Abstract
To test the hypothesis that intestine is a major site of action for D-4F, LDLR−/− mice were fed a Western diet (WD) and administered the peptide subcutaneously (SQ) or orally. Plasma and liver D-4F levels were 298-fold and 96-fold higher, respectively, after SQ administration, whereas peptide levels in small intestine only varied by 1.66 ± 0.33-fold. Levels of metabolites of arachidonic and linoleic acids known to bind with high affinity to D-4F were significantly reduced in intestine, liver and hepatic bile to a similar degree whether administered SQ or orally. However, levels of 20-HETE, which is known to bind the peptide with low affinity, were unchanged. D-4F treatment reduced plasma serum amyloid A (SAA) and triglyceride levels (P < 0.03) and increased HDL-cholesterol levels (P < 0.04) similarly after SQ or oral administration. Plasma levels of metabolites of arachidonic and linoleic acids significantly correlated with SAA levels (P < 0.0001). Feeding 15-HETE in chow (without WD) significantly increased plasma SAA and triglyceride levels and decreased HDL-cholesterol and paraoxonase activity (P < 0.05), all of which were significantly ameliorated by SQ D-4F (P < 0.05). We conclude that D-4F administration reduces levels of free metabolites of arachidonic and linoleic acids in the small intestine and this is associated with decreased inflammation in LDL receptor deficient mice.
Keywords: apolipoprotein A-I, apolipoprotein A-I mimetic peptides, lipoproteins, hydroxyeicosatetraenoic acid, hydroxyoctadecadienoic acid, small intestine
Apolipoprotein A-I (apoA-I) mimetic peptides have been studied in animals and humans for their ability to improve biomarkers of inflammation and in animal models of atherosclerosis for their ability to decrease lesions (1). The mechanism of action of these peptides has been thought to be due to their remarkable ability to bind oxidized lipids compared with apoA-I (2). The classes of oxidized lipids bound with high affinity by these mimetic peptides include oxidized phospholipids, oxidized metabolites of arachidonic and linoleic acids, and oxidized sterols (2). Oxidized metabolites of arachidonic and linoleic acids can be generated by enzymatic systems such as lipoxygenases or cyclooxygenases, or they can be generated by nonspecific oxidation. Except for 20-hydroxyeicosatetraenoic acid (HETE), all of the oxidized metabolites of arachidonic and linoleic acids that we studied bound to the peptide with much higher affinity than was the case for the binding to apoA-I (2, 3).
We recently reported that the dosage of the apoA-I mimetic peptide 4F administered to apoE−/− mice determined efficacy, but plasma and hepatic levels of peptide did not (1). Because efficacy was similar at the same dosages but plasma and hepatic levels were dramatically higher when the peptide was administered by subcutaneous injection (SQ) compared with oral administration, we suspected that there might be a compartment outside of the liver or plasma where peptide concentration would be similar. We found that the concentration of D-4F in the feces was the same regardless of whether the peptide was administered SQ or orally, suggesting that the intestine maybe a major site of action for the peptide regardless of the route of administration (1).
The concentration of free 15-HETE and 13-hydroxyoctadecadienoic acid (HODE) in the plasma of apoE−/− mice was significantly higher than that of wild-type mice (3). After administration of the 4F peptide, plasma levels of free oxidized fatty acids that bound with higher affinity to the mimetic peptide compared with apoA-I (e.g., 5-HETE, 15-HETE, 9-HODE, and 13-HODE) significantly decreased, but the levels of 20-HETE, which bound with equal low affinity to apoA-I and 4F, did not decrease (3). These studies focused on the plasma levels of free oxidized fatty acids, which are only a small fraction (<10%) of the total plasma oxidized fatty acids. Interestingly, only the free oxidized fatty acid plasma levels decreased after the administration of the apoA-I mimetic peptide; esterified oxidized fatty acid levels were unchanged (3).
In other studies, apoE−/− mice were made diabetic, resulting in a significant increase in the hepatic content of free arachidonic acid and free 12-HETE, 15-HETE, 13-HODE, PGD2, and PGE2. This was associated with a significant increase in aortic atherosclerosis. Oral administration of D-4F significantly decreased the hepatic content of free arachidonic acid and free oxidized fatty acids derived from arachidonic and linoleic acids and significantly decreased aortic atherosclerosis without affecting other plasma lipid or lipoprotein levels (4).
Subsequently, we reported that HDL from type 2 diabetics contained significantly more free 5-HETE, 12-HETE, 15-HETE, 9-HODE, and 13-HODE than HDL from healthy volunteers. The type 2 diabetic HDL was also proinflammatory in a cell-based assay and was abnormal in a cell-free assay. The HDL content of free 5-HETE, 12-HETE, 15-HETE, 9-HODE, and 13-HODE significantly correlated with the values obtained in the cell-free assay (5).
The experiments reported here were designed to extend our previous studies on the interaction of the D-4F peptide with the intestine (1). These studies confirm our previous studies (1–5) and demonstrate for the first time that D-4F-mediated reductions in free oxidized metabolites of arachidonic and linoleic acids in the small intestine are associated with reduced inflammation in LDLR−/− mice.
MATERIALS AND METHODS
Materials
The peptide 4F (Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2) was synthesized from all D-amino acids (D-4F) by solid-phase synthesis as described (6). Protease inhibitors were purchased from Roche Diagnostics Gmbh (Complete, Mini, EDTA-free tablet) (Catalogue No. 11 836 179 001). In the experiments in which a HETE or HODE were added to mouse chow without the Western diet (WD), 12(S)-HETE (Catalog #34570), 15(S)-HETE (Catalog #34720), and 13(S)-HODE (Catalog #38610) were purchased from Cayman Chemical Co. (Ann Arbor, MI). All other materials were purchased from sources previously described (2, 3, 7).
Mice
Female LDLR−/− mice originally purchased from Jackson laboratories on a C57BL/6J background were obtained from the breeding colony of the Department of Laboratory and Animal Medicine at the David Geffen School of Medicine at UCLA. The mice used in these studies were of different ages, which are stated in each Table legend. The mice were maintained on a chow diet (Ralston Purina) before being switched to a WD (Teklad, Harlan, catalog # TD88137). The WD was kept frozen until it was administered to the mice each night. Peptide was administered to the mice by daily SQ) on the back or was administered orally by providing the peptide in the drinking water. For oral administration, the peptide was freshly prepared by dissolving in water, and the drinking water was changed three times per week. For SQ administration, the peptide was uniformly dissolved in normal saline (pH 7.4) using a glass-glass homogenizer, and each mouse received a daily injection of 1 ml containing the peptide in normal saline (pH 7.4). In all experiments, the daily dose of peptide administered by either route of administration was 900 μg/mouse/day (45 mg/kg/day).
Addition of oxidized fatty acids to mouse chow
The following protocol was used to conveniently and accurately provide free oxidized fatty acids in the chow diet. Three hundred milliliters of water were added to 400 g of chow pellets. The pellets were allowed to soften for 30 min, and a highly uniform mixture was generated using a small industrial-type mixer. The desired quantity of the free oxidized fatty acid was first mixed in saline and was then carefully and gradually added and mixed into the chow using the mixer. Mixing was continued at high speed for 1 min, at which time the mixer was stopped and the material was mixed in the opposite direction using a spatula to ensure a highly uniform distribution of the added free oxidized fatty acid. This process was continued for a total of 5–10 cycles. The resulting mixture containing the oxidized fatty acid was spread and flattened uniformly on a sheet of aluminum foil in a tray to achieve a height of ∼2 mm. The mixture was then cut into blocks of ∼5 g each using a rotary knife. These blocks were stored at −20°C. Each evening, four blocks of the frozen diet containing the oxidized fatty acids were provided to each cage containing four mice. The mice ate all of the diet by morning. The frozen diet contained 1 μg free oxidized fatty acid per gram frozen diet. Thus, on average each mouse received 5 μg of the free oxidized fatty acid each day.
All experiments were performed using protocols approved by the Animal Research Committee at UCLA.
Determination of free arachidonic acid and free metabolites of arachidonic and linoleic acids in plasma, liver, hepatic bile, and small intestine and in the contents of the small intestine
The levels of free arachidonic acid and free metabolites of arachidonic and linoleic acids were determined by LC-ESI-MS/MS as previously described (3). In each instance, a deuterium-labeled internal standard was included to correct for extraction efficiency and to facilitate quantification (3).
Preparation and analysis of plasma.
After an overnight fast, mouse plasma was immediately prepared from heparinized blood obtained from the retroorbital sinus under mild isoflurane anesthesia. For mice receiving subcutaneous injections, the blood was removed 1.5–3 h after the last injection. Plasma was immediately frozen and stored at −80°C until analysis. Plasma serum amyloid A (SAA) levels were determined by ELISA (, catalog # KMA0011; Invitrogen) according to the manufacturer's instructions. Lipid and lipoprotein and protein determinations were determined by methods described previously (1, 6).
Removal of blood before tissue removal.
Under anesthesia, a nick was made in the inferior vena cava, and ice-cold physiological saline with 2 mM EDTA and 20 μM butylhydroxytoluene (BHT) was infused for 10 min at approximately 0.5 ml/min through a needle placed in the left ventricle. The organs appeared pale within a few minutes.
Preparation and analysis of enterocytes from the small intestine.
After removal of blood as described above, the small intestine (i.e., the portion of intestine between the stomach and the cecum) was removed and kept on ice. Enterocytes were isolated using a minor modification of the method of Merchant and Heller (8). The lumen of the small intestine was thoroughly washed with ice-cold PBS containing 0.1 mM PMSF, 1 mM benzamidine, 2 mM EDTA, and 20 μM BHT. The small intestine was carefully cannulated with a steel rod, the distal end was tied, and the rod was carefully removed, inverting the small intestine. The ends of the inverted small intestine were tied. The tied inverted small intestine was incubated in a 15-ml centrifuge tube in 10 ml of buffer A (1.5 mM KC1, 96 mM NaCl, 8 mM KH2PO4, 27 mM sodium citrate, 5.6 mM Na2HPO4 [pH 7.3], 0.1 mM PMSF, and 1 mM benzamidine) for 15 min at 37°C on an eppendorf MixMate™ set at 300 rpm. Buffer A was replaced with buffer B (2.7 mM KC1, 137 mM NaCl, 1.5 mM EDTA, 1.5 mM KH2PO4, 8.1 mM Na2HPO4 (pH 7.4), 0.5 mM dithiothreitol, 0.1 mM PMSF, and 1 mM benzamidine) and incubated for 30 min at 37°C on the MixMate™ at 300 rpm. The small intestine was removed from the centrifuge tube, the tube was centrifuged at 1500 rpm for 15 min at 4°C, the supernatant above the enterocytes was removed, and the pelleted enterocytes were snap frozen. After all of the mice were processed, the enterocyte pellets were disrupted on ice in the presence of 2 mM EDTA, 20 μM BHT, and protease inhibitors using a tissue grinder and centrifuged at 4°C. The supernatant was collected and extracted using solid-phase extraction (Waters Oasis HLB cartridge and methanol). The cartridge eluent was evaporated under argon, resuspended in methanol, and centrifuged, and a portion of the supernatant was analyzed. The remaining supernatant was saved for determination of cell protein.
Collection and analysis of intestinal contents
The small intestine was collected as described above. The contents within the lumen of the small intestine (133 ± 62 mg per mouse) were collected on ice. Pyrogen-free water containing 20 μM BHT and protease inhibitors were added, and the contents were ground on ice and centrifuged at 4°C. The supernatant was extracted and analyzed.
Collection and analysis of hepatic metabolites of arachidonic and linoleic acids
The livers of the mice were removed, snap frozen, and kept at −80°C. The frozen livers were removed from the freezer and kept on ice while samples were collected from each lobe, minced, homogenized, extracted, and analyzed.
Collection and analysis of metabolites of arachidonic and linoleic acids in hepatic bile
Mice were fasted overnight with free access to water and were anesthetized with a mixture of ketamine (100–150 mg/kg) and xylazine (5–10 mg/kg) using a total maximum volume of 10 ml/kg administered by intraperitoneal injection. Laparotomy was performed through an upper midline incision. After the liver and gallbladder were exposed, the lower end of the cystic duct was ligated, and the common bile duct was cannulated below the entrance of the cystic duct using PE-01 tubing (internal diameter, 0.12 mm; outer diameter, 0.25 mm). After successful cannulation using a dissecting microscope, hepatic bile was collected by gravity for 30–60 min into a vessel maintained in ice. Animal body temperature was maintained at 37°C by placing the mouse 18 inches below a heat lamp. The temperature observed at this distance was 37°C. The bile was rapidly frozen and stored at −80°C until analysis.
Collection and analysis of plasma and organs for determination of D-4F levels
Plasma was collected as described above. The tissues were rendered free of blood by perfusion with ice-cold saline for 10 min as described above. The liver was collected and processed as described above. The small intestine was removed, the contents within the lumen of the small intestine were collected, the lumen was washed with ice-cold saline, adventitial fat was rapidly removed, and the length of the small intestine was determined. The duodenum (the proximal 34% of the length of the small intestine between the stomach and the ligament of Treitz), the jejunum (the middle 36% of the length of the small intestine), and the ileum (the most distal 30% of the length of the small intestine) were separated, snap frozen, and kept at −80°C. The plasma, intestinal contents, and tissues were extracted and analyzed for D-4F content by LC-ESI/MS/MS using an internal standard of 15N13C-labeled 4F peptide as previously described (1, 9–11).
Statistical analyses
Statistical analyses were performed by ANOVA or unpaired two-tail t test or by linear regression using GraphPad Prism version 5.03 (GraphPad Software, San Diego, CA).
RESULTS
D-4F levels in plasma and liver were orders of magnitude higher after SQ administration compared with oral administration, whereas D-4F levels in the small intestine were similar after SQ or oral administration
SQ administration of D-4F resulted in ∼300-fold and ∼100-fold higher levels of peptide in the plasma and liver, respectively, compared with the levels found after oral administration (Table 1). In contrast, peptide levels in the contents of the small intestine or in the tissue of the small intestine were similar whether the peptide was administered orally or SQ. The average fold difference in peptide levels in the small intestine and in the contents of the small intestine was only 1.66 ± 0.33-fold (Table 1).
TABLE 1.
D-4F levels in plasma and liver.
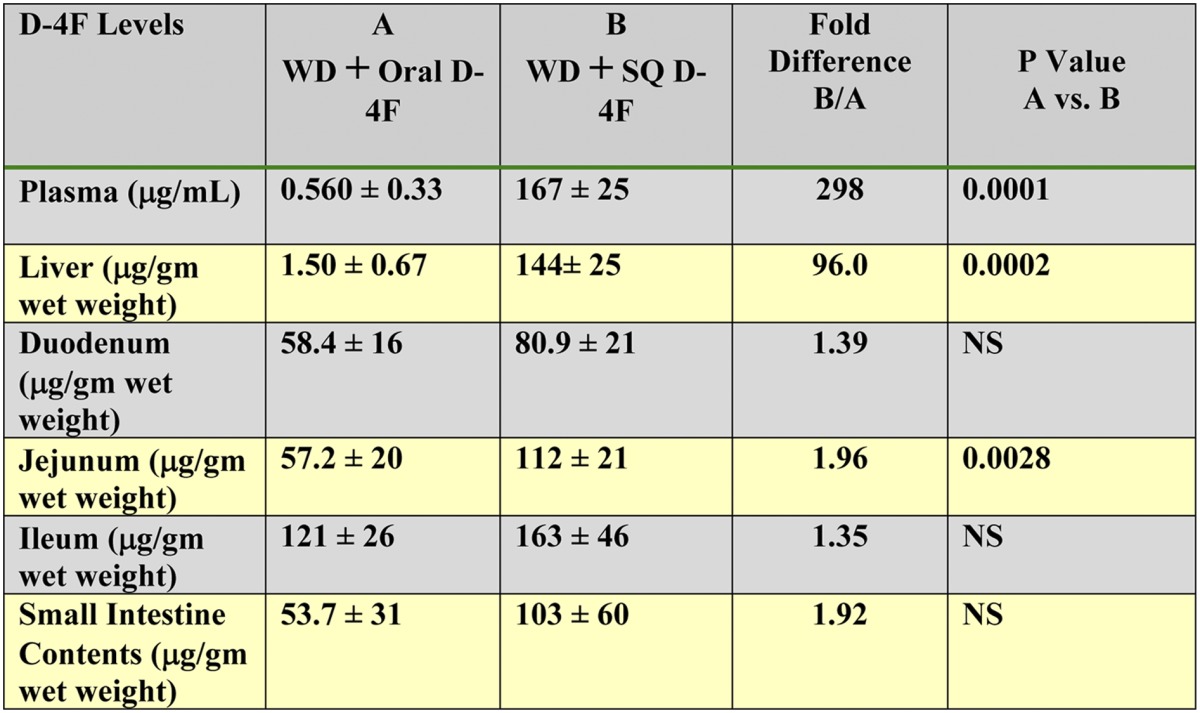
D-4F levels in plasma and liver were orders of magnitude higher after SQ administration compared with oral administration, while D-4F levels in the small intestine were similar after SQ and oral administration. Female LDLR−/− mice (11–12 months of age) were placed on a WD and treated with D-4F. Mice (n = 5 per group) received drinking water with D-4F sufficient to give a daily dose of 900 μg/mouse/day or daily SQ injections of the same dosage on the back. After 2 weeks, the mice were fasted overnight and allowed access to water that did not contain D-4F (SQ group) or did contain D-4F (oral group). In the morning, 90 min after the SQ group received their injection, the mice were anesthetized, plasma was obtained, tissues were rendered free of blood by perfusion, and liver and small intestine were collected, processed, and analyzed by LC-ESI-MS/MS for D-4F as described in Materials and Methods. The data shown are mean ± SD. NS = not significant.
D-4F treatment reduces metabolites of arachidonic and linoleic acids in the contents of the small intestine
Oral administration of D-4F reduced free 5-HETE, 15-HETE, 9-HODE, and 13-HODE in the contents of the small intestine of LDLR−/− mice fed a WD for 1 week (Table 2). The oral and SQ experiments were performed sequentially due to the number of mice processed and the amount of work required for flushing the blood from the mice before removing the small intestine and the time required for collecting the contents of the small intestine from each mouse. Administration of D-4F SQ also reduced the content of free 5-HETE, 15-HETE, 9-HODE, and 13-HODE in the contents of the small intestine of LDLR−/− mice fed a WD (Table 3) . Although the results of the experiment described in Tables 2 and 3 were qualitatively similar, the values obtained in the second experiment (SQ) were much higher than in the first experiment (oral), suggesting variability between different mice studied at different times or variability in the processing of the contents of the small intestine. Supplementary Table I indicates that the free HETEs and HODEs measured in the contents of the small intestine could not be accounted for by undigested WD.
TABLE 2.
Oral D-4F reduced free 5-HETE, 15-HETE, 9-HODE, and 13-HODE in the contents of the small intestine
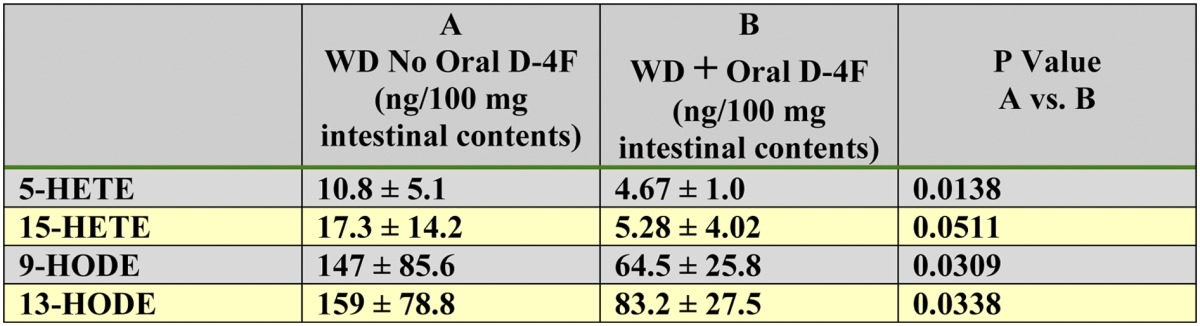
Female LDLR−/− mice 6–7 months of age were placed on a WD and treated or not treated with D-4F. Mice (n = 8 per group) received drinking water without or with D-4F sufficient to give a daily dose of 900 μg/mouse/day. After 1 week the mice were anesthetized, and the contents of the small intestine were collected and analyzed by LC-ESI-MS/MS for free 5-HETE, 15-HETE, 9-HODE, and 13-HODE as described in Materials and Methods. Data are mean ± SD.
TABLE 3.
SQ D-4F reduced free 5-HETE, 15-HETE, 9-HODE, and 13-HODE in the contents of the small intestine
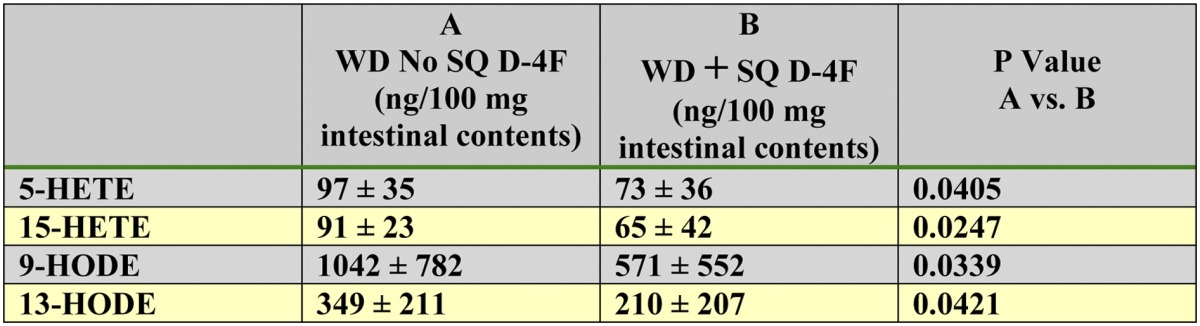
Female LDLR−/− mice 6–7 months of age were placed on a WD and treated or not treated with D-4F. Mice (n = 20 per group) received drinking water without D-4F and received SQ saline without or with D-4F at a daily dose of 900 μg/mouse/day. After 1 week, 2–3 h after the last SQ injection, the mice were anesthetized, and the contents of the small intestine were collected and analyzed by LC-ESI-MS/MS for free 5-HETE, 15-HETE, 9-HODE, and 13-HODE as described in Materials and Methods. The data shown are mean ± SD.
D-4F treatment reduces the levels of free arachidonic acid and free metabolites of arachidonic and linoleic acids in enterocytes of the small intestine and in the liver
In other experiments, instead of collecting the contents of the small intestine, enterocytes and livers were collected from each mouse and analyzed. The oral and SQ experiments were performed sequentially due to the number of mice that needed to be processed, the work required for flushing the blood from each mouse before removing the liver and small intestine, and the time required for harvesting the enterocytes. Oral administration of D-4F significantly reduced enterocyte and hepatic levels of free arachidonic acid, 5-HETE, 12-HETE, 15-HETE, 9-HODE, 13-HODE, PGD2, PGE2, and thromboxane (TX)B2 (Table 4). The level of arachidonic acid was not significantly different in enterocytes compared with liver in the mice that did not receive oral D-4F. In the mice that did receive oral D-4F, there was a significant decrease in free arachidonic acid levels in enterocytes and liver. The values for free arachidonic acid after D-4F administration were significantly less in the enterocytes compared with liver (P = 0.0005). However, the levels of free metabolites of arachidonic and linoleic acids in enterocytes were much higher than in the liver regardless of whether the mice received oral D-4F or not (P < 0.0001 for enterocyte values versus hepatic values in Table 4). The exception was 20-HETE and levels of 20-HETE were not reduced by oral D-4F treatment consistent with our previous observations in the plasma of apoE−/− mice (3).
TABLE 4.
Oral administration of D-4F reduced the levels of free arachidonic acid and, except for 20-HETE, reduced the levels of free metabolites of arachidonic and linoleic acids in enterocytes of the small intestine and in the liver
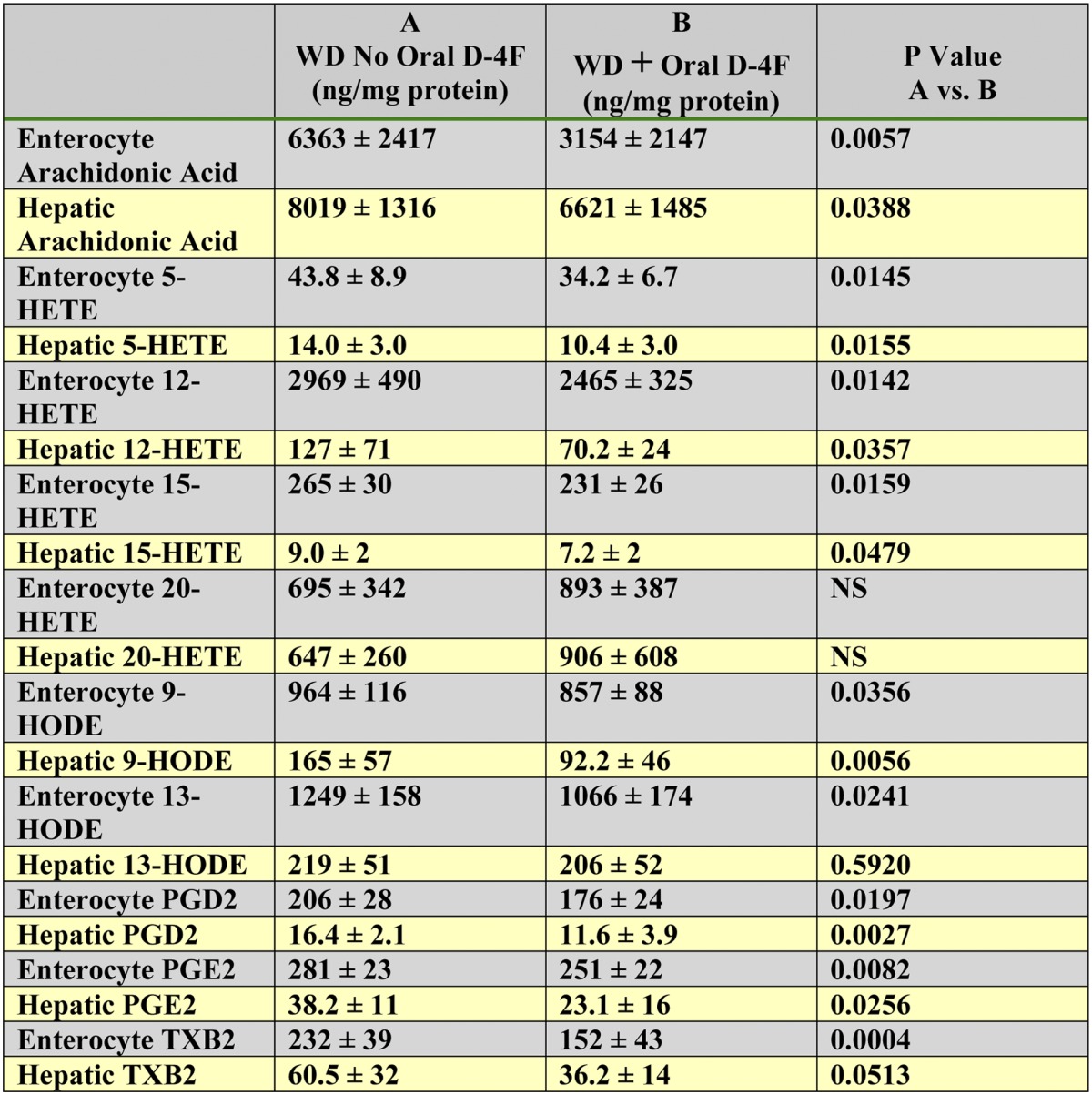
Female LDLR−/− mice (6–7 months of age) were placed on a WD and treated or not treated with D-4F. Mice (n = 10 per group) received drinking water without or with D-4F sufficient to give a daily dose of 900 μg/mouse/day. After 2 weeks, the mice were anesthetized, and enterocytes from the small intestine and livers were harvested and analyzed by LC-ESI-MS/MS for free arachidonic acid, 5-HETE, 12-HETE, 15-HETE, 20-HETE, 9-HODE, 13-HODE, PGD2, PGE2, and TXB2 as described in Materials and Methods. The data shown are mean ± SD. NS = not significant.
In contrast to the large difference in values obtained for the contents of the small intestine in mice not receiving D-4F in Tables 2 and 3, the values for mice not receiving D-4F in Tables 4 and 5 were much more similar. The level of free arachidonic acid was also not significantly different in enterocytes compared with liver in the mice that did not receive SQ D-4F (Table 5). In the mice that received SQ D-4F, there was a significant decrease in free arachidonic acid levels in enterocytes and liver. The values after D-4F administration for free arachidonic acid were significantly less in the enterocytes compared with the liver (P = 0.0101), as was the case for the mice receiving oral D-4F (Table 4). As was also the case in the experiment described in Table 4, the levels of the free metabolites of arachidonic and linoleic acids in enterocytes were much higher than in the liver regardless of whether the mice received SQ D-4F or not (P < 0.0001 for enterocyte values versus hepatic values in Table 5). The exception was again 20-HETE and levels of 20-HETE were not reduced by SQ D-4F treatment, as was the case for oral D-4F treatment.
TABLE 5.
SQ administration of D-4F reduced the levels free arachidonic acid and, except for 20-HETE, reduced the levels of free metabolites of arachidonic and linoleic acids in enterocytes of the small intestine and in the liver
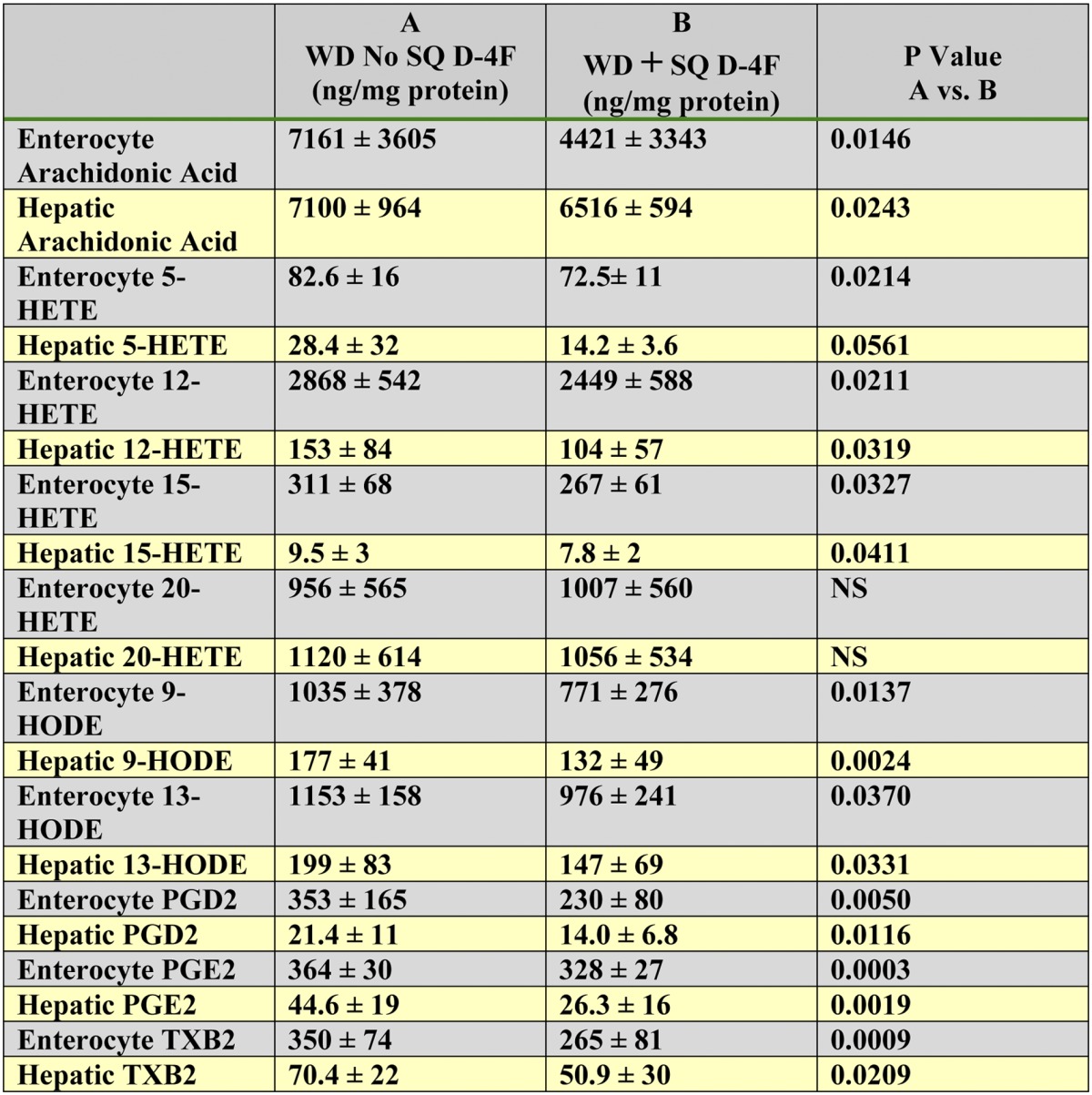
Female LDLR−/− mice 6–7 months of age were placed on a WD and treated or not treated with D-4F. Mice (n = 21 per group) received drinking water without D-4F and received SQ saline without or with D-4F at a daily dose of 900 μg/mouse/day. After 2 weeks, 2–3 h after the last SQ injection, the mice were anesthetized and enterocytes from the small intestine and livers were harvested and analyzed by LC-ESI-MS/MS for free arachidonic acid, 5-HETE, 12-HETE, 15-HETE, 20-HETE, 9-HODE, 13-HODE, PGD2, PGE2, and TXB2 as described in Materials and Methods. The data shown are mean ± SD. NS = not significant.
The Mean ± SD for the percent reduction in levels of free metabolites of arachidonic and linoleic acids (excluding 20-HETE) in the enterocytes and livers of the mice treated with D-4F orally in Table 4 were 17 ± 8% and 31 ± 14%, respectively, for enterocytes and liver. The Mean ± SD for the percent reduction for the mice treated with D-4F SQ in Table 5 were 19 ± 9% and 32 ± 10% for enterocytes and liver, respectively. The difference in the percent reduction between oral and SQ treatments for enterocyte values was not statistically significant. The difference in the percent reduction between oral and SQ treatments for hepatic values was also not statistically significant. However, the greater percent reduction in hepatic values compared with enterocyte values was significant (P = 0.0278 for oral enterocyte values versus oral hepatic values; P = 0.0149 for SQ enterocyte values versus SQ hepatic values).
D-4F treatment reduces free metabolites of arachidonic and linoleic acids in hepatic bile
D-4F treatment reduced the levels of free metabolites of arachidonic and linoleic acids in hepatic bile similarly whether administered orally or SQ (Table 6) .
TABLE 6.
Administration of D-4F reduced free 5-HETE, 12-HETE, 15-HETE, 9-HODE, 13-HODE, and PGE2 in hepatic bile
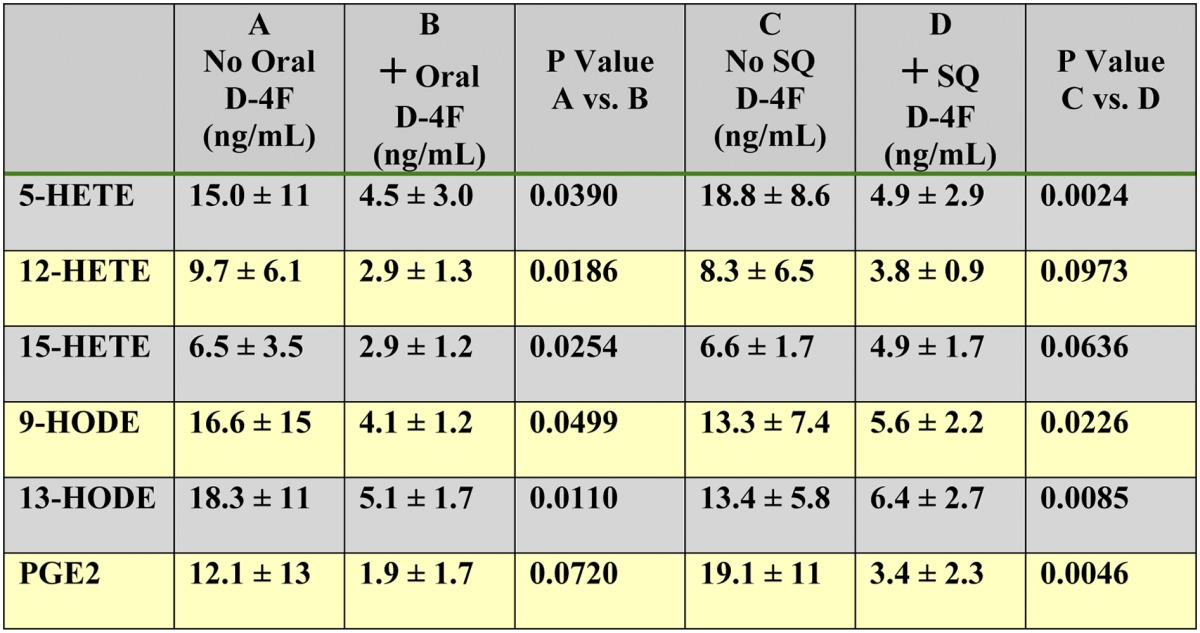
Female LDLR−/− mice (8–10 months) of age were placed on a WD and treated or not treated with D-4F. Mice (n = 8 per group) received drinking water without or with D-4F sufficient to give a daily dose of 900 μg/mouse/day or received drinking water without D-4F, and the mice received SQ injections of saline without or with D-4F at a dose of 900 μg/mouse/day (n = 8 per group). After 2 weeks, 2–3 h after the last SQ injection, the mice were anesthetized, and hepatic bile was obtained and analyzed by LC-ESI-MS/MS as described in Materials and Methods. The data shown are mean ± SD.
D-4F treatment reduces plasma levels of free metabolites of arachidonic and linoleic acids, SAA, and triglycerides and increases HDL-cholesterol without changing total plasma cholesterol
In another experiment, administration of D-4F orally or SQ significantly reduced plasma levels of free 5-HETE, 12-HETE, 15-HETE, 9-HODE, and 13-HODE (Table 7). Plasma SAA levels in these same mice were significantly decreased by D-4F treatment and were not different in mice administered D-4F orally or SQ (Table 7). Supplementary Figs. IA–IE demonstrate that the plasma levels of these free metabolites of arachidonic and linoelic acids significantly correlated with plasma SAA levels.
TABLE 7.
Administration of D-4F reduced plasma levels of free HETEs, HODEs, SAA, and triglycerides and increased HDL-cholesterol levels
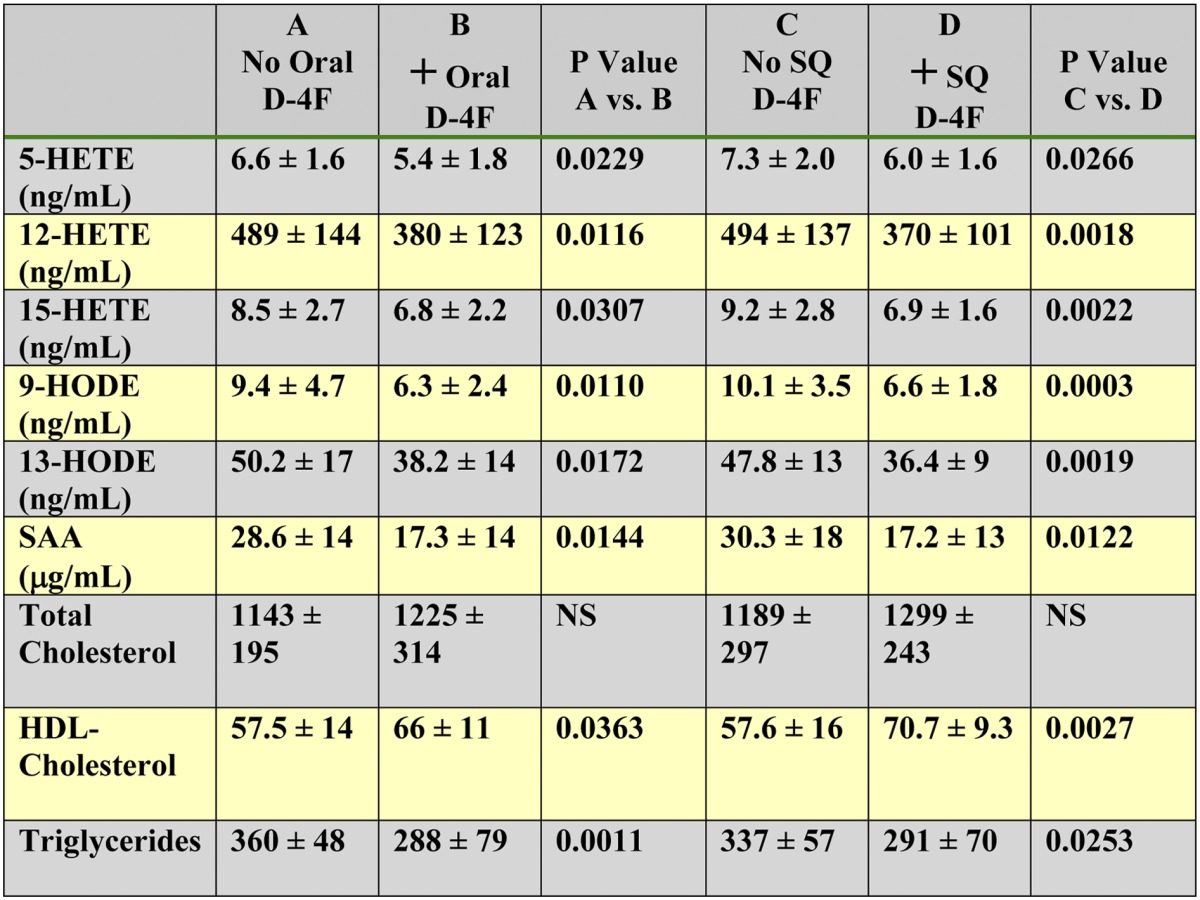
Female LDLR−/− mice (6–7 months of age) were placed on a WD and treated or not treated with D-4F. Mice (n = 21 per group) received drinking water without or with D-4F sufficient to give a daily dose of 900 μg/mouse/day or received drinking water without D-4F, and the mice received SQ injections of saline without or with D-4F at a dose of 900 μg/mouse/day (n = 21 per group). After 2 weeks, 2–3 h after the last SQ injection, the mice were anesthetized, and plasma was obtained and analyzed by LC-ESI-MS/MS for free 5-HETE, 12-HETE, 15-HETE, 9-HODE, and 13-HODE as described in Materials and Methods. SAA levels were determined by ELISA; total cholesterol, HDL-cholesterol, and triglyceride levels were determined as described in Materials and Methods. The data shown are mean ± SD.
D-4F treatment did not alter plasma total cholesterol levels (Table 7). HDL-cholesterol and triglyceride levels were not significantly different in the two groups of mice that did not receive D-4F. However, treatment with oral or SQ D-4F significantly raised HDL-cholesterol levels and lowered triglyceride levels. The HDL-cholesterol values and triglyceride values after oral or SQ D-4F treatment were not significantly different (Table 7).
WD increases plasma levels of free arachidonic acid and the plasma levels of free metabolites of arachidonic and linoleic acids; the increase is ameliorated by D-4F treatment
The experiments described above were performed in mice on the WD. To determine the effect of the WD on the plasma levels of free arachidonic acid and the plasma levels of free metabolites of arachidonic and linoleic acids, mice were fed the chow diet or the WD as described in Supplementary Table II. Feeding the WD increased the levels of free arachidonic acid and increased the levels of the free metabolites of arachidonic and linoleic acids (supplementary Table II). The increases induced by the WD were highly significant except for PGE2 (P = 0.0735) and TXB2 (supplementary Table II). D-4F reduced these plasma levels on a chow diet and on the WD (Table 8). The only free metabolite of arachidonic or linoleic acids that was not increased by the WD and that was not decreased by D-4F treatment on either the chow or WD was 20-HETE (Table 8); this finding is consistent with the findings shown in enterocytes and liver in Tables 4 and 5 and as reported to be the case in apoE−/− mice (3).
TABLE 8.
Effect of D-4F on plasma levels of free arachidonic acid and plasma levels of free metabolites of arachidonic and linoleic acids on the chow diet compared with the WD
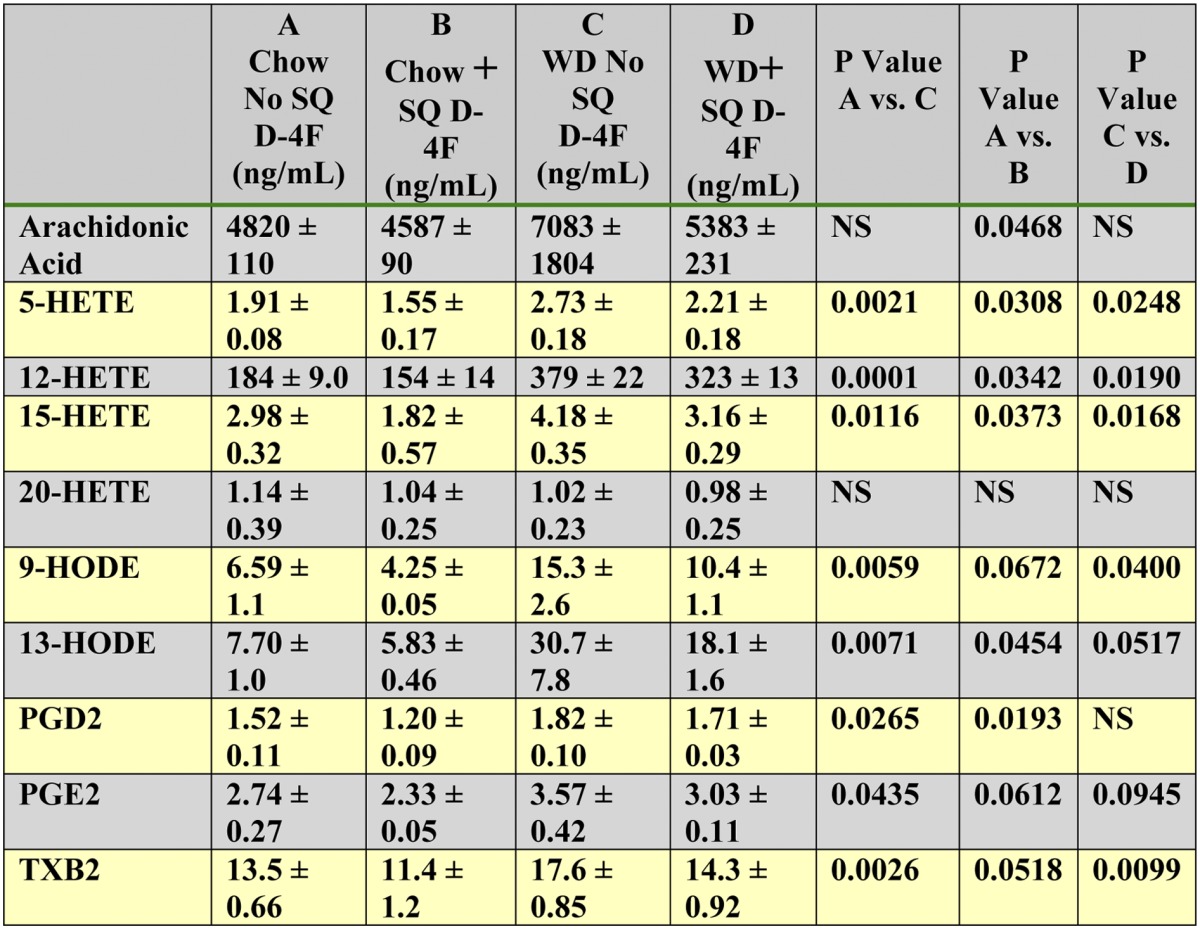
Female LDLR−/− mice (11–12 months of age; n = 3 per group) were maintained on a chow diet or placed on WD for 2 weeks. The mice received daily SQ injections of saline on the back or received saline containing D-4F at 900 μg/mouse/day. Plasma was obtained from fasting mice 2–3 h after the last SQ injection as described in Materials and Methods, and free arachidonic acid and free metabolites of arachidonic and linoleic acids were determined by LC-ESI-MS/MS as described in Methods. The data shown are mean ± SD. NS = not significant.
Addition of metabolites of arachidonic or linoleic acids to mouse chow without the WD significantly lowered plasma HDL-cholesterol levels and paraoxonase activity and increased plasma levels of triglycerides and SAA, all of which were ameliorated by treatment with D-4F
The data in Supplementary Figs. IA–IE are consistent with the hypothesis that free metabolites of arachidonic or linoleic acids can induce systemic inflammation. To test this hypothesis, we incorporated the free oxidized fatty acids shown in supplementary Table III into standard mouse chow and fed it (without WD) to LDLR−/− mice for 2 weeks. This resulted in significantly reduced levels of plasma HDL-cholesterol and paraoxonase (PON) activity and increased plasma levels of triglycerides and SAA (supplementary Table III).
In another experiment, 15-HETE was incorporated into mouse chow and fed to LDLR−/− mice for 2 weeks (without WD). The mice received daily SQ saline or daily SQ saline containing D-4F. Incorporating 15-HETE into the mouse chow again significantly reduced levels of plasma HDL-cholesterol and PON activity and increased plasma levels of triglycerides and SAA (Table 9). Administering SQ D-4F to the mice ameliorated these 15-HETE-mediated changes (Table 9).
TABLE 9.
Feeding LDLRminus/minus mice 15-HETE administered in mouse chow (without the WD) decreased plasma HDL-cholesterol levels, increased plasma triglyceride levels, decreased PON activity, and increased plasma SAA levels, all of which was ameliorated by D-4F treatment
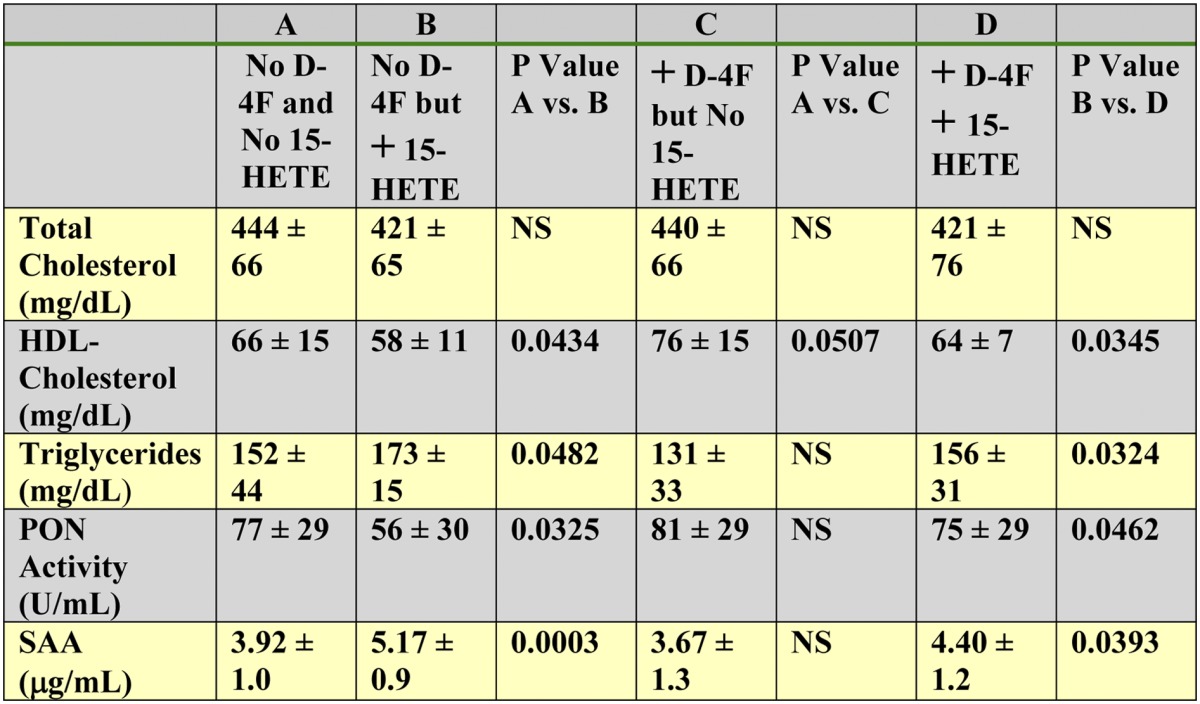
Female LDLR−/− mice (10–11 months of age; n = 20 per group) were fed laboratory rodent chow (Ralston Purina) prepared and presented to the mice as described in Materials and Methods. The chow did or did not contain 15-HETE at a concentration of 1 μg per gram diet to provide each mouse on average with 5 μg of 15-HETE per day. The mice received daily SQ injections of saline or SQ saline containing D-4F at a dosage of 900 μg/mouse/day. After 2 weeks on the diet, 2–3 h after the last SQ dose, the mice were bled, and their plasma was analyzed for lipids, PON activity, and SAA as described in Materials and Methods. The data shown are mean ± SD. NS = not significant.
DISCUSSION
The reduction by D-4F in the levels of free arachidonic acid and free oxidized fatty acids derived from arachidonic and linoleic acids in the small intestine, liver, hepatic bile, and plasma of LDLR−/− mice fed a WD is consistent with previous work from our laboratory showing that the 4F peptide reduced the levels of free oxidized fatty acids in the plasma of apoE−/− mice on a chow diet (3) and reduced hepatic levels of free arachidonic acid and free oxidized fatty acids derived from arachidonic and linoleic acids in diabetic apoE−/− mice on a chow diet (4). Consistent with our previous report (3), levels of 20-HETE, which binds with equal low affinity to apoA-I and D-4F, did not decrease after D-4F treatment (Tables 4, 5, 8).
In apoE−/− mice on either a chow diet or on a WD, the abilities of the peptide to reduce plasma SAA levels, improve anti-inflammatory properties of HDL, reduce aortic atherosclerosis, and reduce plasma levels of lysophosphatidic acid were similar at each dosage tested whether the peptide was administered orally or SQ despite 1,000-fold-higher plasma and hepatic levels when the peptide was administered SQ (1). The data reported here confirm in LDLR−/− mice that plasma and hepatic peptide levels are dramatically higher after SQ administration compared with oral administration (Table 1), but efficacy is similar when the same dosage is administered regardless of the route of administration.
Efficacy of D-4F treatment in the studies reported here was measured by changes in plasma lipid levels, plasma PON activity, and changes in the levels of free arachidonic acid and free metabolites of arachidonic and linoleic acids in plasma, liver, hepatic bile, enterocytes of the small intestine, and in the contents of the small intestine. Assuming that efficacy is related to the ability of the peptide to bind lipids, as is suggested by the failure of the peptide to change 20-HETE levels, these data strongly suggest that the small intestine is the compartment that controls efficacy because this was the only compartment with similar peptide levels. Additionally, except for 20-HETE, the levels of free metabolites of arachidonic and linoleic acids were much higher in enterocytes than in liver, but the percent reduction in the levels of these metabolites was significantly greater in the liver even after oral administration, which resulted in very low hepatic peptide levels. These data are therefore consistent with the hypothesis that D-4F acts to reduce inflammation in LDLR−/− mice by reducing small intestine levels of free arachidonic acid and free metabolites of arachidonic and linoleic acids. However, the experiments reported here are not definitive; they are consistent with the hypothesis but do not prove the hypothesis. This novel hypothesis will be rigorously tested in future studies.
It is not known why 20-HETE binds with low affinity to D-4F and whether this characteristic of 20-HETE separates its actions from other free oxidized fatty acids. Future studies comparing structure with function and the affinity for binding to D-4F of various free oxidized fatty acids may yield important new insights.
The mechanism of action of the peptide may be related to its ability to bind lipids, but the mechanism may not be related to the ability of the peptide to bind the lipids measured here. Levels of free arachidonic acid were reduced by peptide treatment in this study, as in our previous study (4). The reduction of free arachidonic acid levels could account for a reduction in levels of free metabolites derived from arachidonic acid. The reduction in free arachidonic acid levels could have occurred because of a peptide-mediated reduction in phospholipase activities, which were not measured in the studies reported here. We did not measure total arachidonic acid levels, but it is possible that these were altered by peptide treatment, although that was not the case in plasma of apoE−/− mice (3). We also did not measure free linoleic acid in these studies because of technical challenges; however, similar considerations could apply to linoleic acid. There are many possibilities that need to be investigated in future studies. For example, it is not possible to determine from these studies whether the reduction in free metabolites of arachidonic and linoleic acids occurred because of a reduction in substrate (e.g., free arachidonic acid), a decrease in lipoxygenase and cyclooxygenase activities, or a reduction in the activities of other enzyme pathways or whether the decrease resulted from the binding and removal of the final stable products of these pathways. It has been reported that the latter can interact to enhance the former (12, 13), making the distinction difficult to determine. These many possibilities need to be addressed by future studies.
Incorporating free oxidized fatty acids into the chow diet of LDLR−/− mice resulted in reduced levels of HDL-cholesterol and PON activity with increased levels of plasma triglycerides and SAA; administration of D-4F ameliorated these changes. To our knowledge, this is the first demonstration of dietary free oxidized fatty acids modulating these parameters. Together with the strong correlation of plasma SAA levels with plasma levels of free oxidized fatty acids, these data are also consistent with the hypothesis that free oxidized fatty acids may play a role in the inflammatory changes induced by WD. In the studies reported here we did not measure the levels of free oxidized fatty acids in tissues or plasma after feeding them (supplementary Table III and Table 9). Detailed time courses and dose response curves comparing the time after feeding and the dosage of free oxidized fatty acid fed with the resulting level of the fed free oxidized fatty acid in enterocytes, liver, and plasma together with plasma SAA levels are needed to determine the precise mechanisms accounting for the observations reported here.
The increase in SAA levels after administration of single metabolites of arachidonic or linoleic acids in mouse chow to mice not on the WD was statistically significant (Supplementary Table III and Table 9), but the levels achieved were lower than those in mice fed the WD (Table 7). The WD increased the levels of many free metabolites of arachidonic and linoleic acids (Supplementary Table II), which in part may explain the lower increase in SAA levels after administration of only one (Supplementary Table III and Table 9).
The low levels of free HETEs and HODEs contained in the WD suggest that the levels observed in the contents of the small intestine were not from undigested WD. Thus, the free HETEs and HODEs in the contents of the small intestine may have been derived from the enterocytes. The enterocytes may produce free HETEs and HODEs by providing phospholipase activity, which hydrolyzes dietary phospholipids, and/or by enzymatic (e.g., lipoxygenase or cyclooxygenase) or nonenzymatic pathways within the enterocyte that act upon free arachidonic and linoleic acids. Oxidized metabolites of arachidonic and linoleic acids have been reported to be important in the intestine in humans and fish with remarkable conservation of some of the enzyme systems that participate (14–21). It is known that small intestinal ulcers are a complication of treatment with nonsteroidal anti-inflammatory agents. A genetic deficiency of cytosolic phospolipase A2-α, which results in a profound deficiency of metabolites derived from arachidonic acid, has been reported to result in chronic recurrent small intestinal ulcers and small bowel perforations (22), which is consistent with the hypothesis that metabolites of arachidonic acid are required for maintenance of the small intestine.
The small intestine contributes ∼30% of the steady-state plasma HDL-cholesterol pool in mice (23). The HDL particles formed in the small intestine enter the circulation directly; the HDL in lymph is predominantly derived from the plasma compartment (23). We recently reported that in the presence of the 4F peptide metabolites of arachidonic acid, such as free 15-HETE, are rapidly transferred in plasma from LDL to HDL and increase the association of 4F with HDL (24). Future studies are required to determine if 4F transfers oxidized metabolites of arachidonic and linoleic acids in enterocytes to intestinal HDL particles, which are then directly secreted into the circulation.
Bloedon et al. (10) found that administering the 4F peptide orally at dosages of 4.3 and 7.14 mg/kg significantly improved HDL anti-inflammatory properties despite achieving low plasma levels of peptide (8.1 ± 6 or 16 ± 7 ng/ml, respectively). However, doses of 0.43 and 1.43 mg/kg did not alter HDL-anti-inflammatory properties (10). Watson et al. (11) targeted plasma levels of peptide. By administering a dose of 0.43 mg/kg by intravenous infusion, Watson et al. (11) achieved plasma levels of ∼3,000 ng/ml and by SQ administration achieved plasma levels of ∼400 ng/ml of 4F peptide. Despite achieving these high levels of peptide, there was no improvement in HDL anti-inflammatory properties (11). Based on studies in mice (1), we concluded that the efficacy of the peptide was determined by dosage and not by plasma level. Because peptide concentrations were similar in the feces whether administered orally or SQ, we also concluded that the intestine may be a major site of action for the peptide. The data presented here support that hypothesis. If the hypothesis for the mechanism of action of the peptide described above is correct (i.e., the peptide lowers intestinal levels of free metabolites of arachidonic and linoleic acids, and this in turn reduces inflammation), the difference between the results of Bloedon et al. (10) and Watson et al. (11) may have been due to the different levels of peptide in the intestine in the two studies.
The results of the studies reported here together with our previous study (1) suggest that further investigation of the action of apoA-I mimetic peptides on the small intestine is likely to reveal new insights into the mechanism of action of these peptides and into the important role of metabolites of arachidonic and linoleic acids in the small intestine in mediating systemic inflammation.
Supplementary Material
Footnotes
Abbreviations:
- apoE−/−
- ApoE null mice
- D-4F
- the peptide Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2 synthesized from all D-amino acids
- HETE
- hydroxyeicosatetraenoic acid
- HODE
- hydroxyoctadecadienoic acid
- LDLR−/−
- low-density lipoprotein receptor null mice
- PG
- prostaglandin
- SAA
- serum amyloid A
- SQ
- subcutaneous injection
- TX
- thromboxane
- WD
- Western diet
This work was supported in part by US Public Health Service Grants HL-30568, HL-34343, and the Laubisch, Castera, M.K. Grey Funds at UCLA and the Leducq Foundation. MN, STR, GMA, and AMF are principals in Bruin Pharma. AMF is an officer in Bruin Pharma.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three tables and one figure.
REFERENCES
- 1.Navab M., Reddy S. T., Anantharamaiah G. M., Imaizumi S., Hough G., Hama S., Fogelman A. M. 2011. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. J. Lipid Res. 52: 1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Lenten B. J., Wagner A. C., Jung C. L., Ruchala P., Waring A. J., Lehrer R. I., Watson A. D., Hama S., Navab M., Anantharamaiah G. M., et al. 2008. Anti-inflammatory apoA-I mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J. Lipid Res. 49: 2302–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imaizumi S., Grijalva V., Navab M., Van Lenten B. J., Wagner A. C., Anantharamaiah G. M., Fogelman A. M., Reddy S. T. 2010. L-4F differentially alters plasma levels of oxidized fatty acids resulting in more anti-inflammatory HDL in mice. Drug Metab. Lett. 4: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgantini C., Imaizumi S., Grijalva V., Navab M., Fogelman A. M., Reddy S. T. 2010. ApoAI mimetic peptides prevent atherosclerosis development and reduce plaque inflammation in a mouse model of diabetes. Diabetes. 59: 3223–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgantini C., Natali A., Boldrini B., Imaizumi S., Navab M., Fogelman A. M., Ferrannini E., Reddy S. T. 2011. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes. 60: 2617–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Wagner A. C., Frank J. S., Datta G., Garber D., et al. 2004. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 109: 3215–3220. [DOI] [PubMed] [Google Scholar]
- 7.Navab M., Ruchala P., Waring A.J., Lehrer R.I., Hama S., Hough G., Palgunachari M.N., Anantharamaiah G.M., Fogelman A.M. 2009. A novel method for oral delivery of apolipoprotein mimetic peptides synthesized from all L-amino acids. J. Lipid Res. 50: 1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merchant J. L., Heller R. A. 1977. 3-Hydroxy-3-methylglutaryl coenzyme A reductase in isolated villous and crypt cells of the rat ileum. J. Lipid Res. 18: 722–733. [PubMed] [Google Scholar]
- 9.Buga G. M., Navab M., Imaizumi S., Reddy S. T., Yekta B., Hough G., Chanslor S., Anantharamaiah G. M., Fogelman A. M. 2010. L-4F alters hyperlipidemic (but not healthy) mouse plasma to reduce platelet aggregation. Arterioscler. Thromb. Vasc. Biol. 30: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloedon L. T., Dunbar R., Duffy D., Pinell-Salles P., Norris R., DeGroot B. J., Movva R., Navab M., Fogelman A. M., Rader D. J. 2008. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 49: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson C. E., Weissbach N., Kjems L., Ayalasomayajula S., Zhang Y., Chang I., Navab M., Hama S., Hough G., Reddy S. T., et al. 2011. Treatment of patients with cardiovascular disease with L-4F, an apoA-1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 52: 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobrian A. D., Lieb D. C., Cole B. K., Taylor-Fishwick D. A., Chakrabarti S. K., Nadler J. L. 2011. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog. Lipid Res. 50: 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Mari J. F., Saada J. I., Mifflin R. C., Valentich J. D., Powell D. W. 2007. HETEs enhance IL-1-mediated COX-1 expression via augmentation of message stability in human colonic myofibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol. 293: G719–G728. [DOI] [PubMed] [Google Scholar]
- 14.Oleksiak M. F., Wu S., Parker C., Qu W., Cox R., Zeldin D. C., Stegeman J. J. 2003. Identification and regulation of a new vertebrate cytochrome P450 subfamily, CYP2Ps, and functional characterization of CYP2P3, a conserved arachidonic acid epoxygenase/19-hydroxylase. Arch. Biochem. Biophys. 411: 223–234. [DOI] [PubMed] [Google Scholar]
- 15.Wegmann M., Kampen A., Weber S., Seyberth H. W., Kockerling A. 2000. Effect of hydroxyeicosatetraenoic acids on furosemide-sensitive chloride secretion in rat distal colon. J. Pharmacol. Exp. Ther. 295: 133–138. [PubMed] [Google Scholar]
- 16.De Lisle R. C., Meldi L., Flynn M., Jansson K. 2008. Altered eicosanoid metabolism in the cystic fibrosis mouse small intestine. J. Pediatr. Gastroenterol. Nutr. 47: 406–416. [DOI] [PubMed] [Google Scholar]
- 17.Mangino M.J., Anderson C.B., Murphy M.K., Brunt E., Turk J. 1989. Mucosal arachidonate metabolism and intestinal ischemia-reperfusion injury. Am. J. Physiol. (Gastrointest. Liver Physiol. 20); 257: G299–G307. [DOI] [PubMed] [Google Scholar]
- 18.Kamitani H., Ikawa H., Hsi L. C., Watanabe T., DuBois R. N., Eling T. E. 1999. Regulation of 12-lipoxygenase in rat intestinal epithelial cells during differentiation and apoptosis induced by sodium butyrate. Arch. Biochem. Biophys. 368: 45–55. [DOI] [PubMed] [Google Scholar]
- 19.Krilis S. A., Macpherson J. L., de Carle D. J., Daggard G. E., Talley N. A., Chesterman C. N. 1986. Small bowel mucosa from celiac patients generates 15-hydroxyeicosatetraenoic acid (15-HETE) after in vitro challenge with gluten. J. Immunol. 137: 3768–3771. [PubMed] [Google Scholar]
- 20.Morgan A. H., Dioszeghy V., Maskery B. H., Thomas C. P., Clark S. R., Mathie S. A., Lloyd C. M., Kuhn H., Topley N., Coles B. C., et al. 2009. Phosphatidlyethanolamine-esterified eicosanoids in the mouse. Tissue localization and inflammation-dependent formation in Th-2 disease. J. Biol. Chem. 284: 21185–21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins J. F., Hu Z., Ranganathan P. N., Feng D., Garrick L. M., Garrick M. D., Browne R. W. 2008. Induction of arachidonate 12-lipoxygenase (Alox 15) in intestine of iron-deficient rats correlates with the production of biologically active lipid mediators. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G948–G962. [DOI] [PubMed] [Google Scholar]
- 22.Adler D. H., Phillips J. A., III, Cogan J. D., Iverson T. M., Schnetz-Boutaud N., Stein J. A., Brenner D. A., Milne G. L., Morrow J. D., Boutaud O., et al. 2009. The enteropathy of prostaglandin deficiency. J. Gastroenterol. 44(Suppl 19): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunham L. R., Kruit J. K., Iqbal J., Fievet C., Timmins J. M., Pape T. D., Coburn B. A., Bissada N., Staels B., Groen A. K., et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116: 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meriwether D., Imaizumi S., Grijalva V., Hough G., Vakili L., Anantharamaiah G. M., Farias-Eisner R., Navab M., Fogelman A. M., Reddy S. T., et al. 2011. Enhancement by LDL of transfer of L-4F and oxidized lipids to HDL in C57BL/6J mice and human plasma. J. Lipid Res. 52: 1795–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


