Abstract
Epoxyeicosatrienoic acids (EET), the primary arachidonic acid metabolites of cytochrome P450 2J (CYP2J) epoxygenases, possess potent vasodilatory, anti-inflammatory, antiapoptotic, and mitogenic effects. To date, little is known about the role of CYP2J2 and EETs in tumor necrosis factor (TNF)-α–induced cardiac injury. We utilized cell culture and in vivo models to examine the effects of exogenously applied EETs or CYP2J2 overexpression on TNF-α–induced cardiac apoptosis and cardiac dysfunction. In neonatal rat cardiomyocytes, TNF-α–induced apoptosis was markedly attenuated by EETs or CYP2J2 overexpression, leading to significantly improved cell survival. Further studies showed that TNF-α decreased expression of the antiapoptotic proteins Bcl-2 and Bcl-xL, decreased IκBα and PPARγ, and also inhibited PI3K-dependent Akt and EGFR signaling. Both EETs and CYP2J2 overexpression reversed the effects of TNF-α on these pathways. Furthermore, overexpression of CYP2J2 in rats prevented the decline in cardiac function that is normally observed in TNF-α-challenged animals. These results demonstrate that EETs or CYP2J2 overexpression can prevent TNF-α–induced cardiac cell injury and cardiac dysfunction by inhibiting apoptosis, reducing inflammation, and enhancing PPARγ expression. Targeting the CYP2J2 epoxygenase pathway may represent a novel approach to mitigate cardiac injury in diseases such as heart failure, where increased TNF-α levels are known to occur.
Keywords: apoptosis, cytochrome P450, eicosanoids, heart, peroxisome proliferator-activated receptors
Cytochrome P450 epoxygenases of the 2J subfamily (CYP2J) are abundantly expressed in cardiac myocytes and metabolize arachidonic acid (AA) to four biologically active epoxyeicosatrienoic acid (EET) isomers (1). CYP epoxygenase-derived EETs have anti-inflammatory properties (2) and have been reported to possess many potent biological effects in the renal and cardiovascular systems (3–5). For example, EETs inhibit cytokine-induced vascular cell adhesion molecule expression and leukocyte adhesion to the vascular wall (2), inhibit vascular smooth muscle cell migration (6), protect endothelial cells from apoptosis (7), upregulate eNOS (8), and promote endothelial cell proliferation and angiogenesis via activation of MAP kinases and PI3 kinase/Akt signaling (8). 11,12- and 14,15-EETs have been characterized as effective mitogens (1, 9–14) that can enhance proliferation of renal epithelial cells (12, 15, 16). Interestingly, the role of 14,15-EET in mediating the mitogenic responses of EGF and HB-EGF has also been thoroughly documented in cultured LLCPk cells (9, 17).
CYP2J2 is abundantly expressed in the human myocardium; however, little is known about its role in the heart, especially in the context of heart failure. Seubert et al. recently demonstrated a protective role of CYP2J2 overexpression or exogenously administered EETs in ischemia-reperfusion injury in both rodents and dogs via a mechanism that involved opening of sarcolemmal and mitochondrial K+ channels and activation of p42/44 MAPK (18, 19). Importantly, the role of CYP2J2-derived EETs in nonischemic cardiac injury and dysfunction remains unknown. In the present study, neonatal rat cardiomyocytes were used to evaluate the effects of CYP2J2 overexpression and exogenous EET supplementation on tumor necrosis factor (TNF)-α–induced apoptosis and inflammation. An in vivo model of TNF-α–induced cardiac dysfunction was also utilized to evaluate the hypothesis that CYP2J2 could improve cardiac function by inhibiting myocardial inflammation and apoptosis.
MATERIALS AND METHODS
Gene delivery vector construct
Recombinant adeno-associated virus vectors containing green fluorescent protein (GFP) or CYP2J2 were prepared as previously described (20). The CYP2J2 or GFP cDNAs subcloned into the pcDNA3.1 plasmid were prepared as described (2, 21). pcDNA3.1-GFP and pcDNA3.1-CYP2J2 plasmids were purified using the QIAGEN Plasmid Maxi Kit (QIAGEN, Inc., Chatsworth, CA) according to the manufacturer's instructions.
Primary culture and treatment of neonatal cardiomyocytes
Neonatal cardiomyocytes were obtained from one-day-old rats as described previously (22). Experimental protocols were approved by the Institutional Animal Research Committee of Tongji Medical College and complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The cells were incubated with TNF-α (10 ng/ml) in the presence/absence of exogenous 8,9-, 11,12-, or 14,15-EET (100 nM) or the selective CYP epoxygenase inhibitor PPOH (10 µM) for 24 h. Alternatively, the cells were infected with rAAV-GFP or rAAV-CYP2J2 (MOI = 500) for 48 h prior to treatment with TNF-α.
H9C2 cell culture and gene transfection
H9C2 rat heart cells were obtained from American Type Culture Collection (ATCC, Rockville, MD). The 85–90% confluent H9C2 cells were transfected with pcDNA3.1/GFP or pcDNA3.1/CYP2J2 plasmids (2 μg DNA/9.4 cm2) with Fugene 6 Transfection Reagent (Roche Molecular Biochemicals, Indianapolis, IN). At 36 h after transfection, the percentage transfection efficiency was determined by examining pcDNA3.1/GFP-transfected cells for GFP fluorescence under a fluorescence microscope (NIKON). Transfection efficiency was ∼30% (supplementary Fig. IA). The expression of CYP2J2 was confirmed by immunoblotting (supplementary Fig. IB) and was associated with increased production of stable EET metabolites, the DHETs (supplementary Fig. IC).
H9C2 rat heart cell treatments
To investigate the antiapoptotic effect of CYP2J2 or EETs and possible signaling mechanisms involved, transfected H9C2 cells were treated for 45 min with/without the EGFR inhibitor N-(3-chlorophenyl)-6,7-dimethoxy-4-quinazolinamine (AG1478; 100 nM) or the PPARγ inhibitor 2-chloro-5-nitro-N-phenylbenzamide (GW9662; 1 μM), followed by incubation with TNF-α (10 ng/ml) for 24 h. Alternatively, after pretreatment with/without mitogen-activated protein kinase (MAPK) inhibitor apigenin, AG1478, or peroxisome proliferator-activated receptor (PPAR)γ inhibitor GW9662, cells were incubated with 11,12-EET (100 nM) for 30 min prior to TNF-α treatment. Next, cell viability was assessed using an MTT assay as described (23), and apoptosis was assessed by flow cytometry (FACS, Vantage, BD). In addition, caspase-3 activity was measured by colorimetric assay using DEVD-p-nitroanilide as a substrate as described previously (24).
Flow cytometry
Cells were harvested with trypsin/EDTA, resuspended in binding buffer [10 mM HEPES/NaOH (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2], and incubated with FITC-conjugated Annexin V and 1 µg/ml propidium iodide (Annexin V-FITC kit; Bender MedSystems, San Bruno, CA) according to the manufacturer's protocol. Cells were then analyzed with a FACStar-Plus flow cytometer (Becton Dickinson, Franklin Lakes, NJ). The annexin V positive (FL1-H) cells were counted for early stages of apoptosis (the lower right quadrant) and late apoptotic or necrotic cells (the upper right quadrant).
Animal treatment and gene delivery
Forty eight male Sprague-Dawley rats weighing 200–220 g were obtained from the Experimental Animal Center of Tongji Medical College. Experimental protocols were approved by the Institutional Animal Research Committee of Tongji Medical College and complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Rats were randomized to three groups (16 animals per group) and received intravenous injection of saline control, pcDNA3.1-GFP control, or pcDNA3.1-CYP2J2 at a dose of 2.5 mg/kg body weight(25) (2). Two weeks later, 8 rats from each group randomly received a single intravenous injection of TNF-α (10 µg/kg).
Hemodynamic study
At 6 h and 24 h after TNF-α injection, rats were anesthetized by intraperitoneal administration of pentobarbital at 40 mg/kg body weight. A polyethylene catheter (26) was inserted into the left ventricle (LV) through the right carotid artery for measurement of left ventricular end diastolic pressure (LVEDP) and maximum left ventricular pressure ascending speed (+dp/dt max) (26).
14, 15-DHET determination in urine
The 14,15-DHET ELISA kit (Detroit R and D Inc., Detroit, MI) was used to measure 14,15-DHET according to the manufacturer's instructions as previously described (8). EETs can be hydrolyzed to DHETs by acid treatment, and thus, DHET in acidified urine represents total DHETs. The difference between total 14,15-DHET and 14,15-DHET before acidification will be 14,15-EET levels. The concentration of 14,15-DHET and 14,15-EET were expressed as nanograms per milliliter of urine or serum specimen.
Determination of inflammatory mediators by ELISA
The levels of soluble vascular cell adhesion molecule (sVCAM)-1, soluble intercellular adhesion molecule (sICAM)-1, soluble endothelium (sE)-selectin, interleukin (IL)-1β, IL-6, and IL-10 in serum samples were determined using ELISA kits (R and D Systems, Inc., Minneapolis, MN) according to the manufacturer's instructions.
Human umbilical vein endothelial cell culture and adhesion assays
Human umbilical vein endothelial cells (HUVEC) were isolated from fresh human umbilical cords and cultured. The institutional review board of Tongji Hospital approved this study, and written informed consent was obtained from all participants. Experiments were conducted according to the principles expressed in the Declaration of Helsinki. HUVECs were infected with rAAV-CYP2J2 before incubating with TNF-α (10 ng/ml). At 48 h after infection, adhesion assays in peripheral blood mononuclear cells (PBMC) were performed as previously described (27, 28). Briefly, PBMCs from healthy volunteers (n = 8) were isolated and labeled with VybrantTM CFDA SE Cell Tracer Kit (Molecular Probes Inc., OR) following the manufacturer's instructions. HUVECs were washed three times with PBS after incubation for 6 h with TNF-α (10 ng/ml). Labeled PBMCs were added to the medium (1 × 106/ml) and incubated for 30 min, followed by washing with PBS. A video record of adhesive events was made in at least eight video-microscopic fields on each slide after 5 min of washout. For monocytes, the number of adherent cells was counted, averaged per field, and converted to cells per square millimeter using the calibrated field dimensions.
Western blot analysis
Protein concentrations were determined using the Bradford method. Western blots were performed according to methods described previously (29). Mainly, Western blots were probed with antibodies against Bcl-2, Bcl-xl, and Bax (Santa Cruz Biotechnology, Santa Cruz, CA); PI3-kinase; phosphorylated Akt and Akt (Santa Cruz Biotechnology); phospho-ERK1/2 and ERK1/2 (Santa Cruz Biotechnology); NF-κB inhibitor alpha (IkBα) and PPARγ (Cell Signaling Technology, Beverly, MA); and epidermal growth factor receptor (EGFR) and phosphorylated EGFR (Cell Signaling Technology). Then the membranes were treated with secondary antibody conjugated to horseradish peroxidase (Jackson Immunoresearch Laboratories, West Grove, PA) at room temperature for 2 h. Antibody against β-actin was purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO). Polyclonal antibodies against CYP2J2 were developed as described previously (30). The horseradish peroxidase–conjugated secondary antibodies (goat anti-rabbit and goat anti-mice) were bought from (Jackson Immunoresearch Laboratories).
Statistics
All data were expressed as means ± SEM. Comparisons between groups were performed by a one-way analysis of ANOVA with post hoc analyses performed using the Student-Newman-Keuls method. Statistical significance was defined as P < 0.05.
RESULTS
Effect of EETs and CYP2J2 overexpression on apoptosis of primary rat cardiomyocytes
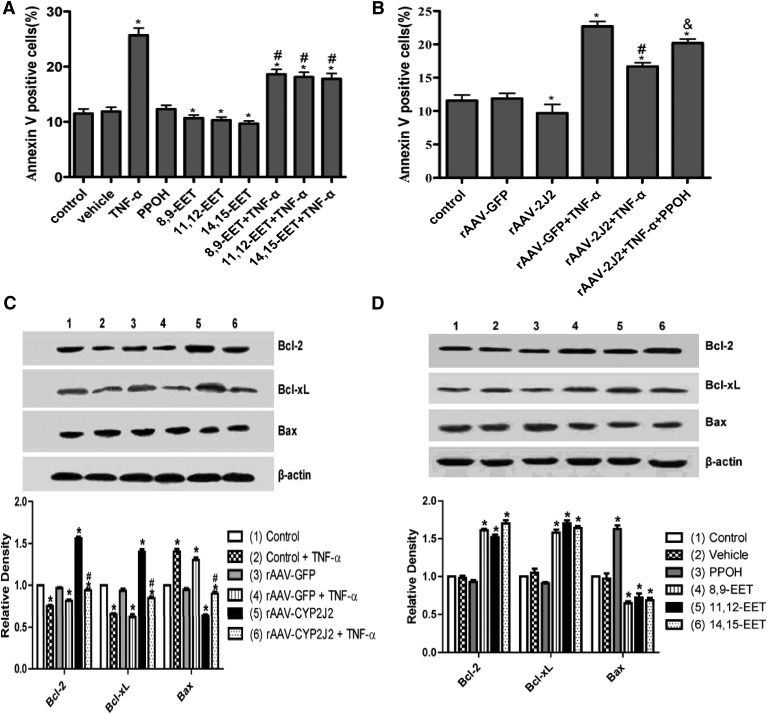
The primary rat cardiomyocytes transfected with rAAV-mediated CYP2J2 epoxygenase had increased CYP2J2 expression and increased DHET levels relative to corresponding cells transfected with rAAV-GFP. But the DHET levels were notably decreased by coincubation with TNF-α or PPOH (supplementary Fig. IA–C). Flow cytometry analysis showed that 8,9-EET, 11,12-EET, and 14,15-EET significantly decreased apoptosis of primary rat cardiomyocytes induced by TNF-α treatment, whereas PPOH, a selective CYP epoxygenase inhibitor, increased apoptosis compared with the corresponding cells without PPOH treatment (Fig. 1A). Furthermore, rAAV-mediated CYP2J2 epoxygenase overexpression reduced spontaneous apoptosis and markedly reduced TNF-α–induced cardiomyocyte apoptosis, an effect that was blocked by PPOH (Fig. 1B). Representative flow cytometry scatterplots from all treatment groups are shown in supplementary Fig. IIA, B. Western blot analysis showed that exogenous EET administration and rAAV-mediated CYP2J2 overexpression in primary rat cardiomyocytes significantly upregulated the antiapoptotic proteins Bcl-2 and Bcl-xL and downregulated the proapoptotic protein, Bax (Fig. 1C, D). Furthermore, PPOH upregulated Bax, providing further support for an antiapoptotic effect of CYP epoxygenase–derived EETs.
Fig. 1.
Effect of epoxygenase overexpression and exogenous EETs on apoptosis of primary rat cardiomyocytes. (A) Exogenous administration of EETs decreases apoptosis in primary rat cardiomyocytes induced by TNF-α treatment, whereas the apoptosis is increased by treatment with PPOH compared with the corresponding cells (PPOH, a selective inhibitor of CYP epoxygenases) (*P < 0.05 versus control; #P < 0.05 versus the corresponding group without TNF-α treatment). (B) Apoptosis of primary rat cardiomyocytes induced by TNF-α is decreased by rAAV-CYP2J2 transfection and increased by PPOH treatment (*P < 0.05 versus control; #P < 0.05 versus rAAV-GFP + TNF-α, and &P < 0.05 versus rAAV-2J2 + TNF-α). (C, D) Representative Western blots and densitometry results showing altered levels of Bcl-2, Bcl-xL, and Bax following exogenous administration of EETs or rAAV-CYP2J2 transfection (*P < 0.05 versus control; #P < 0.05 versus rAAV-GFP + TNF-α).
Effect of 11,12-EET and CYP2J2 overexpression on viability of rat heart H9C2 cells
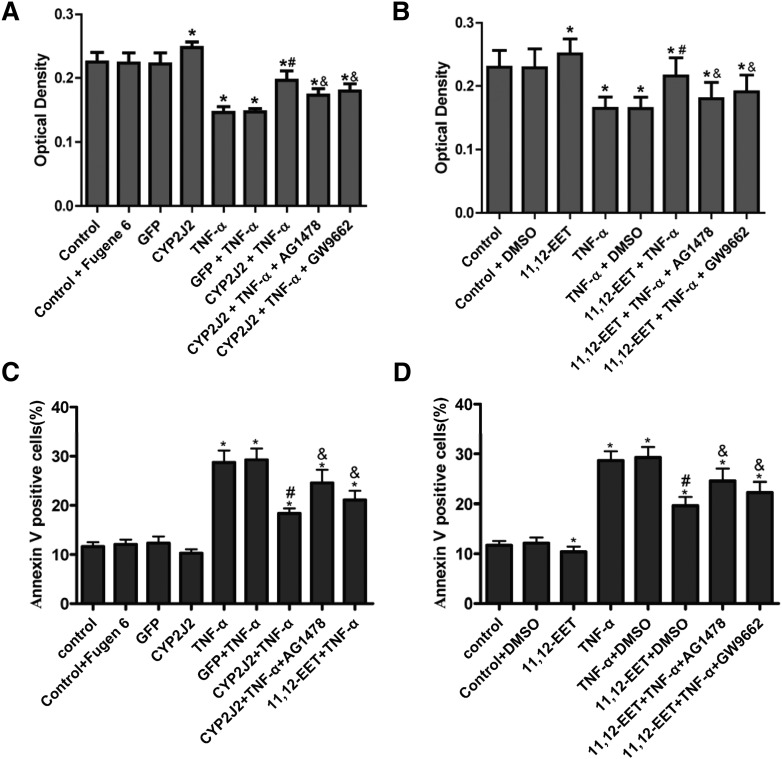
H9C2 cells transfected with pcDNA3.1/CYP2J2 had increased CYP2J2 expression and increased DHET levels relative to H9C2 cells transfected with pcDNA3.1/GFP (supplementary Fig. IIIA–C). These results are similar to those previously reported for endothelial cells (3, 4). The results of MTT assays demonstrate that viability of H9C2 cells transfected with pcDNA3.1/CYP2J2 was significantly greater than control cells transfected with pcDNA3.1/GFP in both the presence and absence of TNF-α (Fig. 2A). The prosurvival effects of CYP2J2 were significantly attenuated by both the EGFR inhibitor AG1478 (100 nM) and the PPARγ inhibitor GW9662 (1 μM). Addition of 11,12-EET to H9C2 cells had similar protective effects on cell viability in both the presence and absence of TNF-α, and these effects were attenuated by AG1478 and GW9662 (Fig. 2B). These results show that the prosurvival effects of CYP2J2 overexpression and 11,12-EET treatment are mediated, at least in part, through activation of EGFR and PPARγ.
Fig. 2.
Effect of CYP epoxygenase overexpression and exogenous EET treatment on viability and apoptosis of H9C2 rat heart cells. (A) CYP2J2 overexpression increases cell viability and inhibits the detrimental effects of TNF-α. The CYP2J2-mediated increase in cell viability is notably attenuated by coincubation with AG1478 or GW9662 (*P < 0.05 versus control; #P < 0.05 compared with GFP + TNF-α; &P < 0.05 compared with CYP2J2 + TNF-α). (B) Viability of H9C2 cells is increased after treatment with 11,12-EET. The EET-mediated increase in cell viability is attenuated by AG1478 and GW9662 (*P < 0.05 versus control; #P < 0.05 versus DMSO + TNF-α; &P < 0.05 compared with 11,12-EET + TNF-α). (C) TNF-α–induced apoptosis of H9C2 cells is inhibited by CYP2J2 overexpression, an effect that is attenuated by coincubation with AG1478 or GW9662 (*P < 0.05 versus control; #P < 0.05 versus GFP + TNF-α; &P < 0.05 versus CYP2J2 + TNF-α). (D) TNF-α–induced apoptosis of H9C2 cells is inhibited by 11,12-EET treatment, an effect that is attenuated by AG1478 or GW9662 (*P < 0.05 versus control; #P < 0.05 versus DMSO + TNF-α; &P < 0.05 versus 11,12-EET + TNF-α).
Effect of 11,12-EET treatment and CYP2J2 overexpression on apoptosis of rat heart H9C2 cells
We also evaluated the effect of EETs and CYP2J2 overexpression on apoptosis of H9C2 cells by flow cytometry. Our results show that cells overexpressing CYP2J2 had significantly reduced apoptosis 24 h after TNF-α treatment compared with GFP-transfected cells treated with TNF-α (Fig. 2C). Exogenous supplementation with 11,12-EET had similar protective effects (Fig. 2D). Representative flow cytometry scatterplots from all treatment groups are shown in supplementary Fig. IVA, B. The antiapoptotic effects of CYP2J2 overexpression or exogenous 11,12-EET supplementation were attenuated by both AG1478 and GW9662 (Fig. 2C, D). These results show that the antiapoptotic effects of CYP2J2 overexpression and 11,12-EET treatment are mediated, at least in part, through activation of EGFR and PPARγ.
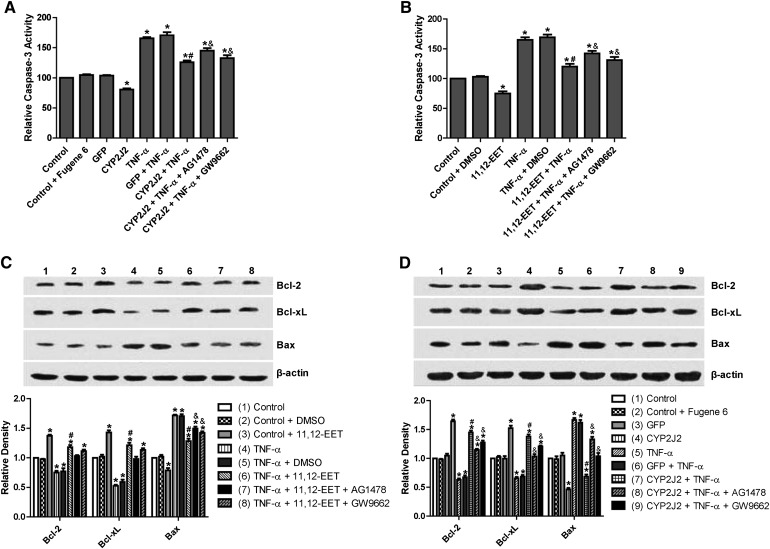
Effect of 11,12-EET treatment and CYP2J2 overexpression on caspase-3 activity in rat heart H9C2 cells
TNF-α treatment of H9C2 cells significantly increased caspase-3 activity. Transfection with pcDNA3.1/CYP2J2 significantly attenuated the TNF-α–induced increase in caspase-3 compared with control and pcDNA3.1/GFP-transfected cells (Fig. 3A). This effect was largely inhibited when cells were pretreated with AG1478 or GW9662. Exogenous supplementation with 11,12-EET had similar protective effects on caspase-3 activity in TNF-α–treated H9C2 cells, an effect that was also attenuated by AG1478 and GW9662 (Fig. 3B). These results show that the effects of CYP2J2 overexpression and 11,12-EET treatment on caspase-3 activity are mediated, at least in part, through activation of EGFR and PPARγ.
Fig. 3.
Effect of CYP2J2 overexpression and exogenous EET treatment on caspase-3 activity and apoptosis-regulating proteins in H9C2 rat heart cells. (A) CYP2J2 overexpression attenuates TNF-α–induced caspase-3 activity, an effect that is attenuated by either AG1478 or GW9662 (*P < 0.05 versus control; #P < 0.05 versus GFP + TNF-α; &P < 0.05 versus CYP2J2 + TNF-α). (B) 11,12-EET treatment attenuates TNF-α–induced caspase-3 activity. The EET-mediated effect is attenuated by AG1478 or GW9662 (*P < 0.05 versus control; #P < 0.05 versus DMSO + TNF-α; &P < 0.05 versus 11,12-EET+ TNF-α). (C, D) Representative Western blots and densitometry results showing altered levels of Bcl-2, Bcl-xL, and Bax following exogenous administration of EETs or overexpression of CYP2J2, and effects of AG1478 and GW9662 (*P < 0.05 versus control; #P < 0.05 versus DMSO + TNF-α or versus GFP + TNF-α; &P < 0.05 versus 11,12-EET + TNF-α or versus CYP2J2 + TNF-α).
Effect of 11,12-EET treatment and CYP2J2 overexpression on pro- and antiapoptotic protein expression in rat heart H9C2 cells
Incubation of H9C2 cells with exogenous 11,12-EET significantly attenuated the downregulation of antiapoptotic proteins Bcl-2 and Bcl-xL and prevented the upregulation of the proapoptotic protein Bax that is normally observed following TNF-α treatment (Fig. 3C). Overexpression of CYP2J2 had similar effects on Bcl-2, Bcl-xL, and Bax expression (Fig. 3D). Furthermore, the effects of 11,12-EET treatment and CYP2J2 overexpression were attenuated by addition of AG1478 or GW9662 (Fig. 3C, D), supporting the roles of EGFR and PPARγ in the EET-mediated regulation of apoptosis signaling.
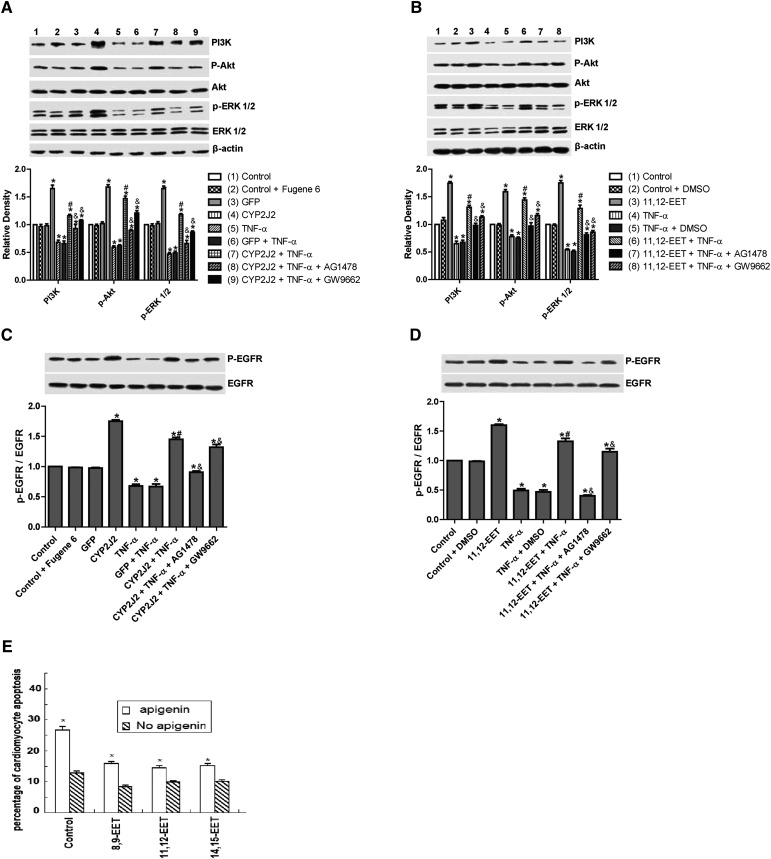
Effect of 11,12-EET treatment and CYP2J2 overexpression on PI3K-dependent Akt phosphorylation and EGFR-ERK pathways in rat heart H9C2 cells
TNF-α treatment inhibited expression of PI3K and PI3K-dependent Akt phosphorylation, whereas CYP2J2 overexpression induced PI3K protein expression and Akt phosphorylation in both the presence and absence of TNF-α (Fig. 4A). The effects of CYP2J2 overexpression were apparently inhibited when cells were pretreated with AG1478 or GW9662. Similarly, exogenous treatment with 11,12-EET increased Akt phosphorylation and enhanced PI3K expression, an effect that was blocked by both AG1478 and GW9662 (Fig. 4B).
Fig. 4.
Effect of CYP2J2 overexpression and 11,12-EET on activity of PI3K/Akt, ERK1/2 and EGFR in cardiac myocytes. (A) Representative Western blots and densitometry results of PI3K/β-actin, phospho-Akt/Akt, and phospho-ERK1/2/ERK1/2 upon CYP2J2 transfection with or without AG1478 or GW9662. (*P < 0.05 versus control; #P < 0.05 versus GFP + TNF-α; &P < 0.05 versus CYP2J2 + TNF-α). (B) Representative Western blots and densitometry results of PI3K/β-actin, phospho-Akt/Akt, and phospho-ERK1/2/ERK1/2 upon 11,12-EET incubation with or without AG1478 or GW9662 (*P < 0.05 versus control; #P < 0.05 versus DMSO + TNF-α; &P < 0.05 versus 11,12-EET + TNF-α). (C) Representative Western blots and densitometry results of phospho-EGFR upon CYP2J2 transfection with or without AG1478 or GW9662 (*P < 0.05 versus control; #P < 0.05 versus GFP + TNF-α; &P < 0.05 versus CYP2J2 + TNF-α. (D) Representative Western blots and densitometry results of phospho-EGFR upon 11,12-EET incubation with or without AG1478 or GW9662. (E) Effect of MAPK1/2 inhibitor apigenin (25 μM) on TNF-α–induced cardiomyocyte apoptosis (*P < 0.05 versus control; #P < 0.05 versus DMSO + TNF-α; &P < 0.05 versus 11,12-EET + TNF-α).
Treatment of cells with TNF-α also led to significantly reduced phosphorylation of extracellular signal-regulated kinases (ERK)1/2 and EGFR in control cells. CYP2J2 overexpression or exogenous 11,12-EET significantly attenuated the TNF-α–induced effects on ERK1/2 and EGFR phosphorylation (Fig. 4AndashD), and ERK1/2 inhibitor apigenin (25 μM) significantly and partially abolished the protective effects of EETs on TNF-α–induced H9C2 cell apoptosis (Fig. 4E). Furthermore, the effects of CYP2J2 overexpression and 11,12-EET treatment on EGFR activation were inhibited by addition of AG1478. GW9662 also attenuated EGFR activation, although the effects were relatively small (Fig. 4C, D). Collectively, these findings suggest that CYP2J2 overexpression or exogenous 11,12-EET activates the EGFR-ERK1/2 signaling pathway, a process that may be involved in the antiapoptotic effects observed in cardiac myocytes.
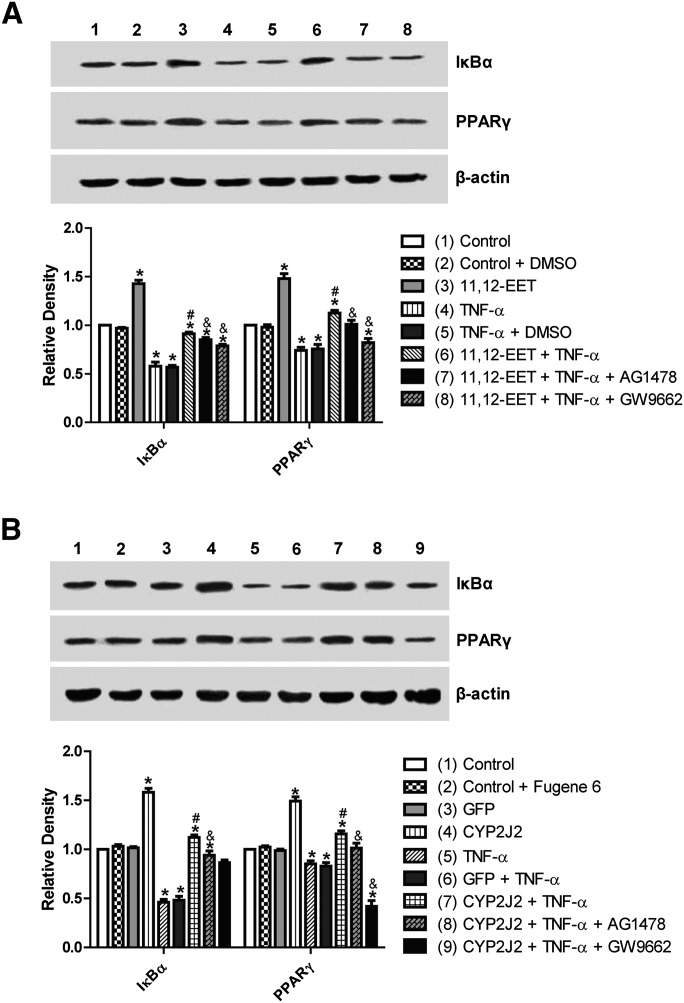
Effect of 11,12-EET treatment and CYP2J2 overexpression on PPARγ and IκBα expression in neonatal rat cardiomyocytes
CYP epoxygenase–derived eicosanoids are known to possess potent anti-inflammatory properties, some of which may be mediated by activation of PPARγ and/or inhibition of IκBα degradation (2, 31). Interestingly, CYP2J2 overexpression or addition of exogenous 11,12-EET significantly upregulated PPARγ and IκBα, both in the presence and absence of TNF-α (Fig. 5A, B). These effects were significantly attenuated by addition of GW9662 or AG1478 prior to TNF-α treatment (Fig. 5A, B). Therefore, the EET/PPARγ/IκBα pathway may represent a novel protective pathway for inhibition of TNF-α–induced effects in cardiomyocytes.
Fig. 5.
Effect of CYP2J2 overexpression and 11,12-EET treatment on PPARγ and IκBα expression in neonatal rat cardiac myocytes. (A) Representative Western blots and densitometry results showing effects of CYP2J2 overexpression on PPARγ and IκBα expression in TNF-α treated neonatal rat cardiomyocytes (*P < 0.05 versus control; #P < 0.05 versus GFP + TNF-α; &P < 0.05 versus CYP2J2 + TNF-α). (B) Representative Western blots and densitometry results showing effects of 11,12-EET treatment on PPARγ and IκBα expression in TNF-α treated neonatal rat cardiomyocytes (*P < 0.05 versus control; #P < 0.05 versus DMSO + TNF-α; &P < 0.05 versus 11,12-EET + TNF-α).
Improvement of hemodynamics and systemic inflammation in TNF-α–treated rats after CYP2J2 gene delivery
Western blot for expression of CYP epoxygenases indicated that CYP2J2 gene delivery induced significant expression of CYP2J2 in vivo in the heart and aorta (supplementary Fig. VA). Overexpression of CYP epoxygenases was associated with a significant increase in urinary 14,15-DHET and serum 14,15-EET levels two weeks after gene delivery compared with levels in rats injected with saline or with pcDNA3.1/GFP (P < 0.05; supplementary Fig. VB, C). These results indicate that CYP2J2 gene delivery induced significant epoxygenase expression and increases in enzyme activity in vivo.
Hemodynamic assessments showed that TNF-α treatment of rats induced a marked deterioration of cardiac function compared with control rats (Table 1). Treatment with pcDNA3.1/CYP2J2 resulted in a significant decrease in LVEDP (P < 0.05) and a notable increase in +dp/dt max (P < 0.05) compared with saline and pcDNA3.1/GFP-treated rats. Together, these results demonstrate an improvement in systolic and diastolic function after CYP2J2 gene delivery.
TABLE 1.
Effect of CYP2J2 gene delivery on hemodynamic parameters in rats
| LVEDP (mm Hg) | +dp/dt max (mm Hg/s) | |
| Control | 1.31 ± 0.22 | 5606.5 ± 277.7 |
| TNF-α | 3.66 ± 0.26a | 1919.5 ± 391.4a |
| GFP | 1.22 ± 0.32 | 5602.5 ± 181.0 |
| GFP + TNF-α | 3.45 ± 0.84a | 1828.7 ± 496.2a |
| CYP2J2 | 1.41 ± 0.39a | 5409.7 ± 57.7a |
| CYP2J2 + TNF-α | 2.55 ± 0.88ab | 2921.5 ± 79.9ab |
Values are expressed as mean ± SEM (n = 8 per group).
+dp/dt max, maximum left ventricular pressure ascending speed.
P < 0.05 versus control.
P < 0.05 versus GFP + TNF-α.
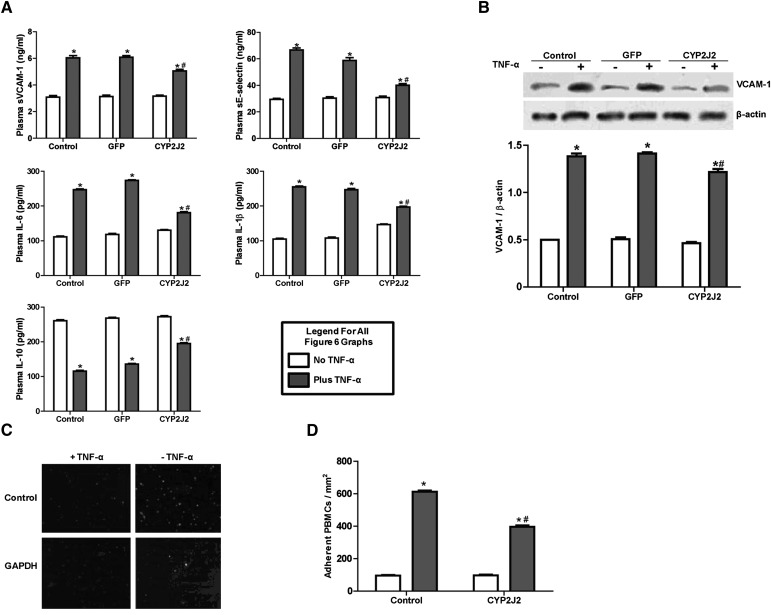
TNF-α injection significantly increased serum levels of proinflammatory cytokines, including sVCAM-1, sE-selectin, IL-1β, and IL-6, but decreased serum levels of IL-10, an anti-inflammatory cytokine (Fig. 6A). Interestingly, the proinflammatory effects of TNF-α were markedly attenuated by CYP2J2 gene delivery (Fig. 6A).
Fig. 6.
Effect of CYP2J2 gene delivery on in vivo inflammatory cytokine production and in vitro adhesion of PBMCs to endothelial cells. (A) Inflammatory cytokine levels in serum of rats treated with TNF-α (n = 8 for each group; *P < 0.05 versus control without TNF-α; #P < 0.05 versus GFP with TNF-α). (B) Representative Western blots and densitometry results of VCAM-1 in HUVECs treated with TNF-α (n = 8 for each group; *P < 0.05 versus control; #P < 0.05 versus GFP + TNF-α). (C, D) Representative image (×200) and numbers of PBMCs adhering to TNF-α treated HUVECs (n = 8 for each group; *P < 0.05 versus control; #P < 0.05 versus TNF-α).
Effect of CYP2J2 overexpression on TNF-α–induced adhesion of PBMC to endothelial cells
CYP2J2 overexpression inhibited TNF-α–induced VCAM-1 expression in cultured HUVECs (Fig. 6B). Likewise, TNF-α greatly increased the number of PBMCs which adhered to HUVECs. This effect was significantly attenuated by CYP2J2 overexpression (Fig. 6C, D).
DISCUSSION
In the present study, we show that CYP2J2 transfection or exogenous addition of 11,12-EET significantly increases cell viability and protects against TNF-α–induced apoptosis in primary neonatal cardiomyocytes and in the H9C2 rat cardiac cell line. Furthermore, CYP2J2 overexpression and exogenous addition of 11,12-EET significantly attenuate the TNF-α–induced increase in caspase-3 activity and decrease in expression of prosurvival proteins Bcl-2 and Bcl-xL, a process which involves activation of PI3K/Akt, EGF/ERK, and PPARγ signaling pathways. Finally, in vivo overexpression of CYP2J2 significantly inhibits inflammation and ameliorates the cardiac dysfunction induced by TNF-α.
Cardiomyocyte apoptosis and inflammation induced by cytokines such as TNF-α are important in the pathogenesis of acute and chronic myocardial injury and heart failure (32–35). Apoptosis plays an important role in the basic pathogenic mechanisms that underlie a variety of heart diseases. Owing to the chronic nature of heart failure, a very low level of cellular apoptosis over a period of months or years can be detrimental. Importantly, cardiac myocytes are terminally differentiated and have little potential for division as a rescue mechanism. As a result, it is appealing to develop therapeutic strategies for heart failure that enhance cell viability and inhibit apoptosis.
The cardioprotective effects of 11,12-EET and CYP2J2 overexpression that we observed in this study are consistent with the observation by Seubert et al. that CYP2J2 overexpression in transgenic mice protects against postischemic myocardial dysfunction (18). EETs and CYP2J2 overexpression have also been shown to attenuate cytokine-induced endothelial cell adhesion molecule expression, and endogenous EETs prevented leukocyte adhesion to the vascular wall by a mechanism involving transcription factor NF-κB and IκB kinase (2). The current study also demonstrated inhibitory effects of CYP2J2 overexpression on cytokine-induced leukocyte adhesion to endothelial cells. In vivo experiments showed that CYP2J2 overexpression attenuates production of VCAM, E-selectin, IL-1β, and IL-6 while enhancing production of the anti-inflammatory cytokine IL-10. In addition, CYP2J2 gene delivery via intravenous injection significantly increased CYP2J2 expression in vivo in most organs, mainly aorta, heart, liver, and kidney (3, 5, 6, 36, 37), which may also explain some effects of heart protection. These results support the view that CYP2J2-derived EETs protect the heart via their anti-inflammatory, antiapoptotic, and prosurvival effects.
Additional experiments demonstrate an important role for PPARγ and EGFR signaling in mediating the cardioprotective effects of EETs and CYP2J2 overexpression. Treatment of H9C2 cells with GW9662 (a PPARγ inhibitor) or AG1478 (an EGFR inhibitor) significantly inhibited the antiapoptotic and prosurvival effects of 11,12-EET treatment and CYP2J2 overexpression, with EGFR inhibition being especially detrimental. These results demonstrate that EET-induced EGFR transactivation and PPARγ signaling is an essential event in preventing cardiac myocyte apoptosis and enhancing survival. These results are supported by previous studies in other cell types showing EGFR transactivation in response to EET treatment (9, 38).
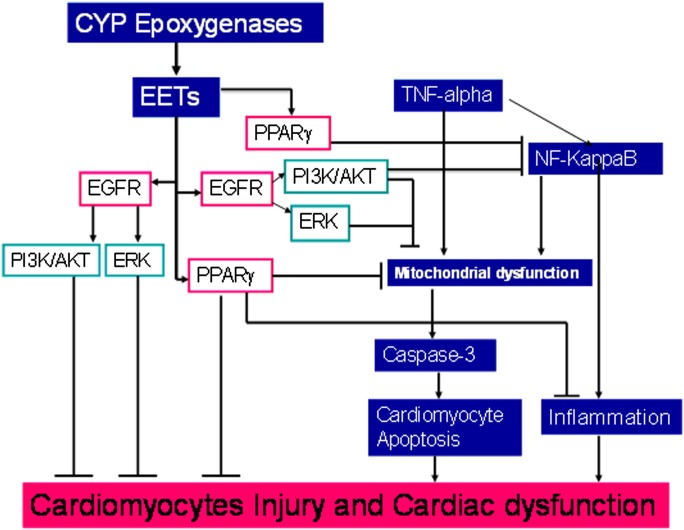
PPARγ agonists have consistently been shown to decrease both chemokine secretion and matrix metalloproteinase (MMP) expression by inhibiting NF-κB activation (39–41). PPARγ activation is also known to attenuate inflammation and apoptosis during cardiac ischemia and injury (42). A study by Liu et al. showed that PPARγ inhibition abolished the EET/AUDA-mediated anti-inflammatory effect in endothelial cells subjected to laminar flow (31). In addition, PPARγ is an effector of EETs via competition and direct binding assays, and PPARγ mediated anti-inflammatory effects of EETs in endothelium (31). PPARγ agonist reduced lipopolysaccharides (LPS)-induced NF-κB activation by antagonizing NF-κB DNA activation (43). In addition, PPARγ agonists similarly inhibit cardiomyocyte inflammatory responses (44, 45). Consistent with these findings, we found that 11,12-EET and CYP2J2 overexpression partially but significantly prevented the loss of PPARγ expression that is normally induced by TNF-α. Furthermore, we found that the PPARγ inhibitor GW9662 hindered the 11,12-EET– and CYP2J2-mediated downregulation of caspase-3 activity and Akt/ERK activation, which may be linked to increase in heparin-binding epithelial growth factor (HB-EGF) release (9, 38). Together, these findings suggest that PPARγ activation plays some role in EET-mediated cardioprotection (Scheme 1). In our study, CYP2J2 overexpression or EET activated MAPK1/2, but PPARγ inhibitor GW9662 blocked this effect; However, PPARγ inhibitor GW9662 had much less inhibitory effect on EET-mediated EGFR activation. These results suggest that PPARγ in heart may have cross-talk with EGFR downstream pathway.
Scheme 1.
Signaling pathways of heart protective effects of EETs. A stop (⊥) indicates inhibitory or blocking effects, and an arrow (→) indicates increasing or enhancing effects.
In previous studies, we demonstrated that overexpression of epoxygenases can reduce systolic blood pressure in hypertensive rats via enhancing atrial natriuretic peptide production in cardiomyocytes (36) and can attenuate pulmonary artery hypertension with significantly decreased inflammatory cytokines in monocrotaline-induced pulmonary artery hypertension model. EETs protect endothelial cells from apoptosis and increased eNOS activity and expression level (46). Dr. Liao demonstrated that EETs inhibited leukocyte adhesion to endothelial cells and reduced IL-1 via inhibition of NF-κB in endothelial cells (2). These data indicate that EETs really have systemic effects and that these cardiovascular protective effects should be able to exert beneficial effects on the heart, especially by decreasing inflammatory cytokines. In the present study, artery blood pressure was not high; therefore, overexpression of epoxygenase had no hypotensive effect.
This study has some limitations. Further studies are needed to figure out whether cardioprotective effects of EETs in anti-inflammation and antiapoptosis involve activation of G-protein coupled receptors and their hyperpolarization role via activating calcium-activated potassium channels, as well as more detailed molecular mechanisms and targets of EETs.
In summary, the present study provides insight on the protective role of EETs against cardiac dysfunction and myocardial injuries. The potency of EET-mediated cardiac protection suggests that abundant CYP2J2 expression and EET production in the myocardium are important protective factors whose mechanisms are likely mediated via EGFR and PPARγ activation. Our data support the development of tools to enhance endogenous EETs and/or to deliver exogenous EETs as a novel approach to the treatment of cardiovascular complications, including heart failure.
Supplementary Material
Footnotes
Abbreviations:
- AA
- arachidonic acid
- rAAV
- recombinant adeno-associated viral vector
- AG1478
- N-(3-Chlorophenyl)-6,7-dimethoxy-4-quinazolinamine
- AKT
- protein kinase B
- CYP2J2
- cytochrome P450 2J2
- sE-selectin
- soluble endothelium-selectin
- EET
- epoxyeicosatrienoic acid
- EGFR
- epidermal growth factor receptor
- ERK
- extracellular signal-regulated kinase
- GFP
- green fluorescent protein
- GW9662
- 2-Chloro-5-nitro-N-phenylbenzamide
- HUVEC
- human umbilical vein endothelial cell
- sICAM-1
- soluble intercellular adhesion molecule-1
- IκBα
- inhibitory subunit of NF-κBα
- IL
- interleukin
- LVEDP
- left ventricular end diastolic pressure
- MAPK
- mitogen-activated protein kinase
- NF-κB
- nuclear factor-κB
- eNOS
- endothelial nitric-oxide synthase
- PBMC
- peripheral blood mononuclear cell
- PI3K
- phosphoinositide 3-kinase
- PPARγ
- peroxisome proliferator-activated receptor γ
- PPOH
- 2-(2-propynyloxy)-benzenehexanoic acid
- PVDF
- polyvinylidene difluoride
- TNF-α
- tumor necrosis factor-α
- VCAM-1
- vascular cell adhesion molecule-1
- sVCAM-1
- soluble VCAM-1
This work was supported by National Natural Science Foundation of China Grant Nos. 31130031, 30770882, 81070236 and 3113031; by National Basic Research Program of China (973 Program) Grant No. 2007CB512004; and by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures.
REFERENCES
- 1.Capdevila J. H., Falck J. R., Harris R. C. 2000. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 41: 163–181. [PubMed] [Google Scholar]
- 2.Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D. C., Liao J. K. 1999. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 285: 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miura H., Gutterman D. D. 1998. Human coronary arteriolar dilation to arachidonic acid depends on cytochrome P-450 monooxygenase and Ca2+-activated K+ channels. Circ. Res. 83: 501–507. [DOI] [PubMed] [Google Scholar]
- 4.Fisslthaler B., Popp R., Kiss L., Potente M., Harder D. R., Fleming I., Busse R. 1999. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 401: 493–497. [DOI] [PubMed] [Google Scholar]
- 5.Casolari C., Rossi T., Baggio G., Coppi A., Zandomeneghi G., Ruberto A. I., Farina C., Fabio G., Zanca A., Castelli M. 2004. Interaction between saquinavir and antimycotic drugs on C. albicans and C. neoformans strains. Pharmacol. Res. 50: 605–610. [DOI] [PubMed] [Google Scholar]
- 6.Sun J., Sui X., Bradbury J. A., Zeldin D. C., Conte M. S., Liao J. K. 2002. Inhibition of vascular smooth muscle cell migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ. Res. 90: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 7.Yang S., Lin L., Chen J. X., Lee C. R., Seubert J. M., Wang Y., Wang H., Chao Z. R., Tao D. D., Gong J. P., et al. 2007. Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 293: H142–H151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang J. G., Chen R. J., Xiao B., Yang S., Wang J. N., Wang Y., Cowart L. A., Xiao X., Wang D. W., Xia Y. 2007. Regulation of endothelial nitric-oxide synthase activity through phosphorylation in response to epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat. 82: 162–174. [DOI] [PubMed] [Google Scholar]
- 9.Chen J. K., Capdevila J., Harris R. C. 2002. Heparin-binding EGF-like growth factor mediates the biological effects of P450 arachidonate epoxygenase metabolites in epithelial cells. Proc. Natl. Acad. Sci. USA. 99: 6029–6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C., Harder D. R. 2002. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic acid. Stroke. 33: 2957–2964. [DOI] [PubMed] [Google Scholar]
- 11.Medhora M., Daniels J., Mundey K., Fisslthaler B., Busse R., Jacobs E. R., Harder D. R. 2003. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 284: H215–H224. [DOI] [PubMed] [Google Scholar]
- 12.Michaelis U. R., Fisslthaler B., Medhora M., Harder D., Fleming I., Busse R. 2003. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR). FASEB J. 17: 770–772. [DOI] [PubMed] [Google Scholar]
- 13.Pratt P. F., Medhora M., Harder D. R. 2004. Mechanisms regulating cerebral blood flow as therapeutic targets. Curr. Opin. Investig. Drugs. 5: 952–956. [PubMed] [Google Scholar]
- 14.Michaelis U. R., Falck J. R., Schmidt R., Busse R., Fleming I. 2005. Cytochrome P4502C9-derived epoxyeicosatrienoic acids induce the expression of cyclooxygenase-2 in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 25: 321–326. [DOI] [PubMed] [Google Scholar]
- 15.Graber M. N., Alfonso A., Gill D. L. 1997. Recovery of Ca2+ pools and growth in Ca2+ pool-depleted cells is mediated by specific epoxyeicosatrienoic acids derived from arachidonic acid. J. Biol. Chem. 272: 29546–29553. [DOI] [PubMed] [Google Scholar]
- 16.Chen J. K., Capdevila J., Harris R. C. 2000. Overexpression of C-terminal Src kinase blocks 14, 15-epoxyeicosatrienoic acid-induced tyrosine phosphorylation and mitogenesis. J. Biol. Chem. 275: 13789–13792. [DOI] [PubMed] [Google Scholar]
- 17.Chen J. K., Wang D. W., Falck J. R., Capdevila J., Harris R. C. 1999. Transfection of an active cytochrome P450 arachidonic acid epoxygenase indicates that 14,15-epoxyeicosatrienoic acid functions as an intracellular second messenger in response to epidermal growth factor. J. Biol. Chem. 274: 4764–4769. [DOI] [PubMed] [Google Scholar]
- 18.Seubert J., Yang B., Bradbury J. A., Graves J., Degraff L. M., Gabel S., Gooch R., Foley J., Newman J., Mao L., et al. 2004. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ. Res. 95: 506–514. [DOI] [PubMed] [Google Scholar]
- 19.Gross G. J., Falck J. R., Gross E. R., Isbell M., Moore J., Nithipatikom K. 2005. Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc. Res. 68: 18–25. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J. G., Chen C. L., Card J. W., Yang S., Chen J. X., Fu X. N., Ning Y. G., Xiao X., Zeldin D. C., Wang D. W. 2005. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 65: 4707–4715. [DOI] [PubMed] [Google Scholar]
- 21.Yang B., Graham L., Dikalov S., Mason R. P., Falck J. R., Liao J. K., Zeldin D. C. 2001. Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol. Pharmacol. 60: 310–320. [DOI] [PubMed] [Google Scholar]
- 22.Prasad A. M., Ma H., Sumbilla C., Lee D. I., Klein M. G., Inesi G. 2007. Phenylephrine hypertrophy, Ca2+-ATPase (SERCA2), and Ca2+ signaling in neonatal rat cardiac myocytes. Am. J. Physiol. Cell Physiol. 292: C2269–C2275. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Wei X., Xiao X., Hui R., Card J. W., Carey M. A., Wang D. W., Zeldin D. C. 2005. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J. Pharmacol. Exp. Ther. 314: 522–532. [DOI] [PubMed] [Google Scholar]
- 24.Kotamraju S., Konorev E. A., Joseph J., Kalyanaraman B. 2000. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J. Biol. Chem. 275: 33585–33592. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C., Wang P., Xiao X., Chao J., Chao L., Wang D. W., Zeldin D. C. 2003. Gene therapy with human tissue kallikrein reduces hypertension and hyperinsulinemia in fructose-induced hypertensive rats. Hypertension. 42: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 26.Xu X., Wan W., Ji L., Lao S., Powers A. S., Zhao W., Erikson J. M., Zhang J. Q. 2008. Exercise training combined with angiotensin II receptor blockade limits post-infarct ventricular remodelling in rats. Cardiovasc. Res. 78: 523–532. [DOI] [PubMed] [Google Scholar]
- 27.Luu N. T., Rainger G. E., Nash G. B. 1999. Kinetics of the different steps during neutrophil migration through cultured endothelial monolayers treated with tumour necrosis factor-alpha. J. Vasc. Res. 36: 477–485. [DOI] [PubMed] [Google Scholar]
- 28.Tsouknos A., Nash G. B., Rainger G. E. 2003. Monocytes initiate a cycle of leukocyte recruitment when cocultured with endothelial cells. Atherosclerosis. 170: 49–58. [DOI] [PubMed] [Google Scholar]
- 29.Wang H., Lin L., Jiang J., Wang Y., Lu Z. Y., Bradbury J. A., Lih F. B., Wang D. W., Zeldin D. C. 2003. Up-regulation of endothelial nitric-oxide synthase by endothelium-derived hyperpolarizing factor involves mitogen-activated protein kinase and protein kinase C signaling pathways. J. Pharmacol. Exp. Ther. 307: 753–764. [DOI] [PubMed] [Google Scholar]
- 30.Wu S., Moomaw C. R., Tomer K. B., Falck J. R., Zeldin D. C. 1996. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J. Biol. Chem. 271: 3460–3468. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Zhang Y., Schmelzer K., Lee T. S., Fang X., Zhu Y., Spector A. A., Gill S., Morisseau C., Hammock B. D., et al. 2005. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA. 102: 16747–16752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottlieb R. A., Burleson K. O., Kloner R. A., Babior B. M., Engler R. L. 1994. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J. Clin. Invest. 94: 1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krown K. A., Page M. T., Nguyen C., Zechner D., Gutierrez V., Comstock K. L., Glembotski C. C., Quintana P. J., Sabbadini R. A. 1996. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J. Clin. Invest. 98: 2854–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubota T., McTiernan C. F., Frye C. S., Slawson S. E., Lemster B. H., Koretsky A. P., Demetris A. J., Feldman A. M. 1997. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ. Res. 81: 627–635. [DOI] [PubMed] [Google Scholar]
- 35.Horton J. W., Maass D. L., Ballard-Croft C. 2005. Rho-associated kinase modulates myocardial inflammatory cytokine responses. Shock. 24: 53–58. [DOI] [PubMed] [Google Scholar]
- 36.Xiao B., Li X., Yan J., Yu X., Yang G., Xiao X., Voltz J. W., Zeldin D. C., Wang D. W. 2010. Overexpression of cytochrome P450 epoxygenases prevents development of hypertension in spontaneously hypertensive rats by enhancing atrial natriuretic peptide. J. Pharmacol. Exp. Ther. 334: 784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng C., Wang L., Li R., Ma B., Tu L., Xu X., Dackor R. T., Zeldin D. C., Wang D. W. 2010. Gene delivery of cytochrome p450 epoxygenase ameliorates monocrotaline-induced pulmonary artery hypertension in rats. Am. J. Respir. Cell Mol. Biol. 43: 740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng L. M., Jiang J. G., Sun Z. Y., Chen C., Dackor R. T., Zeldin D. C., Wang D. W. 2010. The epoxyeicosatrienoic acid-stimulated phosphorylation of EGF-R involves the activation of metalloproteinases and the release of HB-EGF in cancer cells. Acta Pharmacol. Sin. 31: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricote M., Li A. C., Willson T. M., Kelly C. J., Glass C. K. 1998. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 391: 79–82. [DOI] [PubMed] [Google Scholar]
- 40.Chinetti G., Fruchart J. C., Staels B. 2001. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors with functions in the vascular wall. Z. Kardiol. 90(Suppl. 3): 125–132. [DOI] [PubMed] [Google Scholar]
- 41.Moraes L. A., Piqueras L., Bishop-Bailey D. 2006. Peroxisome proliferator-activated receptors and inflammation. Pharmacol. Ther. 110: 371–385. [DOI] [PubMed] [Google Scholar]
- 42.Liu H. R., Tao L., Gao E., Lopez B. L., Christopher T. A., Willette R. N., Ohlstein E. H., Yue T. L., Ma X. L. 2004. Anti-apoptotic effects of rosiglitazone in hypercholesterolemic rabbits subjected to myocardial ischemia and reperfusion. Cardiovasc. Res. 62: 135–144. [DOI] [PubMed] [Google Scholar]
- 43.Takano H., Nagai T., Asakawa M., Toyozaki T., Oka T., Komuro I., Saito T., Masuda Y. 2000. Peroxisome proliferator-activated receptor activators inhibit lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal rat cardiac myocytes. Circ. Res. 87: 596–602. [DOI] [PubMed] [Google Scholar]
- 44.Ding G., Cheng L., Qin Q., Frontin S., Yang Q. 2006. PPARdelta modulates lipopolysaccharide-induced TNFalpha inflammation signaling in cultured cardiomyocytes. J. Mol. Cell. Cardiol. 40: 821–828. [DOI] [PubMed] [Google Scholar]
- 45.Ye P., Fang H., Zhou X., He Y. L., Liu Y. X. 2004. Effect of peroxisome proliferator-activated receptor activators on tumor necrosis factor-alpha expression in neonatal rat cardiac myocytes. Chin. Med. Sci. J. 19: 243–247. [PubMed] [Google Scholar]
- 46.Xu X., Zhang X. A., Wang D. W. 2011. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv. Drug Deliv. Rev. 63: 597–609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.