Abstract
We investigated the effects of the cholesteryl ester (CE) transfer protein inhibitor anacetrapib (ANA) on plasma lipids, lipoprotein subfraction concentrations, and lipoprotein composition in 30 healthy individuals. Participants (n = 30) were randomized to ANA 20 mg/day, 150 mg/day, or placebo for 2 weeks. Changes in concentration of lipoprotein subfractions were assessed using ion mobility, and compositional analyses were performed on fractions separated by density gradient ultracentrifugation. ANA 150 mg/day versus placebo resulted in significant decreases in LDL-cholesterol (26%) and apo B (29%) and increases in HDL-cholesterol (82%). Concentrations of medium and small VLDL, large intermediate density lipoprotein (IDL), and medium and small LDL (LDL2a, 2b, and 3a) decreased whereas levels of very small and dense LDL4b were increased. There was enrichment of triglycerides and reduction of CE in VLDL, IDL, and the densest LDL fraction. Levels of large buoyant HDL particles were substantially increased, and there was enrichment of CE, apo AI, and apoCIII, but not apoAII or apoE, in the mid-HDL density range. Changes in lipoprotein subfraction concentrations and composition with ANA 20 mg/day were similar to those for ANA 150 mg/day but were generally smaller in magnitude. The impact of these changes on cardiovascular risk remains to be determined.
Keywords: cholesteryl ester transfer protein inhibitor, lipid, low density lipoprotein
Elevated plasma levels of LDL cholesterol (LDL-C)and reduced levels of HDL cholesterol (HDL-C) are major risk factors for the development of coronary heart disease (CHD). A high residual risk of cardiovascular events persists despite intensive LDL-C lowering with statins, especially among subjects with CHD (1) or diabetes (2). The identification of novel agents that increase HDL-C may offer a promising therapeutic strategy for further reducing cardiovascular risk.
Cholesteryl ester transfer protein (CETP) is a hydrophobic plasma protein that promotes the bi-directional transfer of cholesteryl esters (CE) and triglycerides (TG) between HDL particles and atherogenic apo B-containing lipoproteins, predominantly TG-rich VLDL, intermediate density lipoprotein (IDL), and LDL particles (3–5). Enhanced CETP activity may be proatherogenic and inhibition of CETP activity could be atheroprotective. However, torcetrapib, the first CETP inhibitor tested in a clinical outcomes trial known as ILLUMINATE, was shown to increase cardiovascular events and overall mortality (6). Torcetrapib also produced off-target, compound-specific effects on blood pressure and secretion of adrenal hormones, which may have accounted for the adverse effects observed in the ILLUMINATE trial (6–8).
Anacetrapib (ANA; MK-0859) is an orally active, potent, and selective CETP inhibitor currently in Phase III development. Early studies conducted in healthy and dyslipidemic volunteers demonstrated that single and multiple doses of ANA resulted in dose-dependent decreases in LDL-C (up to ∼40%) and apoB (up to ∼30%), and increases in HDL-C (up to ∼139%) and apoAI (up to ∼47%) (9, 10). Effects on LDL-C and HDL-C also were observed when ANA was coadministered with statins for up to 1.5 years of treatment (11). To date, studies indicate that ANA has been well tolerated and has had no effects on blood pressure, serum electrolytes, and aldosterone levels (9, 11).
The present report describes the effects of ANA 20 mg/day and 150 mg/day versus placebo on concentrations and composition of lipoprotein subfractions in healthy individuals. A novel gas-phase differential electrophoretic macromolecular mobility-based method (ion mobility [IM]) was used for direct quantification of lipoprotein particles as a function of their size. This methodology enables the measurement of lipoprotein concentrations and their distributions across the entire spectrum from smaller, more dense HDL particles to larger, more buoyant VLDL. Lipoprotein composition was determined in IDL, LDL, and HDL subfractions separated by density gradient ultracentrifugation.
METHODS
Study design
This was a single-center, parallel-group, 3-arm, Phase I trial conducted in healthy subjects between April and July of 2007 (Merck and Co., Inc. Protocol number 015). Eligible participants were randomized in equal proportions to receive one of the following three once-daily oral treatments for 14 consecutive days: ANA 20 mg (administered as 1 × 20-mg capsule; n = 10), 150 mg (administered as 1 × 150-mg capsule; n = 10), or placebo (administered as 1 × placebo capsule to match ANA; n = 10). Subjects received their randomly assigned treatment based on a computer-generated allocation schedule. On Day 1, participants were fasted for predose lipid analysis followed by dose administration with a moderate-fat meal. On Days 2 through 13, study medication was self-administered with a meal. On Day 14, subjects returned to the clinic to receive a standard moderate-fat meal and the final dose of study medication after the collection of predose blood samples. Blood samples for lipid and lipoprotein analyses were collected predose on Day 1, and 24 h postdose on Day 14. The blood was collected in tubes containing dipotassium EDTA 0.15% (final concentration), sodium azide 0.01%, gentamycin sulfate 50 mg/ml, chloramphenicol 0.05 mg/ml, aprotinin 50 KU/ml, and PPACK 1 mM. Plasma was prepared and shipped overnight at 4°C to the Lipoprotein Analysis Core Laboratory at Children's Hospital Oakland Research Institute for the analyses described below.
Study participants
All study participants provided written informed consent before enrollment. The study was conducted in accordance with principles of Good Clinical Practice and was approved by the site's institutional review board (Ohio Valley Institutional Review Board) and regulatory agencies.
Healthy men and women with a body mass index of ≤35 kg/m2 and between 18 and 45 years of age were eligible for enrollment. Individuals bearing a clinically significant medical condition and those with a known history of alcohol and/or drug abuse or requiring use of comedications were excluded from the study. Participants were required to meet the following lipid entry criteria at the screening visit: LDL-C values ≥100 and ≤200 mg/dl; HDL-C values ≥35 and ≤70 mg/dl; and TG ≤300 mg/dl. Thirty participants received treatment and completed the study. One man and one woman in the ANA 150-mg treatment group were discontinued by the primary investigator after randomization, but prior to any dose administration, due to inadequate venous access. These individuals were immediately replaced with two others (both men) who met all of the predefined study entry criteria.
Analytical methods
Plasma lipids, lipoproteins, apo B, apo AI, and apo(a).
TG and cholesterol were measured by enzymatic end-point assays utilizing reagent kits (Ciba-Corning Diagnostics Corp., Oberlin, Ohio) and a Ciba-Corning Express 550 automated analyzer. HDL-C was measured after precipitation of apo B- and apo E-containing lipoproteins with dextran sulfate (12) and LDL-C was calculated by the formula of Friedewald et al. (13). All measurements were standardized through the Center for Disease Control-National Heart, Lung, and Blood Institute (CDC-NHLBI) Lipid Standardization Program.
Particle concentrations of VLDL, IDL, LDL, and HDL subfractions were analyzed in specific particle-size intervals using IM, which uniquely allows for direct particle quantification as a function of particle diameter following a procedure to remove other plasma proteins (14). The IM instrument utilizes an electrospray to create an aerosol of particles, which then pass through a differential mobility analyzer coupled to a particle counter. Particle concentrations (nmol/L) were determined for subfractions defined by the following size intervals (nm): VLDL: large (42.40–54.70), medium (33.50–42.39), small (29.60–33.49); IDL: large (25.00–29.59), small (23.33–24.99); LDL: large LDL1 (22.46–23.32), medium LDL2a (22.20–22.45) and LDL2b (21.41–22.19), small LDL3a (20.82–21.40) and LDL3b (20.49–20.81), very small LDL4a (19.90–20.48) and LDL4b (19.00–19.89); HDL: large HDL2b (10.50–14.50); smaller HDL2a+3 (7.65–10.49). Interassay variation was reduced by inclusion of two in-house controls in each preparatory process and duplicate analysis. CV <15% for each subfraction measurements was maintained throughout.
Plasma apoB, apoAI, and apo(a) were measured by sandwich-style ELISA. Both the apoAI and apoB primary antibodies were from Biodesign International (Saco, ME). Goat antiserum to human apoliprotein (a) (International Immunology Corporation, Murrieta, CA) was purified by caprylic acid precipitation and conjugated to horse radish peroxidase. All controls were validated by Northwest Lipid Laboratory, Seattle, WA, and all immunoassays were performed in triplicate with an inter-assay variation of <10%.
Ultracentrifugal isolation and compositional analysis of lipoproteins and lipoprotein subfractions.
Ultracentrifugational isolation of VLDL, IDL, and seven LDL subfractions was performed as described in the supplementary Materials. The mean densities of the LDL subfractions were (all g/ml): LDL subfraction (LF) 1: 1.0233; LF2: 1.0274; LF3: 1.0323; LF4: 1.0359; LF5: 1.0401; LF6: 1.0465; and LF7: 1.0556. Based on density and size criteria for the major LDL particle subclasses (15), LF1 contains large LDL1, LF2 and LF3 contain medium sized LDL 2, LF4 and LF5 contain small LDL3, and LF6 and LF7 contain very small LDL 4.
HDL and six HDL subfractions (HF) were prepared by ultracentrifugation from 4 ml of plasma as described in supplementary Materials. The mean densities of the subfractions were (all g/ml): HF1: 1.0990; HF2: 1.1074; HF3: 1.1145; HF4: 1.248; HF5: 1.1391; and HF6: 1.1576. Based on recently defined density and size criteria for the major HDL subclasses (16), HF1 contains very large HDL (also designated HDL2b); HF2 contains large HDL (also designated HDL2a); HF3 and HF4 contain medium-sized HDL (also designated HDL3a); HF5 contains small HDL (also designated HDL3b); and HF6 contains very small HDL (also designated HDL3c).
Free cholesterol (FC), CE, TG, phospholipids (PL), and total protein were analyzed in each ultracentrifugally isolated lipoprotein fraction as described in supplementary Materials. ApoAI, apoAII, apoCIII, and apoE were measured in triplicate by sandwich-style ELISA with CVs <10%. The apoAI antibody was the same as that described for the whole plasma assay above. The apoAII antiserum (International Immunology Corporation, Murrieta, CA) was purified by precipitation. The apoCIII primary antibody was purchased from Academy Bio-Medical Co., Inc. (Houston, Texas) and the apoE primary antibody was purchased from Biodesign (Saco, ME). The conjugated detection antibody (International Immunology Corporation, Murrieta, CA) was purified by precipitation. Control samples obtained from healthy individuals were validated by Northwest Lipid Laboratory, Seattle, WA.
Safety assessments
Safety was assessed by clinical evaluation of adverse experiences (AEs) and inspection of other study parameters at various scheduled time points throughout the study. Clinical evaluation included physical examination and measurement of vital signs, laboratory safety tests (i.e., blood chemistry, hematology, and urinalysis), 12-lead electrocardiograms, and AE assessments. For each AE, the intensity (mild, moderate, severe) and relationship to study drug (definitely not, possibly, probably, definitely related to study medication) were recorded by the study investigator.
Statistical analyses
The efficacy analyses utilized a modified intention-to-treat approach including all participants who had a baseline measurement, had taken at least one dose of study medication, and had at least one on-treatment measurement. The primary analyses, described herein, were post hoc analyses that examined the effects of ANA 150 mg versus placebo on whole plasma parameters and lipoprotein subfraction concentrations and composition. The primary endpoint for each analysis was change from baseline to Week 2. Percent change from baseline was also assessed for whole plasma lipid parameters and particle concentration data. Secondary analyses were performed to examine the effects of ANA 20 mg versus placebo and ANA 150 mg–20 mg dose response.
For the analyses of lipoprotein fractions and subfractions, data normality was first assessed using a Shapiro-Wilks test. For normally distributed values, an ANCOVA model was used with treatment as a factor and baseline lipid level as a covariate. Body mass index and age were also assessed as covariates but not included in the final model. Treatment differences in least squares mean change from baseline and corresponding two-sided 95% confidence intervals (CIs) were determined for normally distributed data. For nonnormally distributed values, Hodges-Lehman estimates of the differences in median change from baseline values and distribution-free CIs were determined. Descriptive statistics of baseline and Week 2 data were performed by treatment group using means, standard errors, medians, and interquartile ranges as appropriate. The method of Benjamini and Hochberg (17) for controlling the false discovery rate was employed to limit the proportion of false positives to no more than 5%. Control of the false discovery rate was applied across each set of whole fraction and subfraction analyses (lipoprotein concentration, and composition) and across testing of the whole plasma parameters. For each secondary analysis (ANA 20 mg vs. placebo, and ANA 150 mg–20 mg dose response), significance testing with control for the false discovery rate was performed in a step down manner across those whole plasma parameters where significant placebo-adjusted differences with ANA 150 mg were observed.
RESULTS
Baseline demographics and efficacy variables
Baseline demographic and clinical characteristics for all 30 randomized participants who received treatment are shown in supplementary Table I. All were judged by the study investigator to be in good general health based on routine medical history, physical examination, vital signs, and laboratory data. In total, 30 subjects received treatment and completed the study. The treatment groups were generally well balanced with respect to baseline demographics, although there were no women in either the ANA 150 and placebo groups and only two in the ANA 20 mg group.
Whole plasma lipid and lipoprotein analyses
At Week 2, treatment with ANA 150 mg led to statistically significant placebo-adjusted decreases in LDL-C of 26%, and increases in HDL-C of 82%, respectively when compared with baseline values (Table 1). The observed placebo-adjusted changes in LDL-C and HDL-C were accompanied by a 29% decrease in apoB and a 21% increase in apoAI. A 43% placebo-adjusted decrease from baseline in Lp(a) was also observed. There were no significant changes in total cholesterol or TG relative to placebo. Results for the 20 mg group are presented in supplementary Table II.
TABLE 1.
Standard lipid and lipoprotein measurements in whole plasma for the ANA 150 mg and placebo groups
| Placebo (N=10) | ANA 150 mg (N=10) | Difference in LS mean percent change from baseline (95%CI)a | |||
| Parameter(mg/dl) | Mean baseline ± SE | Week 2 ± SE | Mean baseline ± SE | Week 2 ± SE | |
| TC | 197 ± 8 | 185 ± 8 | 186 ± 8 | 188 ± 10 | 5 (−4, 14) |
| LDL-C | 120 ± 7 | 112 ± 9 | 119 ± 8 | 83 ± 12 | −26 (−43, −9)b |
| HDL-C | 52 ± 3 | 50 ± 3 | 42 ± 2 | 78 ± 5 | 82 (60, 105)bc |
| TGd | 102 ± 93 | 115 ± 40 | 124 ± 48 | 130 ± 87 | 14 (−6, 39) |
| Apo B | 84 ± 7 | 84 ± 7 | 79 ± 5 | 57 ± 3 | −29 (−42, −15)b,c |
| Apo AI | 129 ± 7 | 120 ± 4 | 118 ± 6 | 143 ± 5 | 21 (8, 35)b |
| Lp(a)d | 2 ± 5 | 2 ± 5 | 5 ± 6 | 4 ± 5 | −43 (−70, −20)b |
Least squares mean and 95% confidence interval from ANCOVA model with treatment as a factor and baseline lipid level as a covariate.
Significant with adjustment for false discovery rate less than 5%.
Significant dose-response with adjustment for false discovery rate less than 5%. Testing performed in step-down fashion.
Hodges-Lehman estimate of median and distribution free confidence interval presented for change from baseline results; median ± IQR presented at baseline and Week 2.
CI, confidence interval; IQR, interquartile range; Lp(a), lipoprotein(a); TG, triglyceride; TC = total cholesterol.
IM lipoprotein particle analyses
Baseline and absolute change from baseline in VLDL, IDL, LDL, and HDL particle concentrations as measured by IM methodology for the ANA 150 mg and placebo groups are shown in Table 2. In general, there were no meaningful differences in lipoprotein particle concentrations between the groups at baseline. The only exceptions included slightly higher baseline particle concentrations of large LDL1, large HDL2b, and smaller HDL2a+3 in the placebo compared with the ANA 150 mg groups.
TABLE 2.
Absolute change from baseline to Week 2 in lipoprotein particle concentrations (nmol/L) for the placebo and ANA 150 mg groups as measured by IM methodology
| Placebo (N=10) | ANA 150 mg (N=10) | Differences in LS mean change from baseline(95% CI)aPlacebo vs.ANA 150 mg | |||
| Lipoprotein size fractions | Mean baseline (SE) | Mean Week 2 (SE) | Mean baseline (SE) | Mean Week 2 (SE) | |
| Total VLDL | 156.51 (5.89) | 147.45 (15.82) | 154.57 (13.71) | 101.80 (6.93) | −44.95 (−74.43, −15.46)c |
| Largeb | 25.94 (15.59) | 21.24 (17.67) | 28.36 (10.15) | 18.64(6.36) | −6.86 (−13.68, −0.66) |
| Medium | 65.18 (3.86) | 60.58 (6.86) | 66.14 (6.45) | 42.66 (3.46) | −18.31 (−30.93, −5.70)c |
| Small | 66.55 (2.87) | 63.12 (7.24) | 61.40 (5.20) | 39.67 (3.18) | −21.95 (−35.82, −8.08)c |
| Total IDL | 365.05 (18.13) | 327.55 (36.81) | 303.94 (27.32) | 260.10 (22.15) | −58.49 (−151.2, 34.22) |
| Large | 155.69 (6.89) | 146.53 (19.23) | 142.25 (13.02) | 83.22 (7.22) | −60.87 (−96.59, -25.15)c |
| Small | 209.37 (20.52) | 181.02 (20.66) | 161.69 (16.89) | 176.88 (16.18) | 6.88 (−58.94, 72.69) |
| Total LDL | 1355.3 (82.00) | 1227.5 (158.17) | 1254.0 (84.39) | 767.10 (38.74) | −419.3 (−682.0, −156.6)c |
| Large 1b | 346.07 (179.07) | 226.94 (134.86) | 254.60 (188.69) | 149.80 (41.29) | −75.84 (−189.9, 50.57) |
| Medium 2ab | 229.05 (103.64) | 208.15 (92.56) | 255.64 (190.67) | 99.79 (11.48) | −126.4 (−192.2, −31.25)c |
| Medium 2bb | 297.33 (187.92) | 243.02 (155.97) | 307.32 (94.16) | 134.82 (17.17) | −126.3 (−204.0, −52.12)c |
| Small 3a | 266.41 (59.76) | 276.43 (76.87) | 227.02 (35.13) | 121.42 (5.99) | −127.8 (−223.1, −32.55)c |
| Small 3bb | 41.58 (38.60) | 40.07 (110.02) | 41.41 (26.15) | 49.05 (15.21) | 6.79 (−41.49, 33.02) |
| Very small 4a | 63.38 (6.13) | 64.35 (10.59) | 52.60 (3.48) | 84.81 (5.11) | 28.23 (2.12, 54.34) |
| Very small 4b | 84.98 (9.52) | 73.48 (8.85) | 67.07 (6.66) | 117.09 (7.17) | 57.52 (32.38, 82.66)c |
| Total HDL | 5769.8 (355.82) | 4785.7 (362.07) | 4589.3 (558.16) | 7658.7 (595.16) | 3364.6 (1994.8, 4734.4)c,d |
| Large 2b | 1346.9 (223.29) | 997.01 (159.35) | 738.57 (108.85) | 3382.2 (348.97) | 2851.9 (2056.7, 3647.2)c,d |
| Smaller 2a+3b | 4403.2 (2184.1) | 3806.4 (1101.2) | 3509.3 (956.80) | 4051.3 (1078.4) | 1254.8 (−610.7, 2197.2) |
Least squares mean and 95% confidence interval from ANCOVA model with treatment as a factor and baseline lipid level as a covariate.
Change from baseline data not normally distributed; Hodges-Lehman estimate of median and distribution free confidence interval presented; median (IQR) presented at baseline and Week 2.
Significant with false discovery rate less than 5%
Significant dose-response with adjustment for false discovery rate less than 5%. Testing performed in step-down fashion.
CI, confidence interval; IDL, intermediate density lipoprotein; IM, ion mobility; IQR, interquartile range.
Treatment with ANA 150 mg resulted in large, statistically significant placebo-adjusted reductions in mean particle concentrations of medium VLDL (18 nmol/L, 22%), small VLDL (22 nmol/L, 31%), large IDL (61 nmol/L, 35%), medium LDL2a (126 nmol/L, 35%), and 2b (126 nmol/L, 39%), and small LDL3a (128 nmol/L, 28%) compared with placebo (Table 2). There was also a significant treatment-related placebo-adjusted increase in particle concentration of very small LDL4b (58 nmol/L, 75%), which represented 5.3% of the total LDL fraction at baseline and 15.3% of total LDL after treatment in the ANA 150 mg group. Within the HDL fraction, ANA 150 mg produced a significant placebo-adjusted increase in large HDL2b (2852 nmol/L, 373%) but no significant change in smaller HDL2a+3 particle concentration.
The effects of ANA 20 mg on VLDL, IDL, and HDL lipoprotein particle concentrations were directionally consistent to those seen in the ANA 150 mg group (Table 2; supplementary Table III). Within the HDL fraction, a significant dose-related increase in HDL2b was observed with ANA 150 mg compared with 20 mg; however, no other subfractions displayed significant dose-related changes in particle concentrations (data not shown).
Compositional analyses
Lipids.
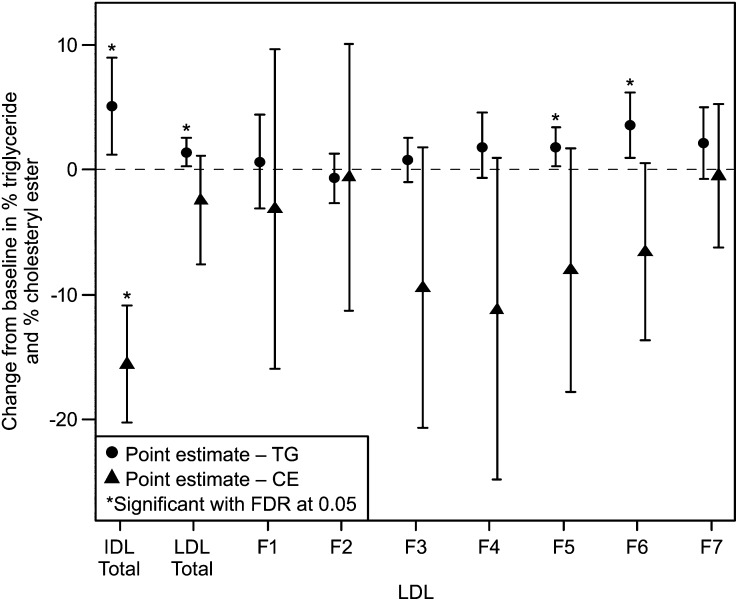
At baseline, the ratios of TG/CE for total VLDL, IDL, LDL, and HDL were similar across the ANA 150 mg and placebo groups (Table 3). Compared with placebo, ANA 150 mg produced significant increases in the TG/CE ratio of VLDL, IDL, and LDL fractions and a statistically significant decrease in the TG/CE ratio of the HDL fraction (Table 3). The magnitude of the increase in the TG/CE ratio with ANA 150 mg/d was larger for the VLDL fraction than for either IDL or LDL. Analyses of TG and CE as a percentage of total lipoprotein mass in the various fractions and subfractions are shown in Fig. 1 and supplementary Table IV. The overall increase in the TG/CE ratio of IDL with ANA treatment (Table 3) was due to enrichment in TG and a reduction in CE content (Fig. 1, supplementary Table IV). The increase in TG/CE ratio of the LDL fraction (Table 3) appears to be driven mainly by larger changes in TG and CE, as a percentage of total mass, for LDL subfractions LF3 through 6 (Fig. 1).
TABLE 3.
Absolute change from baseline in TG/CE ratios for total VLDL, IDL, LDL, and HDL fractions for the placebo and ANA 150 mg groups isolated by ultracentrifugation
| Placebo (N=10) | ANA 150 mg (N=10) | Difference in median change from baseline 95% CI)aPlacebo vs.ANA 150 mg | |||
| Parameter | Median baseline ± IQR | Median Week 2 ± IQR | Median baseline ± IQR | Median Week 2 ± IQR | |
| Total VLDL TG/CE ratio | 8.52 ± 1.73 | 6.78 ± 1.07 | 7.20 ± 0.92 | 22.66 ± 7.40b | 14.67 (2.78, 18.20)c |
| Total IDL TG/CE ratio | 0.99 ± 0.10 | 0.89 ± 0.12 | 0.89 ± 0.11 | 3.85 ± 0.91 | 3.23 (0.95, 3.97)c |
| Total LDL TG/CE ratio | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.01 | 0.13 ± 0.02 | 0.05 (0.00, 0.08)c |
| Total HDLb TG/CE ratio | 0.13 ± 0.08 | 0.13 ± 0.09 | 0.15 ± 0.08 | 0.05 ± 0.02 | −0.13 (−0.13, −0.07)c |
Hodges-Lehman nonparametric estimate of differences in median change from baseline and distribution free confidence interval presented; median ± IQR) presented at baseline and Week 2.
Change from baseline data normally distributed; difference in least squared mean and 95% confidence interval presented from ANCOVA model with treatment as a factor and baseline lipid level as a covariate; mean ± SE presented at baseline and Week 2.
Significant with false discovery rate less than 5%
CE, cholesteryl ester; CI, confidence interval, IDL, intermediate density lipoprotein; IQR, interquartile range; TG, triglyceride.
Fig. 1.
Placebo-adjusted differences in mean change from baseline ± 95% confidence intervals in % TG/mass and %CE/mass content for IDL and LDL subfractions in ANA 150 mg versus placebo groups. For nonparametrically distributed data (TG%: LDL F4; CE%: LDL total, F4), the Hodges-Lehman estimate of median difference and distribution-free confidence interval are presented; otherwise, the least squares mean difference and 95% confidence interval from an ANCOVA model with treatment as a factor and baseline lipid level as a covariate are presented.
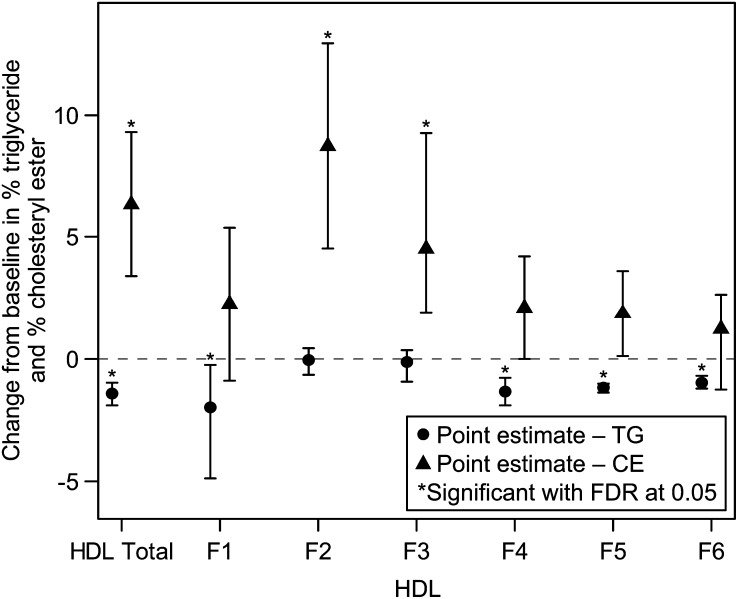
ANA 150 mg produced a statistically significantly greater reduction in the TG/CE ratio of HDL compared with placebo (Table 3). There were statistically significant reductions in TG as a percentage of total mass compared with placebo in all of the HDL subfractions, except for HF2 and HF3, whereas there was a statistically significant increase in CE expressed as a percentage of total lipoprotein mass (%CE) in both HF2 and HF3 but not the other subfractions (Fig. 2 and supplementary Table IV). The effects of ANA 150 mg on %CE in the HDL subfractions were generally more pronounced and more variable than the observed changes in TG expressed as a percentage of total lipoprotein mass. Changes in TG and CE with ANA 20 mg/day (supplementary Table V) were similar to those with ANA 150 mg/day but were generally smaller in magnitude.
Fig. 2.
Placebo-adjusted differences in median change from baseline ± 95% confidence intervals in % TG/mass and %CE/mass content for HDL subfractions in ANA 150 mg versus placebo groups. For normally distributed data (TG%: HDL total, F6; CE%: HDL F1, F2, F4, F5), the least squares mean difference and 95% confidence interval from an ANCOVA model with treatment as a factor and baseline lipid level as a covariate are presented; otherwise, the Hodges-Lehman estimate of median difference and distribution free confidence interval are presented.
Changes in content of FC and PL, each as a percentage of total mass, among the lipoprotein subfractions with ANA treatment were not statistically significant except for modest increases in proportion of %FC in total HDL and %PL in the most buoyant HDL fractions (supplementary Table IV).
Apolipoproteins.
Apo AI and apoAII.
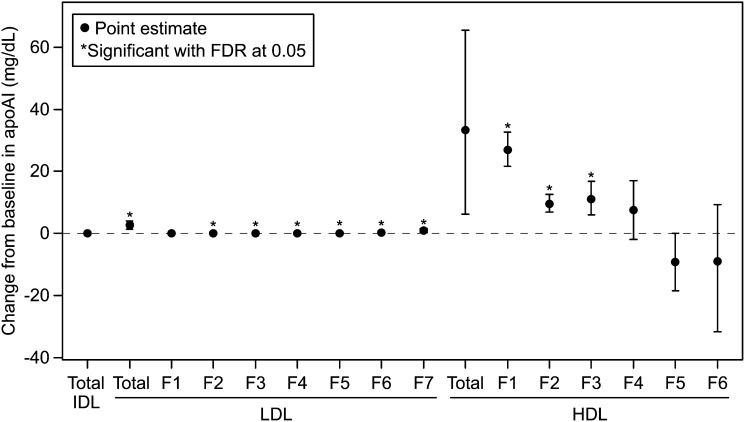
Treatment with ANA 150 mg vs. placebo resulted in small but significant increases in levels of both apoAI and apoAII (Fig. 3 and supplementary Table VI) in total LDL and all LDL subfractions, except LF1, with the greatest increases in those of highest density (Fig. 3). The findings were generally comparable when apoAI and apoAII content were expressed as percent of total LDL mass (supplementary Table VII).
Fig. 3.
Placebo-adjusted differences in median change from baseline ± 95% confidence intervals in ApoAI for IDL, LDL, and HDL subfractions in ANA 150 mg versus placebo groups. For normally distributed data (LDL F2, F5, F6, HDL F5), the least squares mean difference and 95% confidence interval from an ANCOVA model with treatment as a factor and baseline lipid level as a covariate are presented; otherwise, the Hodges-Lehman estimate of median difference and distribution free confidence interval are presented.
As expected, with ANA 150 mg vs. placebo there were large, statistically significant increases in absolute concentrations of ApoAI within total HDL and the most buoyant HDL subfractions HF1-HF3 (Fig. 3). However, HDL apoAI content (weight percent) was significantly increased only for HF1, whereas there was a decrease in apoAI content (weight percent) in HF5, resulting in no net change for total HDL (supplementary Table VII). ApoAII levels were also significantly increased in HF1-HF3, as well as HF6 (supplementary Table VI). but the only significant change in HDL apoAII content expressed as percent mass was a relative decrease in the densest HDL subfraction that appeared to be largely ascribable to an increase in the placebo group (supplementary Table VII).
ApoE and apoCIII.
Content of both apoE and apoCIII within IDL, expressed as percent total mass (supplementary Table VII) were significantly lower with ANA 150 mg versus placebo, whereas there was enrichment of both apolipoproteins in total LDL (supplementary Table VII). The increases in apoE and apoCIII within LDL were confined to the denser subfractions (LF4-LF6), but the changes were not significant for apoCIII, except for a decrease in apoCIII in LF1 (supplementary Table VII). In addition, absolute levels of apoCIII were reduced in LF1 and increased in LF6 (supplementary Table VI).
Interestingly, although absolute apoE levels were increased in HDL subfractions HF1-HF2 (supplementary Table VI), there was significant apoE depletion in HF1-HF3 when expressed as percent total HDL mass (supplementary Table VII). For apoCIII, there were increases in absolute concentrations in subfractions HF1-HF4 (supplementary Table VI), with significant enrichment in HF3 and HF4 (supplementary Table VII).
Safety and tolerability analyses
ANA 20 mg and 150 mg doses were generally well-tolerated in this population of healthy, adult individuals. No serious clinical adverse experiences were reported and no participants discontinued due to an adverse experience. Eight subjects had a total of 15 nonserious clinical adverse experiences during this study (5, 2, and 1 subjects in the ANA 20 mg, 150 mg, and placebo groups, respectively). Headache was the most frequent clinical adverse event reported with all treatments. All clinical adverse experiences were transient in duration and rated by the study investigator as mild or moderate in intensity and definitely not or probably not related to study drug. There were no laboratory adverse experiences reported during this study.
DISCUSSION
This 3-arm, randomized, double-blind, placebo-controlled study compared the effects of two doses of ANA, 20 mg/day and 150 mg/day, with placebo in healthy subjects without dyslipidemia. The results confirmed dose-related decreases in LDL-C and increases in HDL-C, with the largest dose-related effect noted for HDL (9, 10). The changes in LDL-C and HDL-C seen with the ANA at 150 mg/day in this study were less marked than those reported previously (11), possibly reflecting differences in subject characteristics, duration of treatment, drug formulations, and/or analytical methods used for measuring lipoprotein concentrations. Despite the absence of a significant change in plasma TG levels seen with ANA in this study, there was substantial lowering of particle concentrations of the TG-rich lipoprotein fractions medium and small VLDL and large IDL, with the greatest effects on large IDL. This is consistent with TG enrichment of these particles as a result of CETP inhibition, as manifested by a 3- to 4-fold increase in the content of TG relative to CE in ultracentrifugally isolated VLDL and IDL and subsequent lipolytic catabolism and plasma clearance of these particles (18).
The lowering of LDL-C can be attributed primarily to significant reductions of concentrations of medium LDL2a, 2b, and small LDL 3a, coupled with a trend toward CE depletion of ultracentrifugally isolated fractions in the corresponding mid-region of the LDL density distribution. It has been reported that in the case of treatment with the CETP inhibitor torcetrapib, reduction in LDL apo B concentration is due to increased fractional catabolic rate (18). Although the mechanism underlying this effect is not known, it is likely to reflect increased hepatic uptake of LDL particles, possibly due to the upregulation of hepatic LDL receptor activity and/or increased clearance of LDL particles due to their altered composition. Interestingly, although the largest-sized LDL subfraction (LDL 1) was the most abundant in this study population, its levels were not significantly reduced by ANA. In addition, the cholesterol depletion of the most buoyant LDL fractions that contain the largest LDL particles (15) did not appear to be as great as for those in the mid-density range. Whereas there is evidence that the most buoyant LDL have reduced LDL receptor affinity compared with mid-density LDL (19, 20), the similar reductions in relative CE content of TG-enriched IDL and medium density LDL also are consistent with precursor-product relationships between these particles that differ from those for more buoyant LDL (15).
We also observed a modest enrichment of TG content of LDL particles, an effect that was confined to the very dense subfractions. Although the basis for this finding is unclear, it may be related to the observation that the absolute plasma concentrations of very small LDL 4b that are contained in the densest LDL fractions were significantly increased by ANA 150 mg versus placebo. Notably, in subfractions separated by density gradient ultracentrifugation from two patients with extreme CETP deficiency, a distinct subset of particles smaller than the major species was found to extend across the entire density spectrum from IDL to very dense LDL (21). This finding was taken to be consistent with parallel metabolic pathways for production and catabolism of TG-enriched apoB-containing lipoproteins, as discussed elsewhere (15), such that the pathway originating with larger particles is not affected by CETP and gives rise to LDL particles similar in size and density to those found in individuals with normal CETP activity. In the setting of CETP deficiency, particles in the smaller IDL pathway remain TG-enriched and are progressively lipolyzed to yield very small, dense LDL particles. Further evidence for the existence of a specific metabolic pathway giving rise to very small LDL was recently provided by the discovery that levels of these particles are selectively associated with a single nucleotide polymorphism that modulates hepatic production of sortilin, a protein shown to strongly influence hepatic VLDL, TG, and apoB-100 secretion (22). The plasma accumulation of very small LDL with CETP inhibition may be potentiated by their reduced LDL receptor affinity, a property that is shared, as noted above, with large buoyant LDL (19, 20). Finally, the enrichment of very dense LDL with TG may be amplified by their relative resistance to lipolysis by both lipoprotein lipase and hepatic TG lipase (23).
Although small increases of apoAI and apoAII concentrations were observed in LDL subfractions, the composition of these particles differed markedly from that of large HDL, and together with the findings in patients in CETP deficiency (21), this indicates minimal, if any, overlap of the LDL and HDL particle distributions. The concordant enrichment of denser LDL with apoE, and to a lesser extent, apoCIII, is of uncertain significance. Possibilities include retention of these apoproteins during catabolism of TG-rich precursors and/or reduced transfer to HDL in conjunction with reduced TG-CE exchange. It is also possible that, as for HDL, there was redistribution of exchangeable apoproteins as a consequence of ultracentrifugation.
Consistent with previous studies (9, 10), dose-related increases in plasma HDL-C concentrations with ANA were primarily due to effects on very large HDL 2b particles. There was modest TG depletion and PL enrichment in the most buoyant HDL fractions that contain these particles, as well as reduction of TG in the most dense HDL fractions containing smaller HDL. Interestingly, although the largest increases in absolute levels of apoAI, apoAII, apoE, and apoCIII were in the most buoyant HDL subfractions, relative content of apoE, as percent total particle mass, was reduced in these fractions, whereas relative content of both apoCIII and CE were increased in particles in the mid-HDL density range. The basis for these differential changes in lipid and apoprotein content of HDL particles is not known, although they suggest selectivity in the action of CETP and/or the drug on subspecies of HDL particles.
Limitations of this study include the relatively small number of participants, the post hoc nature of the analyses, the restriction of the findings to normolipidemic individuals, and the absence of women in the ANA 150 mg and placebo groups. Also, whereas a 2 week treatment period has been shown to be sufficient for assessing the effects of anacetrapib on LDL-C, further increases in HDL-C have been observed with longer-term treatment (11), and hence, it is possible that this may be accompanied by further changes in HDL subfraction levels and composition.
Although the dramatic increases in HDL-C and reductions in LDL-C induced by ANA as well as other CETP inhibitors suggest a potentially significant benefit on CVD risk, it is not yet known whether the metabolic bases for these effects, and the resultant changes in levels and composition of specific LDL and HDL particle subclasses, might alter their physiological and pathological functions. For example, it has been reported that increased levels of very small LDL are highly associated with angiographic progression of coronary artery disease (24) and the single nucleotide polymorphism discussed above that is selectively associated with very small LDL levels is also predictive of risk of myocardial infarction (25). On the other hand, ANA treatment resulted in a reduction in the total number of LDL particles, most notably those in the medium and small LDL size range as measured by IM that have been associated with increased CVD risk (26). Moreover, it has been shown that capacity of HDL to promote cholesterol cellular efflux, a key determinant of HDL's anti-atherogenic effects (27), is preserved by treatment with ANA (28) as well as with other CETP inhibitors (29, 30). Ultimately, determination of the efficacy of ANA for reducing major coronary events awaits the completion of REVEAL, a clinical outcomes trial in 30,000 patients with cardiovascular disease at high risk for major coronary events (NCT01252953).
Supplementary Material
Acknowledgments
The authors thank the investigators and participants in this study. The authors also thank Kathleen Newcomb for editorial support, Fang Liu for statistical analysis guidance, and Jing Su (all from Merck) for statistical programming support.
Footnotes
Abbreviations:
- AE
- adverse experience
- ANA
- anacetrapib
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- CHD
- coronary heart disease
- CI
- confidence interval
- FC
- free cholesterol
- HDL-C
- HDL cholesterol
- HF
- HDL subfraction
- IDL
- intermediate density lipoprotein
- IM
- ion mobility
- LDL-C
- LDL cholesterol
- LF
- LDL subfraction
- PL
- phospholipid
- TG
- triglyceride
This work was funded by Merck & Co, Inc., Whitehouse Station, NJ. C. A. Pinto, Y. Liu, J. Mabalot Luk, J. A. Wagner, A. O. Johnson-Levonas, M. S. Anderson, and H. M. Dansky are employees of Merck Sharp & Dohme Corp. and may own stock or hold stock options in the company. R. M. Krauss has been a member of the Global Atherosclerosis Advisory Board of Merck and has received grant support from them. Dr. Krauss is also co-inventor on a patent for ion mobility analysis of lipoproteins.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of supplementary Materials and Methods and seven tables.
REFERENCES
- 1.Pepine C. J. 2010. Residual risk for secondary ischemic events in patients with atherothrombotic disease: opportunity for future improvements in patient care. Ann. Med. 42: 19–35. [DOI] [PubMed] [Google Scholar]
- 2.Judge E. P., Phelan D., O'Shea D. 2010. Beyond statin therapy: a review of the management of residual risk in diabetes mellitus. J. R. Soc. Med. 103: 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tall A. R. 1993. Plasma cholesteryl ester transfer protein. J. Lipid Res. 34: 1255–1274. [PubMed] [Google Scholar]
- 4.Koizumi J., Mabuchi H., Yoshimura A., Michishita I., Takeda M., Itoh H., Sakai Y., Sakai T., Ueda K., Takeda R. 1985. Deficiency of serum cholesteryl-ester transfer activity in patients with familial hyperalphalipoproteinaemia. Atherosclerosis. 58: 175–186. [DOI] [PubMed] [Google Scholar]
- 5.Thompson A., Di Angelantonio E., Sarwar N., Erqou S., Saleheen D., Dullaart R. P., Keavney B., Ye Z., Danesh J. 2008. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 299: 2777–2788. [DOI] [PubMed] [Google Scholar]
- 6.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 7.Forrest M. J., Bloomfield D., Briscoe R. J., Brown P. N., Cumiskey A. M., Ehrhart J., Hershey J. C., Keller W. J., Ma X., McPherson H. E., et al. 2008. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br. J. Pharmacol. 154: 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X., Dietz J. D., Xia C., Knight D. R., Loging W. T., Smith A. H., Yuan H., Perry D. A., Keiser J. 2009. Torcetrapib induces aldosterone and cortisol production by an intracellular calcium-mediated mechanism independently of cholesteryl ester transfer protein inhibition. Endocrinology. 150: 2211–2219. [DOI] [PubMed] [Google Scholar]
- 9.Bloomfield D., Carlson G. L., Sapre A., Tribble D., McKenney J. M., Littlejohn T. W., III, Sisk C. M., Mitchel Y., Pasternak R. C. 2009. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am. Heart J. 157: 352–360. [DOI] [PubMed] [Google Scholar]
- 10.Krishna R., Anderson M. S., Bergman A. J., Jin B., Fallon M., Cote J., Rosko K., Chavez-Eng C., Lutz R., Bloomfield D. M., et al. 2007. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet. 370: 1907–1914. [DOI] [PubMed] [Google Scholar]
- 11.Cannon C. P., Shah S., Dansky H. M., Davidson M., Brinton E. A., Gotto A. M., Stepanavage M., Liu S. X., Gibbons P., Ashraf T. B., et al. 2010. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 363: 2406–2415. [DOI] [PubMed] [Google Scholar]
- 12.Warnick G. R., Nguyen T., Albers A. A. 1985. Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin. Chem. 31: 217–222. [PubMed] [Google Scholar]
- 13.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 14.Caulfield M. P., Li S., Lee G., Blanche P. J., Salameh W. A., Benner W. H., Reitz R. E., Krauss R. M. 2008. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin. Chem. 54: 1307–1316. [DOI] [PubMed] [Google Scholar]
- 15.Berneis K. K., Krauss R. M. 2002. Metabolic origins and clinical significance of LDL heterogeneity. J. Lipid Res. 43: 1363–1379. [DOI] [PubMed] [Google Scholar]
- 16.Rosenson R. S., Brewer H. B., Jr, Chapman M. J., Fazio S., Hussain M. M., Kontush A., Krauss R. M., Otvos J. D., Remaley A. T., Schaefer E. J. 2011. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 57: 392–410. [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc, B. 57: 289–300. [Google Scholar]
- 18.Millar J. S., Brousseau M. E., Diffenderfer M. R., Barrett P. H., Welty F. K., Faruqi A., Wolfe M. L., Nartsupha C., Digenio A. G., Mancuso J. P., et al. 2006. Effects of the cholesteryl ester transfer protein inhibitor torcetrapib on apolipoprotein B100 metabolism in humans. Arterioscler. Thromb. Vasc. Biol. 26: 1350–1356. [DOI] [PubMed] [Google Scholar]
- 19.Campos H., Arnold K. S., Balestra M. E., Innerarity T. L., Krauss R. M. 1996. Differences in receptor binding of LDL subfractions. Arterioscler. Thromb. Vasc. Biol. 16: 794–801. [DOI] [PubMed] [Google Scholar]
- 20.Lund-Katz S., Laplaud P. M., Phillips M. C., Chapman M. J. 1998. Apolipoprotein B-100 conformation and particle surface charge in human LDL subspecies: implication for LDL receptor interaction. Biochemistry. 37: 12867–12874. [DOI] [PubMed] [Google Scholar]
- 21.Sakai N., Matsuzawa Y., Hirano K., Yamashita S., Nozaki S., Ueyama Y., Kubo M., Tarui S. 1991. Detection of two species of low density lipoprotein particles in cholesteryl ester transfer protein deficiency. Arterioscler. Thromb. 11: 71–79. [DOI] [PubMed] [Google Scholar]
- 22.Musunuru K., Strong A., Frank-Kamenetsky M., Lee N. E., Ahfeldt T., Sachs K. V., Li X., Li H., Kuperwasser N., Ruda V. M., et al. 2010. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 466: 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musliner T. A., Herbert P. N., Kingston M. J. 1979. Lipoprotein substrates of lipoprotein lipase and hepatic triacylglycerol lipase from human post-heparin plasma. Biochim. Biophys. Acta. 575: 277–288. [DOI] [PubMed] [Google Scholar]
- 24.Williams P. T., Superko H. R., Haskell W. L., Alderman E. L., Blanche P. J., Holl L. G., Krauss R. M. 2003. Smallest LDL particles are most strongly related to coronary disease progression in men. Arterioscler. Thromb. Vasc. Biol. 23: 314–321. [DOI] [PubMed] [Google Scholar]
- 25.Teslovich T. M., Musunuru K., Smith A. V., Edmondson A. C., Stylianou I. M., Koseki M., Pirruccello J. P., Ripatti S., Chasman D. I., Willer C. J., et al. 2010. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 466: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musunuru K., Orho-Melander M., Caulfield M. P., Li S., Salameh W. A., Reitz R. E., Berglund G., Hedblad B., Engstrom G., Williams P. T., et al. 2009. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 29: 1975–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khera A. V., Cuchel M., Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yvan-Charvet L., Kling J., Pagler T., Li H., Hubbard B., Fisher T., Sparrow C. P., Taggart A. K., Tall A. R. 2010. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler. Thromb. Vasc. Biol. 30: 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi J., Okamoto H., Otabe M., Bujo H., Saito Y. 2002. Effect of HDL, from Japanese white rabbit administered a new cholesteryl ester transfer protein inhibitor JTT-705, on cholesteryl ester accumulation induced by acetylated low density lipoprotein in J774 macrophage. Atherosclerosis. 162: 131–135. [DOI] [PubMed] [Google Scholar]
- 30.Yvan-Charvet L., Matsuura F., Wang N., Bamberger M. J., Nguyen T., Rinninger F., Jiang X. C., Shear C. L., Tall A. R. 2007. Inhibition of cholesteryl ester transfer protein by torcetrapib modestly increases macrophage cholesterol efflux to HDL. Arterioscler. Thromb. Vasc. Biol. 27: 1132–1138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.