Abstract
The precise orosensory inputs engaged for dietary lipids detection in humans are unknown. We evaluated whether a common single nucleotide polymorphism (rs1761667) in the CD36 gene that reduces CD36 expression and the addition of orlistat, a lipase inhibitor, to reduce FA release from triacylglycerols (TGs), the main component of dietary fats, would attenuate fat orosensory sensitivity in humans. Twenty-one obese subjects with different rs1761667 genotypes (6 AA, 7 AG, and 8 GG) were studied on two occasions in which oleic acid and triolein orosensory detection thresholds were measured using emulsions prepared with and without orlistat. Subjects homozygous for the G-allele had 8-fold lower oral detection thresholds for oleic acid and triolein than subjects homozygous for the A allele, which associates with lower CD36 expression (P = 0.03). Thresholds for heterozygous subjects were intermediate. The addition of orlistat increased detection thresholds for triolein (log threshold = −0.3 ± 0.2 vs. 0.3 ± 0.1; P < 0.001) but not oleic acid (log threshold = −1.0 ± 0.2 vs. −0.8 ± 0.2; P > 0.2). In conclusion, this is the first experimental evidence for a role of CD36 in fat gustatory perception in humans. The data also support involvement of lingual lipase and are consistent with the concept that FA and not TG is the sensed stimulus.
Keywords: diet and dietary lipids, genetics, lipids, obesity, triglycerides, fat oral sensitivity, taste perception
Obesity is caused by ingesting more energy than is expended over a long period of time. Dietary fat is the most energy-dense macronutrient, and its overconsumption has been linked to obesity (1–4). Obese people prefer foods with higher fat content (5), crave high-fat foods more frequently (6, 7), and consume more fat than lean individuals (8).
Traditionally, perception of fat in the oral cavity is thought to rely almost entirely on textural and aromatic cues activating the somatosensory and olfactory systems. However, there is now increasing evidence to support an important role of the gustatory system in fat perception (9–20) as well as in intestinal lipid metabolism (10, 21). Oral and gastrointestinal fat sensory sensitivity appear to be associated (16) and there is similarity in the chemosensory reception events and their signaling transduction pathways in the tongue and gastrointestinal tract (21). An important requirement for the involvement of a gustatory component in dietary lipid detection is the hydrolysis of triacylglycerols (TGs) to release free FAs, the signaling stimulus, as was demonstrated through the use of the lipase inhibitor orlistat (22). In rodents, lingual lipase is essential for the gustatory perception of dietary fats (22) and the addition of orlistat to fat emulsions diminishes the rat's preference for TG, but not FA (22). Although it is not known whether lingual lipase is important for oral fat perception in humans, data from a recent study suggests that lingual lipase lipolytic activity can produce FA within the concentration range required to activate oral sensors (18).
Several putative fat taste receptor classes have been identified in rodents (12, 23, 24), including the glycoprotein CD36 (25). The presence of CD36, a scavenger receptor that mediates uptake and trafficking of lipids in diverse cell types (26), has been documented in the gustatory papillae of rodents (25, 27), pigs, and humans (28). In rodents, the interaction between CD36 and FA results in signaling events that depend on an intact neuronal gustatory pathway (15, 29). CD36 gene knockout impedes fat detection in mice without affecting sweet or bitter perception and blunts the cephalic phase of pancreatobiliary secretions that are triggered by exposure of specific areas of the tongue to fat (25).
The primary goal of this study was to advance our understanding of fat orosensory perception in humans by evaluating the role of lingual lipase and CD36 on fat detection thresholds. We studied only obese subjects because of their documented preference and consumption of more high-fat foods than lean subjects (5–8), which would tend to neutralize the effect of dietary fat and diminish individual variability. The following two hypotheses were investigated: 1) whether a common variant in the CD36 gene that reduces CD36 expression [i.e., single nucleotide polymorphism (SNP) rs1761667-A allele (30, 31)] will associate with higher oral fat detection thresholds (i.e., lower oral sensitivity to fat) and 2) whether addition of orlistat to a fat emulsion increases the oral detection threshold for TG more than those for FA. Oleic acid and triolein orosensory detection thresholds were measured in obese subjects who were either carriers or noncarriers of the rs1761667-A allele by having subjects taste emulsions prepared with and without orlistat.
MATERIALS AND METHODS
Subjects
Three groups of obese subjects [body mass index (BMI) ≥30 kg/m2] participated in this study (Table 1). Two groups were carriers of the rs1761667-A allele (AA, n = 6 and AG, n = 7) and one group was a noncarrier (GG, n = 8). The three groups were matched on age because there is a generic decline in taste perception with age (32). Potential subjects who smoked cigarettes in the last 6 months, had chronic sinus problems, previous malabsorptive or restrictive intestinal surgery, diabetes, or who were pregnant, breastfeeding, or taking any medication that might affect taste perception were excluded.
TABLE 1.
Subject characteristics
| AA | AG | GG | |
| Age (yrs) | 38.7 ± 3.8 | 39.6 ± 3.5 | 39.1 ± 3.3 |
| Gender | |||
| Female | 6 | 6 | 6 |
| Male | 0 | 1 | 2 |
| Race | |||
| African American | 4 | 7 | 8 |
| Caucasian | 2 | 0 | 0 |
| BMI (kg/m2) | 34.9 ± 2.3 | 38.3 ± 2.1 | 41.5 ± 2.0 |
| Fat Preference Questionnaire | |||
| TASTE | 68.5 ± 7.0 | 67.0 ± 6.5 | 70.1 ± 6.5 |
| FREQ | 48.8 ± 10.5 | 55.5 ± 9.7 | 59.1 ± 9.7 |
| DIFF | 19.7 ± 6.9 | 11.9 ± 6.4 | 11.1 ± 6.4 |
| Food Craving Inventory | |||
| High fats | 2.3 ± 0.3 | 2.3 ± 0.3 | 2.4 ± 0.3 |
| Starches | 2.4 ± 0.4 | 2.1 ± 0.3 | 2.4 ± 0.3 |
| Sweets | 2.2 ± 0.2 | 2.8 ± 0.2 | 2.5 ± 0.2 |
| Fast food fats | 2.8 ± 0.4 | 2.8 ± 0.3 | 2.7 ± 0.3 |
| General food cravings | 2.4 ± 0.3 | 2.5 ± 0.2 | 2.5 ± 0.2 |
| Dietary Intake | |||
| Fat (g/d) | 82 ± 12 | 77 ± 11 | 85 ± 11 |
| Fat (% Kcal) | 37 ± 3 | 37 ± 3 | 39 ± 3 |
| Energy intake (Kcal/d) | 1951 ± 231 | 1882 ± 214 | 1868 ± 214 |
| Number of subjects | 6 | 7 | 8 |
Ethics
All procedures were approved by the Human Research Protection Office at Washington University in St. Louis and each subject gave informed written consent before participation.
Study protocol
CD36 genotyping.
Genomic DNA was isolated from blood (Gentra Puregene Blood Kit, Qiagen) and genotyped for CD36 SNP rs1761667 using Applied Biosystems predeveloped TaqMan SNP Genotyping Assay (Assay ID: C___8314999_10) (33). For each sample, 20 ng of DNA was genotyped in triplicate with negative and positive genotype controls included on the plate (controls were 100% concordant).
Taste testing.
Participants completed taste testing studies on 2 separate days (day 1 and day 2) approximately 1 week apart. For 10 participants, fat taste perception was assessed in the presence of orlistat on day 1 and without orlistat, control day, on day 2. The remaining participants were assessed in the reverse order (i.e., control on day 1 and orlistat on day 2). The type of fat used as the first taste stimuli to measure detection thresholds (i.e., oleic acid or triolein) was counterbalanced within the groups. In addition to the sensory test, all participants but one (from the GG group) were interviewed by a nutritionist to estimate daily fat and energy intake and completed validated questionnaires to assess fat preferences (34) and food cravings (6).
Preparation of oleic acid and triolein emulsions.
Preparation of emulsions followed Chalé-Rush et al. (9) with some modifications. Food grade oleic acid (Sigma Aldrich, St Louis, MO) and food grade triolein (Abitec Corporation; Janesville, WI) were stored in opaque bottles below 4°C. Triolein and oleic acid were added at varying concentrations to double distilled water. Concentrations used ranged from 0.0009 w/v% to 5 w/v% for oleic acid and from 0.006 w/v % to 31.7w/v % for triolein and were prepared in quarter-log dilution steps. All preparations were mixed with 5% (w/v) Gum Arabica (AEP Colloids, Hadley, NY) and white food colorant was added to produce perceptually identical viscosity and color between oil and control samples. For the testing session that used orlistat, emulsions were mixed with 0.5% w/v of orlistat (Glakosmithkline, Parsippany, NJ). All samples were sonicated for 6–9 min using a Branson 250 digital sonicator (Branson Ultrasonic Corporation, Danbury, CT) at 50% power with 30 s on, 60 s off. An ice bath was used during sonication to control for temperature. Samples were stored in opaque polypropylene cylinders and used for testing within 48 h of preparation. Control samples were prepared in the same way but without added oil. To ensure that the emulsions did not alter the nature of the food grade fats used and that no changes occurred that could affect the taste profile of the emulsion, headspace GC analyses were performed on the samples and free FAs were measured with the iodomethane method (35). The values and composition of oils measured on the emulsion samples were within those described in the original food grade product; no product of oxidation was detected, and 100% of the original concentration of oleic acid and triolein was recovered.
Determination of detection thresholds.
Triolein and oleic acid taste detection thresholds were separately assessed using a staircase method (36) implemented in a three-alternative, forced choice paradigm (37). On each trial, subjects were presented with three samples: two were “blank” control and one contained the fat stimulus under evaluation. Subjects were instructed to taste the three samples, without swallowing, and to choose the sample that was different (i.e., the one with fat). The subjects rinsed their mouth with deionized water before and after tasting each sample. The concentration of oleic acid or triolein in the emulsion presented was increased after a single incorrect response and decreased after two correct responses in a row. A reversal was considered to have occurred at points where the concentration sequence changed direction. The procedure was terminated when four reversals that met the following two criteria occurred. First, there were no more than two dilution steps between the two successive reversals. Second, the series of reversals could not form an ascending pattern (i.e., one in which positive and negative reversals are achieved at successively higher concentrations). These additional criteria ensure a more stable measure of the threshold attained (36). The threshold concentration was then calculated as the mean of the log values of the last four reversals. To control for visual and olfactory cues, testing was conducted under red light and participants wore nose clips. Personnel involved in the sensory test were blinded to the genotype groups.
Food consumption, fat preferences, and food-specific cravings
Subjects’ dietary intakes were evaluated by a trained dietitian on each testing day. Dietary intake data was collected and analyzed using Nutrition Data System for Research (NDSR) software version 2009, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. The NDSR software utilizes the 24-h diet recall with a multiple-pass system. The 24-h recall is an in-depth interview that collects detailed information on all foods and beverages consumed by a participant during the previous 24 h. Mean energy intake, total fat consumed (in grams), and macronutrient distribution (% energy from carbohydrate, protein, and fat) were quantified. In addition, subjects completed the Fat Preference Questionnaire (34) and the Food Craving Inventory (6). The Fat Preference Questionnaire is a validated self-administered test that assesses preference for dietary fat. Subjects selected the food that tastes better and is eaten more frequently from 19 sets of food. Each set is comprised of related foods differing in fat content. The percentage of food sets in which high-fat foods are reported to “taste better” (TASTE score) and to be “eaten more often” (FREQ score) is then determined and a measure of dietary fat restriction (DIFF) is created by subtracting TASTE from FREQ. The Food Craving Inventory is a 28-item validated questionnaire designed to measure the frequency of overall food cravings as well as cravings for specific types of foods. Cravings for specific types of foods (i.e., an intense desire for a specific food that is difficult to resist) are measured by four independent subscales, each consisting of four to eight items within the food category: high fats, sweets, carbohydrates/ starches, and fast-food fats. Participants rated how often they experienced a craving for each of the foods over the past month using a 5-point Likert scale (1 = never, 5 = always/almost every day) (6).
Statistical analyses
To determine the acute effects of orlistat and CD36 genotype on fat detection thresholds, a mixed ANOVA was conducted with type of fat (oleic acid vs. triolein) and experimental condition (orlistat day vs. control day) as the within-subjects factor and CD36 genotype (AA, AG, and GG) as the between-subjects factor. In addition, one-way ANOVAs were used to detect differences in habitual energy, macronutrient intakes, fat preferences, and food cravings as a function of CD36 genotype group. Triolein and oleic acid detection thresholds were positively skewed and required logarithmic transformation to approximate a normal distribution. When the ANOVAs revealed significant effects, post hoc Fisher Least Significant Difference analyses were conducted. Data in the tables and figures are presented as means ± SEM. All analyses were performed with STATISTICA 8.0 (StatSoft, Tulsa OK), and criterion for statistical significance was P < 0.05.
RESULTS
Influence of the CD36 common SNP rs1761667 on oleic acid and triolein detection thresholds
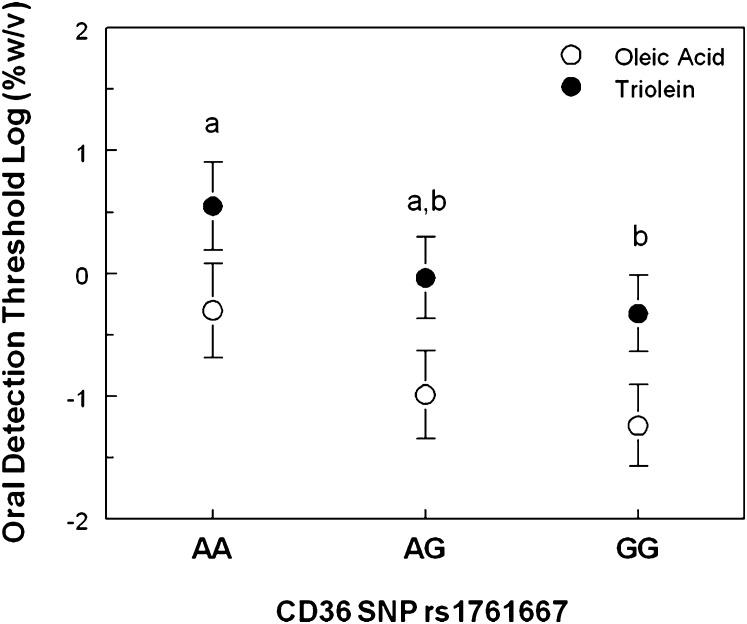
CD36 genotype affected orosensory detection of fats (F(2, 18) = 4.3; P = 0.03). Subjects homozygous for the rs1761667 G-allele had lower detection thresholds for oleic acid and triolein than subjects homozygous for the A allele, which associates with lower CD36 expression (Fig. 1). Detection threshold values for heterozygous subjects were intermediate of the values in homozygous subjects and not statistically different from either group.
Fig. 1.
Oleic acid (open symbol) and triolein (closed symbol) detection thresholds in individuals who are homozygous for the allele associated with low (AA, n = 6) or high (GG, n = 8) CD36 expression levels and in heterozygous subjects (AG, n = 7). Note that the lower the detection threshold, the higher the sensitivity. Different letters signify significant differences at P < 0.05 between groups.
Influence of orlistat on oleic acid and triolein detection thresholds
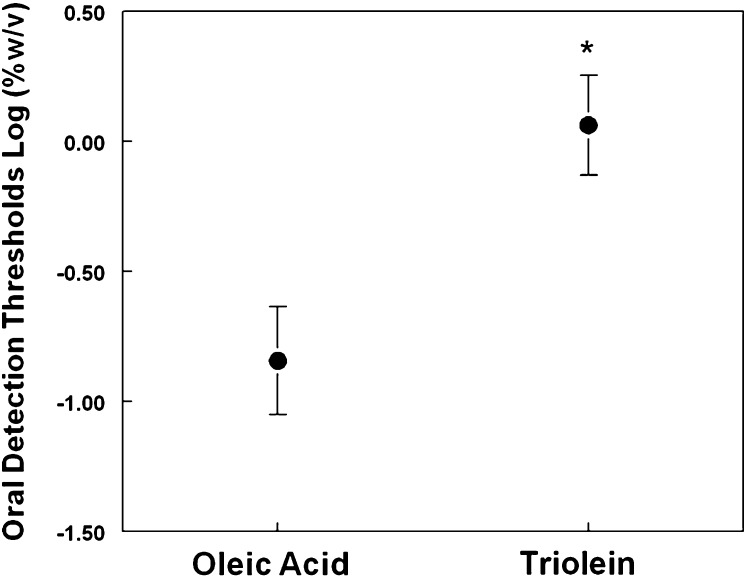
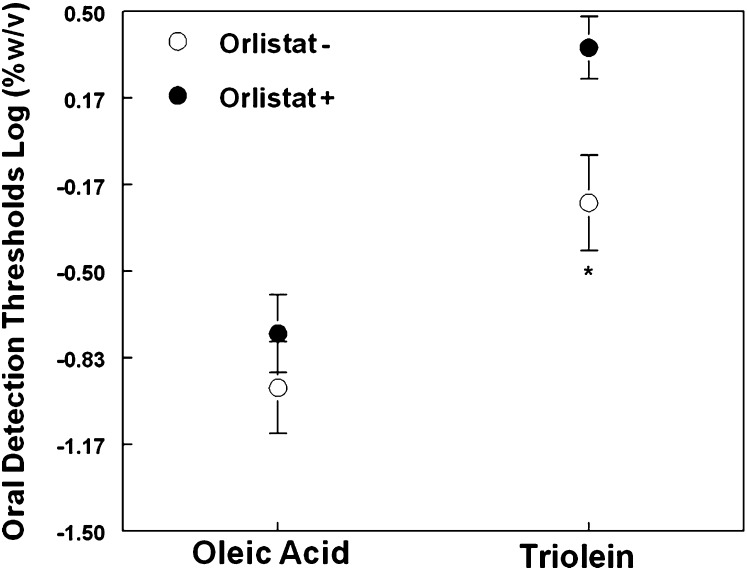
Overall, across all genotype groups, oleic acid was detected at significantly lower concentrations than triolein (F(1,18)=53.4; P < 0.00001; Fig. 2). The presence of orlistat in the emulsion increased fat detection thresholds (F(1,18) = 17.0; P < 0.001). However, this effect was tempered by an almost significant interaction between the effect of orlistat and type of fat (i.e., oleic acid or triolein) (P = 0.10). Based on previous data on animal models (22) and our a priori hypothesis, we further explored whether orlistat had a greater effect on triolein than on oleic acid detection thresholds with simple t-tests corrected for multiple comparisons. As shown in Fig. 3, orosensory detection thresholds for triolein (t(20) = 3.86; P < 0.001) but not for oleic acid (t(20) = 1.24; P > 0.2) were significantly higher in the orlistat day than in the control day.
Fig. 2.
Triolein and oleic acid oral detection thresholds measured in 21 obese subjects. Lower detection thresholds indicate higher sensitivity.
Fig. 3.
Triolein and oleic acid oral detection thresholds measured in 21 obese subjects using emulsions with (Orlistat day; closed symbol) and without (Control day, open symbol) 0.5%w/v orlistat. Lower detection thresholds indicate higher sensitivity.
Food consumption, fat preferences, and food cravings
Total energy, fat consumption, fat preference scores, and food cravings were similar among AA, AG, and GG subjects (all P-values > 0.20) (Table 1).
DISCUSSION
Dietary fat generates textural and aromatic cues that activate somatosensory and olfactory systems, but it is not known whether fat is perceived as a basic taste in humans (17). This issue is particularly important in obesity because obese subjects prefer foods with higher fat content (5) and crave more high-fat foods (6, 7) as compared with lean subjects (8). The data from the present study provide strong support that there is a taste component in the orosensory perception of dietary fat in obese subjects. First, we found that a genetic variant that associates with expression level of CD36, a putative lipid taste receptor, affected fat orosensory detection thresholds. Second, the presence of orlistat, a tasteless substance that is a potent lipase inhibitor, decreased the orosensory detection thresholds of triolein (a TG) more than those of oleic acid (an FA). Third, differences in subjects’ thresholds for detecting triolein and oleic acid were observed under conditions where nongustatory cues were minimized.
A major finding from the present study is that subjects homozygous for the rs1761667 G-allele were more sensitive in detecting oleic acid and triolein than subjects homozygous for the A-allele, which associates with lower CD36 expression levels, whereas subjects heterozygous for this allele were intermediate. These results are consistent with recent data from studies conducted in mice showing an association between CD36 expression level and oral fat detection (38). Mice heterozygous for CD36 deficiency (CD36+/−) have 50% lower CD36 expression in circumvallate taste papillae than wild-type animals (CD36+/+), and like CD36 knockout mice, they fail to exhibit spontaneous preference for fat, suggesting impaired ability to detect FA (38). The current study provides the first experimental evidence to demonstrate that CD36 is involved in fat gustatory perception in humans as observed previously in rodents (25, 27, 38). Although we did not measure CD36 expression in tongue tissue, CD36 has been identified in human taste bud cells (28).
The CD36 gene on human chromosome 7 is located close to the GNAT3 gene, which encodes α-gustducin, the primary G-protein involved in signal transduction of taste for bitter, sweet, and savory. However, it is unlikely that the altered fat detection thresholds we observed in carriers of CD36 rs1761667-A reflect alterations in GNAT3. Rs1761667, which associates with reduced CD36 expression (30, 31), lies between two alternative CD36 promoters, 1C and 1A and is 103.6-kb away from GNAT3, which is transcribed opposite to the direction of CD36 [UCSC gene track (GRCh37/h19)]. It has been shown previously that alterations in CD36 expression do not associate with changes in gustducin expression. Alpha-gustducin expression levels in taste buds are unaffected under conditions of lower CD36 expression or with complete CD36 deletion in mice (38). More importantly, α-gustducin is not involved in fat taste signaling. ΑAlpha-gustducin knockout mice have robust fat preferences that are identical to those of wild-type mice (39). In addition, the signaling mechanisms involved in CD36-mediated fat perception involve pathways distinct from those involving α-gustducin (40). In humans, there does not appear to be any cross-interaction between the effects of CD36 and GNAT3 on taste perception. Detection thresholds for FA are unrelated to the sensitivity to prototypical tastants, such as sweet, sour, or umami (18) where GNAT3 plays a critical role in taste transduction signaling. Conversely, polymorphisms in GNAT3 but not those in CD36, including rs1761667 that we evaluated in our study, affect taste responses to sugar in humans (41).
Addition of orlistat to fat emulsions diminished orosensory sensitivity (i.e., increased detection thresholds) to triolein but not to oleic acid, which is consistent with earlier findings in rodents indicating that the FA is the signaling stimulus (22). These data also suggest that lingual lipase plays a functional role in the gustatory perception of dietary fat in humans. Accordingly, prolonged chewing of food that contains fat before swallowing it should allow greater interaction between lingual lipase and dietary fat, which would increase FA concentration and thereby enhance oral fat perception. The concentration of orlistat used in our study (i.e., 0.5%w/v), which was selected based on its effectiveness in inhibiting lingual lipase in rodents (22), decreased our subjects’ oral sensitivity in detecting triolein, even though it did not annul their capability in detecting it. Additional dose-response studies with lipase inhibitors and chewing time examining how decreasing or increasing oral fat sensitivity affects cephalic phases of fat digestion are needed. In addition, future studies should consider the possibility that orlistat and other lipase inhibitors could interfere with both intestinal fat absorption and gut FA sensing (42, 43).
Variations of several orders of magnitude have been reported for fat orosensory detection thresholds in humans (9, 11, 16, 18, 19). Our data concur with this and identify CD36 genotype as one of the factors contributing to the large individual differences. Other putative fat taste receptors for long chain FA, such as GPR120, have been identified in rodent and human lingual tissue (23, 24, 44) and variation in these fat taste receptors could impact human oral fat perception contributing to further variability.
Our study was conducted in subjects selected for both obesity (presumably with high fat consumption) and the CD36 genotype. BMI affects fat orosensory detection thresholds; the higher the BMI, the lower the oral sensitivity in detecting oleic acid (16, 18), although it remains unknown whether the effect of BMI involves altered expression of CD36 and other putative fat taste receptors. Oral and gastrointestinal sensitivities to oleic acid are related to each other and inversely associated with dietary fat consumption (16). However, whether the decreased oral and gastrointestinal sensory sensitivity to fats is a cause or a consequence of obesity cannot be determined from association studies. Data from recent work in human subjects show that dietary fat manipulations alter oral (20) and gastrointestinal (45) sensitivity to fat. In lean subjects, oral sensitivity to detect the taste of oleic acid is decreased after 4 weeks on a high-fat diet and increased after 4 weeks on a low-fat diet (20). These findings are consistent with studies conducted in rodents showing that a high-fat diet decreases CD36 expression in taste buds cells (38, 46) and reduces intestinal sensory sensitivity to the presence of fat (47). In obese subjects, oral sensitivity to oleic acid is unchanged after 4 weeks on a high-fat diet (20). On the other hand, it is increased after 4 weeks on a low-fat diet (20). Similarly, acute dietary restriction in obese subjects enhances gastrointestinal sensitivity to fat, which is associated with an increased effect of fat on satiation (45). Although we demonstrated the existence of a relationship between fat perception sensitivity and genotype, our study was not able to determine whether oral fat perception sensitivity affects fat intake or body weight. Future studies are needed to answer this important question.
To our knowledge, this is the first study to measure orosensory detection thresholds of a TG (i.e., triolein) and its constituent FA (i.e oleic acid) in the same subjects, which permits a robust comparison of the relative orosensory sensitivity. We could effectively measure FA and TG orosensory sensitivity in our subjects when visual and olfactory cues were eliminated and textural cues minimized. Subjects were less sensitive in detecting triolein than oleic acid, despite triolein having higher viscosity (48), which supports the notion that taste rather than texture is the primary detection mechanism in our threshold measurements. However, interactions between the gustatory and trigeminal pathways might occur in the oral cavity and contribute to the detection thresholds we measured, analogous to the documented olfactory/trigeminal interactions in nasal chemoreception (49). CD36 and other putative fat receptors are present in trigeminal neurons (50), so the potential contribution of the trigeminal pathway, i.e., via sensations of pungency or oral burn, on fat oral perception needs further study.
In summary, our findings support the existence of a taste component in orosensory perception of dietary fat in humans. We found that a genetic variant in the FA translocase gene CD36 and lipase inhibition affect oral taste sensitivity to oleic acid and triolein in obese subjects. These findings have important implications in understanding factors involved in the regulation of food intake. A better understanding of the sensory mechanisms underlying oral and gastrointestinal fat sensing could lead to new strategies in food design and dietary therapy for obesity.
Acknowledgments
The authors thank Bruce Patterson, Jennifer Shew, and Freida Custodio for expert technical assistance in quantitative GC/mass spectrometry analyses of the emulsions, Johanna Sonnenschein and Nancy Allen for preparation of emulsions and sensory testing, Terri Pietka and Timothy Schappe for laboratory analysis of CD36 genotypes, Ann Doyle for subject recruitment, and Faidon Magkos for statistical advice. We thank GlaxoSmithKline (Parsippany, NJ) and Abitec Corporation (Janesville, WI) for kindly providing us with orlistat and triolein samples.
Footnotes
Abbreviations:
- BMI
- body mass index
- CD36
- cluster of differentiation 36
- SNP
- single nucleotide polymorphism
- TG
- triacylglycerol
This work was made possible by Grant UL1 RR024992, sub award KL2RR024994, from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research, by NIH grants DK60022, DK033301, DK 37948, DK56351, and DK 56341 (Nutrition and Obesity Research Center) and by a grant from GlaxoSmithKline Consumer Healthcare Research Program. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or the NIH or other granting agencies. The authors’ responsibilities were as follows: M.Y.P. helped with study design and concepts, subject recruitment, data collection, analysis and interpretation, draft and revision of the manuscript, and had overall responsibility for the project. L.L.G. helped with study design and concepts, subject recruitment, data collection, analysis and interpretation, draft and revision of the manuscript. S.K. and N.A.A. helped with study design and concepts, data analysis and interpretation, draft and revision of the manuscript. All authors approved the final draft of the manuscript. None of the authors declare a conflict of interest.
REFERENCES
- 1.Drewnowski A., Kurth C., Holden-Wiltse J., Saari J. 1992. Food preferences in human obesity: carbohydrates versus fats. Appetite. 18: 207–221. [DOI] [PubMed] [Google Scholar]
- 2.Rolls B. J. 1995. Carbohydrates, fats, and satiety. Am. J. Clin. Nutr. 61: 960S–967S. [DOI] [PubMed] [Google Scholar]
- 3.Bray G. A., Popkin B. M. 1998. Dietary fat intake does affect obesity! Am. J. Clin. Nutr. 68: 1157–1173. [DOI] [PubMed] [Google Scholar]
- 4.Swinburn B. A., Sacks G., Lo S. K., Westerterp K. R., Rush E. C., Rosenbaum M., Luke A., Schoeller D. A., DeLany J. P., Butte N. F., et al. 2009. Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am. J. Clin. Nutr. 89: 1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drewnowski A. 1985. Food perceptions and preferences of obese adults: a multidimensional approach. Int. J. Obes. 9: 201–212. [PubMed] [Google Scholar]
- 6.White M. A., Whisenhunt B. L., Williamson D. A., Greenway F. L., Netemeyer R. G. 2002. Development and validation of the food-craving inventory. Obes. Res. 10: 107–114. [DOI] [PubMed] [Google Scholar]
- 7.Pepino M. Y., Finkbeiner S., Mennella J. A. 2009. Similarities in food cravings and mood states between obese women and women who smoke tobacco. Obesity (Silver Spring). 17: 1158–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller W. C., Lindeman A. K., Wallace J., Niederpruem M. 1990. Diet composition, energy intake, and exercise in relation to body fat in men and women. Am. J. Clin. Nutr. 52: 426–430. [DOI] [PubMed] [Google Scholar]
- 9.Chalé-Rush A., Burgess J. R., Mattes R. D. 2007. Multiple routes of chemosensitivity to free fatty acids in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 292: G1206–G1212. [DOI] [PubMed] [Google Scholar]
- 10.Mattes R. D. 2001. Oral exposure to butter, but not fat replacers elevates postprandial triacylglycerol concentration in humans. J. Nutr. 131: 1491–1496. [DOI] [PubMed] [Google Scholar]
- 11.Mattes R. D. 2005. Fat taste and lipid metabolism in humans. Physiol. Behav. 86: 691–697. [DOI] [PubMed] [Google Scholar]
- 12.Gilbertson T. A., Fontenot D. T., Liu L., Zhang H., Monroe W. T. 1997. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am. J. Physiol. 272: C1203–C1210. [DOI] [PubMed] [Google Scholar]
- 13.Sclafani A., Ackroff K., Abumrad N. A. 2007. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293: R1823–R1832. [DOI] [PubMed] [Google Scholar]
- 14.Fukuwatari T., Shibata K., Iguchi K., Saeki T., Iwata A., Tani K., Sugimoto E., Fushiki T. 2003. Role of gustation in the recognition of oleate and triolein in anosmic rats. Physiol. Behav. 78: 579–583. [DOI] [PubMed] [Google Scholar]
- 15.Gaillard D., Laugerette F., Darcel N., El-Yassimi A., Passilly-Degrace P., Hichami A., Khan N. A., Montmayeur J. P., Besnard P. 2008. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 22: 1458–1468. [DOI] [PubMed] [Google Scholar]
- 16.Stewart J. E., Seimon R. V., Otto B., Keast R. S., Clifton P. M., Feinle-Bisset C. 2011. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am. J. Clin. Nutr. 93: 703–711. [DOI] [PubMed] [Google Scholar]
- 17.Mattes R. D. 2011. Accumulating evidence supports a taste component for free fatty acids in humans. Physiol. Behav. 104: 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart J. E., Feinle-Bisset C., Golding M., Dealhunty C., Clifton P. M., Keast R. S. 2010. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 104: 145–152. [DOI] [PubMed] [Google Scholar]
- 19.Mattes R. D. 2009. Oral detection of short-, medium-, and long-chain free fatty acids in humans. Chem. Senses. 34: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart J. E., Keast R. S. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int. J. Obes. (Lond). Epub ahead of print. August 9, 2011; doi:10.1038/ijo.2011.155. [DOI] [PubMed] [Google Scholar]
- 21.Mattes R. D. 2011. Oral fatty acid signaling and intestinal lipid processing: support and supposition. Physiol. Behav. 105: 27–35. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T., Fushiki T. 2003. Importance of lipolysis in oral cavity for orosensory detection of fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285: R447–R454. [DOI] [PubMed] [Google Scholar]
- 23.Matsumura S., Eguchi A., Mizushige T., Kitabayashi N., Tsuzuki S., Inoue K., Fushiki T. 2009. Colocalization of GPR120 with phospholipase-Cbeta2 and alpha-gustducin in the taste bud cells in mice. Neurosci. Lett. 450: 186–190. [DOI] [PubMed] [Google Scholar]
- 24.Cartoni C., Yasumatsu K., Ohkuri T., Shigemura N., Yoshida R., Godinot N., le Coutre J., Ninomiya Y., Damak S. 2010. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 30: 8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J. P., Besnard P. 2005. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 115: 3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su X., Abumrad N. A. 2009. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol. Metab. 20: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuwatari T., Kawada T., Tsuruta M., Hiraoka T., Iwanaga T., Sugimoto E., Fushiki T. 1997. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 414: 461–464. [DOI] [PubMed] [Google Scholar]
- 28.Simons P. J., Kummer J. A., Luiken J. J., Boon L. 2011. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem. 113: 839–843. [DOI] [PubMed] [Google Scholar]
- 29.El-Yassimi A., Hichami A., Besnard P., Khan N. A. 2008. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J. Biol. Chem. 283: 12949–12959. [DOI] [PubMed] [Google Scholar]
- 30.Love-Gregory L., Sherva R., Schappe T., Qi J. S., McCrea J., Klein S., Connelly M. A., Abumrad N. A. 2011. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum. Mol. Genet. 20: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh A., Murugesan G., Chen K., Zhang L., Wang Q., Febbraio M., Anselmo R. M., Marchant K., Barnard J., Silverstein R. L. 2011. Platelet CD36 surface expression levels affect functional responses to oxidized LDL and are associated with inheritance of specific genetic polymorphisms. Blood. 117: 6355–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mojet J., Christ-Hazelhof E., Heidema J. 2001. Taste perception with age: generic or specific losses in threshold sensitivity to the five basic tastes? Chem. Senses. 26: 845–860. [DOI] [PubMed] [Google Scholar]
- 33.Kutyavin I. V., Afonina I. A., Mills A., Gorn V. V., Lukhtanov E. A., Belousov E. S., Singer M. J., Walburger D. K., Lokjpv S. G., Gall A. A., et al. 2000. 3′-minor groove binder-DNA probes increase sequence specificity at PCR extension temperatures. Nucleic Acids Res. 28: 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ledikwe J. H., Ello-Martin J., Pelkman C. L., Birch L. L., Mannino M. L., Rolls B. J. 2007. A reliable, valid questionnaire indicates that preference for dietary fat declines when following a reduced-fat diet. Appetite. 49: 74–83. [DOI] [PubMed] [Google Scholar]
- 35.Patterson B. W., Zhao G., Elias N., Hachey D. L., Klein S. 1999. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J. Lipid Res. 40: 2118–2124. [PubMed] [Google Scholar]
- 36.Pribitkin E., Rosenthal M. D., Cowart B. J. 2003. Prevalence and causes of severe taste loss in a chemosensory clinic population. Ann. Otol. Rhinol. Laryngol. 112: 971–978. [DOI] [PubMed] [Google Scholar]
- 37.ASTM. 2004. Standard test method E1885-04. In Standard Test Method for Sensory Analysis-Triangle Test WC, PA: ASTM International.). [Google Scholar]
- 38.Martin C., Passilly-Degrace P., Gaillard D., Merlin J. F., Chevrot M., Besnard P. 2011. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS ONE. 6: e24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sclafani A., Zukerman S., Glendinning J. I., Margolskee R. F. 2007. Fat and carbohydrate preferences in mice: the contribution of alpha-gustducin and Trpm5 taste-signaling proteins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293: R1504–R1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan N. A., Besnard P. 2009. Oro-sensory perception of dietary lipids: new insights into the fat taste transduction. Biochim. Biophys. Acta. 1791: 149–155. [DOI] [PubMed] [Google Scholar]
- 41.Fushan A. A., Simons C. T., Slack J. P., Drayna D. 2010. Association between common variation in genes encoding sweet taste signaling components and human sucrose perception. Chem. Senses. 35: 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Little T. J., Feinle-Bisset C. 2011. Effects of dietary fat on appetite and energy intake in health and obesity–oral and gastrointestinal sensory contributions. Physiol. Behav. 104: 613–620. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz G. J. 2011. Gut fat sensing in the negative feedback control of energy balance–recent advances. Physiol. Behav. 104: 621–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galindo M. M., Voigt N., Stein J., van Lengerich J., Raguse J. D., Hofmann T., Meyerhof W., Behrens M. 2011. G protein-coupled receptors in human fat taste perception. Chem. Senses. Epub ahead of print. August 25, 2011; doi: 10.1093/chemse/bjr069. [DOI] [PubMed] [Google Scholar]
- 45.Brennan I. M., Seimon R. V., Luscombe-Marsh N. D., Otto B., Horowitz M., Feinle-Bisset C. 2011. Effects of acute dietary restriction on gut motor, hormone and energy intake responses to duodenal fat in obese men. Int J Obes (Lond). 35: 448–456. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X.J., Zhou L. H., Ban X., Liu D.X., Jiang W., Liu X. M. 2011. Decreased expression of CD36 in circumvallate taste buds of high-fat diet induced obese rats. Acta Histochem. 113: 663–667. [DOI] [PubMed] [Google Scholar]
- 47.Covasa M., Grahn J., Ritter R. C. 2000. Reduced hindbrain and enteric neuronal response to intestinal oleate in rats maintained on high-fat diet. Auton. Neurosci. 84: 8–18. [DOI] [PubMed] [Google Scholar]
- 48.Valeri D., Meirelles A. J. A. 1997. Viscosities of fatty acids, triglycerides, and their binary mixtures. J. Am. Oil Chem. Soc. 74: 1221–1226. [Google Scholar]
- 49.Brand G. 2006. Olfactory/trigeminal interactions in nasal chemoreception. Neurosci. Biobehav. Rev. 30: 908–917. [DOI] [PubMed] [Google Scholar]
- 50.Gilbertson T. A., Yu T., Shah B. P. 2010. Gustatory Mechanisms for Fat Detection. Fat Detection: Taste, Texture, and Post Ingestive Effects. Montmayeur J. P., le Coutre J. CRC Press Boca Raton, FL: 83–97. [Google Scholar]