Abstract
A series of 2-(3-aryl-1-oxo-2-propenyl)-3-methylquinoxaline-1,4-dioxides 1a–l and 2-acetyl-3-methyl-quinoxaline-1,4-dioxide 2 were evaluated against Mycobacterium tuberculosis H37Rv. With the exception of the 4-nitro analog 1k, significant antitubercular potencies were observed in series 1 and 2 which have IC50 values in the range of 1–23 μM. Negative correlations were noted between the IC50 values of 1a–j, l towards M. tuberculosis and both the σ and π constants of the substituents in the benzylidene aryl ring. In particular, 1h emerged as a lead compound having IC50 and IC90 figures of 1.03 μM and 1.53 μM, respectively. This molecule affected respiration in rat liver mitochondria which is likely one way that 1h and the bioactive analogs exert their antitubercular properties. The quinoxaline 2, which lacks an α,β-unsaturated group, has no effect on mitochondrial respiration using concentrations which inhibit the growth of M. tuberculosis.
Keywords: Antitubercular, Quinoxalines, Structure–activity relationships, Drug design, Dual agents, Mitochondria
1. Introduction
Tuberculosis is currently a major health problem worldwide. Mycobacterium tuberculosis has latently infected approximately one-third of the population of the world [1] and currently this disease causes the death of two million people annually [2]. This regrettable situation is caused by a variety of influences including the following problems. First, the ability of the widely disseminated human immunodeficiency virus weakens the immune system which exacerbates the spread of tuberculosis. Second, the emergence of multidrug-resistant (MDR) strains of M. tuberculosis and more recently extremely drug-resistant (XDR) mutants poses major difficulties in treatment. Third, drug therapy is generally for at least six months [3] and the side effects of many of the current antitubercular drugs are severe. Thus new drugs with modes of action divergent from those of contemporary medication are urgently required.
A recent approach taken in this laboratory in regard to the synthesis of compounds designed to combat tuberculosis is twofold in nature. First, the new molecules contain a conjugated unsaturated keto group which interacts with cellular thiols [4] and a number of these molecules target mitochondrial function and other biochemical processes [5]. These modes of action are quite distinct from those of current antitubercular drugs such as rifampin and isoniazid which act by inhibition of DNA-dependent RNA polymerase and the biosynthesis of mycolic acids, respectively [6]. Thus conjugated enones may not be cross resistant to established antitubercular drugs. Second, the compounds are designed to have both antineoplastic and antitubercular properties. The reason for this strategy is based on the premise that individuals suffering with cancer are susceptible to tubercular infections and vice versa, i.e., tuberculosis patients are prone to the development of neoplastic diseases. A review of the literature afforded ample support for the importance of developing dual agents possessing both antitubercular and antineoplastic properties. Thus the risk of tuberculosis developing in cancer patients has been described [7,8], while concern has been expressed for the appearance of non-Hodgkin’s lymphoma developing in tuberculosis patients [9].
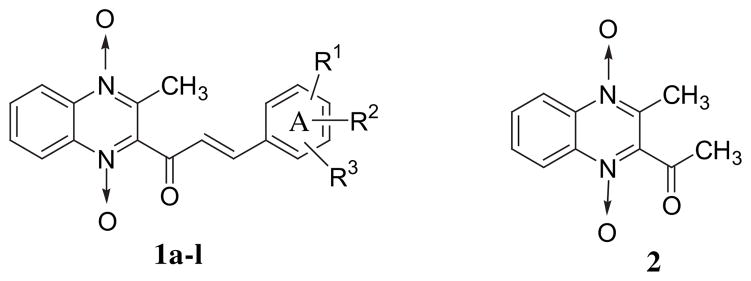
The specific compounds 1 (Fig. 1) were designed for the following reasons. A 3-aryl-1-oxo-2-propenyl (ARCH=CHCO) group was considered which will enable thiol alkylation to take place at the 3-alkenyl carbon atom of this group. The placement of different substituents in the benzylidene aryl ring (subsequently referred to as ring A) leads to variations of the electronic, hydrophobic and steric properties of the molecule which may influence the magnitude of the bioactivity. In addition, this group is present in a number of antitubercular [10,11] and antineoplastic [12,13] agents. In order to enhance antitubercular properties, the 3-aryl-1-oxo-2-propenyl group was attached to the 3-methylquinoxaline-1,1-dioxide moiety, which has been incorporated into a number of antimycobacterials [14]. In addition, extensive studies by the Monge group have revealed the antitubercular properties of a variety of quinoxaline-1,4-dioxides [15–24].
Fig. 1.
The structures of the compounds in series 1 and 2.
The objective therefore of the current study is to (1) ascertain whether the compounds in series 1 display antitubercular properties; (2) develop structure–activity relationships for further designing of potent compounds; (3) gain some insight into the mode of action by which antitubercular properties are mediated. This investigation assumes some importance in view of our recent disclosure that most of the compounds in series 1 have significant cytotoxic potencies towards a wide range of human tumour cell lines [25] which has been reported earlier. Consequently the potential for the future development of series 1 will be enhanced greatly should antitubercular properties be displayed by these compounds.
2. Results
The compounds in series 1 were prepared by the reaction of benzofuran-1-oxide with acetonylacetone in the presence of base to produce 2-acetyl-3-methylquinoxaline-1,4-dioxide 2. Condensation of 2 with various aryl aldehydes under basic conditions afforded the desired unsaturated ketones 1a–l. The antitubercular activity of 1 was compared with 2 which lacks a 3-aryl-1-oxo-2-propenyl group in order to reveal the contribution of this substituent to antitubercular properties. All of the compounds in series 1 and 2 were examined against M. tuberculosis H37Rv. The concentrations required to inhibit 50% and 90% of the growth of this microorganism are presented in Table 1. The effect of a promising lead compound 1h on respiration of rat liver mitochondria is presented in Fig. 2.
Table 1.
Evaluation of 1a–l and 2 against M. tuberculosis H37Rva.
| Compound | Substituents in 1
|
IC50 (μM) | IC90 (μM) | log Pb |
|---|---|---|---|---|
| R1, R2, R3 | ||||
| 1a | H | 3.88 | 6.12 | 1.44 |
| 1b | 4-OCH3 | 23.1 | 40.2 | 1.39 |
| 1c | 3,4-(OCH3)2 | 9.15 | 17.2 | 1.27 |
| 1d | 3,4,5-(OCH3)3 | 6.88 | 11.9 | 1.03 |
| 1e | 3,4-OCH2O | 19.7 | 35.8 | 1.57 |
| 1f | 4-CH3 | 5.58 | 9.44 | 1.91 |
| 1g | 4-Cl | 2.64 | 4.12 | 1.97 |
| 1h | 3,4-Cl2 | 1.03 | 1.53 | 2.45 |
| 1i | 4-Br | 3.71 | >250 | 2.34 |
| 1j | 4-F | 5.83 | 10.7 | 1.45 |
| 1k | 4-NO2 | >250 | >250 | 1.22 |
| 1l | 3-NO2 | 2.18 | 2.39 | 1.35 |
| 2 | – | 6.83 | 7.89 | −0.90 |
Fig. 2.
The effect of 1h and 2 on respiration in rat liver mitochondria.
3. Discussion
The biodata presented in Table 1 reveal that, with the exception of 1k, the compounds in series 1 and 2 display significant antitubercular properties. The unsubstituted compound 1a displays good antitubercular potency. The placement of a 4-methoxy group into ring A led to 1b and a six fold reduction in potency. However the insertion of one or two additional methoxy substituents in the meta location of ring A gave rise to 1c and 1d, respectively, with a stepwise reduction in IC50 values. Hence the importance of meta substitution is suggested. The insertion of a 4-chloro atom into ring A (1g) lowered the IC50 figure relative to 1a while the addition of a second chloro atom to the meta location (1h) led to the compound with the lowest IC50 figure in series 1. The chloro atom in the para position (1g) seems preferable to bromo (1i) and fluoro (1j) substituents. The importance of a group in the meta position was demonstrated further by the huge differential between the IC50 values of 1k and 1l. These generalizations between the nature of the substituents in ring A of series 1 and IC50 values were also noted by an examination of the IC90 data presented in Table 1. In the future, other structural isomers of 1g and 1h along with analogs with multiple chloro atoms in ring A should be synthesized and evaluated for antitubercular properties in attempts to find compounds with increased potencies.
The lack of activity of 1k is a curious phenomenon. There is a 4-nitro group present in 1k which presumably has a major impact on the lack of bioactivity of this compound. This substituent is the most electron-attracting and, along with 1l, has the most hydrophilic group in ring A, the σ and π values of the 4-nitro group being 0.78 and −0.28, respectively [26]. However, the corresponding figures for the 3-nitro group present in 1l are 0.71 and −0.28, respectively [26], which are unlikely to explain the huge differences in potencies between 1k and 1l. In regard to the size of the 4-nitro substituent, the molecular refractivity (MR) figure is 8.39 which is larger than the groups in ring A in 1a,f,g,j and smaller than the substituents in 1b–e,h,i and the same as is present in 1l. Hence the lack of bioactivity of 1k does not appear due to any unique electronic, hydrophobic or steric properties of the 4-nitro group. One may also note that the potency of 1k towards six human neoplastic and transformed cell lines was invariably much lower than was displayed by 1a–j,l [25]. For example, while the average IC50 values of 1a–j,l towards human Molt 4/C8 and CEM T-lymphocytes are 13.4 and 16.1 μM, respectively, the corresponding figures for 1k are 261 and 164 μM, respectively [25]. Thus in regard to the bioassays undertaken to date, the insertion of a nitro group in the 4 position of ring A in series 1 has a deleterious effect on both antitubercular and antineoplastic properties.
An investigation was made to determine if the magnitude of antitubercular potency was correlated with one or more of the physicochemical constants of the substituents in ring A of 1a–j,l. Consequently linear and semilogarithmic plots were constructed between the IC50 values of 1a–j,l and the σ, π and MR figures of the aryl groups in ring A. Semilogarithmic plots revealed negative correlations between the IC50 figures and both the σ values (p < 0.01) and the π constants (p < 0.05). No correlations were noted with the MR values (p > 0.05). Hence antitubercular potency is favoured by the presence of electron-attracting and hydrophobic aryl substituents. Thus in developing this cluster of novel anti-mycobacterials, consideration should be given to creating molecules with higher σ and π values such as inserting three or more chloro or bromo atoms into ring A. In addition, linear, semi-logarithmic and logarithmic plots were made between the IC50 values of 1a–j,l and the log P figures (which are presented in Table 1). A negative trend towards significance (p < 0.1) was noted in the case of the semilogarithmic plots. Thus in developing these compounds in the future, the hydrophobicity of the molecules should be increased.
In a further attempt to discern QSAR, multilinear regression (MLR) analyses were undertaken using a data set of 12 compounds in series 1 and three physicochemical descriptors (σ, π, and log P). When the ring A is di- or tri-substituted, the sum of individual parameters (Σσm,p, Σπ) was used. The results indicate that a statistically significant correlation exists between the antitubercular activities of the compounds in series 1 and the three physico-chemical descriptors (Eq. (1)).
| (1) |
In this equation, n represents the number of data points, r is the correlation coefficient, radj is the adjusted r, s is the standard deviation of the regression equation, and the F value is related to the F-statistic analysis (Fisher test).
The data in Table 1 reveal that 3-methylquinoxaline-1,4-dioxide 2 has moderate antitubercular potency. A comparison was made between the potencies of 1a–j,l and 2 in order to determine the contribution of the 3-aryl-1-oxo-2-propenyl group to antitubercular properties. When the IC50 data are considered, 1a,f–j,l have lower IC50 values than 2 while 1b,c,e are less potent and the figures for 1d and 2 are the same. The average σ, π and MR values of the aryl substituents in ring A of 1a,f–j,l are 0.24, 0.49 and 7.90, respectively, while for 1b,c,e, the relevant data are −0.25, −0.04 and 12.23, respectively. Thus the placement of electron-withdrawing, hydrophobic and relatively small substituents in ring A of series 1 leads to compounds with greater potencies than 2. The IC90 figures reveal a similar trend since 1a,g,h,l have lower IC90 values than 2 (the average σ, π and MR figures are 0.39, 0.46 and 8.42, respectively) while the other compounds in series 1 are less potent than 2 (the average σ, π and MR values for 1b–f,i,j are −0.09, 0.20 and 11.70, respectively). Hence in considering both the IC50 and IC90 figures, approximately half of the compounds in series 1 are more potent than 2 which may be attributed to a large extent to the incorporation of small, hydrophobic, electron-withdrawing groups into ring A of series 1.
The IC50 values are less than 5 μM in the case of 1a,g–i,l. With the exception of the 4-bromo derivative (1i), the IC90 figures of 1a,g,h,l are very encouraging and are clearly lead molecules. However an important feature in considering future development is whether these compounds have drug likeness properties [27,28]. Various physicochemical parameters which are considered of importance in conferring these characteristics are indicated in Table 2 and the data for the active compounds 1a,g,h,l reveal that there are no violations. This observation greatly enhances their potential as candidate antitubercular agents and as leads for further development.
Table 2.
Evaluation of drug likeness properties.
| Compounds | MW <500 | C log P <5 | Number of O and N <10 | Number of OH and NH <5 | N rotatable bonds <10 | N-violations ≯1 |
|---|---|---|---|---|---|---|
| 1a | 306.32 | 1.44 | 5 | 0 | 3 | 0 |
| 1g | 340.76 | 1.97 | 5 | 0 | 3 | 0 |
| 1h | 375.20 | 2.45 | 5 | 0 | 3 | 0 |
| 1l | 351.31 | 1.35 | 8 | 0 | 4 | 0 |
The question arises as to whether correlations exist between the potencies of 1a–j,l in the antitubercular bioassays and the IC50 values in the cytotoxic screens which have been reported earlier [25]. Consequently linear and semilogarithmic plots were made between the IC50 values of 1a–j,l towards M. tuberculosis and the IC50 figures of these compounds towards human Molt 4/C8, CEM, HL-60, HSC-2, HSC-3 and HSC-4 cells. Linear plots established positive correlations between the IC50 values of 1a–j,l in the antitubercular screen and the IC50 figures towards Molt 4/C8 (p < 0.01) and CEM (p < 0.05) T-lymphocytes. No correlations (p > 0.05) were observed with HL-60 promyelocytic leukemic cells nor HSC-2, HSC-3 and HSC-4 squamous cell carcinomas. Thus in the case of the Molt 4/C8 and CEM T-lymphocytes, the trends in potency towards these cells and M. tuberculosis are the same and in these cases the compounds may be said to be acting as dual agents. However, the behaviour of 1a–j,l towards HL-60, HSC-2, HSC-3 and HSC-4 cells reveals that binary actions occur with specific cell lines only. Nevertheless the correlations that are established afford some encouragement for attempting to develop compounds which are toxic to both cancers and the tuberculosis bacillus.
Finally, an attempt was made to ascertain whether the compounds in series 1 and 2 affect respiration in mitochondria. If so, then this toxic property being different from the principal ways in which current antimycobacterials exert their actions could enable the compounds in series 1 and 2 to be of value in treating drug-resistant mutants of M. tuberculosis. Consequently the compound with the lowest IC50 and IC90 values, namely 1h, as well as 2 having less than one-fifth of the potency of 1h, were examined in isolated rat liver mitochondria. Using concentrations of 1, 5 and 10 μM of 1h, respiration was stimulated in a concentration-dependent manner as indicated in Fig. 2. Two mechanisms whereby a compound can stimulate respiration are by acting as an uncoupler [29] or by inducing the mitochondrial permeability transition [30]. The former case produces cytotoxicity by impairing ATP production while the latter triggers apoptosis. The enone 1h lacks an acidic group which is considered to be required for uncoupling properties [29]. Hence it is likely that this compound induces a mitochondrial permeability transition. On the other hand, the same concentrations of 2 had no effect on respiration. This result suggests that a contributing factor to the noteworthy antitubercular properties of 1h is by interference with mitochondrial respiration and it may also explain, at least in part, its greater potency than 2. Furthermore, while additional experimentation is necessary, the possibility exists that a 3-aryl-2-propenoyl group is required for an effect on mitochondrial respiration to be demonstrated.
4. Conclusions
The importance of finding novel compounds which are structurally divergent from contemporary antitubercular drugs is axiomatic. This study revealed that in general a series of 2-(3-aryl-1-oxo-2-propenyl)-3-methylquinoxaline-1,4-dioxides 1 display excellent potencies towards M. tuberculosis. In particular, 1h emerged as a potent lead compound for further studies. Quantitative structure–activity relationships revealed the importance of the electronic and hydrophobic properties of the substituents in ring A which afford guidelines for expanding the project. The observation that some correlations were noted between antitubercular and cytotoxic potencies afford some hope that dual action chemotherapeutics may evolve from this study. The lead compound 1h stimulated respiration in rat liver mitochondria which may indicate that the compounds in series 1 exert their action in a different way from available antimycobacterials thus revealing the possibility of their use against drug-resistant bacteria.
5. Experimental
5.1. Synthesis of compounds
The preparation of 1a–l and 2 has been described previously [25].
5.2. Statistical analyses
The σ, π and MR values of the aryl substituents in ring A of the compounds in series 1 were culled from the literature [26] except that the σ constant for the 3,4-methylenedioxy group was obtained from a different reference [31]. The MR value of the hydrogen atom is 1.03 not 0.00. Hence in computing the steric effect of the aryl substituents, the figures of 1.03 and 2.06 were added to the disubstituted and monosubstituted compounds, respectively. The MR value of the unsubstituted analog 1a is 3.09. The linear and semilogarithmic plots were made using a commercial software package [32]. The Pearson’s coefficients obtained from the semi-logarithmic plots between the IC50 values of 1a–j,l against M. tuberculosis and the σ and π figures of the aryl substituents are 0.892 and 0.609, respectively. The Pearson’s coefficients generated when linear plots were constructed between the IC50 values of 1a–j,l against M. tuberculosis and the IC50 figures against Molt 4/C8 and CEM cells are 0.758 and 0.704, respectively.
5.3. Antitubercular assay
The compounds were evaluated against M. tuberculosis H37Rv (ATCC 27294) in BACTEC 12B medium using the broth micro-dilution Microplate Alamar Blue Assay [33].
5.4. Evaluation of 1h and 2 on respiration of rat liver mitochondria
A rat was anesthetized with isoflurane and decapitated. The mitochondria were isolated from the liver using a literature methodology [34]. The consumption of oxygen by mitochondria was determined by polarography using a previously reported procedure [35]. Compounds 1h and 2 were dissolved in dime-thylsulfoxide (4 μL) and the solvent control (dimethylsulfoxide, 4 μL) caused a stimulation of respiration of 8.22 ± 0.74%.
Acknowledgments
The authors thank the Canadian Institutes of Health Research for an operating grant to J. R. Dimmock. The antitubercular data were provided by the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) through a research and development contract with the U.S. National Institute of Allergy and Infectious Diseases. Erin Watson is thanked for undertaking some of the literature retrieval.
References
- 1.Nayyar A, Jain R. Curr Med Chem. 2005;12:1873–1886. doi: 10.2174/0929867054546654. [DOI] [PubMed] [Google Scholar]
- 2.Dye C, Sheele S, Dolin P, Pathania V, Raviglione MC. J Am Med Assoc. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 3.Bass JB, Jr, Farer LS, Hopewell PC, O’Brien R, Jacobs RF, Ruben F, Snider DE, Jr, Thornton G. Am J Respir Crit Care Med. 1994;149:1359–1374. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 4.Pati HN, Das U, Sharma RK, Dimmock JR. Mini Rev Med Chem. 2007;7:131–139. doi: 10.2174/138955707779802642. [DOI] [PubMed] [Google Scholar]
- 5.Das U, Sharma RK, Dimmock JR. Curr Med Chem. 2009;16:2001–2020. doi: 10.2174/092986709788682218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin AR. Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry. 10. Lipincott-Raven; Philadelphia: 1998. p. 208.p. 205. [Google Scholar]
- 7.Barnadas MA, Baselga E, Curell R, Margall N, de Moragas JM. Int J Dermatol. 1996;35:221–222. doi: 10.1111/j.1365-4362.1996.tb01648.x. [DOI] [PubMed] [Google Scholar]
- 8.Kamboj M, Sepkowitz RA. Clin Infect Dis. 2006;42:1592–1595. doi: 10.1086/503917. [DOI] [PubMed] [Google Scholar]
- 9.Askling J, Ekborm A. Br J Cancer. 2001;84:113–115. doi: 10.1054/bjoc.2000.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh N, Pandey J, Yadav A, Chaturvedi V, Bhatnagar S, Gaikwad AN, Sinha SK, Kumar A, Shukla PK, Tripathi RP. Eur J Med Chem. 2009;44:1705–1709. doi: 10.1016/j.ejmech.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Lin YM, Zhou Y, Flavin MT, Zhou LM, Nie W, Chen FC. Bioorg Med Chem. 2002;10:2795–2802. doi: 10.1016/s0968-0896(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 12.Das U, Doroudi A, Das S, Bandy B, Balzarini J, De Clercq E, Dimmock JR. Bioorg Med Chem. 2008;16:6261–6268. doi: 10.1016/j.bmc.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis R, Das U, Mackay H, Brown T, Mooberry SL, Dimmock JR, Lee M, Pati H. Arch Pharm Chem Life Sci. 2008;341:440–445. doi: 10.1002/ardp.200800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carta A, Paglietti G, Nikookar MER, Sarina P, Sechi L, Zanetti S. Eur J Med Chem. 2002;37:355–366. doi: 10.1016/s0223-5234(02)01346-6. [DOI] [PubMed] [Google Scholar]
- 15.Jaso A, Zarranz B, Aldana I, Monge A. Eur J Med Chem. 2003;38:791–800. doi: 10.1016/s0223-5234(03)00137-5. [DOI] [PubMed] [Google Scholar]
- 16.Ortega MA, Sainz Y, Montoya ME, de A, Lopez C, Monge A. Pharmazie. 1999;54:24–25. [PubMed] [Google Scholar]
- 17.Sainz Y, Montoya ME, Martinez-Crespo FJ, Ortega MA, de A, Lopez C, Monge A. Arzneim-Forsch. 1999;49:55–59. doi: 10.1055/s-0031-1300359. [DOI] [PubMed] [Google Scholar]
- 18.Ortega MA, Montoya ME, Jaso A, Zarranz B, Tirapu I, Aldana I, Monge A. Pharmazie. 2001;56:205–207. [PubMed] [Google Scholar]
- 19.Ortega MA, Sainz Y, Montoya ME, Jaso A, Zarranz B, Aldana I, Monge A. Arzenim-Forsch. 2002;52:113–119. doi: 10.1055/s-0031-1299866. [DOI] [PubMed] [Google Scholar]
- 20.Zarranz B, Jaso A, Aldana I, Monge A. Bioorg Med Chem. 2003;11:2149–2156. doi: 10.1016/s0968-0896(03)00119-6. [DOI] [PubMed] [Google Scholar]
- 21.Villar R, Vicente E, Solano B, Perez-Silanes S, Aldana I, Maddry JA, Lenaerts AJ, Franzblau SG, Cho SH, Monge A, Goldman RC. J Antimicrob Chemother. 2008;62:547–554. doi: 10.1093/jac/dkn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vicente E, Villar R, Burguete A, Solano B, Perez-Silanes S, Aldana I, Maddry JA, Lenaerts AJ, Franzblau SG, Cho SH, Monge A, Goldman RC. Antimicrob Agents Chemother. 2008;52:3321–3326. doi: 10.1128/AAC.00379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicente E, Pérez-Silanes S, Lima LM, Ancizu S, Burguete A, Solano B, Villar R, Aldano I, Monge A. Bioorg Med Chem. 2009;17:385–389. doi: 10.1016/j.bmc.2008.10.086. [DOI] [PubMed] [Google Scholar]
- 24.Ancizu S, Moreno E, Solano B, Villar R, Burguete A, Torres E, Perez-Silanes S, Aldana I, Monge A. Bioorg Med Chem. 2010;18:2713–2719. doi: 10.1016/j.bmc.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Das U, Pati HN, Panda AK, De Clercq E, Balzarini J, Molnár J, Baráth Z, Ocsovszki I, Kawase M, Zhou L, Sakagami H, Dimmock JR. Bioorg Med Chem. 2009;17:3909–3915. doi: 10.1016/j.bmc.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansch C, Leo AJ. Substituent Constants for Correlation Analysis in Chemistry and Biology. John Wiley and Sons; New York: 1979. p. 49. [Google Scholar]
- 27.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv Drug Deliv Rev. 1979;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 28.Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. J Med Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 29.Terada H. Environ Health Perspect. 1990;87:213–218. doi: 10.1289/ehp.9087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green DR, Kroemer G. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 31.Perrin DD, Dempsey B, Serjeant EP. pKa Prediction for Organic Acids and Bases. Chapman and Hall; London: 1981. p. 112. [Google Scholar]
- 32.Statistical Package for Social Sciences, SPSS Statistics 17.0 Release 17.0.1. SPSS Inc; Dec 1, 2008. [Google Scholar]
- 33.Collins L, Franzblau SG. Antimicrob Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowaltowski AJ, Castilho RF, Grijalba MT, Bechara EJ, Vercesi AE. J Biol Chem. 1996;271:2929–2934. doi: 10.1074/jbc.271.6.2929. [DOI] [PubMed] [Google Scholar]
- 35.Estabrook RW. Methods Ezymol X. 1967:41–47. [Google Scholar]
- 36.ACD/ChemSketch Freeware Version. ACD/Labs Release 12.0, Product Version 12.01. 2009 Feb 10; http://www.acdlabs.com.