Abstract
Introduction
Since the initial development of the defibrillator, there has been concern that, while delivery of a large electric shock would stop fibrillation, it would also cause damage to the heart. This concern has been raised again with the development of the biphasic defibrillator.
Objective
To compare defibrillation efficacy, postshock cardiac function, and troponin I levels following 150-J and 360-J shocks.
Methods
Nineteen swine were anesthetized with isoflurane and instrumented with pressure catheters in the left ventricle, aorta, and right atrium. The animals were fibrillated for 6 minutes, followed by defibrillation with either low-energy (n = 8) or high-energy (n = 11) shocks. After defibrillation, chest compressions were initiated and continued until return of spontaneous circulation (ROSC). Epinephrine, 0.01 mg/kg every 3 minutes, was given for arterial blood pressure <50 mmHg. Hemodynamic parameters were recorded for four hours. Transthoracic echocardiography was performed and troponin I levels were measured at baseline and four hours following ventricular fibrillation (VF).
Results
Survival rates at four hours were not different between the two groups (low-energy, 5 of 8; high-energy, 7 of 11). Results for arterial blood pressure, positive dP/dt (first derivative of pressure measured over time, a measure of left ventricular contractility), and negative dP/dt at the time of lowest arterial blood pressure (ABP) following ROSC were not different between the two groups (p = not significant [NS]), but were lower than at baseline. All hemodynamic measures returned to baseline by four hours. Ejection fractions, stroke volumes, and cardiac outputs were not different between the two groups at four hours. Troponin I levels at four hours were not different between the two groups (12 ± 11 ng/mL versus 21 ± 26 ng/mL, p = NS) but were higher at four hours than at baseline (19 ± 19 ng/mL versus 0.8 ± 0.5 ng/mL, p < 0.05, groups combined).
Conclusion
Biphasic 360-J shocks do not cause more cardiac damage than biphasic 150-J shocks in this animal model of prolonged VF and resuscitation.
Keywords: defibrillation, resuscitation, ventricular fibrillation
INTRODUCTION
Since the initial development of the defibrillator, there has been a concern that delivery of a large electric shock would stop ventricular fibrillation (VF) but would also damage the heart to the extent that it would not be able to adequately pump blood following defibrillation.1 With the development of biphasic external defibrillators, this concern has been raised again. Several studies have compared the efficacy of biphasic shocks with that of monophasic shocks. Following short durations of VF, transthoracic biphasic shocks have been shown to defibrillate with lower peak currents and delivered energies than monophasic waveforms.2,3 Following prolonged durations of VF, studies have shown that return of spontaneous circulation (ROSC) is more likely following a biphasic shock than a monophasic shock, but survival is either not improved or improved only slightly compared with that following monophasic waveforms.4–7
Several studies have shown that delivery of multiple 400-J transthoracic shocks for cardioversion of atrial fibrillation does not cause damage to the heart as measured by release of cardiac enzymes.8–10 Studies have also shown that transthoracic shocks up to 400 J can be delivered to large animals without significant decreases in cardiac function as measured by left ventricular pressures and echocardiography.11,12 A 2002 study using isolated rat hearts and very large epicardial shocks has shown that ischemic hearts may be more likely to show cardiac dysfunction than nonischemic hearts.13 The shock energy level at which this difference becomes apparent is approximately two to five times the size of shocks normally given from epicardial patches to defibrillate a heart.
There are a number of situations in which a shock larger than 150 J may be necessary to defibrillate the heart, including increased shock impedance,14 poor electrode placement,15 or pathologic conditions that raise the defibrillation threshold, including spontaneous arrhythmias secondary to acute ischemia16,17or in patients taking drugs such as amiodarone.18
This study tested the hypothesis that high-energy biphasic shocks cause more damage and a greater decrease in cardiac function following defibrillation after prolonged VF than do low-energy biphasic shocks. To test the hypothesis, we delivered either 150-J or 360- J biphasic shocks to swine following 6 minutes of electrically induced VF.
METHODS
Animal Preparation
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. Further, all preoperative and operative care for animals complied with section 6 of the Animal Welfare Act of 1989 and adhered to the principles outlined in the Guide for the Care and Use of Laboratory Animals, National Institutes of Health publication No. 85-23.19
Nineteen domestic swine of both genders, weighing 25–40 kg, were studied. The animals were pre- anesthetized with Telazol/xylazine, 4.4 mg/kg of each, and atropine, 0.04 mg/kg. The animals were intubated, anesthetized with isoflurane, 1.2%–3%, and supported on a pressure-controlled mechanical ventilator (Ohmeda, Madison, WI) at a rate of 10–15 mL/kg/min. The animals received normal saline at a rate of 5–10 mL/kg/h. Blood gas and electrolyte measurements were performed every half hour and corrections in respiratory parameters and infusion fluid makeup were made as necessary. Electrocardiography (ECG) lead II was monitored throughout the study.
After induction of anesthesia, the animal underwent baseline transthoracic echocardiography to determine cardiac function. Left ventricular measurements were made from a short-axis view at the level of the papillary muscles. The animal was placed in dorsal recumbency. The left and right chest walls were shaved. Self-adhesive defibrillation electrodes were placed on the anterior left and right chest walls. The right jugular vein was isolated and a high-fidelity pressure catheter (Mikro-Tip, Millar Instruments, Houston, TX) was advanced under fluoroscopy to the junction of the right atrium and superior vena cava. A quadripolar electrophysiology (EP) catheter (MAP catheter, EP Technologies, San Jose, CA) was inserted into the left jugular vein and advanced into the apex of the right ventricle for VF induction. The left carotid artery was isolated and a high-fidelity pressure catheter was inserted and advanced into the left ventricular cavity. The left femoral artery was isolated and a high-fidelity pressure catheter was advanced into the descending aorta. Blood was drawn for baseline determination of cardiac troponin I level just prior to fibrillation induction.
The heart was fibrillated using 60-Hz current applied to the right ventricular endocardium and the animal was allowed to fibrillate with the ventilator off for 6 minutes. The animal was then randomized to receive either 150-J biphasic shocks or 360-J biphasic shocks (Lifepak-12, Medtronic Physio-Control, Redmond, WA). Up to three shocks were delivered in rapid succession until the animal maintained a nonshockable rhythm for 5 seconds (successful shock).20 If the animal did not convert to a nonshockable rhythm with the three shocks, chest compressions at 100 beats/min using a mechanical cardiopulmonary resuscitation (CPR) device (Thumper, Michigan Instruments, Grand Rapids, MI) was performed for 1 minute. The animal was ventilated during resuscitation at the same rate and tidal volume as delivered before induction of VF by a mechanical ventilator. Up to three shocks at the same energy level were repeated until the animal maintained a nonshockable rhythm. During chest compressions, defibrillation shocks were delivered every minute until the animal was in a nonshockable rhythm. Epinephrine, 0.01 mg/kg, was given after 3 minutes of chest compression and every 3 minutes thereafter until the animal had ROSC and systolic arterial blood pressure was above 50 mmHg. If the animal refibrillated, it was defibrillated at the next whole- minute mark timed from the start of resuscitation using the same-strength shocks. Chest compressions continued until the animal had ROSC or 30 minutes elapsed from the start of chest compressions and the experiment was stopped. If the animal had ROSC but systolic blood pressure dropped below 50 mmHg, CPR was restarted and the animal was given epinephrine. If after one hour from the beginning of resuscitation the animal did not maintain a systolic arterial blood pressure >50 mm Hg, dobutamine, 5 µg/kg/min, was started, continued for two hours, and then stopped one hour prior to the end of the study. The animal was monitored for four hours from the beginning of resuscitation. At the end of four hours, a second transthoracic echocardiogram was performed to assess cardiac function. Blood was also drawn for cardiac troponin I determination.
At the end of the study, the anesthetized animal was sacrificed by applying 60-Hz current to the catheter in the right ventricle. The heart was removed, weighed, and examined for any abnormal gross pathology by the investigator performing the study.
Data Collection and Analysis
Electrocardiography lead II, left ventricular pressure, aortic pressure, and central venous pressure were recorded using a WinDaq data recorder (DATAQ, Akron, OH) at a sampling rate of 250 samples/sec starting 10 seconds prior to VF induction and extending throughout the entire study.
Coronary perfusion pressure was calculated for the period of chest compressions as the maximum thoracic arterial pressure minus central venous pressure measured during the decompression phase of chest compressions. Coronary perfusion pressure was measured for each compression, and the maximum value during chest compressions is reported. Left ventricular dP/dt (first derivative of pressure measured over time), a measure of left ventricular contractility, was calculated using a five-point derivative. Maximum and minimum values were determined for each beat. All calculations were performed using MATLAB software (MathWorks, Natick, MA).
Statistics
Fisher's exact test was used to determine whether there was a difference in survival between the two groups. An unpaired t-test was used to compare hemodynamic, echocardiographic, and troponin I values between the two groups. Bonferroni correction for multiple comparisons was performed. Differences were considered significant if the p-value was less than 0.05. The study was powered to show a 20% difference in mean values with a standard deviation of 25% using a = 0.50 and p = 0.05. We studied more animals in the high-energy group than the low-energy group to better define the high-energy group results.
RESULTS
Nineteen animals, weighing 35 ± 5 kg, were studied. Animal weights were not different between the two groups (35 ± 6.5 kg for the 150-J group versus 36 ± 5.1 kg for the 360-J group), p = not significant [NS]). Gender distributions were not different between the two groups (5 males and 3 females in the 150-J group versus 6 males and 5 females in the 360-J group, p = NS). Defibrillation dose was 4.3 ± 0.6 J/kg for the 150-J group and 10.2 ± 1.3 J/kg for the 360-J group.
Five of the eight animals (62%) that were defibrillated with 150-J shocks had ROSC and seven of the 11 animals (63%) that were defibrillated with 360-J shocks had ROSC (p = NS). First-shock impedance values were not different for the two shock strengths (55 ± 10 ohms for 150 J versus 63 ± 13 ohms for 360 J). All animals that had ROSC survived for four hours. All animals that died without ROSC were in a nonshockable rhythm at the end of resuscitation. There was no significant difference in the time to ROSC in survivors in each group (5.4 ± 2.8 min for 150 J versus 3.7 ± 2.0 min for 360 J). Maximum coronary perfusion pressures during CPR were not significantly different between the two groups (21 ± 10 mmHg for 150 J versus 25 ± 10 mmHg for 360 J). Average heart rates over the first 30 seconds following defibrillation were not significantly different between the two groups (52 ± 33 beats/min for 150 J versus 45 ± 15 beats/min for 360 J). There was no significant difference in the amount of epinephrine required by the survivors for the two groups (0.9 ±1.1 mg for the 150-J group versus 0.8 ± 1.1 mg for the 360-J group). First-shock success rate was significantly higher for 360-J shocks (9 of 11, 82%) compared with that for 150-J shocks (2 of 8, 25%). First-two-shock success rate was significantly higher for 360-J shocks (11 of 11, 100%) compared with that for 150-J shocks (5 of 8, 62%). Significantly fewer shocks were required during resuscitation of survivors defibrillated with 360 J (1.6 ± 0.5) than with 150 J (4.2 ± 2.3), leading to no difference in the total amount of energy that was delivered to each group of survivors (630 ± 180 J versus 600 ± 300 J, p = NS).
Following ROSC, all animals had an increase in hemodynamic parameters, followed by a gradual decline, followed again by improvement (either naturally or drug-induced, depending on how low arterial blood pressure dropped) (Fig. 1). There was no difference in the number of animals requiring dobutamine to maintain arterial blood pressure above 50 mmHg (0 of 8 for the 150-J group versus 1 of 11 for the 360-J group; p = NS). There was no difference in arterial blood pressure between the two groups at baseline (Fig. 2). Arterial blood pressure at its minimum following ROSC and the subsequent surge in arterial blood pressure was significantly lower than baseline. Arterial blood pressure at four hours (one hour after the last dose of any ionotropes) was not significantly different from baseline. There was no difference in minimum blood pressure between the two groups either following ROSC and the subsequent surge or at four hours.
Figure 1.
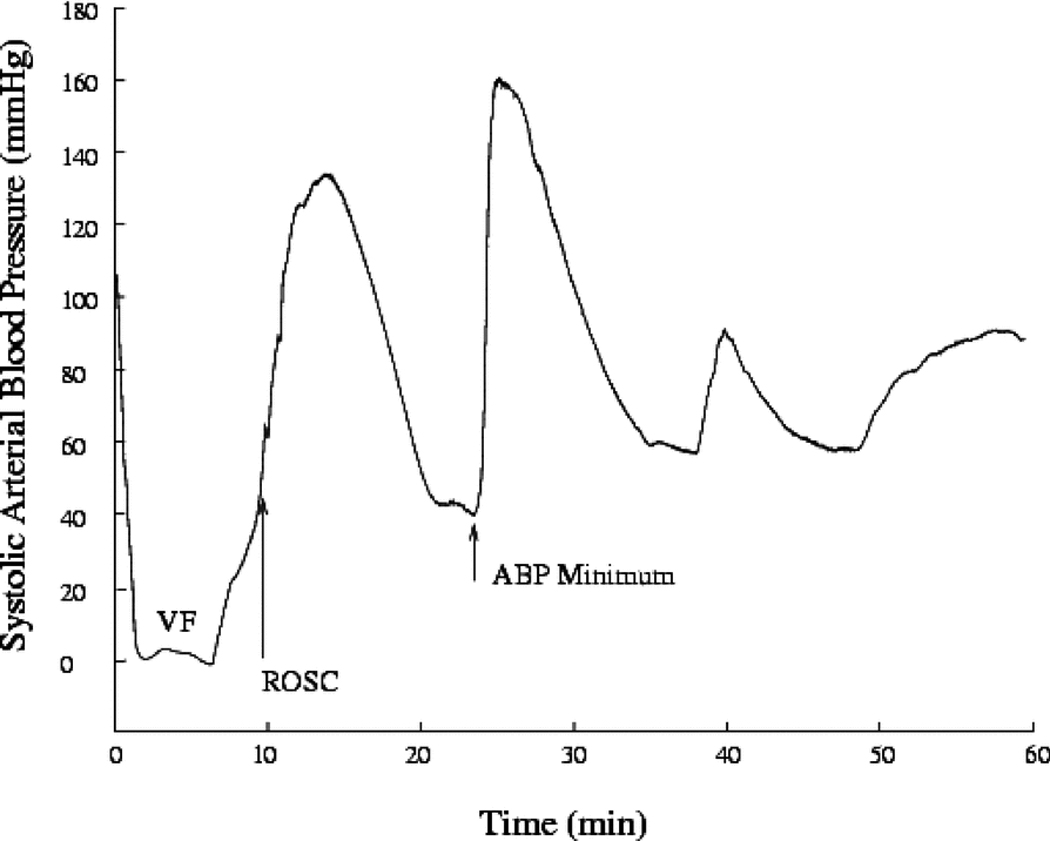
Example of systolic arterial pressure over the first hour of recorded data for an animal defibrillated with two 360-J shocks. Initially, systolic arterial pressure is ~110 mmHg but then drops to close to ~10 mmHg during ventricular fibrillation (VF). Systolic arterial pressure slowly rises during cardiopulmonary resuscitation until return of spontaneous circulation (ROSC) occurs. It then quickly rises to ~170 mmHg. Systolic arterial pressure then slowly drops until it falls below 50 mmHg. Epinephrine is given to the animal and the blood pressure recovers. The time of ROSC and minimum arterial blood pressure (ABP) are labeled.
Figure 2.

Arterial blood pressure measured at baseline, at minimum arterial blood pressure following resuscitation, and at four hours following resuscitation. The group defibrillated with 150-J shocks is shown with white symbols. The group defibrillated with 360-J shocks is shown with black symbols. There was no significant difference between the two groups at any of the three time points. Systolic pressure is shown with a circle. Mean pressure is shown with a square. Diastolic pressure is shown with a diamond.
Peak +dP/dt and peak −dP/dt were significantly lower at minimum arterial pressure following ROSC and the subsequent surge than at baseline or four hours (Fig. 3). Left ventricular end-diastolic pressure was significantly increased at minimum arterial pressure compared with baseline for both shock strengths. There was no significant difference in the change in left ventricular end-diastolic pressure from baseline between the two groups. There was no difference between peak +dP/dt or peak −dP/dt measurements at baseline and at four hours. There was no difference in peak +dP/dt or −dP/dt between animals defibrillated with 150-J shocks and animals defibrillated with 360-J shocks.
Figure 3.
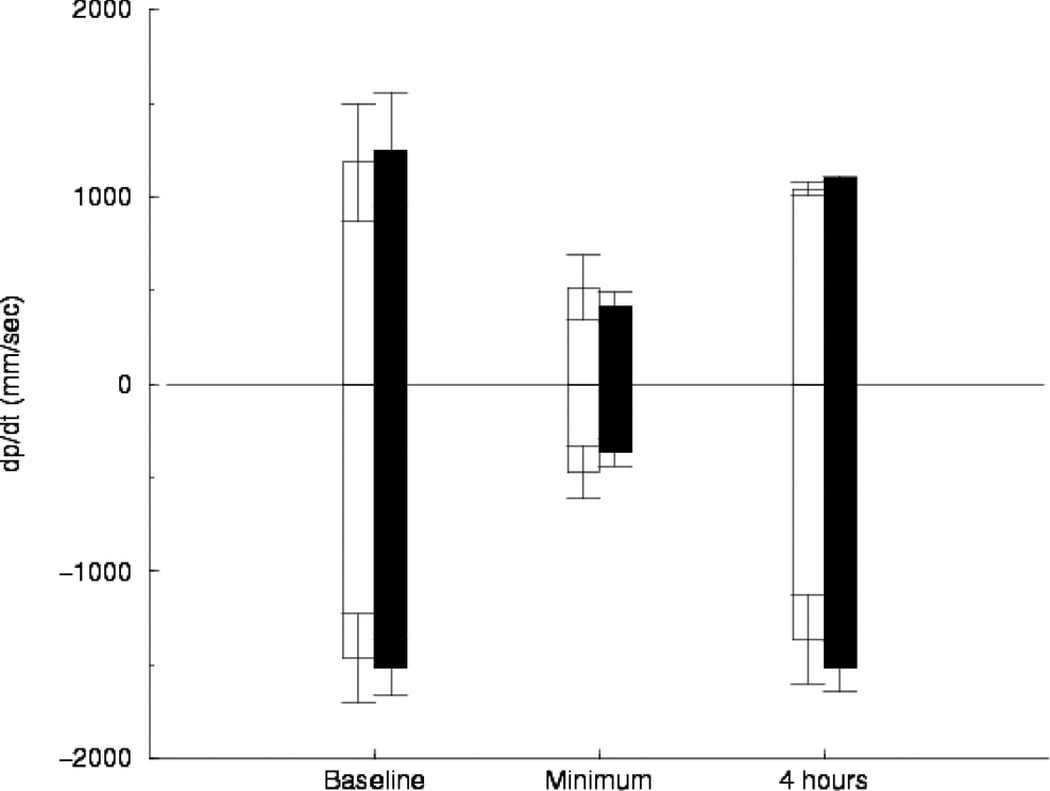
Left ventricular peak positive dP/dt (first derivative of pressure measured over time, a measure of left ventricular contractility) and negative dP/dt measured at baseline, at minimum arterial blood pressure following resuscitation, and four hours following resuscitation. The group defibrillated with 150-J shocks is shown with white bars. The group defibrillated with 360-J shocks is shown with black bars. Error bars show standard deviation. There was no significant difference between the two groups at any of the three time points.
Echocardiography was performed at baseline and four hours after initiation of fibrillation in survivors of both groups (Table 1). There was no difference in echocardiographic measurements between the two groups either at baseline or at four hours.
TABLE 1.
Echocardiography Data
| Ejection Fraction | Stroke Volume | Cardiac Output | ||||
|---|---|---|---|---|---|---|
| Treatment | Pre— Baseline | Post— 4 hours | Pre— Baseline | Post— 4 hours | Pre— Baseline | Post— 4 hours |
| 150 J | 51% ± 9% | 57% ± 5% | 26 ± 2 mL | 34 ± 8 mL | 2.9 ± 1.0 L/min | 4.1 ± 1.5 L/min |
| 360 J | 55% ± 9% | 56% ± 12% | 37 ± 10 mL | 43 ± 10 mL | 3.6 ± 0.9 L/min | 4.7 ± 1.4 L/min |
Plasma troponin I levels were significantly higher following fibrillation and defibrillation than at baseline (17 ± 20 ng/mL following fibrillation and defibrillation versus 0.8 ± 0.5 ng/mL baseline [0.7 ± 0.4 ng/mL for 150 J and 0.8 ± 1.0 ng/mL for 360 J]; p = NS) (Fig. 4). There was no significant difference in plasma troponin I levels between the survivors in the two groups (12 ± 11 ng/mL for 150 J versus 21± 26 ng/mL for 360 J, p = NS). There was no correlation between plasma troponin I level and time to ROSC.
Figure 4.

Troponin I levels at four hours for the animals who achieved return of spontaneous circulation. Data points indicate values for individual animals. Mean ± standard deviation is show for each shock strength.
DISCUSSION
The primary findings of this study are that there was no difference in survival or postresuscitation cardiac function between animals defibrillated with 150-J and 360-J biphasic waveforms following 6 minutes of unsupported VF. Previous studies have compared low-energy biphasic shocks with high-energy monophasic shocks, and low-energy biphasic waveforms with escalating-energy biphasic shocks. This study is, to our knowledge, the first study to explicitly compare low-energy and high-energy biphasic shocks in an animal model of prolonged VF and resuscitation.
Several animal studies have been performed comparing survival and postresuscitation cardiac function following "high-energy" monophasic and "low- energy" biphasic waveform defibrillation. Leng et al. studied 26 dogs following 10 minutes of unsupported VF. Shock strength was progressively increased until the animals were defibrillated.21 They showed that the energy necessary to defibrillate was lower for a biphasic shock than for a damped sinusoidal monophasic shock. Further, postshock myocardial dysfunction as measured by left ventricular +dP/dt was greater in the monophasic shock group than in the biphasic shock group. There was a trend toward improved survival in the animals defibrillated with biphasic shocks compared with monophasic shocks, but this difference was not significant. Tang et al. studied 20 swine following 10 minutes of unsupported VF and then defibrillated with either a 150-J biphasic waveform or 200–360-J damped sinusoidal monophasic waveform.6 There was no difference in survival to 72 hours. Cardiac output was higher in the first hour in the group de- fibrillated with the biphasic waveform. End-diastolic volume was decreased in the group defibrillated with the monophasic waveform for the first six hours following defibrillation. No hemodynamic parameter was significantly different between the two groups at 72 hours. Niemann et al. compared postresuscitation cardiac function in swine following 5 minutes of unsupported VF followed by defibrillation with either a monophasic truncated exponential waveform, 200–300–360 J, or one of two biphasic waveform protocols, 150 J or 200–300–360 J.23 There was no difference in survival among the three groups. There was no difference in cardiac output, left ventricular +dP/dt, or systolic left ventricular pressure among the three groups. Of note, the monophasic waveform shape used in this study was truncated-exponential rather than damped-sinusoidal.
In a study of 115 patients who had sudden cardiac death and presented with VF, more patients had ROSC when treated with a 150-J biphasic waveform than when treated with 200–360-J monophasic waveforms.5 There was no difference in survival to hospital discharge in the two groups of patients. All of these studies show that waveform shape does not seem to affect survival. Though not absolute, postresuscitation cardiac function seems generally better when defibrillated with a 150-J biphasic waveform compared with a 200–360-J monophasic waveform.
Biphasic waveforms are used in most new defibrillators. These devices can be divided into two groups. The first group, low-energy, limit the maximum energy delivered by the defibrillator to 200 J or less. The second group, high-energy, deliver energies up to 360 J, similar to current monophasic defibrillators. Success rate with the low-energy devices is good, with up to 95% of patients defibrillated with the first two shocks.5 But these data suggest that a small proportion of patients require either multiple low-energy shocks or shocks of strength greater than 200 J. One possible reason for the difficulty in defibrillating some patients can be found in animal studies examining defibrillation of spontaneous ischemic VF. These studies show that the defibrillation threshold is much higher for ischemically induced VF than it is for electrically induced VF.16–17
There have been fewer comparisons of low-energy and high-energy biphasic waveforms. In the study by Niemann et al., postresuscitation cardiac function was not different between groups defibrillated with 150-J and 200–360-J biphasic shocks.24 Of note, a majority of the 200-J biphasic shocks were successful in this animal model, and so the effect of 360-J shocks on postdefibrillation cardiac function following prolonged VF was not well characterized. Our study explicitly measured the effect of 360-J compared with 150-J biphasic shocks on postdefibrillation cardiac function following prolonged VF. Similar to the Niemann results, there was no difference in any of the hemodynamic or echocardiographic parameters measured in the two groups of animals.
The BIPHASIC trial compared fixed-lower versus escalating-higher energy levels for prehospital defibrillation in humans.25 Patients were randomized to receive either 1) fixed-energy biphasic shocks (150 J) or 2) escalating-energy biphasic shocks (200–300–360 J). Ventricular fibrillation termination rates were higher in the escalating-energy group compared with the fixed- energy group. There was no difference in survival between the two groups. Though the study was not powered to show a survival difference, the authors suggested that the sample size was chosen to "allow a reasonable estimate of outcome event rates in both study groups." Of the 107 patients randomized to the escalating-energy group, 26 received 360-J shocks. Again, the effect of receiving a 360-J shock versus receiving multiple shocks is hard to isolate in this study.
Measurement of cardiac enzymes following prolonged fibrillation and defibrillation has been proposed as a measure of cardiac damage caused by the prolonged ischemia, defibrillation, and subsequent resuscitation. Grubb et al. measured cardiac enzymes in patients resuscitated from out-of-hospital cardiac arrest, including patients who received no shocks.26 A rise in creatine kinase (CK)-MB and cardiac troponin T concentrations occurred in almost all cases. Patients received from 0 to approximately 2,000 J of total defibrillation energy. There was a modest correlation between enzyme release for both troponin T and CK-MB and the total defibrillation energy delivered among patients without electrocardiographic evidence of acute myocardial infarction. The total amount of delivered defibrillation energy was also positively correlated with the duration of CPR. A similar study performed by Mullner et al. examined the influence of chest compressions and external defibrillation on the release of cardiac enzymes in patients resuscitated from out-of-hospital cardiac arrest.27 Using a multivariate stepwise linear regression model, they showed that CK-MB concentrations 12 hours after CPR were positively associated with the presence of acute myocardial infarction, the duration of CPR, and the presence of cardiogenic shock in the postresuscitation period, but were not significantly associated with the number of defibrillation shocks delivered (mean: 3, range: 1–6) or with the amount of epinephrine administered. Likewise, a similar model was constructed for troponin T concentrations 12 hours after resuscitation and, again, the number of defibrillation shocks administered was not significant. These studies suggest that damage caused by defibrillation during CPR is either small or nonexistent compared with the damage and dysfunction caused by the underlying pathology, period of no-flow ischemia, and reperfusion. Our study showed similar results to the two human studies. There was a significant increase in troponin I concentration following 6 minutes of VF and defibrillation at four hours. There was no difference in troponin I concentration at four hours between the animals defibrillated with 360-J shocks and those defibrillated with 150-J shocks.
We have recently performed an animal study that may explain why the 360-J shocks did not cause more damage by function or troponin I measures.28 Shock potential gradient is an estimate of the amount of current delivered to the heart during a defibrillation shock. Shock potential gradient fields were calculated in animals receiving defibrillation shocks after either 20 seconds or 7 minutes of VF.28 When a 360-J shock was delivered after 7 minutes of VF, the maximum potential gradient measured was 45 V/cm (150 J - 12.5 V/cm). In contrast, the maximum shock potential gradient recorded for a tranvenous implantable cardioverter-defibrillator (ICD) shock of 800 V (−32 J) is 80 V/cm.29 Even though the delivered energy is 10 times higher, the peak current density in the heart is approximately one-half the value for the transthoracic shock compared with the transvenous shock. And the potential gradient threshold for causing impulse conduction block with a biphasic shock is 71 V/cm.30
LIMITATIONS
There are several limitations to our study. First, the animals had normal hearts. Though not completely characterized, it is likely that only a small number of patients suffering from prehospital sudden cardiac death have structurally normal hearts. This study did not determine whether there is some combined effect of shock strength and underlying cardiac disease on cardiac function following prolonged VF and defibrillation. Second, the VF in this study was electrically induced rather than being spontaneous. While 60% of sudden death survivors who have VF have evidence of myocardial ischemia following resuscitation, the other 40% of patients do not.27 Electrically induced VF is used as surrogate for nonischemic spontaneous arrhythmias in ICD implantation testing. It is reasonable to consider the arrhythmias of these 40% of sudden death survivors who have VF to be similar to electrically induced VF.
Third, the investigators were not blinded to treatment either during the study or during data analysis. It is true that some of the measures we used are sensitive to investigator bias, such as echocardiography and gross heart examination; others, such as hemodynamic measures and troponin I concentrations, are not subject to investigator bias. Since none of our measures showed a difference between the two groups, we think that the study as a whole supports the conclusion that the high-energy shocks were not causing more damage to the heart than the low-energy shocks. Another limitation was that blood samples for troponin I measurement were drawn at four hours. Only one de- fibrillation waveform shape was tested in this study, and care should be taken in extrapolating the results to other defibrillation waveform shapes. Finally, this was a small study of 19 animals, and the power to show a statistical difference between the two groups is limited.
CONCLUSIONS AND CLINICAL IMPLICATIONS
The results of this study suggest that 360-J biphasic shocks do not cause measurable damage in this animal model of prolonged VF, defibrillation, and resuscitation. When extrapolating these results to humans, it is important to remember that our animal size was one-third to one-half the size of the average human. Several investigators have shown that defibrillation shock success is directly related to body weight with dose, J/kg.31–33 It is likely that the damaging effects of shocks also scale with body size. Therefore, damage or dysfunction is two to three times more likely to be measured in this animal model compared with humans.
The results of this study combined with the results of the human BIPHASIC study would suggest that delivery of 360-J shocks using the defibrillator waveform tested in this study is safe and efficacious for patients in whom defibrillation fails at lower energy levels.
Acknowledgments
Supported in part by NIH grants HL 67961, HL 63775, and HL 42760 and a grant from Medtronic ERS.
Dr. Hampton was an employee of medtronic ERS, and this work was funded in part by an unrestricted grant from medtronic ERS.
References
- 1.Lown B. Defibrillation and cardioversion. Cardiovasc Res. 2002;55:220–224. doi: 10.1016/s0008-6363(02)00416-9. [DOI] [PubMed] [Google Scholar]
- 2.Gliner BE, Lyster TE, Dillion SM, Bardy GH. Transthoracic defibrillation of swine with monophasic and biphasic waveforms. Circulation. 1995;92:1634–1643. doi: 10.1161/01.cir.92.6.1634. [DOI] [PubMed] [Google Scholar]
- 3.Walcott GP, Melnick SB, Chapman FW, Jones JL, Smith WM, Ideker RE. Relative efficacy of monophasic and biphasic waveforms for transthoracic defibrillation after short and long durations of ventricular fibrillation. Circulation. 1998;98:2210–2215. doi: 10.1161/01.cir.98.20.2210. [DOI] [PubMed] [Google Scholar]
- 4.Niemann JT, Burian D, Garner D, Lewis RJ. Monophasic versus biphasic transthoracic countershock after prolonged ventricular fibrillation in a swine model. J Am Coll Cardiol. 2000;36:932–938. doi: 10.1016/s0735-1097(00)00781-6. [DOI] [PubMed] [Google Scholar]
- 5.Schneider T, Martens PR, Paschen H, Kuisma M, Wolcke B, Gliner BE, Russell JK, Weaver WD, Bossaert L, Chamberlain D. Multicenter, randomized, controlled trial of 150-J biphasic shocks compared with 200- to 360-J monophasic shocks in the resuscitation of out-of-hospital cardiac arrest victims. Optimized Response to Cardiac Arrest (ORCA) Investigators. Circulation. 2000;102:1780–1787. doi: 10.1161/01.cir.102.15.1780. [DOI] [PubMed] [Google Scholar]
- 6.Tang W, Weil MH, Sun S, Povoas HP, Klouche K, Kamohara T, Bisera J. A comparison of biphasic and monophasic waveform defibrillation after prolonged ventricular fibrillation. Chest. 2001;120:948–954. doi: 10.1378/chest.120.3.948. [DOI] [PubMed] [Google Scholar]
- 7.Scheatzle MD, Menegazzi JJ, Allen TL, Durham SB. Evaluation of biphasic transthoracic defibrillation in an animal model of prolonged ventricular fibrillation. Acad Emerg Med. 1999;6:880–886. doi: 10.1111/j.1553-2712.1999.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 8.Lund M, French JK, Johnson RN, Williams BF, White HD. Serum troponins T and I after elective cardioversion. Eur Heart J. 2000;21:245–253. doi: 10.1053/euhj.1999.1745. [DOI] [PubMed] [Google Scholar]
- 9.Skulec R, Belohlavek J, Kovarnik T, Kolar J, Gandalovicova J, Dytrych V, Linhart A, Aschermann M. Serum cardiac markers response to biphasic and monophasic electrical cardioversion for supraventricular tachyarrhythmia—a randomised study. Resuscitation. 2006;70:423–431. doi: 10.1016/j.resuscitation.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Bonnefoy E, Chevalier P, Kirkorian G, Guidolet J, Marchand A, Touboul P. Cardiac troponin 1 does not increase after cardioversion. Chest. 1997;111(1):15–18. doi: 10.1378/chest.111.1.15. [DOI] [PubMed] [Google Scholar]
- 11.Kerber RE, Martins JB, Gascho JA, Marcus ML, Grayzel J. Effect of direct-current countershocks on regional myocardial contractility and perfusion. Experimental studies. Circulation. 1981;63:323–332. doi: 10.1161/01.cir.63.2.323. [DOI] [PubMed] [Google Scholar]
- 12.Pansegrau DG, Abboud FM. Hemodynamic effects of ventricular defibrillation. J Clin Invest. 1970;49:282–297. doi: 10.1172/JCI106238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi H, Weil M, Tang W, Kamohara T, Jin X, Bisera J. Myocardial dysfunction after electrical defibrillation. Resuscitation. 2002;54:289–296. doi: 10.1016/s0300-9572(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 14.Kerber RE, Kieso RA, Kienzle MG, Olshansky B, Waldo AL, Carlson MD, Wilber DJ, Aschoff AM, Birger S, Charbonnier F. Current-based transthoracic defibrillation. Am J Cardiol. 1996;78:1113–1118. doi: 10.1016/s0002-9149(96)90062-4. [DOI] [PubMed] [Google Scholar]
- 15.Moulton C, Dreyer C, Dodds D, Yates DW. Placement of electrodes for defibrillation-a review of the evidence. Eur J Emerg Med. 2000;7:135–143. [PubMed] [Google Scholar]
- 16.Qin H, Walcott GP, Killingsworth CR, Rollins DL, Smith WM, 1deker RE. 1mpact of myocardial ischemia and reperfusion on ventricular defibrillation patterns, energy requirements, and detection of recovery. Circulation. 2002;105:2537–2542. doi: 10.1161/01.cir.0000016702.86180.f6. [DOI] [PubMed] [Google Scholar]
- 17.Walcott GP, Killingsworth CR, Smith WM, Ideker RE. Biphasic waveform external defibrillation thresholds for spontaneous ventricular fibrillation secondary to acute ischemia. J Am Coll Cardiol. 2002;39:359–365. doi: 10.1016/s0735-1097(01)01723-5. [DOI] [PubMed] [Google Scholar]
- 18.Khalighi K, Daly B, Leino EV, Shorofsky SR, Kavesh NG, Peters RW, Gold MR. Clinical predictors of transvenous defibrillation energy requirements. Am J Cardiol. 1997;79:150–153. doi: 10.1016/s0002-9149(96)00702-3. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health. Guide for the Care and Use of Laboratory Animals. Bethesda, MD: N1H; 1985. revised. [Google Scholar]
- 20.Gliner BE, White RD. Electrocardiographic evaluation of defibrillation shocks delivered to out-of-hospital sudden cardiac arrest patients. Resuscitation. 1999;41:133–144. doi: 10.1016/s0300-9572(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 21.Leng CT, Paradis NA, Calkins H, Berger RD, Lardo AC, Rent KC, Halperin HR. Resuscitation after prolonged ventricular fibrillation with use of monophasic and biphasic waveform pulses for external defibrillation. Circulation. 2000;101:2968–2974. doi: 10.1161/01.cir.101.25.2968. [DOI] [PubMed] [Google Scholar]
- 22.Poole JE, White RD, Kanz KG, Hengstenberg F, Jarrard GT, Robinson JC, Santana V, McKenas DK, Rich N, Rosas S, Merritt S, Magnotto L, Gallagher JV, 3rd, Gliner BE, Jorgenson DB, Morgan CB, Dillon SM, Kronmal RA, Bardy GH. Low-energy impedance- compensating biphasic waveforms terminate ventricular fibrillation at high rates in victims of out-of-hospital cardiac arrest. LIFE Investigators. J Cardiovasc Electrophysiol. 1997;8:1373–1385. doi: 10.1111/j.1540-8167.1997.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 23.Niemann JT, Garner D, Lewis RJ. Left ventricular function after monophasic and biphasic waveform defibrillation: the impact of cardiopulmonary resuscitation time on contractile indices. Acad Emerg Med. 2003;10:9–15. doi: 10.1111/j.1553-2712.2003.tb01969.x. [DOI] [PubMed] [Google Scholar]
- 24.Niemann JT, Burian D, Garner D, Lewis RJ. Transthoracic monophasic and biphasic defibrillation in a swine model: a comparison of efficacy, ST segment changes, and postshock hemodynamics. Resuscitation. 2000;47:51–58. doi: 10.1016/s0300-9572(00)00197-0. [DOI] [PubMed] [Google Scholar]
- 25.Stiell 1G, Walker RG, Nesbitt LP, Chapman FW, Cousineau D, Christenson J, Bradford P, Sookram S, Berringer R, Lank P, Wells GA. B1PHAS1C trial: a randomized comparison of fixed lower versus escalating higher energy levels for defibrillation in out- of-hospital cardiac arrest. Circulation. 2007;115:1511–1517. doi: 10.1161/CIRCULATIONAHA.106.648204. [DOI] [PubMed] [Google Scholar]
- 26.Grubb NR, Fox KA, Cawood P. Resuscitation from out-of- hospital cardiac arrest: implications for cardiac enzyme estimation. Resuscitation. 1996;33:35–41. doi: 10.1016/s0300-9572(96)00971-9. [DOI] [PubMed] [Google Scholar]
- 27.Mullner M, Oschatz E, Sterz F, Pirich C, Exner M, Schorkhuber W, Laggner AN, Hirschl MM. The influence of chest compressions and external defibrillation on the release of creatine kinase- MB and cardiac troponin T in patients resuscitated from out-of- hospital cardiac arrest. Resuscitation. 1998;38:99–105. doi: 10.1016/s0300-9572(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 28.Allred JD, Killingsworth CR, Allison JS, Dosdall DJ, Melnick SB, Smith WM, Ideker RE, Walcott GP. Transmural recording of shock potential gradient fields, early postshock activations, and refibrillation episodes associated with external defibrillation of long-duration ventricular fibrillation in swine. Heart Rhythm. 2008;5:1599–1606. doi: 10.1016/j.hrthm.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang AS, Wolf PD, Afework Y, Smith WM, Ideker RE. Three- dimensional potential gradient fields generated by intracardiac catheter and cutaneous patch electrodes. Circulation. 1992;85:1857–1864. doi: 10.1161/01.cir.85.5.1857. [DOI] [PubMed] [Google Scholar]
- 30.Yabe S, Smith WM, Daubert JP, Wolf PD, Rollins DL, Ideker RE. Conduction disturbances caused by high current density electric fields. Circ Res. 1990;66:1190–1203. doi: 10.1161/01.res.66.5.1190. [DOI] [PubMed] [Google Scholar]
- 31.Geddes LA, Tacker WA, Rosborough JP, Moore AG, Cabler PS. Electrical dose for ventricular defibrillation of large and small animals using precordial electrodes. J Clin Invest. 1974;53:310–319. doi: 10.1172/JCI107552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Killingsworth CR, Melnick SB, Chapman FW, Walker RG, Smith WM, Ideker RE, Walcott GP. Defibrillation threshold and cardiac responses using an external biphasic defibrillator with pediatric and adult adhesive patches in pediatric-sized piglets. Resuscitation. 2002;55:177–185. doi: 10.1016/s0300-9572(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Clark CB, Davies LR, Karlsson G, Zimmerman MB, Kerber R. Body weight is a predictor of biphasic shock success for low energy transthoracic defibrillation. Resuscitation. 2002;54:281–287. doi: 10.1016/s0300-9572(02)00121-1. [DOI] [PubMed] [Google Scholar]


