Abstract
The elucidation of the role of endocannabinoids in physiological and pathological conditions and the transferability of the importance of these mediators from basic evidence into clinical practice is still hampered by the indefiniteness of their circulating reference intervals. In this work, we developed and validated a two-dimensional LC/MS/MS method for the simultaneous measurement of plasma endocannabinoids and related compounds such as arachidonoyl-ethanolamide, palmitoyl-ethanolamide, and oleoyl-ethanolamide, belonging to the N-acyl-ethanolamide (NAE) family, and 2-arachidonoyl-glycerol and its inactive isomer 1-arachidonoyl-glycerol from the monoacyl-glycerol (MAG) family. We found that several pitfalls in the endocannabinoid measurement may occur, from blood withdrawal to plasma processing. Plasma extraction with toluene followed by on-line purification was chosen, allowing high-throughput and reliability. We estimated gender-specific reference intervals on 121 healthy normal weight subjects fulfilling rigorous anthropometric and hematic criteria. We observed no gender differences for NAEs, whereas significantly higher MAG levels were found in males compared with females. MAGs also significantly correlated with triglycerides. NAEs increased with age in females, and arachidonoyl-ethanolamide correlated with adiposity and metabolic parameters in females. This work paves the way to the establishment of definitive reference intervals for circulating endocannabinoids to help physicians move from the speculative research field into the clinical field.
Keywords: endocannabinoids, validation, reference intervals
The endocannabinoids (ECs) are bioactive lipid mediators derived from membrane phospholipids. Since the discovery of the first lipid mediator of the endocannabinoid system (ECS), arachidonoyl-ethanolamide (AEA), also called anandamide (1), several molecules belonging to this family were identified, the most important being 2-arachidonoyl-glycerol (2AG) and its isomer 1AG among MAGs (2, 3), palmitoyl-ethanolamide (PEA) and oleoyl-ethanolamide (OEA) among the NAEs, to which AEA belongs (4). Both 2AG and AEA act on cannabinoid receptor type 1 (CB1) and type 2 (CB2), whereas PEA and OEA act by influencing AEA metabolism and binding the peroxisome proliferator-activated receptor α (5, 6), thus defined endocannabinoid related compounds (ERC).
To date, the ECS has been implicated in a number of physiological and pathological processes involving different homeostatic mechanisms, mostly related to psychiatric disorders such as depression, anxiety, and schizophrenia, in stress recovery and hypothalamus-pituitary-adrenal axis modulation, neuropathic and inflammatory pain, immune function (7), fertility and reproduction (8). Moreover, based on the evidence on the ECS role in the regulation of behavioral and metabolic processes of energy balance, ECs represent a pharmacological target for the treatment of obesity and related cardiometabolic risk (9–11).
Despite the growing importance of ECs as modulators of the complex immuno-neuro-endocrine system and the proliferation of mass spectrometry-based quantitative methods, circulating reference intervals have not yet been established and poor agreement exists among the levels reported so far (11, 12). Several preanalytical and analytical pitfalls, potentially explaining part of the discrepancy, were described in the last decade (13–18); in addition, the definition of “normality”, and thus, the selection of control subjects, may have represented a major source of variability among the reported values.
In this study we developed a two-dimensional (2D) LC/MS/MS system for the simultaneous measurement of the five major ECs and ERCs in plasma, AEA, 2AG, 1AG, PEA, and OEA, eliminating or minimizing the pitfalls we encountered, mainly acyl-migration causing isomerization of 2AG in 1AG, contamination of PEA and OEA in solvents and disposables, and the stability of ECs in blood and plasma during sample collection and processing. The validation was conducted following the Food and Drug Administration (FDA) guidelines for bioanalytical method validation (19), by using a plasma matrix deprived or with minimal amounts of the analytes. By applying rigorous inclusion and exclusion criteria and by monitoring anthropometric and biochemical parameters, we were able to estimate EC and ERC reference intervals in a selected population of 121 (76 females and 45 males) healthy, drug-free, and normal-weight subjects. We also examined the association between the abovementioned anthropometric and biochemical parameters and circulating ECs and ERCs.
MATERIALS AND METHODS
Chemicals and disposables
The following compounds were used: AEA, 2AG, 1AG, PEA, OEA, O-arachidonoyl-ethanolamide, d8-AEA, d5-2AG, d5-1AG, d4-PEA, d2-OEA (Cayman Chemical, Ann Arbor, MI). Ethanol (EtOH), ethyl acetate, dichloromethane, n-hexane, all HPLC-grade, methanol (MeOH) gradient-grade, ethyl acetate in polyethylene high density bottle, cyclohexane, chloroform, all analysis-grade, and charcoal-activated powder were from Merck (Darmstadt, Germany). Toluene, acetone, acetic acid glacial, diethyl ether, all analysis-grade, and TRIS were from Carlo Erba (Rodano, Italy). Ultrapure water was produced by MilliQ Gradient A10 system (Millipore, Volketswil, Switzerland). Polypropylene 1.5 ml tubes were from Eppendorf (Hamburg, Germany), Pyrex 12 × 75 mm tubes from Bibby Scientific (Riozzo di Cerro, Italy), 12 × 75 mm glass tubes from Laboindustria S.p.A (Arzergrande, Italy), and 13 × 100 mm capped Pyrex tubes from Sigma-Aldrich (Gallarate, Italy).
Standard solutions, calibrators, and in-house quality controls
The chemical purity declared by the supplier was ≥98% for AEA, 1AG, PEA, OEA, d4-PEA, and d2-OEA, ≥95% for 2AG, d8-AEA, and d5-2AG, and a purity ≥97% of the deuterated product was declared for d5-1AG. The occurrence of d0-standard in deuterated compounds was ≤1% for all but d5-2AG, for which it was not specified. AEA, d4-PEA, and d2-OEA (EtOH), (d5-)2AG and (d5-)1AG (acetonitrile), and d8-AEA(methyl acetate) were supplied as stock solution at known concentration declared by the supplier. OEA and PEA were supplied as crystalline solid; OEA was reconstituted to a stock solution of 15.360 µmol/ml by adding 1 ml MeOH and gently mixing. PEA was gravimetrically determined with Mettler Toledo AX105DR balance and diluted in EtOH to a 3.172 µmol/ml stock solution. Working solutions were prepared in MeOH at the following concentrations: AEA and OEA, 60 nmol/ml; 2AG, 1AG, PEA, and d2OEA, 30 nmol/ml.
A standard mix was prepared at concentrations of 250, 500, 500, 2500, and 1250 pmol/ml for AEA, 2AG, 1AG, PEA, and OEA, respectively; from this solution, three serial dilutions at a factor 4 and four further serial dilutions at a factor 2 were prepared for a total of eight calibrators. Stock solutions of d8-AEA, d5-2AG, d5-1AG, and d4-PEA, used as internal standards (IS), were mixed to a final concentration of 6 nmol/ml. All solutions were stored at −80°C. The calibration curve was prepared by adding 3 pmol of IS mix to proper amounts of calibrators in order to produce the following concentrations: A: 10, 20, 20, 100, 50 pmol/ml; B: 2.5, 5, 5, 25, 12.5 pmol/ml; C: 0.625, 1.25, 1.25, 6.25, 3.125 pmol/ml; D: 0.1563, 0.3125, 0.3125, 1.5625, 0.7813 pmol/ml; E: 0.0781, 0.1563, 0.1563, 0.7813, 0.3906 pmol/ml; F: 0.0391, 0.0781, 0.0781, 0.3906, 0.1953 pmol/ml; G: 0.0195, 0.0391, 0.0391, 0.1953, 0.0977 pmol/ml; H: 0.0098, 0.0195, 0.0195, 0.0977, 0.0488 pmol/ml for AEA, 2AG, 1AG, PEA, and OEA, respectively. In the zero point, the calibrator was substituted by MeOH. Quality controls (QCs) were prepared by pooling the discarded plasma of the Central Laboratory of S.Orsola-Malpighi Hospital. The plasma pool was split in two parts: the first used as is and the second processed with activated charcoal for five cycles to produce an EC-depleted plasma. Charcoal was added at 10% (w/v) to the plasma pool and gently mixed for 8 hr at 4°C. Charcoal was removed by centrifugation (90 min, 2,000 g, 4°C). The pH was finally adjusted to 7.5. The stripped plasma was then spiked with known amounts of pure standards in order to produce in house QCs at low, medium, and high ranges of concentration. QCs were stored at −80°C in 0.6 ml aliquots in polypropylene tubes.
Specimens
One-hundred-twenty-one subjects aged 18 to 78 were selected from among more than 800 subjects recruited as part of the Progetto Regione-Università of the Emilia Romagna Region study focusing on the identification and profiling of genetic and circulating biomarkers for the classification and the clinical assessment of patients affected by the metabolic syndrome. The study was approved by the local Ethics Committee. Each subject was selected by telephone interview and examined at the local health service of the town of Massa Lombarda, after informed consent was obtained. All the subjects had a body mass index (BMI) between 18.1 and 25.0kg/m2, a waist circumference below 88 cm in females and 102 cm in males, body weight stability in the last 3 months, systolic and diastolic blood pressure (SBP and DBP, respectively) below 140 and 90mmHg, complete sexual development and menstrual cycle regularity in fertile women, and a normal wake-sleep cycle. Subjects taking any kind of drug (except for antipyretic or anti-inflammatory compounds if taken one month before blood withdrawal), or presenting endocrine, hepatic, renal, tumoral, autoimmune, cardiovascular, hematological, neurological, or psychiatric diseases, sleep disorders, or allergies requiring treatment were excluded. Subjects filled in the questionnaires for the identification of bulimic attitude (Bulimic Investigation test Edinburgh, BITE) (20), cognitive or somatic depressive symptoms (Beck depression inventory) (21), or binge-eating disorder (Binge Eating Scale, BES) (22). A score was attributed for each test, and subjects were excluded with BITE scores >10 for symptoms and 5 for severity, with BECK scores >10 and with BES scores >17. Blood withdrawal was performed after an overnight fasting at 8–10 a.m. following 10 min saline infusion to avoid any stress related alteration, in Vacuette K3-EDTA tubes (Greiner Bio-One, Kremsmunster, Austria). Samples were immediately centrifuged (10 min, 2,000 rcf, 4°C); plasma was immediately stored in 1.5 ml polypropylene tubes at −80°C until analysis. Serum was collected in Vacuette Z serum beads clot activator, allowed to settle for 20 min, and centrifuged (10 min, 2000 rcf, room temperature, RT). Serum parameters (intra- and inter-assay CV) were measured in the Central Laboratory of S.Orsola-Malpighi Hospital by Modular analyzer (Roche Diagnostics, Mannheim, Germany): triglycerides (<1.5 and 1.8%), total cholesterol (<1.0 and 2.7%), HDL cholesterol (<0.95 and 1.3%), insulin (1.5 and 4.9%), uric acid (0.5 and 0.7%). SBP and DBP were recorded with a sphygmomanometer (Gamma G5, Heine, Herrsching, Germany) and glycemia was measured with a glucometer Breeze 2 (BAYER, Leverkusen, Germany, CV range 2.0–4.5%).
Sample preparation
Samples and QCs were thawed in ice and vortexed before 0.5 ml was transferred in 13 × 100 mm Pyrex tubes.
Liquid-liquid extraction (LLE).
For each batch, the IS mix was diluted in EtOH to a working solution of 30 pmol/ml, and 0.1 ml was added to each sample and QC. After 5 min vortex, 2 ml of the following solvents were added: toluene, ethyl acetate, ethyl acetate:n-hexane(2:1), and diethyl ether. After 10 min vortex and centrifugation (10 min, 2,000 rcf, 4°C), samples were dipped for 5 min into acetone bath in dry ice. The upper layer was transferred to 12 × 75 mm Pyrex tubes and evaporated under a gentle stream of nitrogen. Alternatively, samples were extracted by the traditional Folch's modified method (23, 24): 0.5 ml plasma was deproteinized with 1 ml cold acetone and 0.5 ml of 50 mM TRIS, vortexed for 10 min and centrifuged (10 min, 2,000 rcf, 4°C); the upper phase was evaporated to 1 ml before adding 1 ml of a chloroform:MeOH (2:1) solution. After 10 min vortex and centrifugation (10 min, 2,000 rcf, 4°C), the lower layer was transferred to 12 × 75 mm Pyrex tubes and evaporated. One milliliter of chloroform was added to the residual upper layer and the extraction was repeated; the lower layer was added to the previous one and similarly evaporated.
Solid phase extraction (SPE).
IS mix was diluted in MeOH to a final concentration of 12 pmol/ml and 0.25 ml of this solution was added to 0.5 ml of samples and QCs. After vortex and centrifugation (4°C, 10 min, 2,000 rcf), the supernatant was transferred to SPE cartridge IST Isolute C18 100 mg 1cc inserted in an IST VacMaster vacuum manifold (Biotage, Uppsala, Sweden), previously activated with 1 ml MeOH, and conditioned with 1 ml water. Samples were washed with 2 ml water and 1 ml of 60% MeOH solution; analytes were eluted in 12 × 75 mm Pyrex tubes with 1 ml of a cyclohexane:ethyl acetate (1:1) solution. Dried samples were reconstituted with 100 ul MeOH and transferred into 0.3 ml glass vials (Agilent, Cernusco sul Naviglio, Italy) and placed in a Series 200 Autosampler thermostatted at 4°C (PerkinElmer, Waltham, MA).
On-line purification and LC separation
LLE extracts.
The chromatographic separation was operated on a modular HPLC Series 200 by PerkinElmer. Seventy microliters were injected into the 2D-LC-system by a 3 ml/min loading flow of 28% MeOH and directed to the perfusion column POROS R1/20 2.1 × 30 mm (Applied Biosystems, Foster City, CA). At min 1, the sample was backflushed to the analytical column Discovery HS C18 7.5cm × 4.6 mm, 3 µm, equipped with 2 cm × 4.0 mm, 3 µm, 120A guardcolumn (Supelco, Bellafonte, PA) through a ten-port switching valve (VICI, Houston, TX) at 0.9 ml/min of an eluent made with 90% solvent A (0.1% acetic acid) and 10% solvent B (99.7% MeOH, 0.2% H2O, 0.1% acetic acid). The solvent B increased to 86% from min 3.2 to 5.2; from min 8.5 to 9.0, it moved to 89% and at min 12, it reached 92%. The analytical column was washed with 100% solvent B from min 12.5 to 16.5; the system was then reequilibrated at the initial conditions for 5.5 min. The perfusion column was washed at 2 ml/min with 100% MeOH from min 13.1 to min 15.0 and then reequilibrated to the initial conditions until the end of the run. Oven temperature was set at 42°C. The total run time was 22min.
SPE extracts.
SPE extracts were analyzed in a 1D-LC-system by injecting 70 µl of sample in 0.9 ml/min flow at 90% solvent A directed to the analytical column; the gradient program operated as described for the 2D-LC-system. The chromatographic method was tested for the retention time variability across 15 sessions performed over 2 months and for the presence of carryover by injection of blank samples (MeOH) after the highest calibration point.
Mass spectrometry detection
Mass spectrometric measurements were performed by an API 4000-QTrap (AB-Sciex, Toronto, Canada) working in triple-quadrupole mode. Quantitation was performed by multiple reaction monitoring (MRM) mode, choosing the “quantifier” transition and the “qualifier” one for the confirmation for each analyte. The ratio between the two transitions (ion ratio, IR) was monitored for each analyte in each sample: the measurement was considered nonspecific if the sample IR exceeded the IR of the pure standard by ±20%. MRM parameters were optimized by infusing standard solutions at concentrations ranging from 0.3 to 30 nmol/ml into the Turbo-V source through an infusion pump set at 10 µl/min in addition to a makeup flow of 50% MeOH at 400 µl/min (Table 1). The atmospheric pressure chemical ionization probe operated with a Corona discharge current of 3 µA in positive ion mode. Collision activated dissociation gas was nitrogen set at 10mTorr and the other parameter settings were: probe temperature 400°C, curtain gas 45 psi, nebulizing gas 1 and 2 at 45 psi and 30 psi, respectively. The dwell time for each transition was 60 ms. Unit mass resolution was set for both first and third quadrupole.
TABLE 1.
Experimental conditions for the LC/MS/MS detection
| Analyte | MW | Rt | Transition | Q1 | Q3 | DP | CE | CXP | IR |
| AEA | 347.5 | 11.16 | Quantifier | 348.3 | 62.0 | 70 | 35 | 4 | 4.17 |
| Qualifier | 348.3 | 287.0 | 70 | 15 | 7 | ||||
| d8-AEA | 355.6 | 11.11 | IS | 356.6 | 63.0 | 60 | 30 | 4 | |
| 2AG | 378.6 | 11.45 | Quantifier | 379.4 | 287.2 | 60 | 21 | 7 | 2.20 |
| Qualifier | 379.4 | 269.3 | 60 | 25 | 5 | ||||
| d5-2AG | 383.6 | 11.43 | IS | 384.3 | 287.3 | 66 | 20 | 7 | |
| 1AG | 378.6 | 11.79 | Quantifier | 379.4 | 287.2 | 60 | 21 | 7 | 2.56 |
| Qualifier | 379.4 | 269.3 | 60 | 25 | 5 | ||||
| d5-1AG | 383.6 | 11.77 | IS | 384.3 | 287.3 | 66 | 20 | 7 | |
| PEA | 299.5 | 12.05 | Quantifier | 300.6 | 62.1 | 60 | 30 | 4 | 3.90 |
| Qualifier | 300.6 | 57.0 | 60 | 55 | 4 | ||||
| d4-PEA | 303.5 | 12.02 | IS | 304.7 | 62.1 | 70 | 30 | 4 | |
| OEA | 325.5 | 12.51 | Quantifier | 326.5 | 62.1 | 60 | 35 | 5 | 22.93 |
| Qualifier | 326.5 | 121.2 | 60 | 35 | 7 | ||||
| d2-OEA | 327.5 | 12.50 | n.d.a | 328.7 | 62.2 | 70 | 30 | 4 |
Molecular weight (M.W.), retention time (Rt, min), precursor ion (Q1, m/z), fragment ion (Q3, m/z), declustering potential (DP, V), collision energy (CE, eV), cell exit potential (CXP, V), and ion ratio (IR).
d2-OEA was used only in post column infusion experiment.
Quantitation
Data processing and quantitation were performed by Analyst 1.4.2 software package by AB-Sciex. Calibration was done through linear regression: concentrations for each analyte were back calculated by interpolation on the respective regression curve.
Validation
Selection of the extraction procedure.
Acyl-migration evaluation in 2AG and d5-2AG standard.
The isomerization of (d5-)2AG into (d5-)1AG and the recovery of the total area counts of (d5-)2AG plus (d5-)1AG occurring during the evaporation under nitrogen stream were evaluated in different solvents and tubes by spiking 9pmol of (d5-)2AG standard in 1ml of MeOH, toluene, ethyl acetate, ethyl acetate:n-hexane(2:1), diethyl ether, chloroform, chloroform:MeOH(2:1), dichloromethane, and cyclohexane:ethyl acetate(1:1) in glass, Pyrex, and polypropylene tubes. After evaporation, the dried residuals were reconstituted in 100 µl of MeOH and injected in the LC/MS/MS system. The percentage of (d5-)1AG and the sum of peak area from (d5-)2/1AG found in each solvent/tube condition were compared with those found in 9 pmol of pure non evaporated (d5-)2AG standard diluted in 100 µl of MeOH and injected in the LC/MS/MS system. Each condition was performed in triplicate.
Analyte trace assessment in solvents and tubes.
The presence of traces of the monitored analytes was investigated by dispensing 1 ml of ethyl acetate, toluene, chloroform, dichloromethane, n-hexane, cyclohexane, MeOH, and diethyl ether in glass, Pyrex or polypropylene tubes: each tube was vortexed for 2 min before the solvents were dried under a nitrogen stream. The dried residuals were reconstituted and injected as previously described. All solvents were dispensed from sealed new bottles with glass Pasteur previously rinsed with the respective solvent. All conditions were analyzed in six replications.
Recovery comparison.
A total of 0.5 ml of stripped plasma was spiked with 20 µl of calibrator C and extracted by the described LLE and SPE procedures. Each condition was performed in triplicate. The peak area found in each spiked sample, subtracted for the peak area found in basal stripped plasma, was compared with the peak area of the pure standards. The recovery of the on line purification was also valued in spiked stripped plasma extracted with toluene by comparing the peak area of spiked stripped plasma injected with 2D-LC-system with those injected with 1D-LC-system.
Linearity, sensitivity, accuracy.
A 1/x weighting was chosen to ensure higher accuracy and precision at the lower end of the curve. D8-AEA was used as IS for AEA and OEA, d5-2AG for 2AG, d5-1AG for 1AG and d4-PEA for PEA. To avoid any reciprocal interference due to isotopic distribution between OEA and the available isotope compound d2-OEA, we decided to avoid its use for the quantitation. Linearity and lower limit of quantitation (LLOQ) were calculated on three calibration curves, each conducted in triplicate, run over 3 consecutive weeks. LLOQ was defined as the lowest concentration exhibiting a signal to noise ratio (S/N) >5, an accuracy between 80 and 120% of the true value, and a CV <20%. The limit of detection (LOD) was determined as the lowest concentration exhibiting an S/N > 3. We valued linearity and sensitivity in plasma matrix by spiking 20 µl of calibrator A–H and 20 µl of MeOH for the zero level in 0.5ml aliquots of stripped plasma, and then processing each sample with toluene LLE procedure. To assess accuracy, analyte residuals measured in stripped plasma were subtracted at each point, and the resulting concentration was compared with the expected concentration. All conditions were performed in triplicate.
Intra-and inter-assay imprecision.
Method imprecision was estimated on five replicates analyzed in the same run (intra-assay) and in five runs performed on different days (inter-assay) of QCs at low, medium, and high level and in plasma pool, processed by toluene LLE.
Matrix interference.
A postcolumn infusion of a mixture containing the five deuterated compounds at concentrations suitable for generating measurable steady-state signals was performed during injections of several individual samples extracted with the proposed LLE and SPE. Ion suppression was further investigated in toluene extracts by spiking known amounts of standard analytes on preextracted stripped plasma samples to exclude any procedural losses from the yield calculation. Analyte peak areas in plasma matrix, subtracted for the peak areas of residual analytes in stripped plasma extracts, were compared with peak areas of pure standards.
Stability
The stability of the ECs and ERCs in whole blood was determined by collecting several aliquots of blood from 20 subjects, keeping them at 4°C before centrifugation (10 min, 2,000 rcf, 4°C) after 0, 30, 60, 120, and 240 min and immediate storage of plasma at −80°C. The stability of ECs and ERCs in thawed plasma was assessed by incubating plasma aliquots from five subjects for 0, 1, 2, 4, 8, and 24 h at 4°C and at RT. EC stability across multiple freezing-thawing cycles was assessed on multiple aliquots from five subjects. We also evaluated the stability of the extracts kept in the autosampler at 4°C in sealed vials comparing the peak area of the sample immediately injected with the peak area of the same sample injected after 24 h. Each sample was analyzed in duplicate.
Data analysis and statistics
In both genders, median and interquartile range (IQ) were calculated for each parameter and analyte. Nonparametric reference intervals of circulating ECs and ERCs, expressed as 2.5th and 97.5th centiles, were also estimated. Male and female groups were compared by Mann-Whitney test. Spearman rank correlation coefficient was calculated between analytes and the monitored parameters. Data analysis was performed on MedCalc v9.3.7.0 (Mariakerke, Belgium).
RESULTS
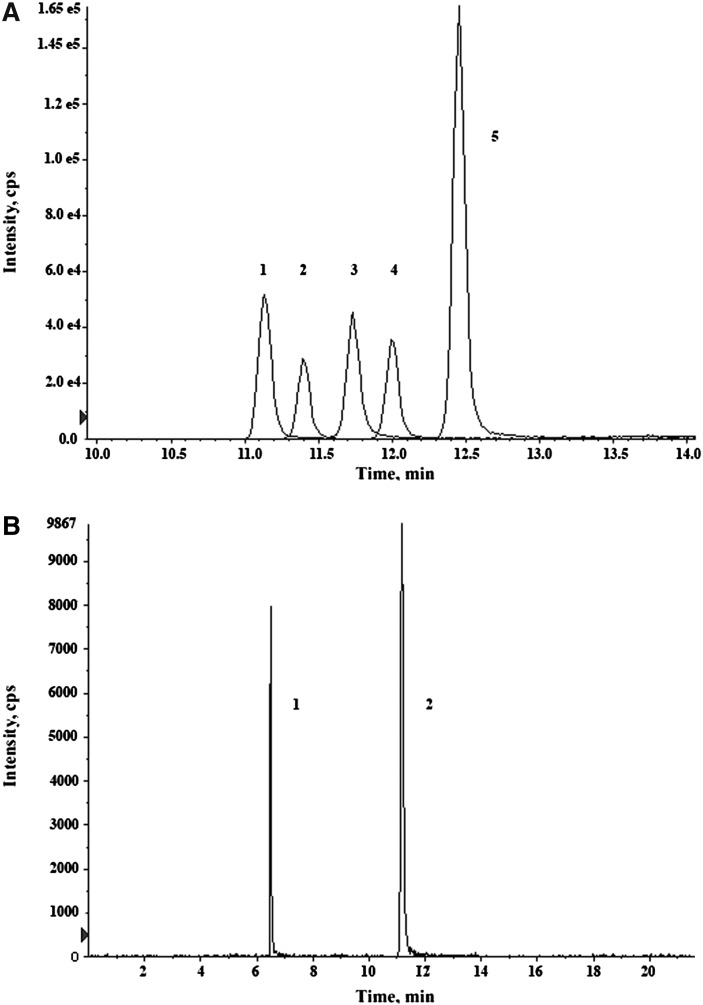
The chromatographic peaks of the five analytes are reported in Fig. 1: the complete resolution of the isobar pairs 2AG – 1AG and O-arachidonoyl-ethanolamide - AEA was achieved. The retention time variability was <0.5% for all analytes, and the carryover tested after the injection of the highest calibration point ranged between 0.3 and 1.2%.
Fig. 1.
Chromatogram of (A) AEA (1), 2AG (2), 1AG (3), PEA (4) and OEA (5); resolution of the isobar pair O-AEA (1)–AEA (2) (B).
The stability of working solutions was assessed by monitoring the signal obtained by periodical injection of 3 pmol of each analyte and IS: the overall variability across 1 year was ≤25% without any significant increase or decrease trend, compatible with the typical variability of the sensitivity of the analytical system. In agreement with what was reported by the supplier, the observed occurrence of d0-standard in each deuterated compound was <1%, except for d8-AEA for which a slightly higher percentage (1.4% of d0-AEA) was noticed by us. Traces of (d5-)1AG of (5.2) 2.9% were found in stock solutions of (d5-)2AG, whereas no traces of (d5-)2AG were observed in (d5-)1AG.
Polypropylene and glass tubes showed the lowest and the highest percentage of (d5-)1AG and, with some exceptions, they also exhibited the highest and the lowest recovery of total peak area of (d5-)2/1AG, respectively. Among the solvents, MeOH-containing solutions gave the highest (d5-)1AG%, and generally poor (d5-)2/1AG total recovery. Notably, when d5-2AG was evaporated in chloroform:MeOH (2:1) solution in glass tubes almost complete isomerization to d5-1AG was observed (97.8%) (Table 2).
TABLE 2.
Recovery of (d5-)2/1AG and isomerization (% of 1AG) occurring during evaporation
| Pyrex |
poly-propylene |
glass |
Pyrex |
poly-propylene |
glass |
|||||||
| 2/1AG % | 1AG % | 2/1AG % | 1AG % | 2/1AG % | 1AG % | d5-2/1AG % | d5-1AG % | d5-2/1AG % | d5-1AG % | d5-2/1AG % | d5-1AG % | |
| Toluene | 79.2 | 7.1 | 90.2 | 3.2 | 63.4 | 27.8 | 75.0 | 8.7 | 87.9 | 6.8 | 74.0 | 33.9 |
| Chloroform | 84.2 | 4.5 | 85.7 | 3.9 | 77.3 | 78.1 | 85.8 | 8.2 | 87.6 | 6.6 | 80.8 | 80.3 |
| Chloroform:MeOH(2:1) | 58.5 | 86.7 | 87.7 | 32.3 | 24.1 | 90.6 | 45.8 | 90.3 | 80.0 | 28.6 | 66.3 | 97.8 |
| Dichloromethane | 82.9 | 5.1 | 85.7 | 4.1 | 79.7 | 31.8 | 81.9 | 8.0 | 87.0 | 6.9 | 79.4 | 33.2 |
| MeOH | 27.3 | 76.0 | 68.6 | 25.3 | 10.5 | 88.7 | 26.1 | 80.7 | 80.9 | 36.1 | 11.5 | 85.9 |
| Ethyl acetate | 83.8 | 4.4 | 89.1 | 2.4 | 81.4 | 14.6 | 83.1 | 7.3 | 84.8 | 5.8 | 75.5 | 13.3 |
| Ethyl acetate:n-hexane(2:1) | 81.5 | 7.4 | 86.2 | 4.1 | 71.6 | 30.7 | 79.1 | 11.9 | 78.0 | 7.5 | 67.7 | 31.3 |
| Ethyl acetate:cyclohexane(1:1) | 77.5 | 7.9 | 86.0 | 4.2 | 81.0 | 19.3 | 83.6 | 11.3 | 80.9 | 6.1 | 80.3 | 22.7 |
| Diethyl ether | 79.1 | 3.9 | 62.3 | 2.1 | 70.8 | 45.2 | 79.2 | 6.5 | 70.7 | 5.5 | 61.9 | 40.4 |
Minimal traces of PEA (S/N between 3 and 8) were detectable in all the tested solvents. A higher signal of PEA was detected in ethyl acetate supplied in polyethylene high density bottles (S/N: 15-25). Both n-hexane and cyclohexane contained consistent traces of PEA (S/N: 9-30 and 152-253, respectively) and traces of OEA (S/N: 7-15 and 8-83, respectively). Among the tubes, those in polypropylene seemed to release additional amounts of PEA and OEA (S/N 2- to 3-fold higher in polypropylene than in Pyrex and glass tubes). Traces of OEA were detected in polypropylene tubes where chloroform and dichloromethane were evaporated (S/N: 4-6). No traces of AEA, 2AG, or 1AG were detected.
Considering the poor preservation of (d5-)2AG during evaporation in glass tubes and the release of PEA and OEA by polypropylene tubes, we chose to use Pyrex tubes for EC extraction.
The residual concentration of total cholesterol, triglycerides and phospholipids after five cycles of charcoal treatment was 88, 80, and 79% of the initial concentration, and was still representative of the concentration found in the normal population: 171, 92, and 191 mg/dl, respectively. This ensured that the complexity of the matrix was not altered. Residual concentrations of 2AG and 1AG (0.275 and 0.467 pmol/ml, respectively) were present and persisted even after additional treatments. The depletion efficiency ranged between 98.2% for 2AG and 100% for NAEs, the original concentration in the discarded plasma pool being 1.90, 15.10, 129.00, 28.05, and 8.23 pmol/ml for AEA, 2AG, 1AG, PEA, and OEA, respectively.
We used spiked stripped plasma for the evaluation of the recovery of the proposed LLE and SPE procedures (Table 3). Because isomerization of 2AG can occur to different extents in different extraction procedures, thus interfering with the recovery calculation, we also calculated the total recovery of 2/1AG, and monitored the occurrence of isomerization by the 1AG/2AG ratio. Among the LLEs, the highest recovery was obtained with toluene for each analyte except 2/1AG, for which the highest recovery was obtained with diethyl ether. The lowest recovery was observed with Folch's modified method. The recovery of the perfusion column was tested only on toluene extracts, because massive dirtiness of the source was observed by injecting samples extracted by other LLEs without on line purification. The overall toluene LLE-on line purification recovery ranged between 67.8% (OEA) and 72.8% (AEA), and was higher than the recovery obtained by SPE for NAEs, ranging from 34.6% (PEA) to 54.1% (AEA). An altered 1AG/2AG ratio, more than 2-fold higher than the ratio of the pure standard, was observed after extraction by SPE and by Folch's modified method.
TABLE 3.
Recovery obtained by different LLE and SPE methods
| 1AG / 2AG |
||||||||
| AEA | PEA | OEA | 2AG | 1AG | 2AG+1AG | pure standard | after extraction | |
| SPE | 54.1 | 34.6 | 51.9 | 52.0 | 130.1 | 94.1 | 1.15 | 2.89 |
| Folch modified method | 54.9 | 48.4 | 50.5 | 29.5 | 86.5 | 60.0 | 1.15 | 3.41 |
| Ethyl acetate | 73.4 | 68.0 | 70.7 | 58.8 | 74.2 | 67.0 | 1.15 | 1.46 |
| Ethyl acetate:n-hexane(2:1) | 80.6 | 79.8 | 83.2 | 73.9 | 98.1 | 86.9 | 1.15 | 1.53 |
| Diethyl ether | 72.3 | 72.4 | 77.4 | 67.1 | 109.7 | 89.9 | 1.15 | 1.87 |
| Toluene | 84.0 | 81.2 | 83.9 | 76.1 | 92.1 | 84.7 | 1.15 | 1.49 |
| On line purification | 86.7 | 84.0 | 80.9 | 80.1 | 83.8 | 81.7 | 1.42a | 1.49 |
| Toluene + on line purification | 72.8 | 68.3 | 67.8 | 61.0 | 77.2 | 69.2 | ||
1AG/2AG ratio referred to sample injected in 1D-LC-system.
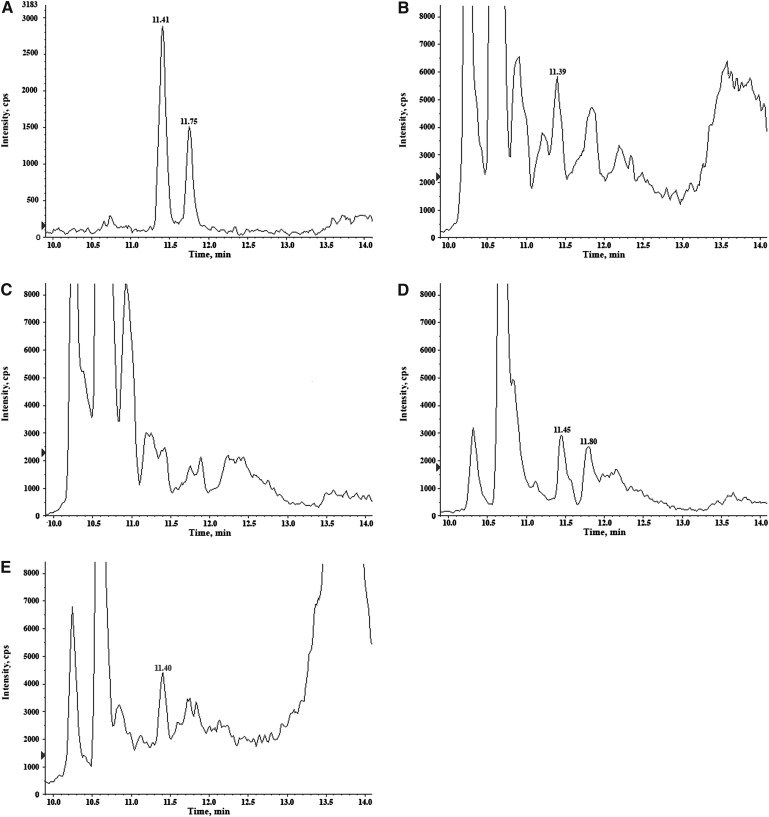
Toluene extracts also showed the lowest baseline noise in several unknown samples, particularly for the transitions 379\287 and 379\269 identifying both 2AG and 1AG (Fig. 2).
Fig. 2.
Chromatograms of 2AG and 1AG MRM transition (379\287) obtained by different LLE extractions of plasma: toluene (A), ethyl acetate (B), Folch's modified method (C), N-hexane:ethyl acetate (D), diethyl ether (E).
Because of 2AG preservation, negligible or absent contamination from PEA and OEA, best recovery and selectivity, toluene LLE-on line purification was definitively chosen and used for the rest of the validation and sample analysis.
Validation parameters calculated in standard curve and plasma matrix are reported in Tables 4 and 5, respectively. Accuracy between 80 and 120% and CV below 20% were achieved when measuring EC standard additions in stripped plasma covering the whole linearity range for AEA, 2AG, 1AG, and PEA, and between 0.1953 and 50.0 pmol/ml for OEA (Table 5). Intra- and inter-assay imprecision for the five analytes ranged between 2.6–9.1% and 3.2–13.6%, respectively (Table 6).
TABLE 4.
Validation parameters of standard curve
| Analyte | Linearity |
LLOQ |
LOD |
||||||||
| slope (mean ± SD) | intercept (mean ± SD) | r2 | SDR | range (pmol/ml) | pmol/ml | accuracy % | CV % | S/N | fmol on column | S/N | |
| AEA | 0.480 ± 0.032 | 0.019 ± 0.002 | 0.995 | 0.0133 | 0.0195-10.0 | 0.0195 | 97.1 | 13.7 | 8.0 | 4.27 | 3.6 |
| 2AG | 0.227 ± 0.023 | 0.001 ± 0.001 | 0.990 | 0.0221 | 0.0781–20.0 | 0.0781 | 118.9 | 12.0 | 6.3 | 17.06 | 4.6 |
| 1AG | 0.228 ± 0.009 | 0.000 ± 0.000 | 0.998 | 0.0068 | 0.0781-20.0 | 0.0781 | 115.5 | 8.6 | 7.3 | 17.06 | 3.9 |
| PEA | 0.196 ± 0.020 | 0.028 ± 0.004 | 0.990 | 0.0772 | 0.1953–100.0 | 0.1953 | 103.4 | 12.4 | 6.3 | 51.40 | 3.4 |
| OEA | 0.958 ± 0.053 | 0.022 ± 0.009 | 0.995 | 0.3559 | 0.0488-50.0 | 0.0488 | 108.6 | 12.9 | 9.0 | 6.42 | 3.4 |
For each analyte slope and intercept coefficients of calibration curves, squared correlation coefficient (r2), standard deviation of residuals (SDR), linearity range, lower limit of quantitation (LLOQ) and limit of detection (LOD) were reported.
TABLE 5.
Accuracy, imprecision, sensitivity and linearity in plasma matrix across the linear range
| Analyte | Standard addition (pmol/ml) | Measured level(mean ± s.d., pmol/ml) | Accuracy %(subtracted basal level) | CV % | r2 |
| AEA | 0.0195 | 0.0188 ± 0.0004 | 96.4 | 2.3 | 0.999 |
| 0.0391 | 0.0420 ± 0.0052 | 107.7 | 12.5 | ||
| 0.0781 | 0.0828 ± 0.0028 | 106.2 | 3.3 | ||
| 0.1563 | 0.1587 ± 0.0046 | 101.7 | 2.9 | ||
| 0.6250 | 0.5660 ± 0.0251 | 90.6 | 4.4 | ||
| 2.5000 | 2.3467 ± 0.0945 | 93.9 | 4.0 | ||
| 10.0000 | 8.9433 ± 0.3134 | 89.4 | 3.5 | ||
| basal level | undetectable | ||||
| 2AG | 0.0781 | 0.3457 ± 0.0227 | 90.6 | 6.6 | 0.999 |
| 0.1563 | 0.4180 ± 0.0173 | 91.7 | 4.2 | ||
| 0.3125 | 0.5760 ± 0.0229 | 96.2 | 4.0 | ||
| 1.2500 | 1.4267 ± 0.0231 | 92.1 | 1.6 | ||
| 5.0000 | 4.8800 ± 0.0889 | 92.1 | 1.8 | ||
| 20.0000 | 17.5667 ± 0.5033 | 86.5 | 2.9 | ||
| basal level | 0.2750 ± 0.0156 | 5.7 | |||
| 1AG | 0.0781 | 0.5497 ± 0.0300 | 106.4 | 5.5 | 0.999 |
| 0.1563 | 0.6127 ± 0.0671 | 93.6 | 11.0 | ||
| 0.3125 | 0.7553 ± 0.0358 | 92.2 | 4.7 | ||
| 1.2500 | 1.5567 ± 0.1250 | 87.2 | 8.0 | ||
| 5.0000 | 4.5000 ± 0.4272 | 80.7 | 9.5 | ||
| 20.0000 | 19.4667 ± 1.3577 | 95.0 | 7.0 | ||
| basal level | 0.4667 ± 0.0023 | 0.5 | |||
| PEA | 0.1953 | 0.2285 ± 0.0255 | 117.2 | 11.1 | 0.999 |
| 0.3906 | 0.4025 ± 0.0580 | 102.9 | 14.4 | ||
| 0.7813 | 0.7332 ± 0.0702 | 93.9 | 9.6 | ||
| 1.5625 | 1.4565 ± 0.0436 | 93.4 | 3.0 | ||
| 6.2500 | 5.9298 ± 0.2721 | 94.9 | 4.6 | ||
| 25.0000 | 23.6298 ± 0.3512 | 94.5 | 1.5 | ||
| 100.0000 | 87.0965 ± 4.2000 | 87.1 | 4.8 | ||
| basal level | undetectable | ||||
| OEA | 0.1953 | 0.1907 ± 0.0126 | 97.8 | 6.6 | 0.999 |
| 0.3906 | 0.3757 ± 0.0220 | 96.1 | 5.9 | ||
| 0.7813 | 0.7400 ± 0.0108 | 94.8 | 1.5 | ||
| 3.1250 | 2.8887 ± 0.0814 | 92.3 | 2.8 | ||
| 12.5000 | 11.7220 ± 0.3464 | 93.8 | 3.0 | ||
| 50.0000 | 44.5887 ± 1.4468 | 89.2 | 3.2 | ||
| basal level | undetectable |
TABLE 6.
Intra-assay and inter-assay imprecision
| Analyte | Unit | low QC | medium QC | high QC | plasma pool |
| AEA | pmol/ml | 0.164 | 0.578 | 2.460 | 1.910 |
| intra-assay CV% | 4.3 | 5.1 | 5.0 | 4.0 | |
| inter-assay CV% | 3.9 | 9.7 | 5.6 | 5.2 | |
| 2AG | pmol/ml | 0.613 | 1.427 | 4.960 | 5.080 |
| intra-assay CV% | 7.5 | 7.9 | 4.0 | 4.9 | |
| inter-assay CV% | 13.6 | 10.2 | 9.9 | 7.2 | |
| 1AG | pmol/ml | 0.829 | 1.634 | 5.687 | 19.146 |
| intra-assay CV% | 3.2 | 9.1 | 4.2 | 7.3 | |
| inter-assay CV% | 10.3 | 5.1 | 3.2 | 8.0 | |
| PEA | pmol/ml | 1.482 | 7.475 | 24.588 | 22.220 |
| intra-assay CV% | 8.3 | 4.6 | 6.8 | 3.1 | |
| inter-assay CV% | 7.6 | 11.4 | 4.8 | 4.9 | |
| OEA | pmol/ml | 0.830 | 1.738 | 11.820 | 7.024 |
| intra-assay CV% | 4.2 | 2.6 | 4.6 | 4.4 | |
| inter-assay CV% | 10.9 | 7.5 | 4.1 | 7.8 |
The post column infusion experiment revealed that none of the extraction procedures caused ion suppression or enhancing phenomena in the signal of the five deuterated compounds at their specific retention time. Negligible deviation from 100% was observed when pure standards were spiked on toluene extracts of stripped plasma (AEA 94.4%, 2AG 96.6%, 1AG 91.3%, PEA 88.4%, OEA 96.4%) confirming the absence of matrix interference.
The stability investigations revealed an increase in AEA, PEA, and OEA concentrations in whole blood kept at 4°C after withdrawal: their levels rose to 183, 142, and 154% in the first 30 min, and increased more until 4 h. The levels of 2AG and 1AG remained stable in whole blood for 2 h after the withdrawal but a reduction to 77.5% of 2AG level was detected after 4 h. NAEs levels were stable in plasma pool incubated at 4°C for 24 h, but they decreased to levels below 80% of the initial value after 24 h at RT. An increase in 2AG and 1AG levels above 120% of the initial plasma value was observed after incubation of 4 h both at RT and at 4°C and increased more subsequently. NAEs and MAGs were not affected by multiple thawing-freezing cycles, with deviation within ± 20% of the value found after the first cycle. When kept in the autosampler at 4°C, sample extracts were stable for at least 24 h, analyte values ranging between 93.8 and 107.7% of the initial value (Table 7).
TABLE 7.
ECs and ERCs stability across plasma collection, storage and processing
| Matrix | Time (min) / cycles | Temperature | AEA | 2AG | 1AG | PEA | OEA |
| whole blood | 0 | 4°C | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 30 | 4°C | 182.9 | 95.7 | 88.6 | 142.0 | 154.4 | |
| 60 | 4°C | 218.7 | 88.8 | 85.5 | 164.5 | 181.6 | |
| 240 | 4°C | 272.4 | 83.3 | 92.1 | 195.8 | 217.6 | |
| 480 | 4°C | 317.0 | 77.5 | 92.0 | 224.5 | 252.2 | |
| plasma | 0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |
| 60 | 4°C | 92.0 | 95.4 | 95.6 | 97.0 | 95.0 | |
| 120 | 4°C | 90.6 | 106.5 | 119.8 | 97.0 | 94.4 | |
| 240 | 4°C | 94.8 | 130.3 | 149.8 | 95.4 | 93.6 | |
| 480 | 4°C | 93.4 | 145.7 | 203.4 | 97.5 | 87.3 | |
| 1440 | 4°C | 94.6 | 157.3 | 348.8 | 106.0 | 89.7 | |
| 60 | RT | 98.6 | 117.9 | 110.6 | 93.7 | 93.3 | |
| 120 | RT | 92.1 | 107.4 | 115.7 | 96.2 | 92.5 | |
| 240 | RT | 88.9 | 124.0 | 150.9 | 93.7 | 82.7 | |
| 480 | RT | 86.8 | 152.0 | 225.9 | 92.1 | 81.3 | |
| 1440 | RT | 78.5 | 147.6 | 421.5 | 78.5 | 66.0 | |
| thawing-freezing | 1 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |
| 2 | 99.2 | 107.0 | 105.6 | 101.4 | 97.0 | ||
| 3 | 96.7 | 104.2 | 107.7 | 99.6 | 93.5 | ||
| 4 | 99.0 | 109.6 | 117.2 | 100.3 | 96.4 | ||
| sample extracts | 0 | 4°C | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 1440 | 4°C | 98.5 | 104.7 | 107.7 | 105.8 | 93.8 |
None of the samples tested during the validation procedures nor among the healthy subjects exhibited an altered IR, thus confirming the specificity of the method.
The main features of the healthy population selected for the estimation of reference intervals are described in Table 8. Female and male groups had similar ages. Compared with males, females were leaner and had lower blood pressure and showed lower fasting levels of triglycerides, glucose, uric acid, and a lower cholesterol to HDL ratio (Chol/HDL) (Table 8).
TABLE 8.
Main features of the healthy normal weight female (F; n = 76) and male (M; n = 45) population
| Parameter | Gender | Median (IQ) | Lowest - Highest value |
| Age (years) | F | 44.5 (35.0–52.0) | 18.0–78.0 |
| M | 43.0 (30.5–52.5) | 18.0–73.0 | |
| BMI (Kg/m ) | F | 21.5 (20.3–23.8) | 18.1–25.0 |
| M | 23.5 (22.4–24.1)** | 18.6–25.0 | |
| Waist circumference (cm) | F | 76.5 (71,0–82.0) | 61.0–88.0 |
| M | 84.0 (78.0–88.3)*** | 61.0–97.0 | |
| DBP (mmHg) | F | 80.0 (70.0–80.0) | 60.0–90.0 |
| M | 80.0 (79.5–85.0)* | 65.0–90.0 | |
| SBP (mmHg) | F | 115.0 (110.0–120.0) | 95.0–140.0 |
| M | 120.0 (110.0–130.0)** | 100.0–140.0 | |
| Triglycerides (mg/dl) | F | 59.0 (48.0–73.0) | 33.0–148.0 |
| M | 70.0 (58.3–85.3)** | 48.0–142.0 | |
| Cholesterol/HDL ratio | F | 3.0 (2.6–3.4) | 1.5–4.4 |
| M | 3.4 (2.8–3.8)** | 2.0–4.8 | |
| Glucose (mg/dl) | F | 86.0 (79.0–92.3) | 69.0–104.0 |
| M | 92.0 (87.0–96.3)*** | 69.0–107.0 | |
| Insulin (uU/ml) | F | 5.0 (3.9–6.3) | 1.0–10.4 |
| M | 5.4 (3.9–6.6) | 2.2–9.8 | |
| Uric acid (mg/dl) | F | 3.6 (3.2–4.0) | 1.6–5.9 |
| M | 5.1 (4.6–5.5)*** | 3.6–7.0 |
Interquartile range (IQ). M versus F *: P < 0.050; **: P < 0.010; ***: P < 0.001.
Circulating EC and ERC levels are reported in Table 9. NAE levels were similar in both genders; however, higher median values of 2AG (1.73 and 1.43 pmol/ml, P = 0.007) and, with a border line significance, 1AG (0.58 and 0.49 pmol/ml, P = 0.051) were detected in males compared with females.
TABLE 9.
Descriptive values and reference interval (RI) of circulating ECs and ERCs (pmol/ml) in healthy normal weight females (F, n = 76) and males (M, n = 45)
| EC | Gender | Median (IQ) | Lowest - Highest value | RI2.5–97.5 P |
| AEA | F | 0.99 (0.79–1.18) | 0.40–1.65 | 0.57–1.59 |
| M | 0.98 (0.88–1.10) | 0.41–1.88 | 0.54–1.66 | |
| PEA | F | 14.35 (12.70–18.00) | 7.28–28.8 | 9.93–23.88 |
| M | 15.20 (13.3–17.05) | 8.01–21.0 | 8.70–21.00 | |
| OEA | F | 4.74 (4.03–5.91) | 2.41–10.20 | 2.72–8.07 |
| M | 4.54 (3.66–5.86) | 2.41–9.06 | 2.58–8.61 | |
| 2AG | F | 1.43 (1.06–1.90) | 0.69–3.44 | 0.71–3.12 |
| M | 1.73 (1.37–2.39)** | 0.71–5.40 | 0.73–4.23 | |
| 1AG | F | 0.49 (0.38–0.64) | 0.21–1.09 | 0.25–0.95 |
| M | 0.58 (0.43–0.76)* | 0.26–1.78 | 0.28–1.43 |
Interquartile range (IQ). M versus F * P = 0.051; **: P < 0.010.
AEA, PEA, and OEA levels strongly correlated (P < 0.0001) with each other, with similar extents in both genders: AEA versus PEA: r = 0.814 and r = 0.759; PEA versus OEA: r = 0.848 and r = 0.747; AEA versus OEA: r = 0.701 and r = 0.693, in females and males, respectively. In analogy, 2AG strongly correlated with its isomer 1AG: r = 0.611 and 0.700 in females and males (P < 0.0001), respectively.
NAEs showed a tendency to increase with age in females (r = 0.205, P = 0.076 and r = 0.207, P = 0.073 for AEA and OEA, respectively) but only for PEA was statistical significance reached (r = 0.249, P = 0.031). Also, 2AG, but not 1AG, displayed a positive correlation with age in females (r = 0.246, P = 0.033). By contrast, no correlation was observed between MAGs or NAEs and age in males.
In females, AEA correlated with BMI (r = 0.257, P = 0.026), waist circumference (r = 0.250, P = 0.030), and fasting insulin (r = 0.246, P = 0.033) and displayed a tendency to increase with triglyceride levels (r = 0.225, P = 0.051). In males, 1AG correlated with Chol/HDL levels (r = 0.337, P = 0.026), and 2AG displayed a tendency to increase with uric acid (r = 0.296, P = 0.050). Both in females and, to a higher extent, in males, MAGs were found to correlate with triglycerides (2AG: r = 0.272, P = 0.018 and r = 0.522, P = 0.001; 1AG: r = 0.274, P = 0.018 and r = 0.700, P < 0.001; respectively). Interestingly, in males, the extent of the correlation between MAGs and triglycerides, representing the precursors of this family of mediators, was similar to the correlation observed between 2AG and 1AG. No significant associations were observed for OEA with all the parameters analyzed.
DISCUSSION
In the last decade information has been obtained from EC circulating levels in relation to ECS involvement in several physiologic and pathologic contests affecting different districts: mostly brain and immune system stress responses, neurologic and psychiatric diseases, inflammatory state, and fertility (7, 8, 25). Considerable emphasis has been recently placed on the role of ECS in energy balance control presumably reflecting the fact that CB1 receptor antagonists entered in pharmacological clinical development to tackle obesity and related comorbidities (11, 26–30).
However, the data published so far on the circulating levels of ECs are often very discordant, and dramatic incoherence still exists on the EC values referring to normal subjects. The definition of EC reference intervals has therefore been recently addressed by many authors as an urgent issue (11, 12). For this reason, we developed and validated an LC/MS/MS method for the reliable measurement of circulating ECs and ERCs in a cohort of subjects selected according to their health status. Particular care was paid to the procedure assessment to avoid the alteration of EC and ERC levels during collection and storage.
Different extraction approaches were tested to achieve the best recovery and stability, minimizing contamination derived from common solvents and disposables. First, we assessed the stability of 2AG in terms of isomerization to 1AG and of total loss of 1/2AG during evaporation in different solvents, observing that both effects were promoted by the protic ambient typical of MeOH (18). The huge loss observed in MeOH and MeOH-containing mixtures could be explained only in part by the adhesion to the tube surface, but mostly by degradation, probably by hydrolysis to arachidonic acid and glycerol. Interestingly, we observed that also the tube material could contribute to determine isomerization and loss. Indeed, glass tubes showed a greater extent of these phenomena than polypropylene and Pyrex tubes, and this is probably due to the presence of catalytic silanol groups on the surface of glass tubes. We also found that a further underestimated pitfall is represented by the contamination of PEA and, less frequently, of OEA not only in solvents, as mostly n-hexane and cycle-hexane, but also in plastic bottles, disposables, and tubes. This issue was first raised by Skonberg et al. (17) referring exclusively to chloroform. The extent of the contamination we observed may not represent a problem when measuring PEA and OEA in plasma, due to their normal circulating concentrations; however, this issue will represent a major problem for reliable detection in tissues or in other biological fluids where small amounts of these mediators are presumed to be present. According to the observations described above, we decided to use Pyrex tubes to minimize both 2AG instability and PEA and OEA contamination. Several extraction methods were compared. Among them, LLE based on toluene was definitively chosen by us because of several key advantages: poor or absent contamination and minimal isomerization, best recovery and selectivity. A similar recovery was reported by Zoerner et al. (18) for 2AG when extracted with toluene, but we achieved a higher recovery for AEA. The further on line purification helped in avoiding any phenomena of matrix interference, guaranteeing the vigorousness of the system across large batches. The recovery we observed for Folch's modified method is poor and generally lower than what has been reported by others for AEA and 2AG (18). We consider Folch's modified method not to be suitable for EC extraction because of poor selectivity and relevant 2AG isomerization induction, leading to a worsening of the sensitivity and repeatability of the 2AG measurement (13, 18). Some authors reported good stability when using acetonitrile, acetone or diethyl-ether in their SPE protocol, indicating that SPE still remains a valid alternative to LLE (13, 16). However, the SPE performed by us showed evidence of isomerization that can be partly explained by the evaporation in the elution solvent cyclohexane:ethyl acetate and partly by the protic ambient induced in the sample by the MeOH added as deproteinizating agent (unpublished observation) and for this reason we did not pursue this approach. Our method was then validated following the FDA guidelines, using a plasma matrix NAE-free and with reduced amounts of MAGs (19). The limiting step in the validation procedures of quantitative methods for endogenous molecules is usually represented by the lack of a blank matrix on which to conduct the experiments. For this reason, we tried to assess a stripping procedure to efficiently remove NAEs and MAGs without altering the complexity of the matrix. This method seemed to work very well for NAEs, whereas low levels of 2AG and 1AG were still detectable in the stripped plasma due to the huge initial amount in the plasma. The elevated levels of MAGs in discarded plasma are consistent with what was observed in the stability evaluation and will be discussed below. Our method proved to have optimal specificity, accuracy, vigorousness, and sensitivity, far below the circulating levels observed in our population. In addition, the overall procedure is not expensive because it uses common solvents and is quite fast: a batch of 60 samples can be processed in about 2 h, but could be faster if automated handlers are used, and the same number of samples can be measured in 24 h.
The assessment of collection, storage, and handling conditions is a central issue to be carefully defined in the method development in order to preserve the accuracy of the measurement. NAEs could be synthesized ex vivo during the collection procedure as first demonstrated in cerebral tissues (31) and also in blood (14, 32–35); however, pitfalls related to the collection and storage of plasma have often been underestimated in the literature. After having observed an immediate dramatic increase in NAE levels after blood withdrawal, we were very keen to immediately centrifuge and store samples, positively avoiding variability and inaccuracy. This is not what usually happens in normal routine procedures, when blood samples are often kept at 4°C for hours before being processed and analyzed. We further observed that MAGs dramatically increase in plasma after 4 h incubation both at RT and even at 4°C, explaining the huge amounts observed in the discarded plasma used for the stripping procedures. For this reason, plasma samples should undergo assisted thawing and should be processed on ice and as fast as possible. Similar to what was previously reported (35), we found that NAEs and MAGs are stable after multiple plasma freezing-thawing cycles if correctly performed, and that extracted samples are stable until 24 h in the autosampler.
It is probable that the pitfalls mentioned above are responsible for a large part of the disagreement existing among the normal levels reported so far in the literature. However, the large discrepancies in the described levels of circulating ECs in normal subjects may also derive from a nonhomogeneous shared concept of normality.
In this study, we proposed gender-specific preliminary reference intervals of ECs and ERCs estimated on 121 healthy subjects fulfilling rigorous inclusion and exclusion criteria selection. Because several studies reported circulating EC levels to be influenced by BMI and by the presence of metabolic dysfunction (9, 26–30, 36, 37), we defined normality not only as the absence of overweight or obesity, but also as the absence of isolated metabolic alterations and hypertension, by tolerating only borderline alterations of blood pressure. Furthermore, because ECs and ERCs were involved in behavioral regulation of feeding (38) as well as in psychiatric disorders (39, 40), we purposely excluded from recruitment subjects with eating disorders such as anorexia nervosa and binge-eating disorders (41) or subjects showing symptoms of anxiety and depression (42), according to the results of psychometric tests. Moreover, as ECs and ERCs were involved in fertility and in sleep regulation, we included only subjects who reported a regular wake-sleep cycle and females with a regular menstrual cycle (43, 44).
We observed that the levels of the analytes belonging to the same family are strongly correlated with each other, but they do not correlate between the different families. These data confirm that NAE molecular machinery is modulated independently by MAGs. Furthermore, they are differentially associated with age and anthropometric and metabolic parameters with some gender specificities. Evidence of gender differences in EC levels was already described by Bluher et al. (27) who showed that, in lean subjects, females displayed higher levels of AEA, but not of 2AG, compared with males. However, this finding has not been reproduced by others because male and female subjects examined are usually pooled together (29, 30, 36, 45, 46). Intriguingly, our data contrast with those reported by Bluher et al. (27), as we found that MAGs were about 20% higher in males than in females, whereas NAE levels were similar between the two genders. The different levels of triglycerides in our subjects, when compared with the lean subjects recruited by Bluher, may explain the discrepancies in the results.
To the best of our knowledge, no data have been produced regarding circulating levels of ECs and ERCs and aging. We found that age in females seems to positively influence 2AG and PEA, whereas no changes in any of the circulating ECs and ERCs were observed in males of different ages. In females, an association exists between AEA and BMI, waist circumference, fasting insulin, and triglycerides, respectively, indicating that this mediator, among other ECs and ERCs, may have a role as a direct or indirect marker of biochemical and metabolic profile. In males, none of the measured compounds was associated with anthropometric features such as BMI or waist circumference, supporting the idea that the association found in obese patients between waist circumference and 2AG does not occur in normal weight individuals. The most striking result, however, derives from the strong correlation observed in males and, to a lesser extent, in females, between MAGs and triglycerides, which parallels a very similar correlation found in obese patients of both genders (9, 28, 29). In an intriguing paper by Ruby et al. (47) an artificial increase of 2AG plasma levels in mice caused an increase in triglycerides, independently of adiposity or food intake, by modulating the dislocation of ApoE among circulating lipoproteins. These findings may suggest that a strong link between 2AG and triglycerides exists independently of a condition of obesity or overweight and might represent an early trigger for the development of lipid and lipoprotein metabolism alteration. To our knowledge, no evidence of the generation of 2AG in plasma in the absence of blood cells has been previously reported, thus opening questions about the role of membrane-independent biochemical pathways, possibly involving triglycerides, regulating 2AG synthesis in the physiology of the ECS and in diseases in which it is involved.
Table 10 compares the values reported so far for normal weight control subjects. EC and ERC mean or median levels vary for more than one order of magnitude across the cited papers. Several differences exist among these studies: time and condition (fasting or not, supine or seated) in which blood withdrawal was performed, time interval between withdrawal and storage, extraction and analytical strategy (some of them used LC-MS) as well as type of matrix (plasma or serum). Moreover, such information, as well as validation data and method performance, anthropometric and metabolic features of the subjects, was very often simply not reported. Interestingly, 2AG values are usually reported as the sum of 1AG and 2AG signals, in order to cope with isomerization occurring throughout the whole processing. It is not known whether the inactive isomer 1AG is present in vivo or it is just an ex vivo artifact. We preferred to separate 2AG from 1AG values and to report independent metabolic associations until the biological significance of 1AG is elucidated.
TABLE 10.
Mean / median values reported so far in normal weight control groups
| Ref. | N | M/F | Age (Years) | BMI (kg/m2) | AEA pmol/ml | 2AG pmol/ml | PEA pmol/ml | OEA pmol/ml |
| 26 | 20 | 0/20 | mean ± SE 57 ± 1 | mean ± SE 23.5 ± 0.4 | mean about 2.1 # | mean about 16 #,§ | ||
| 41 | 15 | 0/15 | mean ± SD 22.9 ± 3.8 | mean ± SD 22.2 ± 2.3 | mean about 2.5 # | mean about 1 # | ||
| 27 | 10 | 10/0 | mean ± SE 48 ± 13 | mean ± SE 25 ± 2 | mean about 2.0 # | mean; M+F about 5 # | ||
| 27 | 10 | 0/10 | mean ± SE 43 ± 14 | mean ± SE 24 ± 1 | mean about 2.8 # | |||
| 37 | 10 | 4/6 | mean ± SE 56.7 ± 5.9 | mean ± SE 21.3 ± 0.3 | mean about 2.6 # | mean about 0.6 # | ||
| 42 | 16 | 0/16 | mean ± SD 27.9 ± 9.2 | mean ± SD 23.8 ± 2.4 | mean ± s.d. 0.72 ± 0.29 | mean ± s.d. 19.6 ± 12.5 | ||
| 29 | 10 | 4/6 | mean ± SEM 44 ± 2 | mean ± SEM 21.9 ± 0.6 | mean about 4.6 # | mean;about 3.4 #,§ | ||
| 45 | 23 | 11/12 | mean ± SEM 29 ± 2 | mean ± SEM 23 ± 0.6 | mean ± SEM 1.96 ± 0.09 * | mean±SEM 7.92 ± 0.26 *,§ | ||
| 30 | 48 | 17/31 | mean ± SD 66.4 ± 6.7 | mean ± s.d. 24.7 ± 1.2 | mean (95%CI) 12.8 (11.4, 14.5) | mean (95%CI) 5.6 (4.6, 6.8) | mean (95%CI) 213.3 (190.1, 239.3) | mean (95%CI) 107.9 (97.2, 119.8) |
| 36 | 21 | 11/10 | median (IQ) 40.0 (33.5-46.5) | median (IQ) 21.1 (20.5-22.6) | median (IQ) 1.61 (1.35-1.90)* | median (IQ)5.28 (2.1-15.6)* ,§ | ||
| 46 | 12 | 2/10 | mean ± SEM 39.1 ± 3.7 | mean ± SEM 21.0 ± 0.6 | < 0.7 # | < 5 # |
#Data extrapolated from graphs; §sum of 2AG + 1AG is reported; *data converted from ng/ml to pmol/ml.
The depicted discrepancies clearly indicate that the establishment of definitive, shared reference intervals for ECs and ERCs is urgently required. Our 2D-LC/MS/MS method proved to have the high-throughput and the low cost per sample suitable for routine application or for large cohort studies, and is supported by the definition of reference intervals based on careful sample processing and subject selection. Our data indicated that even in a homogeneous cohort of healthy subjects, some associations with circulating ECs and ERCs exist, and that the kind and the extent of these associations are strictly gender-dependent. However, until certified material for calibrations and quality controls are available, the establishment of shared reference intervals is difficult to achieve. In these concerns, an effort toward the harmonization of preanalytical and analytical procedures would help in improving the agreement and in moving the EC field out from basic to clinical research.
Acknowledgments
The authors thank Fondazione Cassa di Risparmio, Bologna, Italy for supporting the Centre of Applied Biomedical Research. We also thank Dr. P. Chieco for the laboratory organization, Dr. A.M. Morselli-Labate for the help in statistical analysis, and Dr. Susan West for language editing of the manuscript.
Footnotes
Abbreviations:
- 1AG
- 1-arachidonoyl-glycerol
- 2AG
- 2-arachidonoyl-glycerol
- 2D
- two-dimension
- AEA
- arachidonoyl-ethanolamide
- BES
- Binge Eating Scale
- BITE
- Bulimic Investigation test Edinburgh
- BMI
- body mass index
- CB
- cannabinoid receptor
- Chol/HDL
- cholesterol to HDL ratio
- DBP
- diastolic blood pressure
- EC
- endocannabinoid
- ECS
- endocannabinoid system
- ERC
- endocannabinoid related compounds
- EtOH
- ethanol
- FDA
- Food and Drug Administration
- IQ
- interquartile range
- IR
- ion ratio
- IS
- internal standard
- LLE
- liquid-liquid extraction
- LLOQ
- lower limit of quantitation
- LOD
- limit of detection
- MAG
- monoacyl-glycerols
- MeOH
- methanol
- MRM
- multiple reaction monitoring
- NAE
- N-acyl-ethanolamide
- OEA
- oleoyl-ethanolamide
- PEA
- palmitoyl-ethanolamide
- QC
- quality control
- RT
- room temperature
- S/N
- signal to noise ratio
- SBP
- systolic blood pressure
- SPE
- solid phase extraction
This research was supported by grants from the European Union (REPROBESITY FPVII-223713 and NEUROFAST FPVII-KBBE-2009-3-245009) and the Progetto Regione-Università of the Emilia Romagna Region.
REFERENCES
- 1.Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 258: 1946–1949. [DOI] [PubMed] [Google Scholar]
- 2.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N. E., Schatz A. R., Gopher A., Almog S., Martin B. R., Compton D. R., et al. 1995. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50: 83–90. [DOI] [PubMed] [Google Scholar]
- 3.Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 1995. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215: 89–97. [DOI] [PubMed] [Google Scholar]
- 4.Bisogno T., Piscitelli F., Di Marzo V. 2009. Lipidomic methodologies applicable to the study of endocannabinoids and related compounds: Endocannabinoidomics. Eur. J. Lipid Sci. Technol. 111: 53–63. [Google Scholar]
- 5.Lo Verme J., Fu J., Astarita G., La Rana G., Russo R., Calignano A., Piomelli D. 2005. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 67: 15–19. [DOI] [PubMed] [Google Scholar]
- 6.Guzmán M., Lo Verme J., Fu J., Oveisi F., Blázquez C., Piomelli D. 2004. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha). J. Biol. Chem. 279: 27849–27854. [DOI] [PubMed] [Google Scholar]
- 7.Hillard C. J., Weinlander K. M., Stuhr K. L. 2011. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. Epub ahead of print. November 17, 2011. [DOI] [PMC free article] [PubMed]
- 8.Bari M., Battista N., Pirazzi V., Maccarrone M. 2011. The manifold actions of endocannabinoids on female and male reproductive events. Front. Biosci. 16: 498–516. [DOI] [PubMed] [Google Scholar]
- 9.Di Marzo V., Côté M., Matias I., Lemieux I., Arsenault B. J., Cartier A., Piscitelli F., Petrosino S., Alméras N., Després J. P. 2009. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 52: 213–217. [DOI] [PubMed] [Google Scholar]
- 10.Pagotto U., Marsicano G., Cota D., Lutz B., Pasquali R. 2006. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. 27: 73–100. [DOI] [PubMed] [Google Scholar]
- 11.Quarta C., Mazza R., Obici S., Pasquali R., Pagotto U. 2011. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends Mol. Med. 17: 518–526. [DOI] [PubMed] [Google Scholar]
- 12.Zoerner A. A., Gutzki F. M., Batkai S., May M., Rakers C., Engeli S., Jordan J., Tsikas D. 2011. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: a comprehensive review from an analytical and biological perspective. Biochim. Biophys. Acta. 1811: 706–723. [DOI] [PubMed] [Google Scholar]
- 13.Rouzer C. A., Ghebreselasie K., Marnett L. J. 2002. Chemical stability of 2-arachidonylglycerol under biological conditions. Chem. Phys. Lipids. 119: 69–82. [DOI] [PubMed] [Google Scholar]
- 14.Vogeser M., Hauer D., Christina Azad S., Huber E., Storr M., Schelling G. 2006. Release of anandamide from blood cells. Clin. Chem. Lab. Med. 44: 488–491. [DOI] [PubMed] [Google Scholar]
- 15.Vogeser M., Schelling G. 2007. Pitfalls in measuring the endocannabinoid 2-arachidonoyl glycerol in biological samples. Clin. Chem. Lab. Med. 45: 1023–1025. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi S., Irie K., Nakano T., Sakamoto Y., Akitake Y., Araki M., Ohji M., Furuta R., Katsuki M., Yamaguchi R., et al. 2010. Reducing acyl migration during purification of 2-arachidonoylglycerol from biological samples before gas chromatography mass spectrometry analysis. Anal. Sci. 26: 1199–1202. [DOI] [PubMed] [Google Scholar]
- 17.Skonberg C., Artmann A., Cornett C., Hansen S. H., Hansen H. S. 2010. Pitfalls in the sample preparation and analysis of N-acylethanolamines. J. Lipid Res. 51: 3062–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoerner A. A., Batkai S., Suchy M. T., Gutzki F. M., Engeli S., Jordan J., Tsikas D. 2011. Simultaneous UPLC-MS/MS quantification of the endocannabinoids 2-arachidonoyl glycerol (2AG), 1-arachidonoyl glycerol (1AG), and anandamide in human plasma: Minimization of matrix-effects, 2AG/1AG isomerization and degradation by toluene solvent extraction. J. Chromatogr. B Analyt Technol Biomed Life Sci. Epub ahead of print. June 22, 2011.
- 19.US FDA. 2001. Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research, Rockville, MD, USA.
- 20.Henderson M., Freeman C. P. 1987. A self-rating scale for bulimia. The ‘BITE’. Br. J. Psychiatry. 150: 18–24. [DOI] [PubMed] [Google Scholar]
- 21.Beck A. T., Ward C. H., Mendelson M., Mock J., Erbaugh J. 1961. An inventory for measuring depression. Arch. Gen. Psychiatry. 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 22.Gormally J., Black S., Daston S., Rardin D. 1982. The assessment of binge eating severity among obese persons. Addict. Behav. 7: 47–55. [DOI] [PubMed] [Google Scholar]
- 23.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 24.Wang L., Liu J., Harvey-White J., Zimmer A., Kunos G. 2003. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc. Natl. Acad. Sci. USA. 100: 1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centonze D., Battistini L., Maccarrone M. 2008. The endocannabinoid system in peripheral lymphocytes as a mirror of neuroinflammatory diseases. Curr. Pharm. Des. 14: 2370–2382. [DOI] [PubMed] [Google Scholar]
- 26.Engeli S., Böhnke J., Feldpausch M., Gorzelniak K., Janke J., Bátkai S., Pacher P., Harvey-White J., Luft F. C., Sharma A. M., et al. 2005. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 54: 2838–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blüher M., Engeli S., Klöting N., Berndt J., Fasshauer M., Bátkai S., Pacher P., Schön M. R., Jordan J., Stumvoll M. 2006. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 55: 3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Côté M., Matias I., Lemieux I., Petrosino S., Alméras N., Després J. P., Di Marzo V. 2007. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. (Lond). 31: 692–699. [DOI] [PubMed] [Google Scholar]
- 29.Di Marzo V., Verrijken A., Hakkarainen A., Petrosino S., Mertens I., Lundbom N., Piscitelli F., Westerbacka J., Soro-Paavonen A., Matias I., et al. 2009. Role of insulin as a negative regulator of plasma endocannabinoid levels in obese and nonobese subjects. Eur. J. Endocrinol. 161: 715–722. [DOI] [PubMed] [Google Scholar]
- 30.Sipe J. C., Scott T. M., Murray S., Harismendy O., Simon G. M., Cravatt B. F., Waalen J. 2010. Biomarkers of endocannabinoid system activation in severe obesity. PLoS ONE. 5: e8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel S., Carrier E. J., Ho W. S., Rademacher D. J., Cunningham S., Reddy D. S., Falck J. R., Cravatt B. F., Hillard C. J. 2005. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J. Lipid Res. 46: 342–349. [DOI] [PubMed] [Google Scholar]
- 32.Giuffrida A., de Fonseca F. Rodríguez, Piomelli D. 2000. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal. Biochem. 280: 87–93. [DOI] [PubMed] [Google Scholar]
- 33.Schelling G., Hauer D., Azad S. C., Schmoelz M., Chouker A., Schmidt M., Hornuss C., Rippberger M., Briegel J., Thiel M., et al. 2006. Effects of general anesthesia on anandamide blood levels in humans. Anesthesiology. 104: 273–277. [DOI] [PubMed] [Google Scholar]
- 34.Wood J. T., Williams J. S., Pandarinathan L., Courville A., Keplinger M. R., Janero D. R., Vouros P., Makriyannis A., Lammi-Keefe C. J. 2008. Comprehensive profiling of the human circulating endocannabinoid metabolome: clinical sampling and sample storage parameters. Clin. Chem. Lab. Med. 46: 1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jian W., Edom R., Weng N., Zannikos P., Zhang Z., Wang H. 2010. Validation and application of an LC-MS/MS method for quantitation of three fatty acid ethanolamides as biomarkers for fatty acid hydrolase inhibition in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878: 1687–1699. [DOI] [PubMed] [Google Scholar]
- 36.Quercioli A., Pataky Z., Vincenti G., Makoundou V., Di Marzo V., Montecucco F., Carballo S., Thomas A., Staub C., Steffens S., et al. 2011. Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. Eur. Heart J. 32: 1369–1378. [DOI] [PubMed] [Google Scholar]
- 37.Matias I., Gonthier M. P., Orlando P., Martiadis V., De Petrocellis L., Cervino C., Petrosino S., Hoareau L., Festy F., Pasquali R., et al. 2006. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J. Clin. Endocrinol. Metab. 91: 3171–3180. [DOI] [PubMed] [Google Scholar]
- 38.Cota D., Tschöp M. H., Horvath T. L., Levine A. S. 2006. Cannabinoids, opioids and eating behaviour: the molecular face of hedonism? Brain Res. Rev. 51: 85–107. [DOI] [PubMed] [Google Scholar]
- 39.De Marchi N., De Petrocellis L., Orlando P., Daniele F., Fezza F., Di Marzo V. 2003. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lutz B. 2009. Endocannabinoid signals in the control of emotion. Curr. Opin. Pharmacol. 9: 46–52. [DOI] [PubMed] [Google Scholar]
- 41.Monteleone P., Matias I., Martiadis V., De Petrocellis L., Maj M., Di Marzo V. 2005. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology. 30: 1216–1221. [DOI] [PubMed] [Google Scholar]
- 42.Hill M. N., Miller G. E., Ho W. S., Gorzalka B. B., Hillard C. J. 2008. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 41: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jumpertz R., Wiesner T., Blüher M., Engeli S., Bátkai S., Wirtz H., Bosse-Henck A., Stumvoll M. 2010. Circulating endocannabinoids and N-acyl-ethanolamides in patients with sleep apnea–specific role of oleoylethanolamide. Exp. Clin. Endocrinol. Diabetes. 118: 591–595. [DOI] [PubMed] [Google Scholar]
- 44.Karasu T., Marczylo T. H., Maccarrone M., Konje J. C. 2011. The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Hum. Reprod. Update. 17: 347–361. [DOI] [PubMed] [Google Scholar]
- 45.Schroeder C., Batkai S., Engeli S., Tank J., Diedrich A., Luft F. C., Jordan J. 2009. Circulating endocannabinoid concentrations during orthostatic stress. Clin. Auton. Res. 19: 343–346. [DOI] [PubMed] [Google Scholar]
- 46.Gatta-Cherifi B., Matias I., Vallée M., Tabarin A., Marsicano G., Piazza P. V., Cota D. 2011. Simultaneous postprandial deregulation of the orexigenic endocannabinoid anandamide and the anorexigenic peptide YY in obesity. Int. J. Obes. Epub ahead of print. August 16, 2011; doi:. [DOI] [PubMed]
- 47.Ruby M. A., Nomura D. K., Hudak C. S., Mangravite L. M., Chiu S., Casida J. E., Krauss R. M. 2008. Overactive endocannabinoid signaling impairs apolipoprotein E-mediated clearance of triglyceride-rich lipoproteins. Proc. Natl. Acad. Sci. USA. 105: 14561–14566. [DOI] [PMC free article] [PubMed] [Google Scholar]