Abstract
Fasting promotes triglyceride (TG) accumulation in lean tissues of some animals, but the effect in humans is unknown. Additionally, fasting lipolysis is sexually dimorphic in humans, suggesting that lean tissue TG accumulation and metabolism may differ between women and men. This study investigated lean tissue TG content and metabolism in women and men during extended fasting. Liver and muscle TG content were measured by magnetic resonance spectroscopy during a 48-h fast in healthy men and women. Whole-body and hepatic carbohydrate, lipid, and energy metabolism were also evaluated using biochemical, calorimetric, and stable isotope tracer techniques. As expected, postabsorptive plasma fatty acids (FAs) were higher in women than in men but increased more rapidly in men with the onset of early starvation. Concurrently, sexual dimorphism was apparent in lean tissue TG accumulation during the fast, occurring in livers of men but in muscles of women. Despite differences in lean tissue TG distribution, men and women had identical fasting responses in whole-body and hepatic glucose and oxidative metabolism. In conclusion, TG accumulated in livers of men but in muscles of women during extended fasting. This sexual dimorphism was related to differential fasting plasma FA concentrations but not to whole body or hepatic utilization of this substrate.
Keywords: liver fat, muscle fat, magnetic resonance spectroscopy
The primary substrates for energy metabolism in humans are glucose and fatty acids (FAs), with the relative importance of each dependent upon food availability (fed ↔ fasting ↔ starvation) (1, 2). In the fed state, insulin is elevated, glucose utilization predominates, and there is net flow of free FA from liver to adipose tissue. In the transition from fed to fasting, insulin falls, leading to increased release of free FAs from adipose tissue that are used by lean tissues (e.g., muscle and liver) for energy production or stored as triglyceride (TG). The rate of lipolytic release during fasting and starvation exceeds whole-body energy requirements in a sexually dimorphic manner, with women and men having release rates ∼64% and ∼50% greater than oxidation rates, respectively (3). This may result in sexually dimorphic lipid and glucose metabolism in muscle and liver.
A significant portion of whole-body FAs are reesterified to TG in skeletal muscle and liver. In skeletal muscle this TG appears to function as a local energy source (4), whereas in liver most reesterified FAs are exported as very-low-density lipoprotein-bound TG (5). Surprisingly little is known about the magnitude of lipid redistribution to liver and muscle during fasting or its metabolic consequences in humans. In liver, mouse models suggest that as much as a 14-fold increase in TG content during starvation may be normal (6). Such a degree of hepatic steatosis in humans has only been reported in pathological states such as obesity and insulin resistance, called nonalcoholic fatty liver disease (7). Intramyocellular TGs increase during fasting and starvation in women (8) and in endurance-trained male athletes (9, 10), but sexual dimorphism in this response has not been investigated. Moreover, the redistribution of adipose FAs to liver and muscle may also alter glucose homeostasis: 1) increased FA oxidation in liver stimulates gluconeogenesis and 2) increased intramyocellular TGs may reduced insulin sensitivity and impair glucose disposal (11–15). The impact of fasting-induced free FA availability on lean tissue TG content and glucose homeostasis in normal women and men is unknown.
The present study investigated changes in lean tissue TG content during a 48-h fast in healthy men and women using proton magnetic resonance spectroscopy (1H-MRS) and related these findings to changes in carbohydrate, lipid, and energy metabolism determined via biochemical, calorimetric, and MRS-based stable isotope tracer measurements. Multiple stable isotope tracers were used to evaluate the fasting response of hepatic glucose production, glycogenolysis, gluconeogenesis, and metabolic pathways of the tricarboxylic acid (TCA) cycle. Women and men were examined separately to determine whether known sexually dimorphic fasting lipid metabolism (16) extends to lean tissue lipid accumulation and metabolism during fasting.
RESEARCH DESIGN AND METHODS
Participants
Healthy individuals were recruited for study at the University of Texas Southwestern Medical Center. The study population was composed of nine women and nine men whose characteristics are presented in Table 1. All of the women studied were premenopausal, and two were taking oral contraceptives. The protocol and consent form were approved by the UTSW Institutional Review Board, and all participants provided written informed consent before enrollment.
TABLE 1.
Characteristics of subjects at enrollment by sex
| Women (n = 9) | Men (n = 9) | P Value | |
| Age (yr) | 24 (23–42) | 21 (21–23) | 0.189 |
| Ethnicity/race (n) | |||
| Non-Hispanic White | 6 | 7 | 0.599 |
| Non-Hispanic Black | 2 | 1 | 0.527 |
| Hispanic | 1 | 0 | 0.303 |
| Asian | 0 | 1 | 0.303 |
| Body mass index (kg/m ) | 27 (22–32) | 25 (23–27) | 0.427 |
| Glucose (mg/dl) | 86 (84–90) | 90 (85–93) | 0.907 |
| Total cholesterol (mg/dl) | 167 (163–184) | 164 (155–194) | 0.489 |
| HDL-c (mg/dl) | 60 (52–64) | 40 (37–49) | 0.021 |
| LDL-c (mg/dl) | 91 (83–109) | 119 (93–135) | 0.343 |
| Triglycerides (mg/dl) | 67 (58–79) | 52 (50–94) | 0.604 |
| AST (U/l) | 19 (17–22) | 23 (19–24) | 0.176 |
| ALT (U/l) | 16 (11–20) | 20 (18–22) | 0.064 |
Values are median with interquartile range in parentheses. Data analyzed by unpaired t-test and Chi-square test. ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol. Conversions: triglycerides, HDL-c, and LDL-c (mg/dl) × 0.02586 = mmol/l; glucose (mg/dl) × 0.05551 = mmol/l.
Design
Participants were admitted to the Clinical and Translational Research Center (CTRC) where they fasted for 48 h. On day 0, subjects ate a self-prepared lunch at 12:00 and were admitted to the CTRC at 16:00. Subjects remained fasted from the time of admission on day 0 until 12:00 on day 2. Between 08:30 and 09:00 (day 1 and 2), all subjects underwent measurement of respiratory quotient using a Delta Trak II indirect calorimeter (Sensormedics, Yorba Linda, CA). Hepatic and intramyocellular TG content were measured on admission and at 12:00 on day 1 and 2. A subset of participants (women = 6, men = 7) underwent additional studies using stable isotope tracers. Between 22:00 and 09:00 (day 0–1), subjects received two tracers orally: [U-13C]propionate (∼1200 mg) at 08:30 and divided doses of 70% [2H]water (5 g/kg body water, calculated as 60% of body weight in men and 50% of body weight in women) at 22:00, 02:00, and 06:00. Subjects were allowed to drink 0.5% [2H]water ad libitum for the remainder of the fast. Subjects were then given a 2.25 mg/kg bolus of [3,4-13C]glucose intravenously followed by a 2-hour infusion (0.0225 mg/kg/min). At the end of the infusion period, a 50-ml blood draw was performed. These procedures were repeated on day 2 of the fast, except for overnight loading with 70% [2H]water.
Dietary macronutrient intake before fasting
Dietary records were collected for 3 days before the study and evaluated by the CTRC dietician. Diets were composed of 48% (range, 43–50%) carbohydrates, 38% (range, 37–40%) fats, and 15% (range, 14–17%) proteins. Dietary macronutrient composition was comparable between the sexes; however, daily energy intake was significantly greater in men compared with women (2,825 [range, 2,654–3,140] vs. 1,563 [1,424–2,100] kcal/day; P = 0.010).
Isotopes and other materials
Seventy-percent [2H]water and 99% [U-13C]propionate (sodium salt) were obtained from Cambridge Isotopes (Andover, MA). Sterility and pyrogen tested [3,4-13C]glucose was obtained from Omicron Biochemicals, Inc. (South Bend, IN). Other common reagents were purchased from Sigma (St. Louis, MO).
Blood samples
Every 4 h, 3 ml of blood was drawn from each subject. Blood was collected in nonheparinized, EDTA-containing tubes and immediately centrifuged to isolate plasma. Samples were immediately frozen and maintained at −80°C, after which they were thawed once and analyzed. Plasma glucose, cholesterol, TG, and high-density lipoprotein cholesterol (HDL-c) concentrations were determined using a Vitros 250 spectrophotmetric analyzer (Ortho-Clinical Diagnostics, Rochester, NY). Enzyme-linked immunosorbent assay kits were used to measure plasma insulin, leptin, and adiponectin concentrations (Millipore, Billerica, MA) as well as plasma-free fatty acid (FA) concentrations (Wako Chemicals USA, Richmond, VA). Plasma ketone bodies were determined using a commercial kit (Wako Chemicals, Richmond, VA). Other chemistries were performed by an outside laboratory (Quest Diagnostics, Madison, NJ).
Measurement of hepatic glucose and energy metabolism
Plasma was extracted with perchloric acid and the glucose was purified as previously described (17, 18). Purified plasma glucose was converted to 1,2-isopropylidene glucofuranose (monoacetone glucose) before 2H and 13C NMR analysis as detailed previously (17–19). Samples were analyzed on a 14.1 Tesla Varian Inova spectrometer (Varian Instruments, Palo Alto, CA) equipped with a 3 mm broadband probe tuned to 92 MHz for 2H spectra or 150 MHz for 13C spectra. Resonance areas were determined using ACD/Labs 12.0 (Advanced Chemistry Development, Inc., Toronto, Ontario, Canada).
The relative deuterium enrichments in glucose H2, H5 and H6s were assessed by 2H-NMR and these values were used to determine the fractional contribution of gluconeogenesis and glycogenolysis to endogenous glucose production as previously detailed (17). Pathways intersecting the TCA cycle were evaluated by 13C-NMR analysis of glucose C2 isotopomers formed as a consequence of metabolism of [U-13C]propionate (20). Assumptions regarding this model have been previously reported (21). Endogenous glucose production was measured by dilution of [3,4-13C]glucose as previously described (22). Fractional glycogenolysis and gluconeogenesis measured by 2H-NMR was normalized with pyruvate carboxylase (PC)/phosphoenolpyruvate carboxykinase (PEPCK), pyruvate cycling and gluconeogenesis relative to TCA cycle flux measured by 13C-NMR and the rate of endogenous glucose production (µmol/kg/min) was used to calculate the absolute fluxes through each of these pathways (23).
Measurement of hepatic and intramyocellular TG
Planning images were obtained and localized 1H-NMR spectra were acquired using a 3.0 Tesla ACHIEVA whole-body MR system (Philips Medical Systems, Best, The Netherlands) with subjects in the supine position. For liver, a 2 × 2 × 2 cm volume of interest was positioned in the right hepatic lobe, avoiding major blood vessels, intra-hepatic bile ducts, and the lateral margins of liver. For muscle, a 2 × 2 × 2 cm volume of interest was positioned in the soleus, ensuring consistent orientation of muscle fibers and avoiding major blood vessels, bone, and subcutaneous fat. Soleus muscle was chosen due to the predominance of oxidative (type II) fibers in this muscle group (8) and the larger anticipated TG signal relative to other muscle groups (24). After the system was tuned and shimmed, spectra were collected using a combination of whole-body and SENSE torso (liver) or knee (muscle) coils for radio frequency signal transmission and reception and a STEAM sequence with interpulse delay Tr = 1.6 s, spin echo time Te = 14 ms, mixing time Tm = 18 ms, 16 acquisitions, and 2048 data points over a 1,500-Hz spectral width. The TG content is expressed as a percent of the methylene groups resonance at 1.40 ppm referenced to the combined signal from methylenes and water (25, 26). The typical error for measurement of hepatic and intramyocellular TG was ±0.13% (r2 = 0.938; slope = 0.89) and ±0.16% (r2 = 0.953; slope = 0.98), respectively. To obtain these values, hepatic (n = 6) and intramyocellular (n = 5) TG measurements were performed twice during the same session in some subjects.
Statistical analysis
Statistical analyses were performed using SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA). Differences between the two groups were evaluated using the unpaired t-tests. Proportions were evaluated with the Chi-square test. The significance of trends over the fast was determined using 1-factor repeated-measures ANOVA. Comparisons of multiple measurements between groups were performed using 2-factor repeated-measures ANOVA. Tukey's test was used for post hoc analysis of significant findings on ANOVA. Unless otherwise indicated, values are presented as median and interquartile range. Statistical significance was taken at P < 0.05.
RESULTS
Changes in plasma metabolite concentrations over a 48-h fast
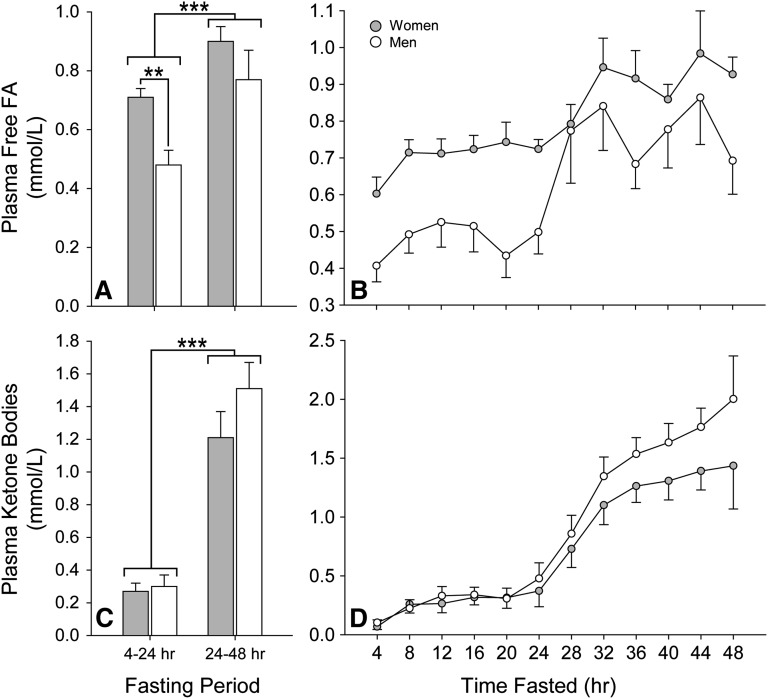
A comparison of metabolic parameters in women and men over the 48-h fast is presented in Table 2. Consistent with prior reports, differences in lipid metabolites were apparent between the sexes (16). There was a small but significant rise in plasma cholesterol with fasting that was independent of sex. Plasma TGs were highest in the immediate postprandial period and declined with fasting in women and men; however, a significant disordinal interaction was encountered between sex and duration of fasting. Men had higher plasma TGs after 4 h of fasting but maintained a lower concentration for the majority of the fast. Plasma-free FA concentrations were higher in women than men from 4 to 24 h of fasting (Fig. 1A; average 24 h values shown). Although there was a significant rise in plasma-free FAs in both sexes between 20 and 32 h of fasting, the rate of increase was 4-fold greater in men than in women (Fig. 1B). Despite the sex differences in plasma FA concentrations, there was no significant difference in plasma ketones, with each sex exhibiting similar ketosis during the fasting period (Fig. 1C, D). We also examined the relationship between plasma-free FA and ketones in each gender (Fig. 1E, F; average 24 h values shown). ANCOVA revealed a significant positive within-subjects correlation between these metabolites in women (r = 0.654; P < 0.001) and men (r = 0.606; P < 0.001) over the 48-h fast. As a group, women also exhibited a significant positive relationship between plasma-free FA and ketone concentrations over the initial (r = 0.799; P = 0.008) and final (r = 0.732; P = 0.025) day of the fast. However, a similar between subjects relationship was not present in men during either 24-h period (initial, r = −0.259; P = 0.501; final, r = −0.031; P = 0.937). Plasma lactate, glucose, insulin, adiponectin, and transaminases were also comparable between the sexes. Plasma leptin was significantly higher in women than in men. Taken together, these data are consistent with a sexually dimorphic induction of lipolysis and FA metabolism over the fasting period.
TABLE 2.
Characteristics of women and men during a 48-h fast
| Women (n = 9) |
Men (n = 9) |
||||||||
| Time Fasted |
P Value |
||||||||
| Parameter | 4 hr | 24 h | 48 h | 4 h | 24 h | 48 h | Sex | Time | Sex × Time |
| Plasma cholesterol (mg/dl) | 145 | 158 | 176 | 155 | 177 | 166 | 0.460 | 0.002 | 0.438 |
| (144–186) | (150–203) | (154–195) | (135–184) | (154–184) | (161–193) | ||||
| Plasma triglycerides (mg/dl) | 110 | 85 | 75 | 115 | 100 | 75 | 0.514 | <0.001 | 0.023 |
| (80–180) | (65–135) | (75–110) | (95–230) | (80–105) | (65–85) | ||||
| Plasma free FA (mmol/l) | 0.58 | 0.71 | 0.94 | 0.41 | 0.56 | 0.71 | 0.007 | <0.001 | 0.006 |
| (0.51–0.61) | (0.65–0.78) | (0.87–0.98) | (0.35–0.52) | (0.30–0.65) | (0.47–0.86) | ||||
| Plasma ketones (mmol/l) | 0.05 | 0.33 | 1.22 | 0.09 | 0.41 | 1.94 | 0.647 | <0.001 | 0.620 |
| (0.04–0.08) | (0.21–0.57) | (1.11–1.56) | (0.07–0.11) | (0.29–0.45) | (1.42–2.13) | ||||
| Plasma lactate (mmol/l) | 0.9 | 0.7 | 0.8 | 0.9 | 0.7 | 0.9 | 0.612 | <0.001 | 0.502 |
| (0.8–1.1) | (0.6–0.8) | (0.8–1.0) | (0.9–0.9) | (0.7–0.7) | (0.8–1.0) | ||||
| Plasma glucose (mg/dl) | 90 | 87 | 79 | 88 | 90 | 73 | 0.393 | <0.001 | 0.972 |
| (85–96) | (78–90) | (67–84) | (87–95) | (88–93) | (71–79) | ||||
| Plasma insulin (μU/ml) | 16 | 5 | 3 | 10 | 2 | 1 | 0.422 | <0.001 | 0.592 |
| (5–20) | (2–5) | (2–4) | (7–19) | (2–3) | (1–2) | ||||
| Plasma leptin (ng/ml) | 19.3 | 14.6 | 3.8 | 5.9 | 3.1 | 1.8 | 0.036 | <0.001 | 0.194 |
| (7.8–57.9) | (4.9–27.5) | (2.5–9.1) | (3.4–6.2) | (2.8–4.0) | (1.6–2.0) | ||||
| Plasma adiponectin (ng/ml) | 15.9 | 15.2 | 13.8 | 14.1 | 13.1 | 13.0 | 0.927 | <0.001 | 0.139 |
| (10.5–20.4) | (9.2–17.3) | (8.1–17.0) | (9.7–20.0) | (9.8–18.8) | (8.5–17.3) | ||||
| Plasma AST (U/l) | 21 | 19 | 20 | 21 | 20 | 21 | 0.742 | 0.637 | 0.162 |
| (18–25) | (16–26) | (16–22) | (18–21) | (18–24) | (17–24) | ||||
| Plasma ALT (U/l) | 16 | 16 | 15 | 21 | 22 | 20 | 0.387 | 0.723 | 0.868 |
| (12–20) | (10–20) | (10–20) | (14–24) | (15–22) | (16–24) | ||||
Values are median with interquartile range. Data for the entire 48-h period were analyzed by 2-factor repeated-measures ANOVA. ALT, alanine aminotransferase; AST, aspartate aminotransferase. Conversions: total cholesterol and VLDL-TG (mg/dl) × 0.02586 = mmol/l; glucose (mg/dl) × 0.05551 = mmol/l.
Fig. 1.
Change in plasma-free FA and ketone body concentrations with short-term fasting in women and men. Data were analyzed using 2-factor repeated-measures ANOVA and are presented as mean with SEM (gray: women; white: men). A: The average plasma-free FA concentrations over the initial 24 h of fasting were significantly higher among women (P = 0.007). This value was determined by summing all free FA concentration values for a subject over the 24-h period and dividing by the number of measurements. B: Although there was a significant rise in plasma-free FA in both sexes between 20 and 32 h of fasting (P < 0.001), the rate of increase was 4-fold greater in men than in women (P = 0.006). C: There was no significant difference in the average plasma ketone concentrations between women and men during the initial and final 24 h of fasting. D: Each sex exhibited a similar rise in concentration over the fasting period. The average plasma ketone concentration was determined by summing all plasma ketone concentration values for a subject over the 24-h period and dividing by the number of measurements. ** P < 0.01; *** P < 0.001.
Changes in calorimetric measurements with fasting
Inasmuch as the differential changes in lipidemia in response to fasting suggested distinct substrate oxidation in women and men, we investigated whole-body fuel sources using indirect calorimetry. Regardless of sex, CO2 production (VCO2) was unchanged from day 1 to day 2 of the fast (165 [136–180] vs. 171 [133–183] ml/min; P = 0.402), whereas there was a significant increase in O2 consumption (VO2) (202 [167–214] vs. 222 [178–234] ml/min; P = 0.014). As expected, respiratory quotient (VCO2/VO2) declined over the 48-h fast (0.81 [0.80–0.83] vs. 0.77 [0.75–0.81]; P < 0.001), indicating increasing fat oxidation with fasting. Women had lower VCO2 (P = 0.027), VO2 (P = 0.006), and energy expenditure (P = 0.008) when compared with men; however, these differences were not present when normalized to lean body weight. The lack of difference in respiratory quotients between the sexes throughout the fast suggested that the altered metabolic response to fasting was not mediated by differential whole-body substrate oxidation.
Effect of fasting on lean tissue TG content
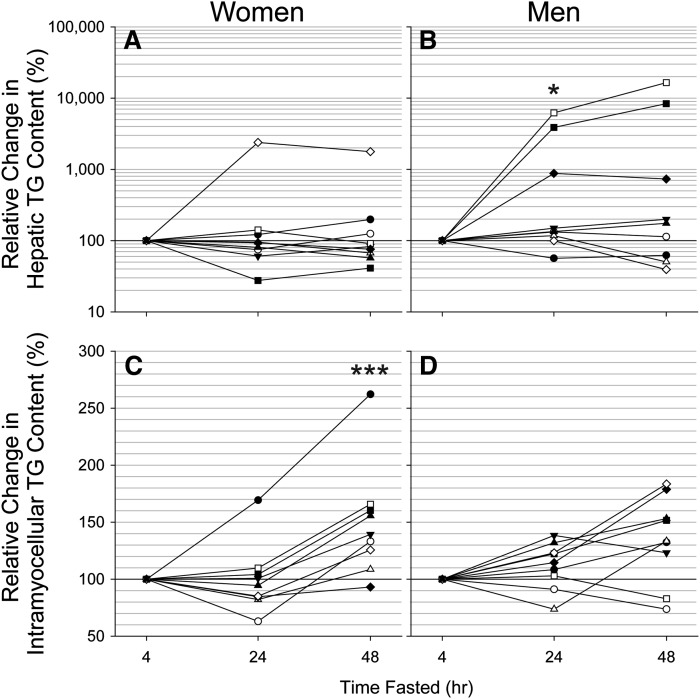
Given the increased FA metabolism during fasting, we determined if a redistribution of this substrate as stored TGs was apparent in lean tissues. Hepatic TG content was similar in women and men at admission to the CTRC (0.9% [0.6–1.9%] vs. 0.8% [0.2–1.2%]; P = 0.254). Fig. 2A and 2B show the relative change in liver TGs for women and men from 4 to 24 h and from 24 to 48 h of fasting. Together these data demonstrated a significant disordinal interaction between sex and duration of fasting for hepatic TG content (P = 0.047). The interaction increased in significance when the hepatic TG content after 4 and 24 h of fasting was compared between women and men separately (P = 0.017); however, neither the interaction nor main effects were significant on separate comparison of the 24 and 48 h fasting data. Closer inspection of hepatic TG content at 4 and 24 h of fasting demonstrated that men experienced a modest but significant increase in liver fat (P = 0.043), whereas liver fat levels in women tended to decrease (P = 0.092).
Fig. 2.
Relative change in lean tissue TG content with fasting in women and men. Measurements were obtained using a Philips 3.0 Tesla ACHIEVA whole-body MR system and a SENSE torso or knee coil with volume selective spectroscopy (STEAM, 2 × 2 × 2 cm voxel). Data were analyzed using 2-factor repeated-measures ANOVA. A and B: A significant disordinal interaction between sex and duration of fasting for hepatic TG content was encountered (P = 0.047). This was due to a significant increase in hepatic TG content from 4 to 24 h in men (P = 0.043) in conjunction with a tendency for levels in women to decrease (P = 0.092). Further changes in hepatic TG content from 24 to 48 h of fasting were not apparent in women or men. C and D: No significant change in intramyocellular TG content was apparent in either sex from 4 to 24 h of fasting. Intramyocellular TG content exhibited an ordinal interaction between sex and duration of fasting (P < 0.001) from 24 to 48 h due to a significant increase in intramyocellular TGs in women but not in men (P = 0.127). Results were similar when the single apparent outlier among the women was excluded from the analysis. * P < 0.05; *** P < 0.001.
Intramyocellular TG content was also comparable between women and men at the onset of fasting (2.4% [1.9–3.5] vs. 1.6% [1.5–2.7%]; P = 0.191). The relative change in intramyocellular TGs in these groups from 4 to 24 h and from 24 to 48 h of fasting is shown in Fig. 2C, D. These data exhibited an ordinal interaction between sex and duration of fasting (P = 0.019). Upon separate analysis of the 4- and 24-h data, there was no significant difference in intramyocellular TGs between women and men and no significant change in levels over this period. However, an ordinal interaction was encountered from 24 to 48 h of fasting (P = 0.028). Post hoc analysis revealed that women had a significant increase in intramyocellular TGs by 48 h of fasting (P < 0.001), whereas men did not (P = 0.127). Together, MRS of muscle and liver indicated a sexually dimorphic response of lean tissue lipid accumulation during fasting, with women but not men favoring a modest accumulation of lipid in skeletal muscle during short-term fasting and men but not women favoring a modest accumulation of lipid in liver.
Insulin and glucose excursion after refeeding
In light of the differences in intramyocellular TG content between women and men by the end of the 48-h fast, we determined if this was accompanied by differences in glycemic control upon refeeding. Some individuals (women = 6, men = 5) were re-fed a low-fat meal (60% carbohydrate, 25% fat, and 15% protein; women = 567 and men = 667 kcal), and insulin and glucose excursion were assessed. In the 4 h after refeeding, peak insulin was significantly higher in women (46 [37–47] vs. 20 [15–25] μU/ml; P = 0.011), whereas peak glucose concentration did not differ between sexes (P = 0.233). These findings indicated reduced insulin sensitivity in women after short-term fasting.
Endogenous glucose production, glycogenolysis, and gluconeogenesis during fasting
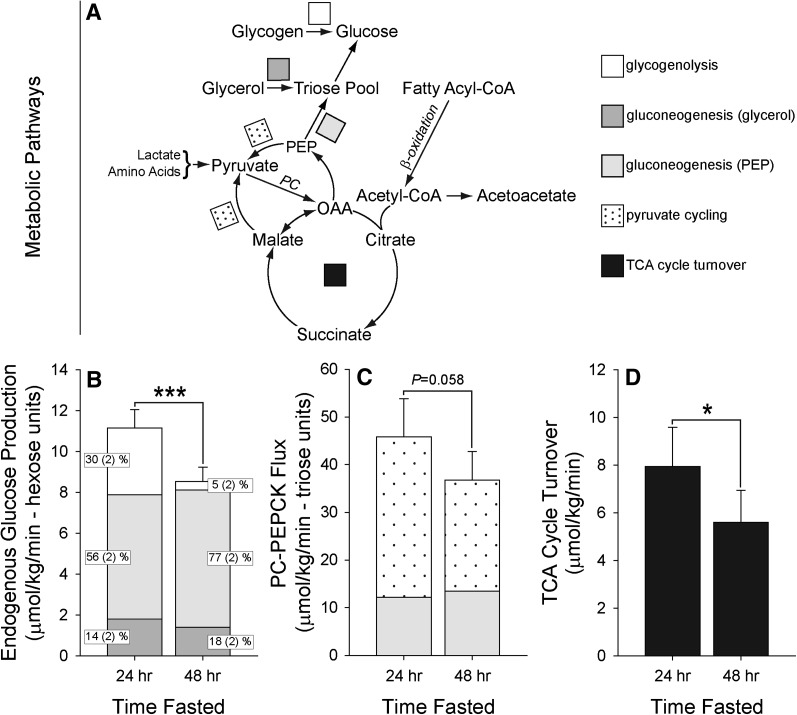
We next examined how short-term fasting altered hepatic glucose metabolism in a subset of subjects using stable isotope tracers (women = 6, men = 7). Enrollment characteristics of the subset did not differ from that of the study population (data not shown). Subjects were loaded orally with [2H]water to determine the origins of endogenous glucose production (EGP). In addition, subjects received a 2-h tracer infusion of [3,4-13C]glucose to determine rates of EGP. No differences in hepatic metabolic fluxes were observed between women or men, so the data were combined to examine the effect of fasting on these pathways. EGP declined with fasting duration due to reduced glycogenolysis (P < 0.001) (Fig. 3A and 3B). Although the fraction of circulating glucose derived from gluconeogenesis increased significantly with fasting (P < 0.001), the absolute rate of gluconeogenesis remained constant throughout the fast (P = 0.704). In addition, the contribution of the precursor from which glucose was synthesized (glycerol vs. lactate/amino acids) remained constant throughout the fast (P > 0.05). Similar results were obtained when the data were normalized to lean body weight (data not shown). These data demonstrated that, despite differences in hepatic lipid accumulation, the rate of gluconeogenesis from all precursors was remarkably stable over the entirety of the fast in women and in men.
Fig. 3.
Change in hepatic glucose and energy metabolism with fasting. Measurements were obtained using a combination of 2H and 13C (oral and intravenous) stable isotope tracers and NMR spectroscopy. No differences in hepatic metabolic fluxes were observed between men or women, so the data were combined to examine the effect of fasting on these pathways. Data were analyzed using 1-factor repeated-measures ANOVA and are presented as mean with SEM. A: The metabolic pathways studied included hepatic glucose production, composed of glycogenolysis, as well as gluconeogenesis occurring from glycerol, lactate, and amino acids. The techniques used also allowed pathways centered on the TCA cycle to be investigated, including cycle turnover, anaplerosis (PC-PEPCK flux), and pyruvate cycling. B: Endogenous glucose production declined significantly from 24 to 48 h of fasting. This reduction was solely due a decrease in glycogenolysis (white) (P < 0.001). Gluconeogenesis occurring from glycerol (dark gray) as well as lactate/amino acids (light gray) did not change over the fasting period (fractional values [inset] are presented as mean and SD). C: Flux through PC and PEPCK tended to decline from 24 to 48 h of fasting. Nonetheless, the rate of anaplerosis (PC-PEPCK flux) was several-fold higher than the actual rate of gluconeogenesis occurring from OAA (light gray) (day 1: 3.6-fold vs. day 2: 2.6-fold; P = 0.009). The excess production of OAA and PEP was accounted for by pyruvate cycling (dots), a futile cycle usually attributed to pyruvate kinase and malic enzyme activity, with pyruvate returning to the TCA cycle via pyruvate carboxylase. The rate of pyruvate cycling decreased significantly with fasting (P = 0.029), completely accounting for the decrease in anaplerotic flux. D: In contrast with a massive induction of ketones with fasting and in spite of an increase in FA availability as the duration of fasting increased, TCA cycle turnover (black) diminished significantly from 24 to 48 h of fasting. * P < 0.05; *** P < 0.001.
Effect of fasting on mitochondrial anaplerosis in liver
To examine the impact of fasting on hepatic anaplerosis, we analyzed the 13C isotopomers of plasma glucose formed from the [U-13C]propionate by 13C NMR. This approach measures flux through the combined pathways of pyruvate carboxylase (PC) and phosphoenolypyruvate carboxykinase (PEPCK) (20, 21). Anaplerosis tended to decrease with fasting (Fig. 3A, C), suggesting that there was an unexpected decrease in PC-PEPCK flux. Nonetheless, the rate of anaplerosis was several-fold higher than the actual rate of gluconeogenesis occurring from oxaloacetate (OAA) (day 1: 3.6-fold vs. day 2: 2.6-fold; P = 0.009). As previously described (21, 27), the excess production of OAA and PEP was accounted for by pyruvate cycling, a futile cycle usually attributed to pyruvate kinase (PEP → pyruvate) and malic enzyme activity (OAA → malate → pyruvate), with pyruvate returning to the TCA cycle via PC (pyruvate → OAA) (Fig. 3A). The rate of pyruvate cycling decreased significantly with fasting (P = 0.029), completely accounting for the decrease in anaplerotic flux. These data indicated that constant gluconeogenesis from OAA in the fasting human (P = 0.492) is maintained by decreased pyruvate kinase or malic enzyme activity rather than an induction of PC and/or PEPCK flux.
Hepatic TCA cycle activity with fasting
To determine the effect of short-term fasting on terminal substrate oxidation in liver, we assessed hepatic TCA cycle activity by 2H and 13C isotopomer analysis of glucose (28). In contrast to marked fasting ketosis and in spite of an increase in FA availability, TCA cycle turnover diminished during fasting (P = 0.021) (Fig. 3A, D). Moreover, there was no difference in TCA cycle activity between the sexes at any time during the fast, even when differences in lean body weight were taken into account.
DISCUSSION
In this study, we examined the effect of fasting on lipid and glucose metabolism in healthy women (n = 9) and men (n = 9), examining for the first time physiologic changes that occur in hepatic TG content. The classic metabolic effects of fasting began after 24 h and were marked by a rapid rise in plasma-free FA and ketones with a concomitant decrease in plasma glucose, insulin, and leptin. Consistent with prior reports (16), significant differences in plasma-free FA concentration were apparent between the sexes (Fig. 1A, B), with women having higher free FAs in the postabsorptive period and men having a 4-fold greater rate of increase in concentration over a 48-h fast. In vivo 1H-MRS also revealed a previously unknown sexually dimorphic response in lean tissue distribution of lipids during a 48-h fast, with more TG accumulation in liver of men (Fig. 2A) but in muscle of women (Fig. 2D). Despite sex differences in plasma FA concentrations and tissue TG distribution during extended fasting, glucose and oxidative metabolism were remarkably similar between women and men (Fig. 1C, 1D, and 3DFig. 1C, 1D, and 3D). As expected, increased plasma-free FAs and a near 20-fold induction of ketonemia during 48 h of fasting (Table 2) was accompanied by a 20% reduction in plasma glucose and a 25% decrease in glucose production and utilization. This reduction in glucose production was secondary to suppressed glycogenolysis (Fig. 3B), with no compensatory increase in gluconeogenesis. Despite increased hepatic fat oxidation, as indicated by the onset of ketosis, energy production by the TCA cycle declined significantly with fasting as hepatic redox state became progressively reduced (Fig. 3D).
The findings of the present study are reminiscent of differences in fasting metabolism between male C57BL/6J and SJL/J mice previously reported by Guan et al. (6). Like women, SJL/J mice were resistant to fasting-induced increases in liver TGs. This resistance was due to enhanced uptake and oxidation of free FA by skeletal muscle during fasting, sparing liver from FA overload. Consistent with enhanced uptake and storage of FA by skeletal muscle, women demonstrated a significant increase in intramyocellular TG content with fasting that was not observed in men (Fig. 3). It has been shown that fatty acid transporter (FAT/CD36) and β-oxidation enzyme protein content is higher in skeletal muscle of women (29, 30). Likewise, Mittendorfer et al. (31) have shown significantly greater lean tissue free FA uptake and oxidation in untrained women during exercise-induced lipolysis. As with SJL/J mice, the lack of increase in hepatic TG content with fasting in women may be explained, in part, by a greater capacity for uptake and processing of circulating FA by skeletal muscle.
The failure of serum concentrations of free FA to increase with fasting in SJL/J mice in conjunction with a modest increase in serum ketone bodies (∼30% of C57BL/6J) was the initial clue that most free FAs released from adipose tissue were removed by skeletal muscle before entering the portal circulation (6). In contrast, women have basally higher lipolysis and postabsorptive plasma-free FA concentrations than men (32–34) (Fig. 2B) even though hepatic TG content tended to decline. However, we also observed a more rapid and greater induction of plasma-free FA in men after 20–32 h of fasting (Fig. 1B). This comparably blunted rise in free FA in women during this period may have contributed to the relatively static levels of hepatic TG during the 48-h fast. In support of this, women with the lowest serum-free FA concentration by 48 h of fasting (r = 0.900; P < 0.001) or the smallest increase in serum-free FAs (r = 0.683; P = 0.036) experienced a decrease in hepatic TGs. Together, these metabolic attributes may explain the relative resistance of women to accumulation of hepatic TGs with fasting. These findings may provide insight into the observation that the risk of pathologic accumulation of hepatic TGs (fatty liver disease) is modestly, but significantly, higher in men (35). To our knowledge, these are the first data demonstrating sexual dimorphism in the hepatic response to lipid excess in humans.
FA esterification is greater in women than in men during the postabsorptive period (3). The rise in intramyocellular TG content in women but not in men supports this finding and suggests that skeletal muscle is an important site for reesterification of lipolytic FAs in women. Koutsari et al. (3) discounted muscle as a possible site for sex differences in nonoxidative FA disposal because women in that study did not demonstrate insulin resistance. In the present study, women had a greater insulin excursion upon refeeding after the 48-h fast, consistent with impaired insulin sensitivity. However, this sex difference was not present in the postprandial period, suggesting that the increased insulin excursion in women was related to higher concentrations of plasma FAs, intramyocellular TGs, or both (36). The lack of increased intramyocellular TG in men who fasted in our study contrasts with data presented by Stannard et al. (10); however, those subjects were endurance-trained athletes and the duration of the fast was 24 h greater.
Despite sex differences in FA metabolism and distribution to lean tissues with fasting, whole-body and hepatic energy metabolism were similar between women and men. We found no evidence for differences in respiratory quotient or VO2 per lean mass between the sexes. Plasma ketone body concentrations were similar throughout the fast (Fig. 1C), suggesting that men and women have similar rates of ketogenesis (37). Similarly, there were no differences in the rates of hepatic oxidative metabolism at the level of the TCA cycle (Fig. 3A, D). Among all subjects, there was a reciprocal relationship between ketosis and TCA cycle activity over the course of the fast: Ketosis increased, whereas TCA cycle turnover decreased. Similar findings have been found in animal studies (38) and have been attributed to the inhibition of citrate synthase by high ATP and suppression of the forward dehydrogenase reactions of the TCA cycle by mitochondrial redox (NADH:NAD+) (39). Indeed, liver became progressively more reduced over the 48-h fast, as indicated by a steady increase in the β-hydroxybutyrate/acetoacetate ratio in both sexes (P < 0.001). Taken together, these data suggest that the uptake of FA by liver for delivery to mitochondria to undergo β-oxidation followed by terminal oxidation in the TCA cycle and/or ketogenesis is remarkably similar in women and men. The key difference between the sexes appears to be in the FA esterification and lipoprotein export pathways.
The transition to fasting ketosis occurred in tandem with a decline in EGP. This decrease was caused by reduced glycogenolysis (Fig. 3A, B), similar to previous studies in fasting humans (40, 41). In contrast, gluconeogenesis and its precursors (glycerol versus lactate/amino acids) was stable across 48 h of fasting, similar to other studies in fasting humans (as reviewed in Ref. 42). However, this finding differs from the expected transcriptional upregulation of genes, such as PEPCK by fasting (43). Because PC-PEPCK flux tended to decline during fasting, the transcriptional regulation of PEPCK appears less important for controlling gluconeogenic flux than previously thought (12, 44) and may serve mainly to defend the pathway against shrinking gluconeogenic substrate supply and/or altered lipid metabolism.
Gluconeogenic flux is increased by hepatic lipid oxidation (14, 15) via the production of gluconeogenic cofactors (i.e., ATP, NADH) and allosteric activation of PC by acetyl-CoA (45, 46). However, the initiation of gluconeogenic flux by oxidative metabolism appears to apply strictly to TCA cycle oxidation rather than to β-oxidation per se (13). Despite increased β-oxidation and massive ketosis during fasting, suppression of TCA cycle activity (Fig. 3D) was attended by a decline in PC-PEPCK flux (Fig. 3A and 3C). Only a concomitant decrease in pyruvate cycling prevented a decline in gluconeogenesis. Pyruvate cycling has contributions from malic enzyme and/or pyruvate kinase (21). Both are important in liver, but malic enzyme activity is unresponsive to nutritional state (47), whereas pyruvate kinase activity is suppressed 4-fold during fasting (48). Regardless, substrate cycling provides an important form of metabolic control under rapidly changing environmental conditions (49). In this case, decreased hepatic pyruvate cycling in fasting humans may support a metabolic buffering system to assure ample availability of PEP and therefore gluconeogenic potential regardless of nutritional state.
In conclusion, lean tissue TG content increased in a sexually dimorphic manner with short-term fasting. The resistance of women to fasting-induced increases in hepatic TG content may be related to the blunted induction of plasma-free FA during extended fasting and an increased propensity for uptake and storage of plasma FAs by skeletal muscle. Our findings may indicate a greater susceptibility of men to pathological accrual of hepatic TGs, providing a potential explanation for the greater prevalence of hepatic steatosis among men (35). Likewise, these findings may help to explain differences in nonoxidative FA metabolism (3) among women and men. In contrast, no sex differences were observed in hepatic and whole-body glucose or energy metabolism. This finding suggests that the major sex differences in hepatic FA metabolism are at the level of reesterification and/or lipoprotein export. Short-term fasting did not lead to increased rates of gluconeogenesis and total endogenous glucose production decreased due to diminishing glycogenolysis during the fast. Reduced TCA cycle flux during fasting ketosis appears to limit PC-PEPCK flux, but gluconeogenesis was spared by reduced pyruvate cycling, a potentially important metabolic control point for gluconeogenesis.
Acknowledgments
The authors thank Sonya Rios, Carol Parcel, and Janet Jerrow from the Advanced Imaging Research Center at UT Southwestern who were critical support staff in the conduct of this study and Jay D. Horton for critical review of the manuscript.
Footnotes
Abbreviations:
- CTRC
- Clinical and Translational Research Center
- EGP
- endogenous glucose production
- 1H-MRS
- proton magnetic resonance spectroscopy
- OAA
- oxaloacetate
- PC
- pyruvate carboxylase
- PEPCK
- phosphoenolpyruvate carboxykinase
- STEAM
- stimulated echo acquisition mode
- TG
- triglyceride
This work was supported by the Clinical and Translational Science Award (CTSA) at UT Southwestern (UL1RR024982), the Task Force for Obesity Research (TORS) at UT Southwestern (UL1DE019584), the TORS Human Biology Core (PL1DK081183), and the TORS Molecular and Metabolic Mouse Phenotyping Core (PL1DK081182). This work was also supported by National Institutes of Health Grants RL1DK081187 (J.D.B., S.C.B.), K23DK074396 (J.D.B.), R01DK087977 (J.D.B.), RR02584 (S.C.B.), R01DK078184 (S.C.B.), and ADA7-09-BS-24 (S.C.B.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.McGarry J. D., Foster D. W. 1980. Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem. 49: 395–420. [DOI] [PubMed] [Google Scholar]
- 2.Cahill G. F., Jr 1970. Starvation in man. N. Engl. J. Med. 282: 668–675. [DOI] [PubMed] [Google Scholar]
- 3.Koutsari C., Basu R., Rizza R. A., Nair K. S., Khosla S., Jensen M. D. 2011. Nonoxidative free fatty acid disposal is greater in young women than men. J. Clin. Endocrinol. Metab. 96: 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boden G., Lebed B., Schatz M., Homko C., Lemieux S. 2001. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 50: 1612–1617. [DOI] [PubMed] [Google Scholar]
- 5.Coppack S. W., Jensen M. D., Miles J. M. 1994. In vivo regulation of lipolysis in humans. J. Lipid Res. 35: 177–193. [PubMed] [Google Scholar]
- 6.Guan H. P., Goldstein J. L., Brown M. S., Liang G. 2009. Accelerated fatty acid oxidation in muscle averts fasting-induced hepatic steatosis in SJL/J mice. J. Biol. Chem. 284: 24644–24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browning J. D., Horton J. D. 2004. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wietek B. M., Machann J., Mader I., Thamer C., Haring H. U., Claussen C. D., Stumvoll M., Schick F. 2004. Muscle type dependent increase in intramyocellular lipids during prolonged fasting of human subjects: a proton MRS study. Horm. Metab. Res. 36: 639–644. [DOI] [PubMed] [Google Scholar]
- 9.Johnson N. A., Stannard S. R., Rowlands D. S., Chapman P. G., Thompson C. H., O'Connor H., Sachinwalla T., Thompson M. W. 2006. Effect of short-term starvation versus high-fat diet on intramyocellular triglyceride accumulation and insulin resistance in physically fit men. Exp. Physiol. 91: 693–703. [DOI] [PubMed] [Google Scholar]
- 10.Stannard S. R., Thompson M. W., Fairbairn K., Huard B., Sachinwalla T., Thompson C. H. 2002. Fasting for 72 h increases intramyocellular lipid content in nondiabetic, physically fit men. Am. J. Physiol. Endocrinol. Metab.283: E1185–1191. [DOI] [PubMed]
- 11.Hausler N., Browning J., Merritt M., Storey C., Milde A., Jeffrey F. M., Sherry A. D., Malloy C. R., Burgess S. C. 2006. Effects of insulin and cytosolic redox state on glucose production pathways in the isolated perfused mouse liver measured by integrated 2H and 13C NMR. Biochem. J. 394: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgess S. C., He T., Yan Z., Lindner J., Sherry A. D., Malloy C. R., Browning J. D., Magnuson M. A. 2007. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 5: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browning J. D., Weis B., Davis J., Satapati S., Merritt M., Malloy C. R., Burgess S. C. 2008. Alterations in hepatic glucose and energy metabolism as a result of calorie and carbohydrate restriction. Hepatology. 48: 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X., Iqbal N., Boden G. 1999. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J. Clin. Invest. 103: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staehr P., Hother-Nielsen O., Landau B. R., Chandramouli V., Holst J. J., Beck-Nielsen H. 2003. Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis. Diabetes. 52: 260–267. [DOI] [PubMed] [Google Scholar]
- 16.Mittendorfer B. 2005. Sexual dimorphism in human lipid metabolism. J. Nutr. 135: 681–686. [DOI] [PubMed] [Google Scholar]
- 17.Landau B. R., Wahren J., Chandramouli V., Schumann W. C., Ekberg K., Kalhan S. C. 1995. Use of 2H2O for estimating rates of gluconeogenesis. Application to the fasted state. J. Clin. Invest. 95: 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess S. C., Nuss M., Chandramouli V., Hardin D. S., Rice M., Landau B. R., Malloy C. R., Sherry A. D. 2003. Analysis of gluconeogenic pathways in vivo by distribution of 2H in plasma glucose: comparison of nuclear magnetic resonance and mass spectrometry. Anal. Biochem. 318: 321–324. [DOI] [PubMed] [Google Scholar]
- 19.Burgess S. C., Weis B., Jones J. G., Smith E., Merritt M. E., Margolis D., Dean Sherry A., Malloy C. R. 2003. Noninvasive evaluation of liver metabolism by 2H and 13C NMR isotopomer analysis of human urine. Anal. Biochem. 312: 228–234. [DOI] [PubMed] [Google Scholar]
- 20.Jones J. G., Naidoo R., Sherry A. D., Jeffrey F. M., Cottam G. L., Malloy C. R. 1997. Measurement of gluconeogenesis and pyruvate recycling in the rat liver: a simple analysis of glucose and glutamate isotopomers during metabolism of [1,2,3-(13)C3]propionate. FEBS Lett. 412: 131–137. [DOI] [PubMed] [Google Scholar]
- 21.Magnusson I., Schumann W. C., Bartsch G. E., Chandramouli V., Kumaran K., Wahren J., Landau B. R. 1991. Noninvasive tracing of Krebs cycle metabolism in liver. J. Biol. Chem. 266: 6975–6984. [PubMed] [Google Scholar]
- 22.Jin E. S., Jones J. G., Burgess S. C., Merritt M. E., Sherry A. D., Malloy C. R. 2005. Comparison of [3,4–13C2]glucose to [6,6–2H2]glucose as a tracer for glucose turnover by nuclear magnetic resonance. Magn. Reson. Med. 53: 1479–1483. [DOI] [PubMed] [Google Scholar]
- 23.Burgess S. C., Hausler N., Merritt M., Jeffrey F. M., Storey C., Milde A., Koshy S., Lindner J., Magnuson M. A., Malloy C. R., et al. 2004. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 279: 48941–48949. [DOI] [PubMed] [Google Scholar]
- 24.Vermathen P., Kreis R., Boesch C. 2004. Distribution of intramyocellular lipids in human calf muscles as determined by MR spectroscopic imaging. Magn. Reson. Med. 51: 253–262. [DOI] [PubMed] [Google Scholar]
- 25.Szczepaniak L. S., Babcock E. E., Schick F., Dobbins R. L., Garg A., Burns D. K., McGarry J. D., Stein D. T. 1999. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am. J. Physiol. 276: E977–E989. [DOI] [PubMed] [Google Scholar]
- 26.Longo R., Pollesello P., Ricci C., Masutti F., Kvam B. J., Bercich L., Croce L. S., Grigolato P., Paoletti S., de Bernard B., et al. 1995. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J. Magn. Reson. Imaging. 5: 281–285. [DOI] [PubMed] [Google Scholar]
- 27.Jin E. S., Jones J. G., Merritt M. E., Burgess S. C., Malloy C. R., Sherry A. D. 2004. Glucose production, gluconeogenesis, and hepatic TCA cycle fluxes measured by NMR analysis of a single glucose derivative. Anal. Biochem. In press. [DOI] [PubMed] [Google Scholar]
- 28.Jones J. G., Solomon M. A., Cole S. M., Sherry A. D., Malloy C. R. 2001. An integrated (2)H and (13)C NMR study of gluconeogenesis and TCA cycle flux in humans. Am. J. Physiol. Endocrinol. Metab. 281: E848–E856. [DOI] [PubMed] [Google Scholar]
- 29.Kiens B., Roepstorff C., Glatz J. F., Bonen A., Schjerling P., Knudsen J., Nielsen J. N. 2004. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J. Appl. Physiol. 97: 1209–1218. [DOI] [PubMed] [Google Scholar]
- 30.Maher A. C., Akhtar M., Vockley J., Tarnopolsky M. A. 2010. Women have higher protein content of beta-oxidation enzymes in skeletal muscle than men. PLoS ONE. 5: e12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittendorfer B., Horowitz J. F., Klein S. 2002. Effect of gender on lipid kinetics during endurance exercise of moderate intensity in untrained subjects. Am. J. Physiol. Endocrinol. Metab. 283: E58–E65. [DOI] [PubMed] [Google Scholar]
- 32.Jensen M. D., Johnson C. M. 1996. Contribution of leg and splanchnic free fatty acid (FFA) kinetics to postabsorptive FFA flux in men and women. Metabolism. 45: 662–666. [DOI] [PubMed] [Google Scholar]
- 33.Jensen M. D., Cryer P. E., Johnson C. M., Murray M. J. 1996. Effects of epinephrine on regional free fatty acid and energy metabolism in men and women. Am. J. Physiol. 270: E259–E264. [DOI] [PubMed] [Google Scholar]
- 34.Mittendorfer B., Horowitz J. F., Klein S. 2001. Gender differences in lipid and glucose kinetics during short-term fasting. Am. J. Physiol. Endocrinol. Metab. 281: E1333–E1339. [DOI] [PubMed] [Google Scholar]
- 35.Browning J. D., Szczepaniak L. S., Dobbins R., Nuremberg P., Horton J. D., Cohen J. B., Grundy S. M., Hobbs H. H. 2004. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 40: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 36.Kelley D. E., Mokan M., Simoneau J. A., Mandarino L. J. 1993. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J. Clin. Invest. 92: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichard G. A., Jr, Owen O. E., Haff A. C., Paul P., Bortz W. M. 1974. Ketone-body production and oxidation in fasting obese humans. J. Clin. Invest. 53: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krebs H. A. 1970. Rate control of the tricarboxylic acid cycle. Adv. Enzyme Regul. 8: 335–353. [DOI] [PubMed] [Google Scholar]
- 39.Williamson J. R., Scholz R., Browning E. T. 1969. Control mechanisms of gluconeogenesis and ketogenesis. II. Interactions between fatty acid oxidation and the citric acid cycle in perfused rat liver. J. Biol. Chem. 244: 4617–4627. [PubMed] [Google Scholar]
- 40.Landau B. R., Wahren J., Chandramouli V., Schumann W. C., Ekberg K., Kalhan S. C. 1996. Contributions of gluconeogenesis to glucose production in the fasted state. J. Clin. Invest. 98: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothman D. L., Magnusson I., Katz L. D., Shulman R. G., Shulman G. I. 1991. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 254: 573–576. [DOI] [PubMed] [Google Scholar]
- 42.Nuttall F. Q., Ngo A., Gannon M. C. 2008. Regulation of hepatic glucose production and the role of gluconeogenesis in humans: is the rate of gluconeogenesis constant? Diabetes Metab. Res. Rev. 24: 438–458. [DOI] [PubMed] [Google Scholar]
- 43.Chakravarty K., Cassuto H., Reshef L., Hanson R. W. 2005. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit. Rev. Biochem. Mol. Biol. 40: 129–154. [DOI] [PubMed] [Google Scholar]
- 44.Ramnanan C. J., Edgerton D. S., Rivera N., Irimia-Dominguez J., Farmer B., Neal D. W., Lautz M., Donahue E. P., Meyer C. M., Roach P. J., et al. 2010. Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes. 59: 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jomain-Baum M., Hanson R. W. 1975. Regulation of hepatic gluconeogenesis in the guinea pig by fatty acids and ammonia. J. Biol. Chem. 250: 8978–8985. [PubMed] [Google Scholar]
- 46.Williamson J. R., Browning E. T., Scholz R. 1969. Control mechanisms of gluconeogenesis and ketogenesis. I. Effects of oleate on gluconeogenesis in perfused rat liver. J. Biol. Chem. 244: 4607–4616. [PubMed] [Google Scholar]
- 47.Shrago E., Lardy H. A., Nordlie R. C., Foster D. O. 1963. Metabolic and hormonal control of phosphoenolpyruvate carboxykinase and malic enzyme in rat liver. J. Biol. Chem. 238: 3188–3192. [PubMed] [Google Scholar]
- 48.Rognstad R., Katz J. 1977. Role of pyruvate kinase in the regulation of gluconeogenesis from L-lactate. J. Biol. Chem. 252: 1831–1833. [PubMed] [Google Scholar]
- 49.Newsholme E. A., Crabtree B. 1976. Substrate cycles in metabolic regulation and in heat generation. Biochem. Soc. Symp. 41: 61–109. [PubMed] [Google Scholar]