Abstract
Members of the pancreatic lipase family exhibit both lipase activity toward triacylglycerol and/or phospholipase A1 (PLA1) activity toward certain phospholipids. Some members of the pancreatic lipase family exhibit lysophospholipase activity in addition to their lipase and PLA1 activities. Two such enzymes, phosphatidylserine (PS)-specific PLA1 (PS-PLA1) and phosphatidic acid (PA)-selective PLA1α (PA-PLA1α, also known as LIPH) specifically hydrolyze PS and PA, respectively. However, little is known about the mechanisms that determine their substrate specificities. Crystal structures of lipases and mutagenesis studies have suggested that three surface loops, namely, β5, β9, and lid, have roles in determining substrate specificity. To determine roles of these loop structures in the substrate recognition of these PLA1 enzymes, we constructed a number of PS-PLA1 mutants in which the three surface loops are replaced with those of PA-PLA1α. The results indicate that the surface loops, especially the β5 loop, of PA-PLA1α play important roles in the recognition of PA, whereas other structure(s) in PS-PLA1 is responsible for PS preference. In addition, β5 loop of PS-PLA1 has a crucial role in lysophospholipase activity toward lysophosphatidylserine. The present study revealed the critical role of lipase surface loops, especially the β5 loop, in determining substrate specificities of PLA1 enzymes.
Keywords: lysophospholipid, lysophospholipase, lipase, surface loop, lid, phospholipases, phospholipids, phospholipids/phosphatidic acid, phospholipids/phosphatidylserine
Phospholipase A1 (PLA1) is an enzyme that hydrolyzes fatty acid bound at the sn-1 position of phospholipids. There are several classes of PLA1 isozymes that differ in their structure and cellular localization. In mammals intracellular PLA1 consists of three members, iPLA1α [also known as phosphatidic acid (PA)-preferential PLA1, PA-PLA1] (1–3), iPLA1β (also known as p125) (4) and iPLA1γ (also known as KIAA0725) (5), whereas extracellular PLA1 consists of at least six members [phosphatidylserine (PS)-specific PLA1 (PS-PLA1), phosphatidic acid (PA)-selective PLA1α (PA-PLA1α, also known as LIPH), PA-PLA1β (also known as LIPI), hepatic lipase (HL), endothelial lipase (EL) and pancreatic lipase-related protein 2] (6–8). The latter six belong to the pancreatic lipase family, which hydrolyzes triglyceride (TG), phospholipids or both. Based on amino acid sequences and their substrate specificities, PS-PLA1, PA-PLA1α, and PA-PLA1β form a subfamily within the pancreatic lipase family (8). Interestingly, these members show unique substrate preference toward specific phospholipids such as PS and PA, but not TG (9–11). PS-PLA1 is specific to PS, whereas PA-PLA1α and β are specific to PA. The enzymes produce lysophospholipid mediators such as lysophosphatidylserine (LPS) and lysophosphatidic acid (LPA) from PS and PA, respectively, and are considered to be responsible for production of these lysophospholipid mediators (12–14). However, little is known about the mechanism underlying the strict substrate specificity. In addition to its PLA1 activity, PS-PLA1 shows lysophospholipase activity, which cleaves the fatty acid bound at the sn-1 position of LPS (9, 15). Among the members of the pancreatic lipase family, HL and EL were also reported to have lysophospholipase activity (16, 17), whereas other members have not been tested.
A crystallographic study of human pancreatic lipase (PL) (18) showed that it possesses three surface loops called the β5, β9, and lid loops that cover the active site. Because the lid loop of PL was found to undergo a conformational change upon contact with its substrate to allow the substrate to access the active site, it has been postulated that the lid loop is involved in substrate specificity (6, 19). In fact, the substrate specificities of lipoprotein lipase (LPL) and EL can be switched by exchanging their lid loops (20). The crystallographic studies of PL also suggested that the β5 and β9 loops need conformational changes to allow full substrate entry (18). Furthermore, the importance of these three loops in substrate recognition was supported by the following evidence: the β9 and lid loops of PLA1s (PS-PLA1, PA-PLA1α and β) are much shorter (composed of 12 amino acids) than those of TG lipases such as PL, HL, and LPL (composed of 22 or 23 amino acids) (8, 21). These notions raise the possibility that the surface loops are involved in the substrate recognition of lipases.
In this study, to test this hypothesis, we constructed a number of chimeric molecules between PS-PLA1 and PA-PLA1α in which the three loop structures, β5, β9, and lid, were interexchanged and examined the substrate specificity. The results indicated that the surface loops of PA-PLA1α, especially β5 and lid, participate in the recognition of PA, whereas other domain(s) are responsible for PS recognition in PS-PLA1. In addition, we found that the β5 loop of PS-PLA1 is crucial for the lysophospholipase activity of the enzyme toward LPS.
MATERIALS AND METHODS
Construction of PS-PLA1 mutants
A series of cDNAs encoding PS-PLA1 mutants were constructed by overlap extension PCR method (22). The strategy is illustrated in supplementary . In the first step, two independent PCR reactions were carried out using mouse PS-PLA1 cDNA in pCAGGS as a template. One reaction was performed using Primer 1 and an overlap reverse primer, corresponding to the β5, β9, or lid domain of PA-PLA1α, to amplify the 5′-half of mutant PS-PLA1 cDNA. The other reaction was performed using Primer 2 and an overlap forward primer, corresponding to the β5, β9, or lid domain of PA-PLA1α, to amplify the 3′-half of mutant PS-PLA1 cDNA. To produce the full-length mutant PS-PLA1 cDNA, the resulting two fragments were gel purified and used as templates for the second PCR reaction using Primer 1 and Primer 2. The resulting DNA fragments were subcloned into the KpnI/XhoI site of a plasmid expression vector pCAGGS-c-myc, which harbors an in-frame myc tag sequence followed by a stop codon at 3′ position of the XhoI site. Nucleotide sequences were confirmed by a standard dideoxy method (Fasmac, Japan). The oligonucleotide DNA primers used for PCR are as follows: Primer 1; gactccggtacc accatgcgtcctggcctc, Primer 2; aggctc ctcgag cacgcaggctatttt, β5 overlap fwd; ccaacaggctcccctccttcttggatcgac, β5 overlap rev; aggggagcctgttggcctgaatccatgaat, β9 overlap fwd; gacgcactaggctacaaggaagccctaggacatgtggactac, β9 overlap rev; cttgtagcctagtgcgtcagtgtcagagtgtggatggcttctac, lid overlap fwd; tttggaggtataaagtacttcaagtgtgatcacatgagg, lid overlap rev; ctttatacctccaaatattgtcttagggcatccaggctg.
PS-PLA1 single amino acid mutants of β5 loop
By the overlap extension PCR method (see supplementary Fig. I), we constructed four PS-PLA1 β5 loop mutants in which an amino acid residue of the β5 loop of PS-PLA1 was replaced with that of PA-PLA1α (namely, A93P, L94T, T96S, and K97P). The first PCR was carried out using mouse PS-PLA1 cDNA in pCAGGS as a template. In the first step, one reaction was performed with Primer 1 and a single-mutation reverse primer, A93P, L94T, T96S, or K96P rev primer, to amplify the 5′-end of PS-PLA1. The other reaction was performed with Primer 2 and a single-mutation forward primer, A93P, L94T, T96S, or K96P fwd primer, to amplify the 3′-end of PS-PLA1. The second PCR was carried out using the products of the first PCR reactions with Primer 1 and Primer 2. These DNA fragments were subcloned into the KpnI/XhoI site of a plasmid expression vector pCAGGS-c-myc. Nucleotide sequences were confirmed by a standard dideoxy method (Fasmac, Japan). The oligonucleotide DNA primers used for PCR are as follows: Primer 1; gactccggtaccaccatgcgtcctggcctc, Primer 2; aggctcctcgagcacgcaggctatttt, A93P fwd; attattcatggattcaggccactcgga, A93P rev; agaaggctttgttccgagtggcctgaa, L94T fwd; agggcgacaggaacaaagccttcttgg, L94T rev; tgttcctgtcgccctgaatccatgaat, T96S fwd; ctcggatccaagccttcttggatcgac, T96S rev; aggcttggatccgagcgccctgaatcc, K97P fwd; ggaacacctcctcttggatcgacaag, K97P rev; agaaggaggtgttccgagcgccctgaa.
Cell culture and transfection
HEK293 cells were maintained in DMEM (Nissui Pharmaceutical) supplemented with 10% fetal bovine serum (GIBCO), 100 U/ml penicillin (Sigma-Aldrich) and 100 μg/ml streptomycin (GIBCO) in a 37°C incubator with 5% CO2. For transfections, HEK293 cells were seeded at 2.0 × 105 per well in 12-well plate and cultured for 24 h. The cells were transfected using LipofectamineTM 2000 (Invitrogen) according to the manufacturers. Twenty-four hours after transfection, the medium was replaced with 600 μl of serum-free ExCell 302 medium (JRT) and incubated for another 24 h. Conditioned media were collected, clarified by low-speed centrifugation and used as an enzyme source.
Western blotting
Protein in conditioned media was TCA-precipitated. Briefly, 100 μl of conditioned media was mixed with 10 μl of 100% (w/v) TCA. After a brief vortex, the samples were incubated at 4°C for 1 h and centrifuged at 20,000 g for 20 min at 4°C. The supernatant was aspirated and the pellet was washed with 100 μl of cold acetone. The samples were centrifuged, as above, and the pellet was again washed with cold acetone. After centrifugation, the pellet was air-dried at room temperature and resuspended in 15 μl of SDS-PAGE sample buffer (62.5 mM Tris-HCl (pH 6.8), 5% 2-mercaptoethanol, 2% SDS and 10% Glycerol). The cells were extracted in 75 μl of lysis buffer (10 mM HEPES (pH 7.3), 10% Glycerol, 1% Triton X-100, 1 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 10 μg/ml PMSF, 20 μg/ml leupeptin and 2.5 mM p-NPP). Cell lysates were centrifuged at 20,000 g and the resulting supernatants were collected and added with 4 x SDS-PAGE sample buffer.
Before loading the samples on SDS-PAGE, they were heated to 100°C. Fifteen microliters of the samples were applied and separated by SDS-PAGE. Protein samples were transferred to nitrocellulose membranes using the Bio-Rad protein transfer system. The membranes were blocked with Tris-buffered saline containing 5% (w/v) skimmed milk and 0.05% (v/v) Tween-20, incubated with 1:50 anti-myc antibody (9E10) and treated with 1:2000 anti-mouse IgG-horseradish peroxidase. Proteins bound to the antibodies were visualized with an enhanced chemiluminescence system (GE Health Science).
PLA1 assays
Substrates [phosphatidylethanolamine (PE), PA, PS, phosphatidylinositol (PI), phosphatidylcholine (PC), LPA and LPS)] were purchased from Avanti Polar Lipids. These substrates were dried under nitrogen gas and dissolved at 400 μM in 100 mM Tris-HCl (pH 7.5) using water bath sonication and stocked in −20°C. We confirmed that phospholipid substrates stored at −20°C gave similar results to those obtained using freshly prepared phospholipid substrates. Substrates (final 80 μM) were added to 10 μl of conditioned media in a total volume of 100 μl in a 96-well plate and incubated at 37°C for several hours (PS, LPA, and LPS: 1 h; PE and PA: 1.5 h; PI: 2 h; PC: 4 h). Then, the resulting fatty acid liberated from phospholipids was measured using NEFA C-test Wako (WAKO) according to the manufacturers. In all experiments we confirmed that reaction was linear with time and amount of protein at 80 µM substrate concentration.
PS-PLA1 sandwich ELISA assay
Monoclonal antibodies against mouse PS-PLA1 were established as described previously (23). The amount of PS-PLA1 in the conditioned media was determined by PS-PLA1 sandwich ELISA assay using two anti-mouse PS-PLA1 monoclonal antibodies. A 96-well plate (Nunc) was coated with purified anti-mouse PS-PLA1 monoclonal antibody (clone 4D2). After soaking with PBS containing 3% (w/v) BSA, conditioned media containing recombinant PS-PLA1 was applied, incubated with biotinylated anti-PS-PLA1 monoclonal antibody (clone 4C10) and treated with HRP-conjugated streptavidin. Bound HRP-conjugated streptavidin was visualized with 3,3',5,5'-tetramethylbenzidine (TMB) as the peroxidase substrate. Standard PS-PLA1 was recombinant PS-PLA1 protein expressed by baculovirus system. The standard curve is shown in supplementary Fig. II.
RESULTS
Expression of PS-PLA1 mutants
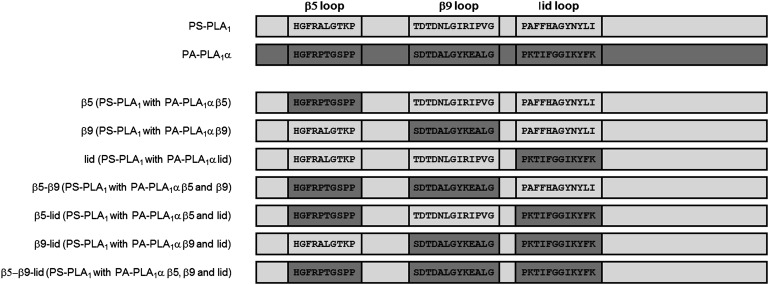
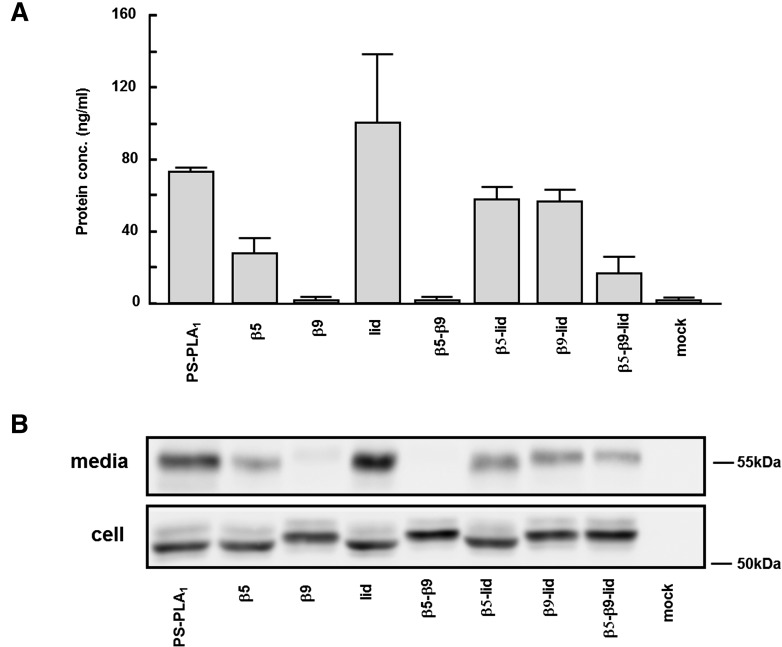
We previously found that PS-PLA1 was exclusively secreted into the culture media when expressed in cultured cells (9, 24), whereas PA-PLA1α was secreted but localized exclusively to the plasma membrane (10, 11). Based on these observations, we introduced the PA-PLA1α loop structures, β5, β9, and/or lid, into the PS-PLA1 backbone. Accordingly, we constructed seven cDNAs encoding PS-PLA1 mutants with PA-PLA1α loop structures (Fig. 1) and transfected HEK293 cells with the resulting plasmids. As is the case for wild-type PS-PLA1, most of the recombinant PS-PLA1 mutant proteins were detected in both cells and conditioned media as judged by sandwich ELISA (Fig. 2A) and Western blotting (Fig. 2B). However, the β9 and β5-β9 mutant proteins were almost exclusively detected in the cells. We therefore examined the enzymatic activity of other five PS-PLA1 mutant proteins.
Fig. 1.
Schematic diagrams of wild-type PS-PLA1, wild-type PA-PLA1α and PS-PLA1 mutants.
Fig. 2.
The expression of PS-PLA1 mutants. HEK 293 cells were transiently transfected with plasmids encoding PS-PLA1 mutants (see Fig. 1). Forty-eight hours after transfection, conditioned media were collected and the protein levels in each conditioned medium were examined by ELISA (A) and by Western blotting (B). In (A), bars indicate mean values and error bars indicate SD.
Substrate preference of PS-PLA1
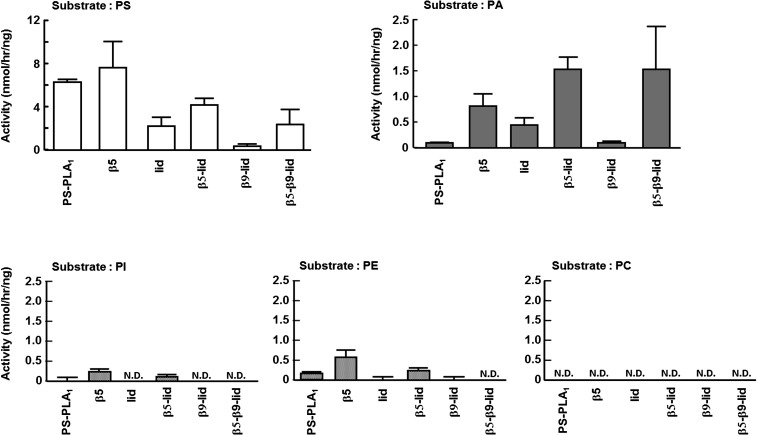
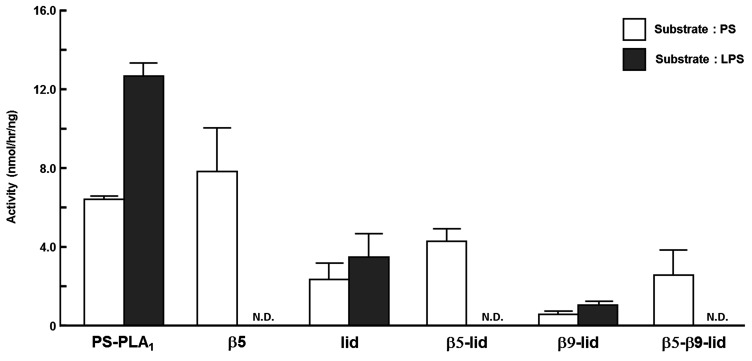
To examine the role of surface loops in determining the substrate specificity of PS-PLA1 and PA-PLA1α, we examined the substrate specificity of the above five PS-PLA1 mutant proteins and wild-type PS-PLA1 using various phospholipid substrates including PS, PA, PC, PE, and PI. PLA1 activities were determined by quantifying the fatty acid liberated as described in Materials and Methods (Fig. 3). All five mutants (β5, lid, β5-lid, β9-lid, and β5-β9-lid) retained catalytic activity toward PS. Especially, four mutants (β5, lid, β5-lid, and β5-β9-lid) hydrolyzed PA efficiently and hydrolyzed PS to a similar extent as in wild-type PS-PLA1. Like wild-type PS-PLA1, most mutants did not hydrolyze PC, PE, and PI. However, two mutants (β5 and β5-lid) slightly hydrolyzed PI and PE in addition to PS and PA (Fig. 3). These results indicate that at least the β5 and lid loops of PA-PLA1α play a role in the recognition of PA but not in the recognition of PS. Because all the mutants still showed a preference for PS, structures of PS-PLA1 other than the three loops must be responsible for the recognition of PS.
Fig. 3.
Substrate specificity of PS-PLA1 mutants. Phospholipid substrates (PS, PA, PI, PE, and PC) were mixed with each PS-PLA1 mutant in 100 mM Tris-HCl (pH 7.5) and incubated at 37°C for several hours. Enzyme activity was determined by quantifying the fatty acids liberated and expressed as calculated enzyme activity per ng protein of PS-PLA1 mutants. Bars indicate mean values and error bars indicate SD. N.D., not detected.
The β5 loop of PS-PLA1 is required for lysophospholipase activity
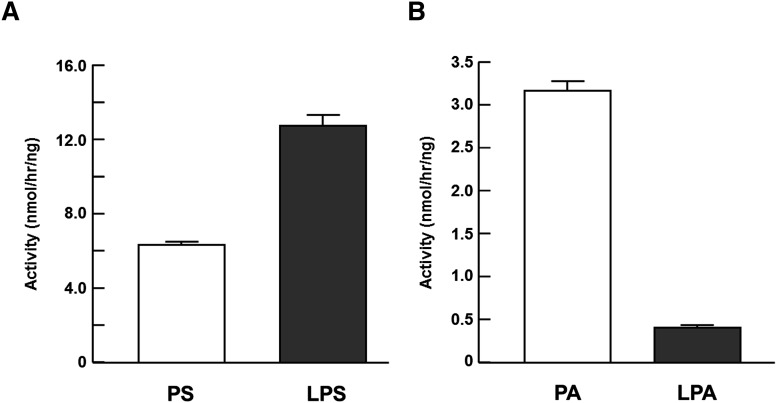
PS-PLA1 liberates fatty acid at the sn-1 position of PS and LPS (Fig. 4A). By contrast we found that PA-PLA1α did not hydrolyze LPA efficiently (Fig. 4B). Accordingly, we tested whether the three surface loops are involved in the discrimination between di-acylphospholipids and lysophospholipids by examining lysophospholipase activity of PS-PLA1 mutants toward LPS because all the five mutants were found to retain activity toward PS (Fig. 3). Like wild-type PS-PLA1, the lid mutants (lid) hydrolyzed LPS. β9-lid mutants showed a very weak lysophospholipase activity (Fig. 5). We found that PS-PLA1 mutants with different combinations of the β5 loop PA-PLA1α (β5, β5-lid and β5-β9-lid) completely lost hydrolysis activity toward LPS, although they hydrolyzed PS efficiently (Fig. 5). Hydrolysis of phospholipids in this assay followed Michaelis-Menten kinetics (supplementary Fig. III). Therefore, we calculated the Michaelis-Menten kinetic parameters of each enzyme from the Michaelis-Menten curve of the phospholipase and lysophospholipase activity and confirmed that the Vmax, LPS/PS ratios of β5 mutants (β5, β5-lid, and β5-β9-lid) were markedly decreased (Table 1). These data show that the β5 loop of PS-PLA1 is essential for lysophospholipase activity and that it is not exchangeable with the β5 loop of PA-PLA1α.
Fig. 4.
Lysophospholipase activities of PS-PLA1 (A) and PA-PLA1α (B). Enzyme activities of PS-PLA1 and PA-PLA1α were determined by quantifying fatty acid liberated and exhibited as calculated enzyme activity per ng protein of PS-PLA1 (A) or PA-PLA1α (B). The culture media of HEK293 cells transfected with PS-PLA1 or PA-PLA1α plasmid were used as an enzyme source. Although PA-PLA1α is localized exclusively to the plasma membrane, it is slightly secreted into conditioned medium. Substrates are PS and LPS for PS-PLA1 and PA and LPA for PA-PLA1α. Bars indicate mean values and error bars indicate SD.
Fig. 5.
The β5 mutants lost lysophospholipase activity toward LPS. Phosphatidylserine (PS, white columns) and lysophosphatidylserine (LPS, black columns) were incubated at 37°C for 1 h with each PS-PLA1 mutant in 100 mM Tris-HCl (pH 7.5). Then, enzyme activity was determined by quantifying fatty acids liberated and exhibited as calculated enzyme activity per ng protein of PS-PLA1 mutants. Bars indicate mean values and error bars indicate SD. N.D., not detected.
TABLE 1.
Kinetic characteristics of PS-PLA1 and PS-PLA1 mutants
| Substrate: LPS |
Substrate: PS |
Vmaxratio | |||
| Enzyme | Vmax(pmol/min/ng protein) | apparent Km(μM) | Vmax(pmol/min/ng protein) | apparent Km(μM) | (LPS/PS) |
| PS-PLA1 | 245 | 38 | 113 | 26 | 2.2 |
| β5 | N.D. | N.D. | 126 | 19 | – |
| Lid | 64.6 | 30 | 40.8 | 13 | 1.6 |
| β5-Lid | N.D. | N.D. | 74.5 | 22 | – |
| β9-Lid | 22.9 | 28 | 10.9 | 11 | 2.1 |
| β5-β9-Lid | N.D. | N.D. | 46.6 | 22 | – |
LPS, lysophosphatidylserine; PS, phosphatidylserine; N.D., not detected.
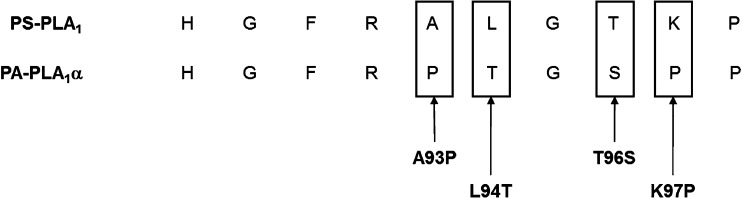
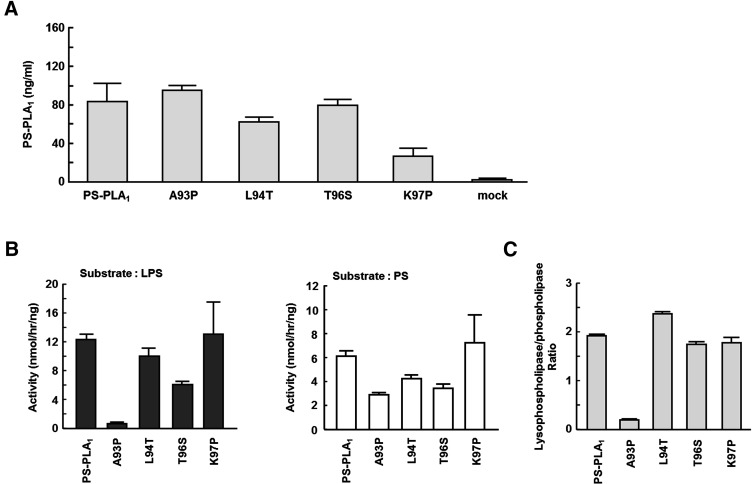
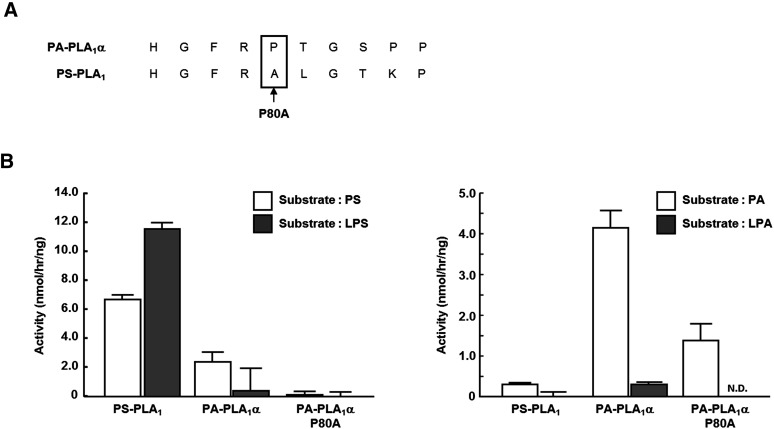
The amino acid sequences of the β5 loops in PS-PLA1 and PA-PLA1α differ by four amino acids (Fig. 6). To identify the amino acid residues responsible for lysophospholipase activity, we generated single amino acid mutants in which each of the four different amino acids of the β5 loop in PS-PLA1 was replaced with that of PA-PLA1α (A93P, L94T, T96S, and K97P; each number represents the amino acid position of PS-PLA1) and examined the lysophospholipase activity of these mutants toward LPS. All the single mutants were detected in conditioned media (Fig. 7A). Interestingly, A93P mutant showed a drastic change in the substrate preference (i.e., little lysophospholipase activity toward LPS) (Fig. 7B, C). The substrate preferences of the other three mutants (L94T, T96S and K97P), which hydrolyzed both PS and LPS, did not differ significantly from that of wild-type PS-PLA1. We also calculated the Michaelis-Menten kinetic parameters of each single amino acid mutant from the Michaelis-Menten curve (supplementary ) and confirmed that the Vmax, LPS/PS ratio of only A93P mutant was markedly decreased (Table 2). In addition, we constructed the single amino acid mutant of PA-PLA1α in which the proline in the β5 loop of PA-PLA1α was replaced with alanine (as in PS-PLA1) (Fig. 8A) and examined the lysophospholipase activity toward LPS and LPA. We found that this mutant did not show lysophospholipase activity toward LPA, although it was active toward PA (Fig. 8B). From these observations, we concluded that the presence of the proline alone in the β5 loop of PA-PLA1α was not enough to explain why PA-PLA1α lacks lysophospholipase activity. Thus, other factor(s) on PS-PLA1 should be involved in exhibiting lysophospholipase activity. A hydrophobic interaction between some amino acids and acyl chains can be such a factor.
Fig. 6.
Alignment of amino acid sequence of the β5 loops of PS-PLA1 and PA-PLA1α. Four amino acids out of nine differ between PS-PLA1 and PA-PLA1α. The positions of single amino acid mutants are also shown.
Fig. 7.
Lysophospholipase activity of single amino acid mutants. A: HEK 293 cells were transiently transfected with plasmids encoding single amino acid PS-PLA1 mutants. Expression of each mutant was examined by sandwich ELISA. B: Phosphatidylserine (PS, left panel) and lysophosphatidylserine (LPS, right panel) were incubated at 37°C for several h with conditioned media in 100 mM Tris-HCl (pH 7.5). Then, enzyme activity was determined by quantifying fatty acids liberated and exhibited as calculated enzyme activity per ng protein of the single amino acid mutants. Results are presented as bar charts of mean values ± SD depicted by error bars. C: The ratio of lysophospholipase to phospholipase was expressed as calculated enzyme activity to LPS per to PS. Bars indicate mean values and error bars indicate SD.
TABLE 2.
Kinetic characteristics of PS-PLA1 and single amino acid mutants
| Substrate: LPS |
Substrate: PS |
Vmaxratio | |||
| Enzyme | Vmax(pmol/min/ng protein) | apparent Km(μM) | Vmax(pmol/min/ng protein) | apparent Km(μM) | (LPS/PS) |
| PS-PLA1 | 245 | 38 | 113 | 26 | 2.2 |
| A93P | 11.6 | 20 | 49.6 | 24 | 0.23 |
| L94T | 192 | 25 | 73.6 | 21 | 2.6 |
| T96S | 118 | 31 | 62.8 | 16 | 1.9 |
| K97P | 259 | 28 | 129.6 | 11 | 2.0 |
LPS, lysophosphatidylserine; PS, phosphatidylserine.
Fig. 8.
Lysophospholipase activity of single amino acid PA-PLA1α mutant. A: The position of single amino acid mutant is shown. B: Phosphatidylserine (PS), lysophosphatidylserine (LPS), phosphatidic acid (PA) and lysophosphatidic acid (LPA) were incubated at 37°C for several hours with conditioned media in 100 mM Tris-HCl (pH 7.5). Then, enzyme activity was determined by quantifying fatty acids liberated and exhibited as calculated enzyme activity per ng protein of PS-PLA1, PA-PLA1α, or PA-PLA1α P80A mutant. Bars indicate mean values and error bars indicate SD.
DISCUSSION
PS-PLA1 and PA-PLA1α specifically recognize PS and PA, respectively (9, 10), although the recognition mechanism is unclear. In this study, we constructed a number of PS-PLA1 mutants in which corresponding the three loop structures of PA-PLA1α were introduced and tested their substrate specificities (Figs. 1, 3). PS-PLA1 mutant with the triple substitutions (β5-β9-lid) hydrolyzed PA in addition to PS. Hydrolysis of PA was also observed in the PS-PLA1 mutant with the PA-PLA1α lid loop (lid) or β5 and lid loops (β5-lid). Therefore, at least the β5 and lid loops of PA-PLA1α appear to act in concert to recognize and hydrolyze PA. Meanwhile, the β9 loop of PA-PLA1α may be involved in regulating membrane association because the β9 and β5-β9 mutant proteins were almost exclusively detected in the cells (Fig. 2). In addition, two mutants, β5 and β5-lid, slightly hydrolyzed PI and PE in addition to PS and PA (Fig. 3), suggesting that the β5 loop plays a critical role in substrate selectivity. We assume that the exchange of the β5 loop changed the structure of catalytic pocket of PS-PLA1 and resulted in temporary substrate promiscuity.
On the other hand, the finding that the preference for PS was not affected by the introduction of the three loops of PA-PLA1α clearly shows that the surface loops of PS-PLA1 are not involved in the recognition of PS. We speculate that amino and carboxyl groups of the serine residue in PS enter into the catalytic pocket of PS-PLA1 and, thus, the amino acid residues in the internal surface of the pocket are involved in the recognition of serine in PS. Given that PS-PLA1 doesn't act on PA, the three surface loops of PS-PLA1 probably play a role in accepting PS and excluding PA into the catalytic pocket.
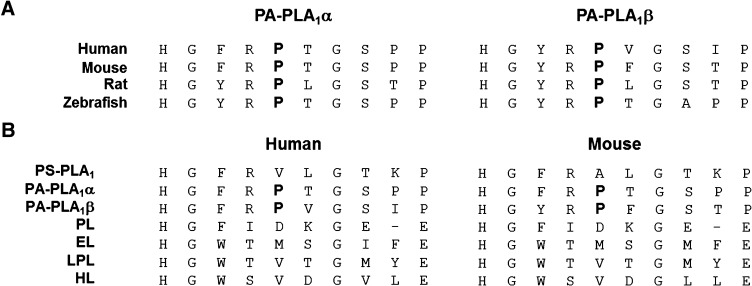
PS-PLA1 hydrolyzes lysophospholipid more effectively than PA-PLA1α (Fig. 4). We found that lysophospholipase activity of PS-PLA1 was dramatically reduced when the β5 loop was replaced with the β5 loop of PA-PLA1α (Fig. 5 and Table 1). Furthermore, among the single amino acid mutants of PS-PLA1 (A93P, L94T, T96S and K97P), only A93P mutant markedly lost lysophospholipase activity against LPS (Fig. 7B and Table 2). Interestingly, Pro 93 in the β5 loop of mouse PA-PLA1α and β is completely conserved among PA-PLA1α and β in other vertebrate species (Fig. 9A). In addition, the corresponding residue in other members of the pancreatic lipase family is not proline (Fig. 9B). Due to its ring structure, Pro 93 may affect the entire structure of the protein molecule. Taken together, this suggests that the conformation of the PA-PLA1α β5 loop is altered by the presence of a proline residue, which as a result, does not allow the enzyme to hydrolyze lysophospholipid in PA-PLA1α. Another possibility is that hydrophobic interaction has a role in exhibiting lysophospholipase activity. This idea is supported by the fact that the corresponding amino acid is valine and methionine in HL and EL, respectively (Fig. 9B), which also show lysophospholipase activity (16, 17).
Fig. 9.
Comparison of the amino acid sequences of PS-PLA1, PA-PLA1α, β and other lipases. A: Comparison of the amino acid sequences of β5 loop of PA-PLA1α and PA-PLA1β from human to zebrafish. The amino acid, Pro93 of mouse PA-PLA1α, is completely conserved in PA-PLA1α and β from several species. B: Comparison of the amino acid sequences of the β5 loop of PS-PLA1, PA-PLA1α, PA-PLA1β, EL, LPL, and HL. The corresponding residue in other members of the pancreatic lipase family (PS-PLA1, EL, LPL and HL) is not Pro.
PS-PLA1 stimulates degranulation of mast cells and participates in the progression of allergy by producing LPS with fatty acid at the sn-2 position of the glycerol backbone (2-acyl-LPS) (25). 2-Acyl-LPS is known to be unstable and is readily converted to 1-acyl-LPS by the quick spontaneous acyl chain migration within a molecule, known as intra molecular acyl migration (26). Thus, it is reasonable to assume that PS-PLA1 has a dual role: production and elimination of LPS. Namely, LPS (2-acyl-LPS) produced by PS-PLA1 is degraded by the same enzyme after the acyl migration reaction occurs. Recent study has identified GPR34 as a cellular receptor for LPS (27). Our preliminary data suggested that 2-acyl-LPS was by far a better ligand for GPR34 than 1-acyl-LPS. This suggests that PS-PLA1 is an activator of GPR34. Hydrolysis of both PS and 1-acyl-LPS by PS-PLA1 may result in an increase in the relative amount of 2-acyl-LPS, which contributes the local action of LPS signaling. Recent studies have shown that PA-PLA1α has a critical role in the formation of hair follicle by producing LPA and activating LPA receptor P2Y5/LPA6 (14). Unlike the LPS system, LPA can be degraded by its dephosphorylation reaction catalyzed by lipid phosphate phosphatases (28). Because of the presence of ipid phosphate phosphatases, PA-PLA1α may not need to have lysophospholipase activity while PS-PLA1 has.
We are now in a position to evaluate the biological importance of the lysophospholipase activity of PS-PLA1 because we have a way to separate its PLA1 and lysophospholipase activities. A93P PS-PLA1 mutant will be a useful tool because it shows only PLA1 activity without any detectable lysophospholipase activity. We suppose that A93P PS-PLA1 mutant is not capable of degrading LPS, thus leading to enhanced LPS-induced effects. These possibilities are now being tested in our laboratory.
Supplementary Material
Footnotes
Abbreviations:
- EL
- endothelial lipase
- LPA
- lysophosphatidic acid
- LPS
- lysophosphatidylserine
- PA
- phosphatidic acid
- PA-PLA1α
- phosphatidic acid-selective PLA1α
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PI
- phosphatidylinositol
- PLA1
- phospholipase A1
- PS
- phosphatidylserine
- PS-PLA1
- phosphatidylserine-specific PLA1
- TG
- triglyceride
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Pete M. J., Ross A. H., Exton J. H. 1994. Purification and properties of phospholipase A1 from bovine brain. J. Biol. Chem. 269: 19494–19500. [PubMed] [Google Scholar]
- 2.Higgs H. N., Glomset J. A. 1994. Identification of a phosphatidic acid-preferring phospholipase A1 from bovine brain and testis. Proc. Natl. Acad. Sci. USA. 91: 9574–9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgs H. N., Han M. H., Johnson G. E., Glomset J. A. 1998. Cloning of a phosphatidic acid-preferring phospholipase A1 from bovine testis. J. Biol. Chem. 273: 5468–5477. [DOI] [PubMed] [Google Scholar]
- 4.Tani K., Mizoguchi T., Iwamatsu A., Hatsuzawa K., Tagaya M. 1999. p125 is a novel mammalian Sec23p-interacting protein with structural similarity to phospholipid-modifying proteins. J. Biol. Chem. 274: 20505–20512. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima K., Sonoda H., Mizoguchi T., Aoki J., Arai H., Nagahama M., Tagaya M., Tani K. 2002. A novel phospholipase A1 with sequence homology to a mammalian Sec23p-interacting protein, p125. J. Biol. Chem. 277: 11329–11335. [DOI] [PubMed] [Google Scholar]
- 6.Wong H., Schotz M. C. 2002. The lipase gene family. J. Lipid Res. 43: 993–999. [DOI] [PubMed] [Google Scholar]
- 7.Inoue A., Aoki J. 2006. Phospholipase A1: structure, distribution and function. Future Lipidol. 1: 687–700. [Google Scholar]
- 8.Aoki J., Inoue A., Makide K., Saiki N., Arai H. 2007. Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie. 89: 197–204. [DOI] [PubMed] [Google Scholar]
- 9.Sato T., Aoki J., Nagai Y., Dohmae N., Takio K., Doi T., Arai H., Inoue K. 1997. Serine phospholipid-specific phospholipase A that is secreted from activated platelets. A new member of the lipase family. J. Biol. Chem. 272: 2192–2198. [DOI] [PubMed] [Google Scholar]
- 10.Sonoda H., Aoki J., Hiramatsu T., Ishida M., Bandoh K., Nagai Y., Taguchi R., Inoue K., Arai H. 2002. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J. Biol. Chem. 277: 34254–34263. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu T., Sonoda H., Takanezawa Y., Morikawa R., Ishida M., Kasahara K., Sanai Y., Taguchi R., Aoki J., Arai H. 2003. Biochemical and molecular characterization of two phosphatidic acid-selective phospholipase A1s, mPA-PLA1alpha and mPA-PLA1beta. J. Biol. Chem. 278: 49438–49447. [DOI] [PubMed] [Google Scholar]
- 12.Hosono H., Aoki J., Nagai Y., Bandoh K., Ishida M., Taguchi R., Arai H., Inoue K. 2001. Phosphatidylserine-specific phospholipase A1 stimulates histamine release from rat peritoneal mast cells through production of 2-acyl-1-lysophosphatidylserine. J. Biol. Chem. 276: 29664–29670. [DOI] [PubMed] [Google Scholar]
- 13.Aoki J., Inoue A., Okudaira S. 2008. Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta. 1781: 513–518. [DOI] [PubMed] [Google Scholar]
- 14.Shinkuma S., Akiyama M., Inoue A., Aoki J., Natsuga K., Nomura T., Arita K., Abe R., Ito K., Nakamura H., et al. 2010. Prevalent LIPH founder mutations lead to loss of P2Y5 activation ability of PA-PLA1alpha in autosomal recessive hypotrichosis. Hum. Mutat. 31: 602–610. [DOI] [PubMed] [Google Scholar]
- 15.Higashi S., Kobayashi T., Kudo I., Inoue K. 1988. Purification and characterization of lysophospholipase released from rat platelets. J. Biochem. 103: 442–447. [DOI] [PubMed] [Google Scholar]
- 16.Bohn E., Gerke V., Kresse H., Loffler B. M., Kunze H. 1992. Annexin II inhibits calcium-dependent phospholipase A1 and lysophospholipase but not triacyl glycerol lipase activities of rat liver hepatic lipase. FEBS Lett. 296: 237–240. [DOI] [PubMed] [Google Scholar]
- 17.Gauster M., Rechberger G., Sovic A., Horl G., Steyrer E., Sattler W., Frank S. 2005. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J. Lipid Res. 46: 1517–1525. [DOI] [PubMed] [Google Scholar]
- 18.Winkler F. K., D'Arcy A., Hunziker W. 1990. Structure of human pancreatic lipase. Nature. 343: 771–774. [DOI] [PubMed] [Google Scholar]
- 19.van Tilbeurgh H., Egloff M. P., Martinez C., Rugani N., Verger R., Cambillau C. 1993. Interfacial activation of the lipase-procolipase complex by mixed micelles revealed by X-ray crystallography. Nature. 362: 814–820. [DOI] [PubMed] [Google Scholar]
- 20.Griffon N., Budreck E., Long C., Broedl U., Marchadier D., Glick J., Rader D. 2006. Substrate specificity of lipoprotein lipase and endothelial lipase: studies of lid chimeras. J. Lipid Res. 47: 1803–1811. [DOI] [PubMed] [Google Scholar]
- 21.Carrière F., Withers-Martinez C., van Tilbeurgh H., Roussel A., Cambillau C., Verger R. 1998. Structural basis for the substrate selectivity of pancreatic lipases and some related proteins. Biochim. Biophys. Acta. 1376: 417–432. [DOI] [PubMed] [Google Scholar]
- 22.Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 77: 61–68. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K., Igarashi K., Ohkawa R., Saiki N., Nagasaki M., Uno K., Hayashi N., Sawada T., Syukuya K., Yokota H., et al. 2010. A novel enzyme immunoassay for the determination of phosphatidylserine-specific phospholipase A(1) in human serum samples. Clin. Chim. Acta. 411: 1090–1094. [DOI] [PubMed] [Google Scholar]
- 24.Nagai Y., Aoki J., Sato T., Amano K., Matsuda Y., Arai H., Inoue K. 1999. An alternative splicing form of phosphatidylserine-specific phospholipase A1 that exhibits lysophosphatidylserine-specific lysophospholipase activity in humans. J. Biol. Chem. 274: 11053–11059. [DOI] [PubMed] [Google Scholar]
- 25.Aoki J., Nagai Y., Hosono H., Inoue K., Arai H. 2002. Structure and function of phosphatidylserine-specific phospholipase A1. Biochim. Biophys. Acta. 1582: 26–32. [DOI] [PubMed] [Google Scholar]
- 26.Plückthun A., Dennis E. A. 1982. Acyl and phosphoryl migration in lysophospholipids: importance in phospholipid synthesis and phospholipase specificity. Biochemistry. 21: 1743–1750. [DOI] [PubMed] [Google Scholar]
- 27.Sugo T., Tachimoto H., Chikatsu T., Murakami Y., Kikukawa Y., Sato S., Kikuchi K., Nagi T., Harada M., Ogi K., et al. 2006. Identification of a lysophosphatidylserine receptor on mast cells. In Biochem Biophys Res Commun, United States. 1078–1087. [DOI] [PubMed]
- 28.Brindley D. N., Pilquil C. 2009. Lipid phosphate phosphatases and signaling. J. Lipid Res. 50(Suppl): S225–S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.