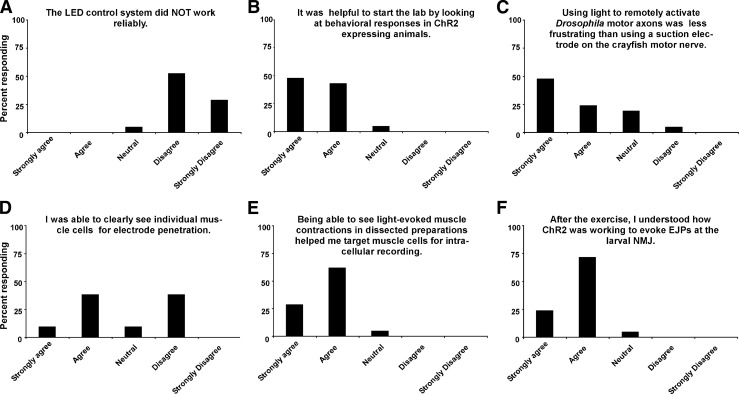
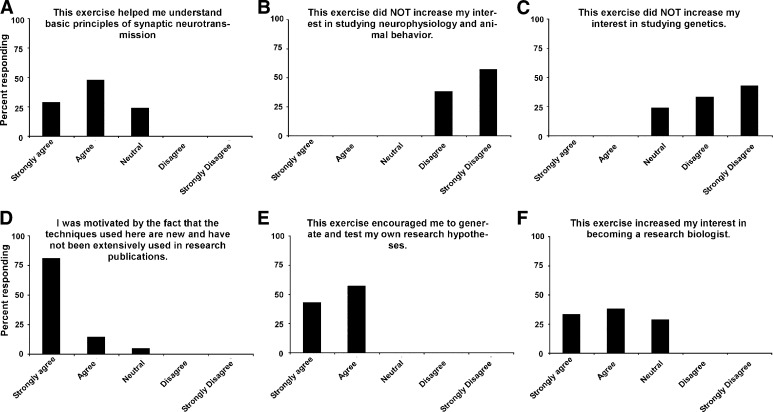
Before running the exercises in the second year, we corrected problems with the LED control system and allocated a second week for student exploration. After the exercises, we quantitatively evaluated student reactions. Figure 6 shows student responses (n = 21) to six questions designed to rank technical features of the exercises. While some students had difficulty clearly seeing muscle fibers for electrode penetration (Fig. 6D), on the whole, students were satisfied with the technical features of the exercises (Fig. 6, A, C, and E). Students also liked starting the laboratory with behavioral analysis (Fig. 6B) and appeared to understand and be excited about what they were doing (Fig. 6F). Figure 7 shows student responses to an additional six questions aimed at evaluating how effective these exercises were at conveying biological concepts and promoting interest in biological research. Students indicated that these exercises helped them understand principles of synaptic transmission (Fig. 7A) while also stimulating interest in studying neural mechanisms of behavior and genetics (Fig. 7, B and C). Students were extremely excited about using new optogenetic technology and doing experiments that have not yet been done by researchers (Fig. 7D). Overall, the exercises helped students learn how to implement the scientific method and heightened student interest in pursuing careers as research scientists (Fig. 7, E and F).
Fig. 6.
Student evaluation of the technical aspects of the ChR2 behavior and physiology exercises. A–F: responses to six queries (shown above each plot) ranked on a Leikert scale. n = 21 students.
Fig. 7.
Student evaluation of the conceptual and motivational aspects of ChR2 exercises. A–F: responses to six queries (shown above each plot) ranked on a Leikert scale. n = 21 students.
DISCUSSION
Behavior experiments.
In a teaching exercise, it is important that any behavioral phenotypes being studied are robust. We reasoned that activating glutamatergic neurons with ChR2 might produce phenotypes appropriate for teaching laboratories. Glutamate is the primary neurotransmitter at NMJs in Drosophila (13, 14). The demonstration and student data (Fig. 2 and Table 1) clearly show that despite longer-term adaptations (25), activation of glutamatergic neurons with ChR2 leads to an immediate and dramatic decrease in larval locomotion. Quantification of student feedback suggests that it was instructive to start the exercise by examining ChR2-mediated behavioral responses (Fig. 5B), thus providing a behavioral context for the following physiology. This is probably because the behavior responses are so unambiguous; they produce immediate positive reinforcement for students early on in the exercise.
Activating glutamatergic neurons provides a reliable and easily interpretable phenotype (motor neuron activation = muscle contraction = tetanic paralysis). However, these experiments also provide a solid jumping off point for additional behavioral studies aimed at analysis of other ensembles of fly neurons. With the genetic tools currently available in Drosophila, students can remotely stimulate a variety of transmitter systems and neuronal subpopulations. For example, GAL4 drivers currently exist for labeling various aminergic systems (28), peptidergic cells (31), and cholinergic neurons (27). Other drivers target the peripheral nervous system and identified sensory cells (11, 30). To date, the functions of some identified neuronal populations have been examined with ChR2 (12, 25, 28, 29, 34), but a large and ever-growing number of GAL4 lines (and, by extension, hypotheses) remain to be tested.
Electrophysiology experiments.
Consistent with previous work (25), in demonstration experiments, we reliably evoked LEJPs in reduced preparations that consisted only of motor axons, nerve terminals, and muscles with stimulus durations of 2.5–10 ms. When evoking EJPs with electrical stimulation, researchers typically use 100-μs to 1-ms duration stimuli (14, 33). Critically, the LEJPs recorded with longer stimulation times were essentially identical to those evoked by 1-ms electrical stimulation of a single low-threshold motor unit innervating larval muscle 6 [most likely the RP3 motor neuron (18, 20)]. Furthermore, increasing the light pulse duration did not affect single LEJP parameters. These results suggest that EJPs resulting from ChR2 initiated action potentials are not essentially different from EJPs evoked by traditional nerve stimulation. There was one obvious difference between the two methods of evoking EJPs: using ChR2, we were not able to recruit both motor units innervating larval muscle 6. One possible explanation for this result is simply that our LED system cannot generate high enough intensity blue light to trigger an action potential in the motor unit with the higher threshold. A second possibility is that ChR2 expression in the two motor neurons is not uniform. The strength of GAL4 expression often varies among cell types within an expression pattern (S. R. Pulver, personal observations). If GAL4 expression is relatively weak in high-threshold motor neurons, then those cells would have fewer functional ChR2 channels and would, in turn, be less responsive to blue light than other ChR2-containing motor neurons. The use of higher-power LEDs and/or alternative motor neuron GAL4 drivers could help resolve this issue.
In our second set of demonstration experiments, we found that leaving the ventral ganglion intact lowered the effective stimulus duration needed to evoke EJPs. This could be a consequence of having intact motor neurons (dendritic regions, cell bodies, and initial spike generation zones) in the ventral ganglion exposed to blue light. It could also be caused by the activation of excitatory glutamatergic interneurons, which, in turn, activate motor neurons through synaptic pathways. Regardless, the leading LEJPs in these CNS-nerve-muscle preparations were similar in amplitude and duration to LEJPs in experiments with only nerve and muscles present.
One prominent feature of preparations with intact ventral ganglia was that they generated multiple EJPs in response to single light pulses with durations longer than 2.5 ms. In addition, in about half the preparations, short light pulses triggered long-lasting trains of spontaneously generated LEJPs. From a teaching perspective, these features provide students and educators with opportunities for further exploration. For example, students can easily examine basic synaptic integration when motor neurons fire high-frequency bursts and postsynaptic potentials summate; students can also compare LEJPs and spontaneously generated EJPs without the use of stimulating electrodes.
In classroom exercises, students recorded EJPs from different body wall muscles. They were encouraged to target any muscles that contracted in response to light pulses (as opposed to specifically targeting only larval muscle 6). While this resulted in heterogeneity across student results, it also increased the chances of students obtaining usable data, because many students had difficulty visualizing individual muscles for electrode penetration (Fig. 6D). Opportunistically targeting muscle areas that contract with light stimulation facilitated student success. For example, all student groups (11 groups/2 laboratory sessions) from our 2010 cohort recorded LEJPs. Once they successfully recorded EJPs, most students focused on examining short-term synaptic plasticity at the larval NMJ (Fig. 5). They were aided by a suite of powerful software tools to analyze the dynamics of synaptic transmission. The data analysis program g-PRIME (http://crawdad.cornell.edu/gprime/) has been optimized and student tested for analyzing many aspects of synaptic transmission at the crayfish NMJ (21). These freely available analysis tools can be immediately and directly applied to analyzing synaptic transmission in Drosophila.
Dissection for electrophysiology experiments: coping with small size.
The largest drawback to the Drosophila NMJ electrophysiology preparation is its small size. Because of this, students have difficulty doing the larval dissection. In particular, they often cannot make a clean initial posterior-to-anterior cut with the spring scissors typically provided in teaching laboratories (10). We have found two solutions to this problem. One option is for teachers and teaching assistants to prepare the dissections ahead of time and provide preparations “on the fly” during a 3- to 4-h laboratory class. With high-quality scissors and a few practice sessions, experienced teaching assistants (and students) can typically complete a dissection in under 5 min. The second approach is to follow a “try one, get one free” policy. Student groups try the dissection once, and if they do not see light-evoked muscle contractions, they receive a fresh preparation from an instructor. Most preparations will provide some data unless large areas of the body wall are obviously damaged. Scotch Tape placed on the under surface of Sylgard-lined petri dishes diffuses transmitted light and increases contrast to more easily visualize target muscles.
Practical advantages of using ChR2.
A major advantage of using ChR2 is that students are able to evoke LEJPs without the use of suction electrodes. Students (and researchers) often have difficulties maneuvering and operating suction or other stimulating electrodes in small working areas, especially with the larval fly preparation. Eliminating the need for a suction electrode potentially eliminates a major source of frustration in the teaching laboratory. Before our fly laboratory sessions, BIONB/BME 4910 students spent 2 wk studying synaptic transmission at the crayfish NMJ. Students used the same equipment as used in our study and had the same primary instructor (B. R. Johnson); use of suction electrodes in the crayfish preparation was required. This gave us the opportunity to test the hypothesis that evoking EJPs with ChR2 in Drosophila was technically easier for students than traditional suction electrode stimulation in crayfish. Indeed, ∼75% of the students agreed that using ChR2 to evoke LEJPs at the larval NMJ was easier than using a suction electrode at the crayfish NMJ (Fig. 6C). This suggests that the ChR2-based exercises demonstrated here offer a technical advantage over at least one traditional NMJ teaching preparation.
A second practical advantage of using ChR2 is that students can get continuous feedback on the health of their preparations and where to insert intracellular electrodes. In dissected preparations, shining blue light on a larval CNS expressing ChR2 causes visible muscle contractions. Therefore, if students see light-evoked contractions, they know that their preparation is healthy and in what muscle area to insert an electrode, even if individual muscle fibers are not distinguishable. Since all motor neurons express ChR2, students can target muscles in any healthy body wall segment of the larvae for intracellular recording.
We noticed that many students had difficulty identifying muscle cells for penetration with recording electrodes (Fig. 6D). Our student evaluations point to a solution to this problem: simply being able to see light-evoked muscle contractions in dissected preparations helped over 90% of students target individual muscles for successful recordings (Fig. 6E). We also noted that seeing these contractions appeared to galvanize students to continue trying to get intracellular recordings even in the face of frustration caused by technical difficulties.
Outlook for student-led research.
The ability to optogenetically evoke EJPs at the larval NMJ opens multiple avenues for further exploration and independent student projects. For example, students can explore indepth fundamental features of ChR2-mediated synaptic transmission and its plasticity, including facilitation, summation, posttetanic potentiation, and depression. They can also examine how these properties vary among identified muscles in larvae (something that has never been done systematically by researchers). Furthermore, since miniature EJPs are visible in larval muscle 6 (13, 14) students can estimate the quantal content of LEJPs [i.e., LEJP amplitude/miniature EJP amplitude (4)]. Finally, students can also examine how acute application of neuromodulatory substances (i.e., neuropeptides and biogenic amines) affect synaptic transmission at the larval NMJ. Overall, many fundamental experiments have yet to be performed using optogenetic methods to evoke LEJPs in fly larvae; therefore, any student projects would be breaking new ground, not just repeating previous work.
Students were clearly motivated by this laboratory exercise. They felt it helped them understand communication within the nervous system, and it enhanced their interest in the intellectual background material (Fig. 7, A–C). Perhaps more importantly, almost all of the students (94%) expressed excitement that they could potentially do novel experiments that have not yet been done by researchers (Fig. 7D). This led most of them to express a positive interest in practicing the scientific method as students and even to consider a career in research (Fig. 7, E and F).
Conclusions.
Here, we present inexpensive methods for remotely activating neural circuits in freely behaving Drosophila larvae with ChR2. We also show how to record ChR2-mediated EJPs at the larval NMJ and show that they are equivalent to EJPs evoked by traditional electrical stimulation. These teaching exercises give reliable results with minimal effort and expense. More importantly, they generate avenues for further research and give students and educators the means to explore them independently.
GRANTS
This work was supported by National Institute of Mental Health Grant R01-MH-067284 (to L. Griffith, Brandeis University), a Royal Society Newton International Fellowship (to S. R. Pulver), an American Physiological Society Teaching Career Enhancement award (to S. R. Pulver), and the Department of Neurobiology and Behavior of Cornell University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Prof. Leslie Griffith (Brandeis University) for providing laboratory space and equipment. The authors gratefully acknowledge the students of “BIONB/BME 4910” at Cornell University for providing constructive feedback on teaching protocols. The authors also thank Jimena Berni and Leslie Griffith for critical reading of the manuscript, graduate teaching assistants Frank Rinkovitch and Gil Menda for help teaching this exercise, Gil Menda for taking the pictures in Fig. 1, and Dr. Julio Ramirez for critiquing the student evaluation.
Glossary
- A/D
Analog-to-digital
- ATR
All-trans-retinal
- CHR2
Channnelrhodopsin-2
- CNS
Central nervous system
- EEJPs
Electrically evoked excitatory juctional potentials
- EJPs
Excitatory juctional potentials
- LED
Light-emitting diode
- LEJPs
Light-evoked excitatory juctional potentials
- NMJ
Neuromuscular junctiion
Footnotes
Supplemental Material for this article is available at the Advances in Physiology Education website.
REFERENCES
- 1. Berni J, Mudal A, Pulver SR. Using the warmth-gated ion channel TRPA1 to study the neural basis of behavior in Drosophila. J Undergrad Neuro Educ 9: A5–A14, 2010. [PMC free article] [PubMed] [Google Scholar]
- 2. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993. [DOI] [PubMed] [Google Scholar]
- 4. Del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol 124: 560–573, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglass AD, Kraves S, Deisseroth K, Schier AF, Engert F. Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Curr Biol 18: 1133–1137, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feng Y, Ueda A, Wu CF. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J Neurogenetics 18: 377–402, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Franks CJ, Murray C, Ogden D, O'Connor V, Holden-Dye L. A comparison of electrically evoked and channel rhodopsin-evoked postsynaptic potentials in the pharyngeal system of Caenorhabditis elegans. Invert Neurosci 9: 43–56, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Hägglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat Neuro 13: 246–52, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454: 217–220, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hornstein NJ, Pulver SR, Griffith LC. Channelrhodopsin2 mediated stimulation of synaptic potentials at Drosophila neuromuscular junctions. J Vis Exp 25: 1133, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol Cell Neurosci 35: 383–396, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol 17: 2105–2116, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jan LY, Jan YN. l-Glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol 262: 215–236, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol 262: 189–214, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol 47: 81–92, 2001. [DOI] [PubMed] [Google Scholar]
- 16. Krans JL, Rivlin PK, Hoy RR. Demonstrating the temperature sensitivity of synaptic transmission in a Drosophila mutant. J Undergad Neuro Educ 4: A27–A33, 2005. [PMC free article] [PubMed] [Google Scholar]
- 17. Liewald JF, Brauner M, Stephens GJ, Bouhours M, Schultheis C, Zhen M, Gottschalk A. Optogenetic analysis of synaptic function. Nat Methods 5: 895–902, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Li H, Peng X, Cooper RL. Development of Drosophila larval neuromuscular junctions: maintaining synaptic strength. Neuroscience 115: 505–513, 2002. [DOI] [PubMed] [Google Scholar]
- 19. Likert R. A technique for the measurement of attitudes. Arch Psychol 140: 1–55, 1932. [Google Scholar]
- 20. Landgraf M, Bossing T, Technau GM, Bate M. The origin, location, and projections of the embryonic abdominal motorneurons of Drosophila. J Neurosci 17: 9642–9655, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lott GK, Johnson BR, Bonow RH, Land BR, Hoy RR. g-PRIME: a free, windows based data acquisition and event analysis software package for physiology in classrooms and research labs. J Undergrad Neuro Educ 8: A50–A54, 2009. [PMC free article] [PubMed] [Google Scholar]
- 22. Mahr A, Aberle H. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr Patterns 6: 299–309, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 15: 2279–2284, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 100: 13940–13945, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol 101: 3075–3088, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia AS, McNamara JO, White LE. Neuroscience. Sunderland, MA: Sinauer, 2008, p. 857. [Google Scholar]
- 27. Salvaterra PM, Kitamoto T. Drosophila cholinergic neurons and processes visualized with Gal4/UAS-GFP. Brain Res Gene Expr Patterns 1: 73–82, 2001. [DOI] [PubMed] [Google Scholar]
- 28. Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol 16: 1741–1747, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Suh GS, Ben-Tabou de Leon S, Tanimoto H, Fiala A, Benzer S, Anderson DJ. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol 17: 905–908, 2007. [DOI] [PubMed] [Google Scholar]
- 30. Suster ML, Bate M. Embryonic assembly of a central pattern generator without sensory input. Nature 416: 174–178, 2002. [DOI] [PubMed] [Google Scholar]
- 31. Taghert PH, Hewes RS, Park JH, O'Brien MA, Han M, Peck ME. Multiple amidated neuropeptides are required for normal circadian locomotor rhythms in Drosophila. J Neurosci 21: 6673–6686, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wyttenbach RA, Johnson BR, Hoy RR. Crawdad: a CD-ROM Lab Manual for Neurophysiology. Sunderland, MA: Sinauer, 1999. [Google Scholar]
- 33. Zhang B, Stewart B. Synaptic electrophysiology of the Drosophila neuromuscular junction. In: Drosophila Neurobiology: a Laboratory Manual, edited by Zhang B, Freeman MR, Waddell S. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2010, p. 171–6673–214. [DOI] [PubMed] [Google Scholar]
- 34. Zhang W, Ge W, Wang Z. A toolbox for light control of Drosophila behaviors through channelrhodopsin-2 mediated photoactivation of targeted neurons. Eur J Neurosci 26: 2405–2416, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.