Abstract
Background
Intermittent preventive treatment (IPT), the main strategy to prevent malaria and reduce anaemia and low birthweight, focuses on the second half of pregnancy. However, intrauterine growth restriction may occur earlier in pregnancy. The aim of this study was to measure the effects of malaria in the first half of pregnancy by comparing the fetal biparietal diameter (BPD) of infected and uninfected women whose pregnancies had been accurately dated by crown rump length (CRL) before 14 weeks of gestation.
Methodology/Principal Findings
In 3,779 women living on the Thai-Myanmar border who delivered a normal singleton live born baby between 2001–10 and who had gestational age estimated by CRL measurement <14 weeks, the observed and expected BPD z-scores (<24 weeks) in pregnancies that were (n = 336) and were not (n = 3,443) complicated by malaria between the two scans were compared. The mean (standard deviation) fetal BPD z-scores in women with Plasmodium (P) falciparum and/or P.vivax malaria infections were significantly lower than in non-infected pregnancies; −0.57 (1.13) versus −0.10 (1.17), p<0.001. Even a single or an asymptomatic malaria episode resulted in a significantly lower z-score. Fetal female sex (p<0.001) and low body mass index (p = 0.01) were also independently associated with a smaller BPD in multivariate analysis.
Conclusions/Significance
Despite early treatment in all positive women, one or more (a)symptomatic P.falciparum or P.vivax malaria infections in the first half of pregnancy result in a smaller than expected mid-trimester fetal head diameter. Strategies to prevent malaria in pregnancy should include early pregnancy.
Introduction
Malaria remains one of the most common parasitic infection of human pregnancy [1]–[4], and it lowers birthweight whether or not maternal symptoms are present [5]. Even a single episode of treated Plasmodium (P.) falciparum or P.vivax malaria during pregnancy has a negative effect on birthweight [6], [7]. The mechanisms of this reduction in birthweight include placental insufficiency by sequestration of malaria parasites leading to intrauterine growth restriction (IUGR), premature labour or a combination of the two [8], [9]. The evidence is less clear in P.vivax infected pregnancies where placental sequestration is probably limited [10]. Difficulties in estimating gestational age (GA) accurately and diagnosing malaria infection in early pregnancy have complicated the interpretation of previous malaria studies on fetal growth [9], [11]. IUGR may start in the first trimester and influence late pregnancy outcomes [12]. Early antenatal ultrasound - which is essential to date pregnancy accurately [13] - is becoming available in developing countries [14]–[16]. The aim of this study was to assess whether malaria infection affects early fetal growth by comparing the fetal biparietal diameter (BPD) before 24 weeks gestation in infected and uninfected women whose pregnancies had been accurately dated by crown rump length (CRL) measurement before 14 weeks.
Methods
Study site and population
The Shoklo Malaria Research Unit (SMRU) is located on the border between Thailand and Burma in Tak province where the majority of people belongs to the Karen ethnic group [17]. P.falciparum [18] and P.vivax [7] transmissions are low and seasonal [19]. Since 1986 the SMRU has offered free antenatal care (ANC) to refugees and later (1998) to migrant women, including weekly malaria screening to detect and treat all parasitaemic episodes during pregnancy in order to prevent maternal death [18]. There is no presumptive treatment of malaria, chemoprophylaxis or intermittent preventive treatment in pregnancy due to drug resistance. Since the inception of this ANC program, all pregnant women have been encouraged to attend as early as possible in the first trimester. In 2001 antenatal ultrasound was introduced to improve pregnancy dating in this population because of low literacy rates and poor recall of the date of the last menstrual period [14], [20]. At enrolment in the antenatal clinic all women are interviewed for demographics and have anthropometric measurements taken. At every visit ferrous sulphate (200 mg daily) and folic acid (5 mg weekly) for anemia prophylaxis and thiamine (Vitamin B1, 100 mg daily) to prevent infant mortality from beri-beri [21] are offered to all women. Anaemic women receive treatment doses of ferrous sulphate (200 mg three times daily) and folic acid (5 mg daily). All medical and obstetric problems in pregnancy are investigated and treated free of charge by locally trained health workers working in SMRU facilities [22], [23].
Pregnancy ultrasound
Locally trained health workers (10 sonographers at 3 clinics) obtain all ultrasound scans using Toshiba Powervision 7000 (since 2006), Dynamic Imaging (since 2001), Fukuda Denshi UF 4100 (since 2002) ultrasound machines. Their practice is supervised by doctors certified in fetal ultrasonography. All women are offered two scans in pregnancy. The 1st scan occurs at the booking visit (between 8–14 weeks gestation) where ultrasound is used to determine viability, identify multiple pregnancy and estimate GA by CRL measurement. Based on this GA estimate women then return for a 2nd scan performed at 18–24 weeks to re-assess viability, measure fetal biometry, identify major fetal abnormalities and determine placental location. Fetal biometry is routinely measured twice in each woman at each scan, as part of an existing quality control [14].
The training manual and protocol for obtaining trans-abdominal CRL and biometry measurements were from the British Medical Ultrasound Society recommendation [24].
The BPD is measured at the cross-sectional view of the fetal head at the level of the ventricles by placing the calipers on the outer border of the upper and the inner border of the lower parietal bones (‘outer to inner’, BPD) across the widest part of the skull. Importantly for this study, operators taking fetal measurements were not aware of the maternal malaria status.
Outcomes and SMRU delivery rooms
All women are encouraged to deliver in the SMRU facilities under supervision of locally trained midwives who speak their language. Women requiring Caesarean section are referred to the nearest Thai hospital; in this study the Caesarean section rate was 3.4% (129/3,779). Each baby is weighed within 24 hours on electronic SECA (Model 336 or 376, accuracy = 10 g) digital newborn scales.
Ethics statement
This is a retrospective hospital record analysis. For those patients in trials a written informed consent was obtained including storing of data and samples. For the women seen in the ANC the routine clinical records were anonymized and these pregnancy records have been routinely entered into a database since 1987. Permission was granted by Oxford Tropical Research Ethics Committee (reference: OXTREC 28–09) to use these records for analysis.
Diagnosis of malaria
Malaria is diagnosed by Giemsa stained thick and thin blood films; 200 fields on the thick film are read before being declared negative. All parasite densities are counted per 500 white blood cells or per 1000 red blood cells. P. vivax or P. falciparum malaria infection is defined by the presence of asexual stages of the respective parasite in the peripheral blood.
Definitions
Severe malaria is defined as per WHO treatment guidelines [25] and hyperparasitaemic malaria by the presence of at least 4% infected red blood cells in the absence of other signs of severity. Anaemia is defined by a haematocrit less than 30%. Symptomatic malaria is defined by a temperature ≥37.5°C or a history of fever [25]. When a women had at least one symptomatic episode between the 1st and the 2nd scan she was classified as symptomatic. Mid Upper Arm Circumference (MUAC) was measured at the first ANC consultation on an unclothed left arm with a SECA measuring tape (model 212) accurate to one mm and low MUAC is defined as <21.0 cm [26]. Maternal height is measured at the first ANC consultation and short stature is defined as <145 cm. Maternal weight of women wearing the lightest possible clothing, is measured at the first consultation and at the time of the biometry ultrasound scan on mechanical SECA weight scales (model 762) with graduation of 500 grams. Weight gain is defined as the difference in maternal weight between the two scans. The weight in the first trimester is used to calculate the body mass index (BMI): a BMI of <18.5 kg/m2 is considered underweight [27]. Pregnancy duration is defined as 280 days post menstruation. Miscarriage is a pregnancy ending before 28 weeks GA and stillbirth a delivery from 28 weeks or ≥800 g birthweight in which the infant displayed no sign of life (gasping, muscular activity, cardiac activity). The 28-week GA, rather than the current WHO 22-week GA cut-off was chosen, as no infant ventilatory support is available in the clinics. This cut-off has been in place since SMRU was established as the lower limit of viability in this area. Congenital abnormality is considered if any major abnormality was present at birth by staff trained in examination of the newborn.
Inclusion and Exclusion criteria
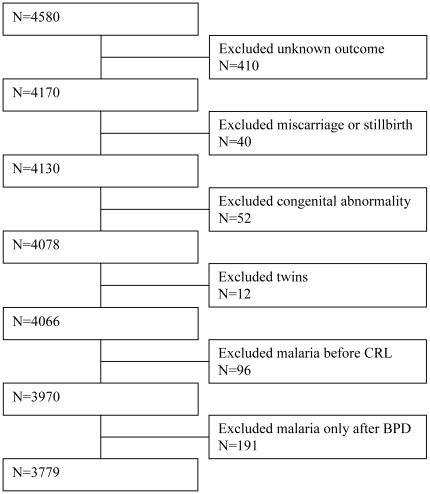
All women who had GA estimated by CRL measurement <14 weeks (1st scan) and BPD measured <24 weeks (2nd scan), were included in the analysis. Twin pregnancies, pregnancies that were complicated by miscarriage, stillbirth or fetal structural abnormalities and pregnancies with an unknown outcome were excluded (Figure 1). Women who had their first malaria episode before or at the time of the 1st scan or after the 2nd scan were also excluded. Therefore in this analysis all malaria infected women had their first malaria episode between the first and the second ultrasound scans. For the analysis of birth outcomes women who had another episode after the second scan were excluded to avoid the confounding effects of malaria outside the window of measurement (14+0–24+0 weeks).
Figure 1. Selection of pregnant women.
Statistical Analysis
Clinical data and the results of the two ultrasound scans were entered into a Microsoft Access database and analyzed using SPSS version 18 for Windows. Student's t-test and Mann-Whitney test were used for comparison of means and ranks respectively. Categorical data were compared using the chi-squared test or the Fisher's exact test, as appropriate. The mean of two CRL measurements was used to calculate GA in days using a well established formula [28]. The average of two BPD measurements was recorded as ‘BPD observed’. For the purpose of this study the ‘expected BPD size’ for each GA was calculated using a reference equation for the Karen population [29]. For validation purposes all analyses were repeated for z-scores derived from a chart based on an ethnic Chinese population [30].
Each observed BPD was then converted into a z-score using the following formula:
where SD is the standard deviation of the BPD at that GA. The advantage of converting measurements into z scores is that it eliminates variability by GA allowing measurements to be compared [31], [32]. A positive z-score indicates a larger and a negative value a smaller than expected BPD.
In order to assess the impact of malaria infection on fetal growth during early pregnancy, the mean BPD z-scores were compared in women who did and did not have peripheral parasitaemia in the window between the 1st and 2nd scans. Other factors associated with BPD were evaluated by univariate analysis; variables with a significance of P<0.1 were kept in the multivariate regression model. A secondary endpoint was the effect of malaria infection between the 1st and 2nd scan only, on GA and birthweight at delivery. For this purpose a population birthweight centiles chart (per gestational age) was computed from all pregnancies in SMRU with accurate ultrasound dating. Only newborns who were weighed within the first 24 hours after delivery were included [11].
Results
Between September 2001 and January 2010, 4,580 women had a 1st (CRL measurement <14 weeks) and a 2nd scan (BPD measurement <24 weeks). Women with an unknown pregnancy outcome (410, 9.0%) were excluded from further analysis; they were more likely to be younger and primigravid, to book at a lower GA and have malaria (data not shown). A further 391 women (8.5%) were excluded for miscarriage/stillbirth (n = 40), congenital abnormality (n = 52), twin pregnancy (n = 12), first malaria before the CRL dating scan (n = 96) or first malaria infection after the BPD scan (n = 191) (Figure 1). There were three maternal deaths: two women died from post-partum hemorrhage after delivering stillborn infants (excluded from analysis due to stillbirth); one woman died of severe malaria five weeks post-partum and was included as the infant was live born. Therefore, a total of 3,779 women remained for analysis.
Frequent intermittent malaria screening in the ANC
The median number of antenatal visits was 23 [range 3–38]. At each visit a malaria smear was obtained and malaria parasites were detected in 930 (1.1%) of the available 86,416 blood slides; these were from 336/3,779 (8.9%) women. The median number of malaria episodes per woman was 2 [range 1–8] for the duration of pregnancy.
Timing of Malaria episodes
The 336 women who had their first malaria infection in the window between the two scans had a lower BMI, a lower Hct and were younger and more likely to smoke, than the 3,443 uninfected women (Table 1). Of the 336 women with malaria, 240 (71.4%) had only P.vivax infections and 96 (28.6%) had P.falciparum or both infections (Table 2). In 233 (69.3%) there was a single malaria infection in the window between the two scans; of these 80.3% (187/233) were P.vivax. Multiple malaria infections [range 2–4] in the window were diagnosed in 103 women (30.7%). There were only six women (1.8%) with a hyperparasitaemic P.falciparum infection in the window and no severe malaria infections (Table 2). Only 114 (33.9%) women had all their episodes of malaria between the two scans and none after the second scan.
Table 1. Demographics of the refugee and migrant women from Thai Burmese border, 2001–2010.
| Malaria+ | No Malaria | P value | |
| n = 336 | n = 3,443 | ||
| Age, years | 24.0 20–30 | 26.0 21–31 | 0.006 |
| Gravida | 3 1–4 | 3 2–4 | 0.187 |
| Parity | 1 [0–3] | 1 [0–3] | 0.036 |
| Nulliparous, % (n) | 28.3 (95) | 24.3 (836) | 0.105 |
| Height, cm$ | 151 [148–155] | 151 [147–155] | 0.349 |
| BMI, kg/m2 $ | 20.0 [18.6–21.5] | 20.5 [19.0–22.4] | <0.001 |
| Weight gain, kg* | 2.0 [1.0–3.0] | 2.0 [1.0–3.0] | 0.265 |
| Hct, % | 28 (26–30) | 30 (28–32) | <0.001 |
| MUAC, cm# | 24.3 [23.0–26.0] | 24.0 [22.8–26.0] | 0.483 |
| Smoker, % (n) | 30.7 (103) | 22.5 (773) | 0.001 |
| NOC during pregnancy | 23 19–28 | 23 17–29 | 0.485 |
Median [IQR], or as indicated.
BMI body mass index, Hct Haematocrit at first consultation, MUAC middle upper arm circumference, NOC number of consultations.
between the 1st and 2nd scans.
*Weight gain from the first to the second scan; available from 301 in malaria and 2,677 in no malaria group.
Available from 292 in malaria and 2,626 in no malaria group.
Available from 314 in malaria and 3,044 in no malaria group.
Table 2. Species, episodes and severity of malaria and mean BPD z-score of women infected between the first and second scan.
| Total malaria episodes | P.falciparum or mixed | P.vivax | Proportion of women with one episode | Proportion women at least one symptomatic episode | Proportion at least one hyper/severe episode | |
| N = 336 | N = 96 | N = 240 | N = 336 | N = 334# | N = 336 | |
| Between 1st and 2nd scan | 1 1–4 | 1 1–3 | 1 1–4 | 69.3% (233) | 49.1% (165) | 1.8% (6) |
| Mean BPD z-score | −0.57 (1.1) | −0.45 (1.2) | −0.62 (1.1) | −0.51 (1.2) | −0.59 (1.1) | n.a. |
Median [min-max], or mean (SD). BPD biparietal diameter; hyper = hyperparasitaemia (≥4% red blood cells infected), n.a. not applicable, P plasmodium.
Missing data n = 2.
Effect of malaria on BPD
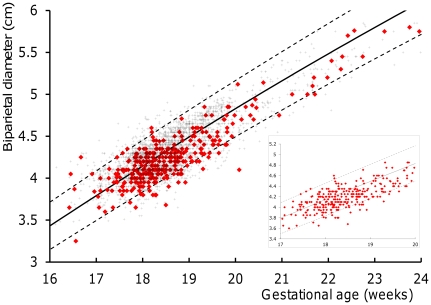
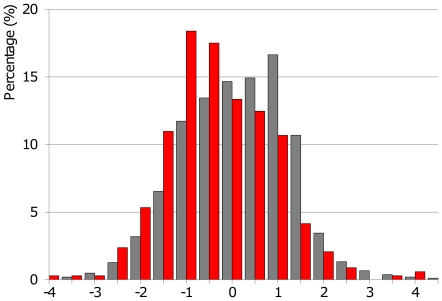
The BPD measurements of malaria infected and uninfected women were superimposed on the 2.5th, 50th, and 97.5th centiles of the reference equation [29] and presented graphically between 16 and 24 weeks GA to allow visual comparison (Figure 2). Most of the BPD measurements of women with malaria (red diamonds) were below the 50th centile (Figure 2 and insert, which shows the BPD measurements between 17 and 20 weeks GA in which 90% (302/336) of the measurements were obtained). The BPD measurements were then converted into z-scores that were normally distributed. There was a statistically significant difference between the mean (SD) BPD z-scores of the fetuses of infected and uninfected women (−0.57 (1.13) versus −0.10 (1.17), p<0.001) (Figure 3, Table 3). Both P.falciparum (or mixed) (n = 96) and P.vivax (n = 240) were associated with a lower mean z-score than uninfected women (−0.45 (1.2), p = 0.006 and −0.62 (1.1) respectively, p<0.001). There was no significant difference in mean (SD) z-scores between women with symptomatic (n = 165) or asymptomatic malaria (n = 169) episodes: −0.59 (1.1) versus −0.54 (1.2) respectively, p = 0.68. Women with multiple malaria infections (n = 103) had the lowest mean (SD) z-scores (−0.72 (1.0), p<0.001) but even a single malaria episode between the 2 scans (n = 233) resulted in a significantly lower mean (SD) BPD z-score: −0.51 (1.2), p<0.001). There was no significant difference in mean z-scores between women with uncomplicated and hyperparasitaemic malaria, but the number of hyperparasitaemic women was too small for analysis (Table 2).
Figure 2. Fetal biparietal diameter measurements in Burmese and Karen pregnant women with and without malaria.
The x-axis shows the gestational age (GA) in weeks, based on first trimester dated pregnancies on the Thai-Burmese border from 2001 to 2010. The y-axis depicts the fetal biparietal diameter measurement (BPD) in centimeters. The fetal BPD in pregnant women with malaria (red diamonds, n = 336) and in women without malaria (+, n = 3,443) between 16 and 24 GA weeks were superimposed on the 2.5th, 50th and 97.5th centiles of a reference equation for this population [29]. Note that the majority of fetal BPD measurements in malaria infected women lie below the 50th centile in both the main figure (16 to 24 GA weeks) and in the inset (17 to 20 GA weeks, where 90% (302/336) of the measurements in malaria infected women were obtained).
Figure 3. Z-scores of fetal biparietal diameter in Burmese and Karen pregnant women with and without malaria.
The x-axis shows the z-score and the y-axis depicts the distribution in percentages. The distribution of z-scores of fetal biparietal diameter in pregnant women with malaria (n = 336, red bars) is significantly lower than in women without malaria (n = 3,443, grey bars) on the Thai-Burmese border from 2001 to 2010.
Table 3. Risk factors associated with mean BPD z-score as a measure of early fetal growth restriction.
| Univariate | Multivariate (n = 2972) | |||||
| Frequency (%) | Coefficient (95% CI) | P-value | Coefficient (95%CI) | P-value | ||
| Teenager | No | 3,210 (84.9) | ||||
| Yes | 569 (15.1) | −0.02 (−0.13, 0.08) | 0.66 | * | NS | |
| Primigravida | No | 2,848 (75.4) | ||||
| Yes | 931 (24.6) | 0.07 (−0.02, 0.16) | 0.10 | * | NS | |
| Smoking | No | 2,893 (76.8) | ||||
| Yes | 876 (23.2) | 0.04 (−0.05, 0.12) | 0.43 | * | NS | |
| Low MUAC | No | 2,801 (96.0) | ||||
| Yes | 116 (4.0) | 0.07 (−0.14, 0.29) | 0.51 | * | NS | |
| Short | No | 2,995 (89.2) | ||||
| Yes | 362 (10.8) | 0.04 (−0.09, 0.16) | 0.58 | * | NS | |
| Low BMI | No | 2,738 (81.6) | ||||
| Yes | 619 (18.4) | 0.18 (0.08, 0.28) | 0.001 | 0.15 (0.04, 0.26) | 0.005 | |
| Weight loss | No | 2,396 (80.5) | ||||
| Yes | 581 (19.5) | 0.09 (−0.02, 0.19) | 0.10 | 0.11 (0.00, 0.21) | 0.05 | |
| Anaemia | No | 2152 (56.9) | ||||
| Yes | 1627 (43.1) | 0.16 (0.09, 0.24) | <0.001 | $ | ||
| Malaria | No | 3,443 (91.1) | ||||
| Yes | 336 (8.9) | 0.47 (0.34, 0.60) | <0.001 | 0.50 (0.36, 0.63) | <0.001 | |
| Symptomatic malaria | No | 169 (50.6) | ||||
| Yes | 165 (49.4) | 0.05 (−0.19, 0.30) | 0.68 | * | NS | |
| Newborn gender | M | 1,936 (51.2) | ||||
| F | 1,843 (48.8) | 0.25 (0.18, 0.33) | <0.001 | 0.25 (0.17,0.34) | <0.001 | |
Significant differences are shown in bold.
BMI body mass index, MUAC middle upper arm circumference, NS not significant.
*Not included in the multivariate analysis.
Anaemia was highly collinear with malaria, and the co-variates in the multivariate model were unchanged when anaemia was adjusted for or not.
Aside from malaria, female sex of the fetus, maternal anaemia and low maternal BMI were also significantly associated with a lower mean z-score in the univariate analysis. These remained independent risk factors in the linear regression model which included 2972 women, the remainder being excluded because of missing values mainly maternal weight at the second scan (Table 3). This significantly lower mean BPD z-score (difference in z-score of 0.47 SD) means that malaria during the period between the two scans reduces the diameter of the fetal head by approximately 1 mm when measured at a GA of 22 weeks. The same analysis was repeated for z-scores derived from a Chinese population and except for a small shift in mean z-score (as expected for different populations) the magnitude and significance of the differences were similar (Supporting File S1 and Supporting Table S1).
Effect of malaria on birth outcomes
Of the 3,083 live born congenitally normal singleton infants weighed within 24 hours of delivery, the z-score for BPD was positively correlated with birthweight centile: B = 0.168 (95% CI 0.128–0.209), p<0.001. Seventy two of the 114 women (63.2%) who had all their malaria infections between the two scans only, had their baby weighted within 24 hours. Neonates from these pregnancies had similar birth outcomes as the ones from uninfected pregnancies (n = 3,011): mean (SD) birthweight 2919 (SD 533) g versus 2973 (SD 433) g, p = 0.40, mean GA 272 (SD 13) days (or 38.9 (SD 1.9) weeks) versus 273 (SD 11) days (or 39.0 (SD 1.6) weeks), p = 0.22 and birthweight centile 0.00 (SD 1.2) versus 0.03 (SD 1.0) respectively. Within this small malaria sub-group (n = 72) there was no association between BPD z-score and birthweight centile.
Discussion
In this study a significantly smaller fetal BPD was observed by ultrasound when malaria infection occurred in the first half of pregnancy, compared to pregnancies unaffected by malaria. Previous studies have shown that malaria early in pregnancy has an impact on birthweight, but they were limited by small numbers of first trimester exposures [33] or by inaccuracy in the dating of gestation [34]. In this analysis the women had a documented and treated episode of malaria during a specific period between two ultrasound scans. By studying this selected group the effects of malaria on fetal growth could be quantified in-utero. Even a single infection of treated P.vivax or P.falciparum was associated with reduced BPD irrespective of whether the woman was symptomatic or not. The mechanisms underlying the adverse effects of malaria in pregnancy are not fully understood [7], [35], [36]. P.falciparum is thought to sequester in the placenta and interfere with materno-fetal exchanges but other mechanisms may also be involved [8], [36]. Systemic or hormonal mechanisms may play a role in P. vivax related growth restriction, as there is little evidence that P.vivax sequesters in the placenta, like P.falciparum does [7], [10], [35], [36]. In non-malaria endemic areas, early pregnancy growth restriction has been associated with miscarriage [37], maternal physical characteristics and lifestyle habits related to early fetal growth [38], and low BPD growth rates between the first and second trimester are associated with increased perinatal mortality and IUGR [39], [40]. In malaria endemic areas ultrasound studies have related malaria in pregnancy to changes in maternal and fetal blood flow [41], [42] and associated malnutrition and malaria in pregnancy with IUGR [43]. So the results presented here are not entirely unexpected but show for the first time the direct evidence of the effect of malaria (both falciparum and vivax) on fetal growth. More surprising perhaps is that low BPD growth was observed after a single (even asymptomatic) infection and despite early treatment.
Studies on the impact of malaria in pregnancy have almost always focused on birthweight. However, for infections that occur in early pregnancy, the size of the fetal head may be a more appropriate indicator of growth restriction. It has been shown that the growth velocity of the fetal head (in mm/day) is maximal during the second trimester [44]–[46]. In contrast the fetal “weight velocity” (in g/week) peaks in the third trimester. This weight gain velocity curve has often been cited wrongly as a fetal “growth velocity” curve [47], [48]. The characteristics of the “fetal head size” and “fetal weight” growth velocity curves are similar but the timing is different. One of the strengths of this study is that the timing of the BPD measurement coincided with the maximal growth velocity of the fetal head, making it a better marker of the effect of malaria in early pregnancy than birthweight.
The reduction in BPD size occurred despite prompt treatment with effective antimalarials in this setting, which highlights the importance of prevention in pregnancy. Multiple episodes of P.vivax are most likely from liver stage relapses instead of newly acquired infections. There is no treatment available in pregnancy for liver stages. Furthermore, in this setting of multidrug resistant parasites there are no safe and effective drugs available to prevent malaria from the start of pregnancy. To protect the developing fetus from growth restriction from both symptomatic and asymptomatic P.vivax and P.falciparum infections, prevention strategies from early pregnancy onwards or even pre-pregnancy interventions should be considered [33], [34], [49], [50].
There are some limitations to this analysis. Firstly, although dating by CRL is generally considered to be the most accurate method of estimating GA, some factors that may have had an impact on CRL dating, for example maternal age [51] or haematocrit [38]. Such factors are difficult to control for in this type of population because the date of the last menstrual period is often not available. The second limitation is that the observed difference in BPD is small and within the range of error for most ultrasound machines and sonographers. The scans were obtained by locally trained technicians who were previously reported to have a mean difference of 0.43 (SD 1.21) mm in their BPD measurements in scans between 18 and 24 weeks, corresponding to 0.12 (SD 0.36) weeks [14]. The observed reduction in BPD in malaria infected pregnancies at 22 weeks is within the range of this measurement error. However the data of this retrospective analysis were derived from the entire population of women attending ANC for their routine ultrasound scans, which minimizes selection bias. In addition the sonographers were not aware whether women had malaria or not during pregnancy and therefore observer bias is unlikely. If there was no true effect of malaria infection on BPD any observed difference would likely be occulted by the expected measurement error of the examiners. In contrast, within this large population of routine ultrasound scans, malaria in the late first and/or second trimester was the largest risk factor for a smaller BPD. Thirdly, in order to examine the effect of the malaria between the scans on birth outcomes, a highly selected group of women was studied for this analysis. This small group (N = 72) had their malaria infections only between the two scans, and was malaria free in the time period where weight gain velocity is highest. Therefore no firm conclusions on the relationship between malaria in early pregnancy, its impact on BPD and birth outcome can be drawn from this analysis. Finally, other biometric parameters, such as fetal head circumference, were not available for most women.
Intermittent preventive treatment, one of the main WHO recommended strategies for malaria prevention and control during pregnancy in areas of stable malaria transmission, aims to provide two or three treatment doses after quickening (around 20 weeks GA) at least one month apart [48]. This approach fails to protect women in the gestational weeks of the highest fetal head growth velocity. Fetal growth has been postulated to be a dynamic system where pulsatile characteristics of saltatory growth events are able to change throughout pregnancy [52]–[55]. Such pulsatile growth events all the way through pregnancy stress the importance of protecting each fetus from the effects of malaria parasites starting as early as possible in pregnancy.
Supporting Information
Calculations derived from the equations based on a Chinese population.
(DOC)
Factors associated with mean BPD z-score when calculated from the Chinese equation.
(DOC)
Acknowledgments
We thank all the staff of the Shoklo Malaria Research Unit, especially Ratree Arunjerdja and the sonographers, midwives, medics, nurses, laboratory technicians (especially Lilly Kiricharoen) and home visitors for the antenatal and delivery care for pregnant women, Khun Tip Ruchaitrakul and Ladda Kajeechewa and their teams for the logistic support, and the computer team for database management. We are grateful for advice on the statistical analysis of these data kindly provided by Eric Ohuma.
Footnotes
Competing Interests: The authors have read the journal's policy and have the following conflicts: Professor Francois Nosten is an academic editor of PLoS ONE. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This research was a part of the Wellcome Trust Mahidol University Oxford Tropical Medicine Research Programme, supported by the Wellcome Trust of Great Britain (Major Overseas Programme–Thailand Unit Core Grant). A T Papageorghiou is supported by the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the Department of Health NIHR Biomedical Research Centres funding scheme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, et al. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 3.Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 2010;7:e1000221. doi: 10.1371/journal.pmed.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rijken MJ, McGready R, Boel ME, Poespoprodjo R, Singh N, et al. Malaria in pregnancy in the Asia-Pacific region. Lancet Infect Dis. 2012;12:75–88. doi: 10.1016/S1473-3099(11)70315-2. [DOI] [PubMed] [Google Scholar]
- 5.Nosten F, McGready R, Mutabingwa T. Case management of malaria in pregnancy. Lancet Infect Dis. 2007;7:118–125. doi: 10.1016/S1473-3099(07)70023-3. [DOI] [PubMed] [Google Scholar]
- 6.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 7.Nosten F, McGready R, Simpson JA, Thwai KL, Balkan S, et al. Effects of Plasmodium vivax malaria in pregnancy. Lancet. 1999;354:546–549. doi: 10.1016/s0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- 8.Brabin BJ, Romagosa C, Abdelgalil S, Menendez C, Verhoeff FH, et al. The sick placenta-the role of malaria. Placenta. 2004;25:359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho BO, Lopes SC, Nogueira PA, Orlandi PP, Bargieri DY, et al. On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis. 2010;202:638–647. doi: 10.1086/654815. [DOI] [PubMed] [Google Scholar]
- 11.Rijken M, Rijken J, Papageorghiou A, Kennedy S, Visser G, et al. Malaria in pregnancy: the difficulties in measuring birthweight. BJOG. 2011;118:671–678. doi: 10.1111/j.1471-0528.2010.02880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith GC. First trimester origins of fetal growth impairment. Semin Perinatol. 2004;28:41–50. doi: 10.1053/j.semperi.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Neilson JP. Ultrasound for fetal assessment in early pregnancy. Cochrane Database Syst Rev. 2000:CD000182. doi: 10.1002/14651858.CD000182. [DOI] [PubMed] [Google Scholar]
- 14.Rijken MJ, Lee SJ, Boel ME, Papageorghiou AT, Visser GH, et al. Obstetric ultrasound scanning by local health workers in a refugee camp on the Thai-Burmese border. Ultrasound Obstet Gynecol. 2009;34:395–403. doi: 10.1002/uog.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris RD, Marks WM. Compact ultrasound for improving maternal and perinatal care in low-resource settings: review of the potential benefits, implementation challenges, and public health issues. J Ultrasound Med. 2009;28:1067–1076. doi: 10.7863/jum.2009.28.8.1067. [DOI] [PubMed] [Google Scholar]
- 16.Hofmeyr GJ. Routine ultrasound examination in early pregnancy: is it worthwhile in low-income countries? Ultrasound Obstet Gynecol. 2009;34:367–370. doi: 10.1002/uog.7352. [DOI] [PubMed] [Google Scholar]
- 17.Carrara VI, Sirilak S, Thonglairuam J, Rojanawatsirivet C, Proux S, et al. Deployment of early diagnosis and mefloquine-artesunate treatment of falciparum malaria in Thailand: the Tak Malaria Initiative. PLoS Med. 2006;3:e183. doi: 10.1371/journal.pmed.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosten F, ter Kuile F, Maelankirri L, Decludt B, White NJ. Malaria during pregnancy in an area of unstable endemicity. Trans R Soc Trop Med Hyg. 1991;85:424–429. doi: 10.1016/0035-9203(91)90205-d. [DOI] [PubMed] [Google Scholar]
- 19.Nosten F, Rogerson SJ, Beeson JG, McGready R, Mutabingwa TK, et al. Malaria in pregnancy and the endemicity spectrum: what can we learn? Trends Parasitol. 2004;20:425–432. doi: 10.1016/j.pt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Carrara VI, Hogan C, De Pree C, Nosten F, McGready R. Improved pregnancy outcome in refugees and migrants despite low literacy on the Thai-Burmese border: results of three cross-sectional surveys. BMC Pregnancy Childbirth. 2011;11:45. doi: 10.1186/1471-2393-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luxemburger C, White NJ, ter Kuile F, Singh HM, Allier-Frachon I, et al. Beri-beri: the major cause of infant mortality in Karen refugees. Trans R Soc Trop Med Hyg. 2003;97:251–255. doi: 10.1016/s0035-9203(03)90134-9. [DOI] [PubMed] [Google Scholar]
- 22.Burmese Border Guidelines. Available: http://www.ibiblio.org/obl/docs4/BBG_2007-Eng.pdf. Accessed 07 February 2011.
- 23.SMRU Malaria Handout. Available: http://www.shoklo-unit.com/smru_meeting/SMRU_Malaria_Handout_English_version_16_Edition.pdf. Accessed 07 February 2011.
- 24.The British Medical Ultrasound Society. Available: http://www.bmus.org. Accessed 14 March 2008.
- 25.WHO. Guidelines for the treatment of malaria. Geneva: WHO; 2010. [Google Scholar]
- 26.TBBC Nutrition Programme 2007. Available: http://www.tbbc.org/resources/2007-nutrition-programme-outline.pdf. Accessed 07 February 2011.
- 27.Liabsuetrakul T. Is international or Asian criteria-based body mass index associated with maternal anaemia, low birthweight, and preterm births among Thai population? An observational study. J Health Popul Nutr. 2011;29:218–228. doi: 10.3329/jhpn.v29i3.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson HP, Fleming JE. A critical evaluation of sonar “crown-rump length” measurements. Br J Obstet Gynaecol. 1975;82:702–710. doi: 10.1111/j.1471-0528.1975.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 29.Rijken MJ. Quality of ultrasound biometry obtained by local health workers in a refugee camp on the Thai-Burmese Border. Ultrasound Obstet Gynecol in press; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung TN, Pang MW, Daljit SS, Leung TY, Poon CF, et al. Fetal biometry in ethnic Chinese: biparietal diameter, head circumference, abdominal circumference and femur length. Ultrasound Obstet Gynecol. 2008;31:321–327. doi: 10.1002/uog.5192. [DOI] [PubMed] [Google Scholar]
- 31.Salomon LJ, Bernard JP, Ville Y. Analysis of Z-score distribution for the quality control of fetal ultrasound measurements at 20–24 weeks. Ultrasound Obstet Gynecol. 2005;26:750–754. doi: 10.1002/uog.2640. [DOI] [PubMed] [Google Scholar]
- 32.Salomon LJ, Duyme M, Crequat J, Brodaty G, Talmant C, et al. French fetal biometry: reference equations and comparison with other charts. Ultrasound Obstet Gynecol. 2006;28:193–198. doi: 10.1002/uog.2733. [DOI] [PubMed] [Google Scholar]
- 33.Huynh BT, Fievet N, Gbaguidi G, Dechavanne S, Borgella S, et al. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am J Trop Med Hyg. 2011;85:214–220. doi: 10.4269/ajtmh.2011.11-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taha Tel T, Gray RH, Mohamedani AA. Malaria and low birth weight in central Sudan. Am J Epidemiol. 1993;138:318–325. doi: 10.1093/oxfordjournals.aje.a116861. [DOI] [PubMed] [Google Scholar]
- 35.Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Warikar N, et al. Adverse pregnancy outcomes in an area where multidrug-resistant plasmodium vivax and Plasmodium falciparum infections are endemic. Clin Infect Dis. 2008;46:1374–1381. doi: 10.1086/586743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umbers AJ, Aitken EH, Rogerson SJ. Malaria in pregnancy: small babies, big problem. Trends Parasitol. 2011;27:168–175. doi: 10.1016/j.pt.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Mukri F, Bourne T, Bottomley C, Schoeb C, Kirk E, et al. Evidence of early first-trimester growth restriction in pregnancies that subsequently end in miscarriage. BJOG. 2008;115:1273–1278. doi: 10.1111/j.1471-0528.2008.01833.x. [DOI] [PubMed] [Google Scholar]
- 38.Mook-Kanamori DO, Steegers EA, Eilers PH, Raat H, Hofman A, et al. Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA. 2010;303:527–534. doi: 10.1001/jama.2010.78. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen NG, Figueras F, Wojdemann KR, Tabor A, Gardosi J. Early fetal size and growth as predictors of adverse outcome. Obstet Gynecol. 2008;112:765–771. doi: 10.1097/AOG.0b013e318187d034. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen NG, Wojdemann KR, Scheike T, Tabor A. Fetal growth between the first and second trimesters and the risk of adverse pregnancy outcome. Ultrasound Obstet Gynecol. 2008;32:147–154. doi: 10.1002/uog.6109. [DOI] [PubMed] [Google Scholar]
- 41.Arbeille P, Carles G, Georgescu M, Tobal N, Herault S, et al. Consequences of reduced umbilical and increased foetal cerebral flow during malaria crisis on foetal behaviour. Parasitology. 2003;126:513–519. [PubMed] [Google Scholar]
- 42.Dorman EK, Shulman CE, Kingdom J, Bulmer JN, Mwendwa J, et al. Impaired uteroplacental blood flow in pregnancies complicated by falciparum malaria. Ultrasound Obstet Gynecol. 2002;19:165–170. doi: 10.1046/j.0960-7692.2001.00545.x. [DOI] [PubMed] [Google Scholar]
- 43.Landis SH, Lokomba V, Ananth CV, Atibu J, Ryder RW, et al. Impact of maternal malaria and under-nutrition on intrauterine growth restriction: a prospective ultrasound study in Democratic Republic of Congo. Epidemiol Infect. 2009;137:294–304. doi: 10.1017/S0950268808000915. [DOI] [PubMed] [Google Scholar]
- 44.Villar J, Belizan JM. The timing factor in the pathophysiology of the intrauterine growth retardation syndrome. Obstet Gynecol Surv. 1982;37:499–506. doi: 10.1097/00006254-198208000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Milani S, Bossi A, Bertino E, di Battista E, Coscia A, et al. Differences in size at birth are determined by differences in growth velocity during early prenatal life. Pediatr Res. 2005;57:205–210. doi: 10.1203/01.PDR.0000148452.98518.D5. [DOI] [PubMed] [Google Scholar]
- 46.Bertino E, Di Battista E, Bossi A, Pagliano M, Fabris C, et al. Fetal growth velocity: kinetic, clinical, and biological aspects. Arch Dis Child Fetal Neonatal Ed. 1996;74:F10–15. doi: 10.1136/fn.74.1.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogerson SJ, Mwapasa V, Meshnick SR. Malaria in pregnancy: linking immunity and pathogenesis to prevention. Am J Trop Med Hyg. 2007;77:14–22. [PubMed] [Google Scholar]
- 48.WHO A Strategic Framework for Malaria Control During Pregnancy in the African Region. Available: http://www.rollbackmalaria.org/toolbox/tool_MalariaPreventionInPregnancy.html. Accessed 2 September 2011.
- 49.Cottrell G, Mary JY, Barro D, Cot M. The importance of the period of malarial infection during pregnancy on birth weight in tropical Africa. Am J Trop Med Hyg. 2007;76:849–854. [PubMed] [Google Scholar]
- 50.McGready R, Lee S, Wiladphaingern J, Ashley E, Rijken M, et al. Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population-based study. 2011. Lancet Infect Dis [Epub 2011/12/12] http://www.ncbi.nlm.nih.gov/pubmed/22169409. [DOI] [PMC free article] [PubMed]
- 51.Bottomley C, Daemen A, Mukri F, Papageorghiou AT, Kirk E, et al. Assessing first trimester growth: the influence of ethnic background and maternal age. Hum Reprod. 2009;24:284–290. doi: 10.1093/humrep/den389. [DOI] [PubMed] [Google Scholar]
- 52.Lampl M, Jeanty P. Timing is everything: a reconsideration of fetal growth velocity patterns identifies the importance of individual and sex differences. Am J Hum Biol. 2003;15:667–680. doi: 10.1002/ajhb.10204. [DOI] [PubMed] [Google Scholar]
- 53.Lampl M, Johnson ML, Frongillo EA., Jr Mixed distribution analysis identifies saltation and stasis growth. Ann Hum Biol. 2001;28:403–411. doi: 10.1080/03014460010016662. [DOI] [PubMed] [Google Scholar]
- 54.Lampl M, Kusanovic JP, Erez O, Gotsch F, Espinoza J, et al. Growth perturbations in a phenotype with rapid fetal growth preceding preterm labor and term birth. Am J Hum Biol. 2009;21:782–792. doi: 10.1002/ajhb.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lampl M, Thompson AL. Growth chart curves do not describe individual growth biology. Am J Hum Biol. 2007;19:643–653. doi: 10.1002/ajhb.20707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calculations derived from the equations based on a Chinese population.
(DOC)
Factors associated with mean BPD z-score when calculated from the Chinese equation.
(DOC)