Abstract
A number of N-4-(2-aminoethoxy)phenylcarbonyl derivatives of various 3,5-bis(benzylidene)-4-piperidones 2–5 demonstrated noteworthy cytotoxic potencies towards human HL-60 leukemic cells as well as human HSC-2 and HSC-4 squamous cell carcinomas. In general, toxicity towards HGF, HPC, and HPLF normal cells was substantially lower. The highest selective toxicity was noted when the terminal base is morpholine. Lead optimization was based on finding compounds which had (i) high cytotoxic potencies, (ii) a greater toxicity to neoplasms than normal cells, and (iii) drug-likeness based on the rule of five. From the biodata generated, 5a evolved as a promising lead compound for further development. The mode of action of 5a included the induction of apoptosis in HL-60 cells in which internucleosomal DNA fragmentation and activation of caspase-3 was noted. In addition, 5a caused autophagy in HSC-2 cells.
Keywords: Conjugated unsaturated ketones, Cytotoxicity, Selectivity toxicity, Structure–activity relationships, Apoptosis
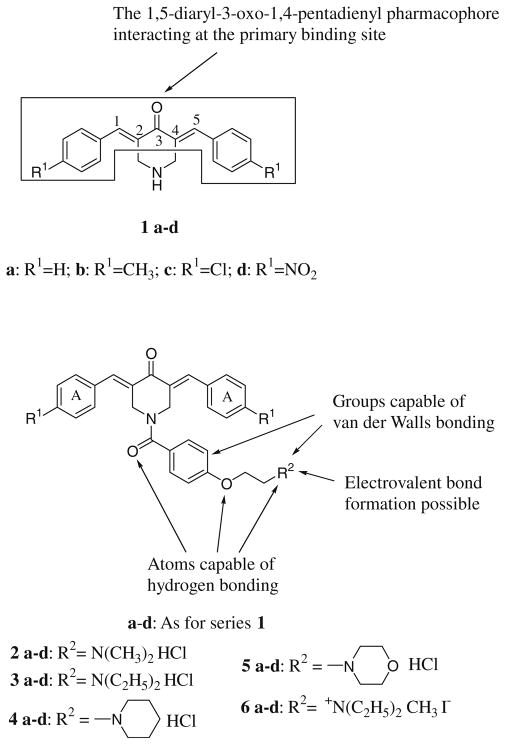
The major interests in these laboratories are the design, syntheses, and bioevaluations of candidate anticancer agents. These compounds generally possess the 1,5-diaryl-3-oxo-1,4-pentadienyl pharmacophore into their structures for the following reasons. First, compounds containing one or more α,β-unsaturated keto groups react preferentially or exclusively with thiols in contrast to amino and hydroxy groups.1,2 Hence reactions with nucleic acids may not occur and these molecules should be devoid of the genotoxic effects associated with a number of anticancer drugs which are used today.3 Second, the presence of two thiol-alkylating groups in such molecules (namely the olefinic carbon atoms) enables sequential attacks of cellular thiols to occur which may be more detrimental to neoplasms than normal cells.4 This phenomenon occurs when an initial chemical interaction in malignant cells creates greater chemosensitivity to a subsequent chemical insult in tumors rather than with normal cells.5,6 Such considerations led to the decision to prepare series 1 which possess promising cytotoxic properties.7–9 The 1,5-diaryl-3-oxo-1,4-pentadienyl group in these molecules is considered to align with a complementary area on a receptor as indicated in Figure 1.
Figure 1.
Structures of the compounds in series 1–6.
N-Acylation of 1a–d leading to series 2–6 was undertaken in order to allow additional types of bonding to occur between the ligands and biological macromolecules as indicated in Figure 1 with the aim of creating more potent cytotoxins. A preliminary evaluation of 2–6 employed human Molt 4/C8 and CEM T-lymphocytes as well as murine L1210 leukemic cells.10 Approximately half of the N-aroyl derivatives 2–5 have increased potencies compared to the analogs in series 1 which have the same aryl substituent while about one-third are equipotent. In general the quaternary ammonium compounds 6 are appreciably weaker in potencies than the compounds in series 1–5.
The objectives of the present study are threefold. First, the identification of a lead molecule for pharmacokinetic and pharmacodynamic studies based on the following criteria, namely high cytotoxic potencies towards neoplasms and substantially lower toxic effects to normal cells. Second, the development of structure– activity relationships (SAR) in terms of antineoplastic properties and selective toxicity was considered important for future expansion of this class of compounds as well as considering those compounds which possess drug-like properties.11 Third, to undertake mode of action studies in order to discern the way in which cytotoxicity is mediated.
In order to achieve the first objective, the compounds in series 2–6 were evaluated against human HL-60 promyelocytic leukemic cells as well as HSC-2 and HSC-4 oral squamous cell carcinomas.12 The data presented in Table 1 reveal that in general the compounds in series 2–5 have promising cytotoxic potencies. On the other hand, the compounds 6a–d possess high CC50 values (and also poor selectivity for neoplasms) and hence further discussion devolves solely on series 2–5.
Table 1.
Evaluation of the compounds in series 2–6 against normal and malignant cells
| Compound | Normal cells, CC50a (μM)
|
Tumor cells, CC50a (μM)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HGF | HPC | HPLF | Aveb | HL-60 | SIc | HSC-2 | SIc | HSC-4 | SIc | |
| 2a | 3.6 | 13 | 8.9 | 8.5 | 1.0 | 8.5 | 3.8 | 2.2 | 2.1 | 4.1 |
| 2b | 7.9 | 7.2 | 3.4 | 6.2 | 0.85 | 7.3 | 1.4 | 4.4 | 2.1 | 3.0 |
| 2c | 23 | 11 | 4.3 | 12.8 | 1.7 | 7.5 | 2.3 | 5.6 | 1.8 | 7.1 |
| 2d | 7.0 | 5.2 | 5.9 | 6.0 | 0.31 | 19 | 0.93 | 6.5 | 0.71 | 8.5 |
| 3a | 4.2 | 12 | 6.0 | 7.4 | 0.61 | 12 | 3.1 | 2.4 | 2.0 | 3.7 |
| 3b | 3.6 | 9.3 | 4.6 | 5.8 | 0.79 | 7.3 | 2.2 | 2.6 | 2.3 | 2.5 |
| 3c | 230 | >400 | >400 | >343 | 46 | >7.5 | 160 | >2.1 | 34 | >10 |
| 3d | 5.0 | 4.5 | 5.9 | 5.1 | 0.26 | 20 | 0.98 | 5.2 | 1.1 | 4.6 |
| 4a | 4.6 | 12 | 7.4 | 8.0 | 1.4 | 5.7 | 3.0 | 2.7 | 2.5 | 3.2 |
| 4b | 6.0 | 15 | 5.5 | 8.8 | 2.3 | 3.8 | 2.9 | 3.0 | 4.1 | 2.2 |
| 4c | >400 | >400 | 340 | >380 | 63 | >6.0 | 110 | >3.5 | 48 | >7.9 |
| 4d | 8.0 | 3.9 | 4.9 | 5.6 | 0.29 | 19 | 0.86 | 6.5 | 0.90 | 6.2 |
| 5a | 83 | 95 | 30 | 69 | 1.2 | 58 | 2.4 | 29 | 2.4 | 29 |
| 5b | 10 | 49 | 12 | 24 | 1.2 | 20 | 1.6 | 15 | 2.0 | 12 |
| 5c | 400 | >400 | 360 | >387 | 36 | >11 | 71 | >5.5 | 18 | >22 |
| 5d | 12 | 8.2 | 9.3 | 9.8 | 0.35 | 28 | 0.76 | 13 | 0.74 | 13 |
| 6a | 160 | 170 | 79 | 136 | 82 | 1.7 | 130 | 1.1 | 82 | 1.7 |
| 6b | 34 | 29 | 8.3 | 24 | 16 | 1.5 | 17 | 1.4 | 17 | 1.4 |
| 6c | >400 | >400 | 9.4 | >270 | 64 | >4.2 | >400 | ~0.7 | 220 | >1.2 |
| 6d | 93 | 130 | 13 | 79 | 29 | 2.7 | 67 | 1.2 | 61 | 1.3 |
| Melphaland | >200 | >200 | >200 | >200 | 6.0 | >33 | 35 | >5.7 | 81 | >2.5 |
The CC50 values are the concentrations of the compounds which kill 50% of the cells and are the average of two independent determinations.
These figures are the average CC50 values of the HGF, HPC, and HPLF normal cells.
The letters SI refer to the selectivity index which was computed by dividing the average CC50 values of the normal cells by the CC50 figure for either the HL-60, HSC-2, or HSC-4 neoplasms.
The data for melphalan is reported previously.25 Copyright (2008) with permission of Elsevier.
The data in Table 1 reveal that 81% of the CC50 values of the compounds in series 2–5 towards HL-60, HSC-2, and HSC-4 cells are less than 5 μM while 29% of these figures are submicromolar. In general these compounds are substantially more potent than a reference anticancer drug melphalan whose average CC50 figure is 41 μM. In order to identify potent cytotoxins, the average CC50 value for each compound towards HL-60, HSC-2, and HSC-4 cells was computed and the results are presented in Figure 2. Clearly many of the compounds in series 2–5 are promising clusters of molecules.
Figure 2.
The average selectivity index (SI) figures of the compounds in series 2–5 (as the hydrochloride salts) are presented along with the average CC50 values in parentheses. The R1 and R2 substituents refer to the groups indicated in Figure 1.
In order to detect those compounds which display preferential cytotoxicity to neoplasms compared to normal cells, all of the compounds were evaluated using three normal human cell lines viz HGF gingival fibroblasts, HPC pulp cells, and HPLF periodontal ligament fibroblasts.12 These results are presented in Table 1. Under clinical conditions, a tumor will be surrounded by different types of normal cells. Hence selectivity index (SI) figures were generated which are the quotients of the average CC50 value of each compound towards HGF, HPC, and HPLF cells and the CC50 figure against a specific malignant cell line. The SI figures are indicated in Table 1. All SI figures for the compounds in series 2–5 are over 1. A SI value of greater than 5 was arbitrarily chosen as evidence of substantial selective toxicity for malignant cells and was noted in two thirds of the SI figures generated. These observations indicate clearly the importance of these molecules as tumor-selective cytotoxins.
With the view of guiding the development of this project, the contributions of the substituents in rings A and the nature of the terminal basic group to selective toxicity were assessed. The average SI values for each compound were obtained and are summarized in Figure 2. The 4-nitro analogs have the highest SI values in series 2–5 with the exception of 5a which displays the greatest median tumor-selectivity among all of the compounds. The results portrayed in Figure 2 reveal that irrespective of the substituent in rings A, the optimal basic center is a 4-morpholinyl group which is present in series 5.
The second phase of the study was to develop SAR. Initially an attempt was made to discern the contributions to cytotoxic potencies of both the nature of the substituents in the arylidene aryl rings and the terminal basic groups. Thus the average CC50 figure for each compound towards HL-60, HSC-2, and HSC-4 cells was computed and the results are presented in Figure 2. The data reveal that the maximum potencies are obtained with the compounds containing a 4-nitro group which are approximately three times more potent than the unsubstituted and 4-methyl analogs. However with the exception of 2c, the other 4-piperidones containing a 4-chloro substituent in rings A, namely 3c, 4c, and 5c, are substantially weaker in potencies. In the absence of the biodata for the 4-chloro analogs, the figures in parentheses in Figure 2 indicate that the nature of the basic group in 2–5 has minimal effects on cytotoxicity. Thus in future, analogs should be prepared in which one or more nitro groups are placed in different locations in rings A as well as introducing other strongly electron-withdrawing groups such as the trifluoromethyl moiety. In addition, the terminal basic group should be excised in order to gauge its importance in regard to cytotoxic potencies. Halogens in rings A (as well as terminal quaternary ammonium groups) should be avoided in the design of further analogs.
In a further attempt to discern correlations between the nature of the aryl substituents and cytotoxic potencies, the following QSAR analysis was undertaken. Linear and semilogarithmic plots13 were made between the Hammett sigma (σ), Hansch pi (π), and molar refractivity (MR) values of the substituents in rings A14 in series 2 with the CC50 figures in each of the HL-60, HSC-2, and HSC-4 bioassays. This procedure was repeated with series 3–5. A negative correlation between the CC50 data of 2a–d in the HSC-4 screen and the σ values was noted (p <0.05). Thus potency rises as the magnitude of the σ figures increases. A negative trend to significance (p <0.1) was found between the MR values and the CC50 figures of 2a–d in the HSC-2 screen. No other correlations or trends to significance were observed among the series 2–5, that is, p >0.1.
A QSAR analysis was also undertaken in regard to the SI values. Linear and semilogarithmic plots were made between the SI values generated in the HL-60, HSC-2, and HSC-4 screens by 2a–d and the σ, π, and MR constants of the substituents in the arylidene aryl rings. The procedure was repeated for 3a–d, 4a–d, and 5a–d. The following positive correlations were noted among series 2–5 (p <0.05). For 2a–d, the SI values and MR constants are correlated in the HSC-2 test and trends towards significance (p <0.1) were noted in regard to the σ values in both the HL-60 and HSC-4 screens. Positive correlations between the SI figures of 4a–d and the σ constants were found in both the HL-60 and HSC-2 screens. The p values are greater than 0.1 in all of the other evaluations. Thus in general terms of enhancing selectivity for malignant cells, large electron-withdrawing groups should be placed in the arylidene aryl rings A.
The remarkable effect of the 4-morpholinyl group in clearly enhancing selectivity but not cytotoxic potencies is intriguing. An investigation was made to determine if basicity was correlated with cytotoxic potencies and/or the SI data. Consequently linear and semilogarithmic plots were made between the pKa values of dimethylamine, diethylamine, piperidine and morpholine15 and first, the CC50 figures of 2a, 3a, 4a, and 5a in each cell line and subsequently with the SI values. The process was repeated for the compounds in series 3–5 which have the same aryl substituents. No correlation or trends towards significance were noted between the pKa values and the CC50 figures. However negative correlations (p <0.05) were observed in every case with the SI values using HL-60, HSC-2 and HSC-4 cells except for 2c, 3c, 4c, and 5c in the HSC-2 assay and 2d, 3d, 4d, and 5d with HSC-4 cells (although in this case p <0.1). Hence selectivity is greatly influenced by the pKa of the terminal basic group and this correlation is of huge importance in the quest for developing further tumor-specific cytotoxins.
In order to identify those compounds which can serve as prototypic molecules guiding further development of series 2–5, the PL10 concept was applied. This criterion refers to Promising Leads which have average SI values of 10 or more and mean CC50 figures of 10 μM or less towards the malignant cell lines. Evaluation of the biodata in Figure 2 reveals that 2d, 4d and 5a, b, d achieved PL10 status. With the aim of identifying which of the five compounds should be examined in greater detail, the drug-like properties of 2d, 4d, 5a, 5b, and 5d were considered based on the rule of five11 as well as two other important molecular descriptors, namely the number of rotatable bonds and polar surface areas.11,16,17 The appropriate data are presented in Table 2 which indicates that 5a has the most favorable physicochemical properties. In addition, 5a has the highest SI values which afford further evidence of identifying this molecule as the lead compound emerging from this study.
Table 2.
Evaluation of candidate lead molecules
| Compound | Average SI value | Average CC50 value | Molecular weight | Clog P | Hydrogen bond
|
Rotatable bonds | PSAa (Å2) | Violations | |
|---|---|---|---|---|---|---|---|---|---|
| Donors | Acceptors | ||||||||
| 2d | 11 | 0.7 | 556.58 | 4.52 | 0 | 11 | 9 | 141.5 | 4 |
| 4d | 11 | 0.7 | 596.64 | 5.42 | 0 | 11 | 9 | 141.5 | 5 |
| 5a | 39 | 2.0 | 508.62 | 4.45 | 0 | 6 | 7 | 59.18 | 1 |
| 5b | 16 | 1.6 | 536.67 | 5.34 | 0 | 6 | 7 | 59.08 | 2 |
| 5d | 18 | 0.6 | 598.61 | 4.36 | 0 | 12 | 9 | 150.7 | 4 |
| Ideal compound | ≮10 | <5 μM | <500 | <5 | <5 | <10 | <8 | <120 | ≯ 1 |
The letters PSA indicate polar surface area.
The cytotoxic potential of 5a was assessed further against 48 human tumor cell lines isolated from nine different malignant conditions. 18 Figure 3 indicates the substantial growth inhibition by 5a against many cell lines at a concentration of 5 μM. Once more the selective toxicity displayed by this compound is noteworthy. In other words, differences in potencies towards various cell lines was observed even within the same neoplastic diseases, for example, against CNS and renal tumors. Thus the possibility exists that this selectivity may be reflected by 5a demonstrating greater toxicity to malignancies than normal cells. The data in Figure 3 provides additional support for further investigations of this prototypic molecule.
Figure 3.
Percentage growth inhibition of a panel of human cancer cell lines by 5 μM of 5a.
The third phase of this investigation was launched in order to glean some appreciation of the way in which 5a exerts its cytotoxic action. Figure 4 clearly reveals that 5a induced the bell-shaped internucleosomal DNA fragmentation, with a maximum at the concentration of 1–2 μM, but not in HSC-2 neoplasms. The relatively higher background level of DNA fragmentation in untreated HL-60 cells may reflect the higher sensitivity of this cell line. Figure 5 indicates that 5a activates caspase-3 in both cell lines but greater activation takes place in HL-60 cells. The CC50 figures in Table 1 indicate that HL-60 cells are more sensitive to 5a than HSC-2 cells and the data provided in Figures 4 and 5 may account for this differential in potency. Both experiments reveal that apoptosis is one way in which the lethal effects of 5a are mediated. Cell death can also be caused by autophagy, that is, the degradation of subcellular constituents by creating acidic organelles (secondary lysosomes) in response to stress caused by such factors as deprivation of nutrients.19 Figure 6 reveals that 5a induced the formation of acidic organelles in HSC-2 cells although to a slightly less extent than is caused by nutritional starvation. Cell cycle analysis was undertaken by flow cytometry using HT29 human colon cancer cells.20,21 The IC50 figure of 5a towards this cell line is 3.75 μM.22 Figure 7 reveals 5a causes cell killing of HT29 cells as indicated by the huge increase in released DNA. In summary the modes of action of a very promising lead compound 5a is by apoptosis and autophagy. The type of cell death induced may depend on the malignancy of the target cells.
Figure 4.
Evaluation of 5a on the induction of internucleosomal DNA fragmentation in HL-60 and HSC-2 cells after 7 h of incubation. M is the molecular weight marker of DNA and the figures are the concentrations of 5a in μM. UV refers to ultraviolet radiation (6 J/m2/min) applied for 1 min followed by incubation of the cells for 3 h.
Figure 5.
Effect of 5a on the induction of caspase-3 after 6 h incubation of HL-60 and HSC-2 cells. UV refers to the radiation used which is indicated in Figure 4.
Figure 6.
Effect of 5a on the formation of acidic organelles in HSC-2 cells after 6 h incubation. HBSS refers to Hank’s balanced salt solution in which the cells were cultured for 1 h.
Figure 7.
Cell cycle analysis of 5 μM of 5a on HT29 cells.
In conclusion, this investigation has revealed that 1-[4-(2-aminoethoxy)phenylcarbonyl]-3,5-bis(benzylidene)-4-piperidone hydrochlorides 2–5 display preferential selective toxicity to neoplasms compared to normal cell lines. As Figure 2 reveals, this selectivity and cytotoxic potencies are favored by having morpholine as the basic group and either the 4-nitro or no substituent in the arylidene aryl rings. Compound 5a emerged as an excellent lead molecule which will initiate further investigations. This compound causes cell death inter alia by the induction of apoptosis and autophagy.
Acknowledgments
The authors thank the Canadian Institutes of Health Research for grants to J. R. Dimmock and R. K. Sharma and the Ministry of Education, Science, Sports and Culture for a Grant-in-Aid (No. 19592156) to H. Sakagami. The National Cancer Institute, USA, kindly evaluated 5a against a panel of 48 human tumor cell lines.
References and notes
- 1.Mutus B, Wagner JD, Talpas CJ, Dimmock JR, Phillips OA, Reid RS. Anal Biochem. 1989;177:237. doi: 10.1016/0003-2697(89)90045-6. [DOI] [PubMed] [Google Scholar]
- 2.Dimmock JR, Raghavan SK, Logan BM, Bigam GE. Eur J Med Chem. 1983;18:248. [Google Scholar]
- 3.Chen EX, Moore MJ. In: Principles of Medical Pharmacology. 7. Kalant H, Grant DM, Mitchell J, editors. Saunders Elsevier; Toronto: 2007. p. 778. [Google Scholar]
- 4.Das U, Sharma RK, Dimmock JR. Curr Med Chem. 2009;16:2001. doi: 10.2174/092986709788682218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Waxman DJ. Biochem Pharmacol. 1994;47:1079. doi: 10.1016/0006-2952(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 6.Tsutsui K, Komuro C, Ono K, Nishidia T, Shibamoto Y, Takahashi M, Abe M. Int J Radiat Oncol Biol Phys. 1986;12:1183. doi: 10.1016/0360-3016(86)90254-3. [DOI] [PubMed] [Google Scholar]
- 7.Dimmock JR, Arora VK, Wonko SL, Hamon NW, Quail JW, Jia Z, Warrington RC, Fang WD, Lee JS. Drug Des Deliv. 1990;6:183. [PubMed] [Google Scholar]
- 8.Dimmock JR, Arora VK, Quail JW, Pugazhenthi U, Allen TM, Kao GY, De Clercq E. J Pharm Sci. 1994;83:1124. doi: 10.1002/jps.2600830811. [DOI] [PubMed] [Google Scholar]
- 9.Dimmock JR, Padmanilayam MP, Puthucode RN, Nazarali AJ, Motoganahalli NL, Zello GA, Quail JW, Oloo EO, Kraatz HB, Prisciak JA, Allen TM, Santos CL, Balzarini J, De Clercq E, Manavathu E. J Med Chem. 2001;44:586. doi: 10.1021/jm0002580. [DOI] [PubMed] [Google Scholar]
- 10.Das U, Alcorn J, Shrivastav A, Sharma RK, De Clercq E, Balzarini J, Dimmock JR. Eur J Med Chem. 2007;42:71. doi: 10.1016/j.ejmech.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv Drug Delivery Rev. 2001;46:3. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 12.Motohashi N, Wakabayashi H, Kurihara T, Fukushima H, Yamada T, Kawase M, Sohara Y, Tani S, Shirataki Y, Sakagami H, Satoh K, Nakashima H, Molnár A, Spengler G, Gyémánt N, Ugocsai K, Molnár J. Phytother Res. 2004;18:212. doi: 10.1002/ptr.1426. [DOI] [PubMed] [Google Scholar]
- 13.Statistical Package for Social Sciences. SPSS for Windows, Release 14.0.0. SPSS Inc; Chicago: 2005. [Google Scholar]
- 14.Hansch C, Leo AJ. Substituent Constants for Correlation Analysis in Chemistry and Biology. John Wiley and Sons; New York: 1979. p. 49. [Google Scholar]
- 15.Albert A, Serjeant EP. The Determination of Ionization Constants. 3. Chapman and Hall; London: 1984. pp. 151–152. [Google Scholar]
- 16.Kelder J, Grootenhuis PD, Bayada DM, Delbressine LP, Ploemen JP. Pharm Res. 1999;16:1514. doi: 10.1023/a:1015040217741. [DOI] [PubMed] [Google Scholar]
- 17.Remko M, Swart M, Bickelhaupt FM. Bioorg Med Chem. 2006;14:1715. doi: 10.1016/j.bmc.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Boyd MR, Paull KD. Drug Dev Res. 1995;34:91. [Google Scholar]
- 19.Broker E, Krujt FAE, Giaccone G. Clin Cancer Res. 2005;11:3155. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 20.HT29 cells were plated and grown for 48 h to reach 50–60% confluency.21 Three concentrations of 5a were added to the cells and after 48 h, the cells were trypsinized; washed in PBS and fixed overnight in 70% ethanol at 4 °C. At the time of harvest, the cultures were 70–90% confluent. After centrifugation, the ethanolic solution was removed and the cells were resuspended in a buffer containing Tris (10 mM, pH 7.5), sucrose (125 mM), magnesium chloride (2.5 mM), NP40 (0.185%), RNase A (0.02 mg/mL), sodium citrate (0.05%) and PI (25 μg/mL). After incubation on ice for 1 h, the cells were subjected to DNA content analysis21 using a FACScan cytometer.
- 21.Lakshmikuttyamma A, Pastural E, Takahaski N, Sawada K, Sheridan DP, De Coteau JF, Geyer CR. Oncogene. 2008;27:3831. doi: 10.1038/onc.2008.8. [DOI] [PubMed] [Google Scholar]
- 22.The IC50 value of 5a towards human HT29 colon cancer cells was determined as follows. The cells were obtained from the American Type Culture Collection (ATCC) and grown in DMEM media containing 10% fetal calf serum. Cells were maintained in an atmosphere of 5% carbon dioxide at 37 °C.23 After detachment by trypsin (2.5 g/L), the cells were resuspended in the tissue culture media to yield a concentration of 1 × 105 cells/mL. The cells were added to 96-well plates (9000 cells/well) and allowed to attach for 24 h at 37 °C. Various concentrations of 5a were added to the wells while control wells contained only culture media. After incubation for 96 h, cell proliferation was estimated based on the cellular reduction of MTT using a microplate reader at 540 nm.24
- 23.Park J, Meisler AI, Cartright CA. Oncogene. 1993;8:2627. [PubMed] [Google Scholar]
- 24.Carmichael J, De Graff WG, Gazdar AF, Minna JD, Mitchell JB. Cancer Res. 1987;47:936. [PubMed] [Google Scholar]
- 25.Pati HN, Das U, Quail JW, Kawase M, Sakagami H, Dimmock JR. Eur J Med Chem. 2008;43:1. doi: 10.1016/j.ejmech.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]