Abstract
The Wnt and Hedgehog signaling pathways are essential for normal development and are misregulated in cancer. The casein kinase family of serine/threonine kinases regulates both pathways at multiple levels. However, it has been difficult to determine whether individual members of this family have distinct functions in vivo, due to their overlapping substrate specificities. In Drosophila melanogaster, photoreceptor differentiation is induced by Hedgehog and inhibited by Wingless, providing a sensitive system in which to identify regulators of each pathway. We used a mosaic genetic screen in the Drosophila eye to identify mutations in genes on the X chromosome required for signal transduction. We recovered mutations affecting the transcriptional regulator CREB binding protein, the small GTPase dynamin, the cytoskeletal regulator Actin-related protein 2, and the protein kinase Casein kinase 1α. Consistent with its reported function in the β-Catenin degradation complex, Casein Kinase 1α mutant cells accumulate β-Catenin and ectopically induce Wingless target genes. In contrast to previous studies based on RNA interference, we could not detect any effect of the same Casein Kinase 1α mutation on Hedgehog signaling. We thus propose that Casein kinase 1α is essential to allow β-Catenin degradation and prevent inappropriate Wingless signaling, but its effects on the Hedgehog pathway are redundant with other Casein kinase 1 family members.
FORWARD genetic screens are a powerful method with which to uncover unanticipated molecular requirements for specific biological processes. Screens for developmental defects in model organisms have identified functions for numerous genes that are conserved throughout evolution and misregulated in human pathologies. Although large-scale RNAi transgenic collections have now made reverse genetic screens possible in Drosophila (Dietzl et al. 2007; Ni et al. 2008; Ni et al. 2011), it is difficult to achieve specific and complete loss of gene activity with this method, making it poorly suited to assigning functions to individual members of gene families. Because development of the complex, yet nonessential, Drosophila eye relies on most of the major signaling pathways (Doroquez and Rebay 2006; Roignant and Treisman 2009), it provides a sensitive system in which to screen for defects indicative of abnormal signaling.
The adult eye consists of an array of 800 ommatidia, each containing eight photoreceptor neurons (R1–R8), and develops from the larval eye imaginal disc. In the third larval instar, photoreceptor differentiation initiates at the posterior margin of the eye disc and propagates toward the anterior under the control of the morphogen Hedgehog (Hh) (Ready et al. 1976; Heberlein et al. 1993; Ma et al. 1993). Hh secreted by differentiating photoreceptors induces immediately anterior cells to form a transient indentation known as the morphogenetic furrow (MF) (Corrigall et al. 2007; Escudero et al. 2007), and to express the bone morphogenetic protein (BMP) family member Decapentaplegic (Dpp) (Heberlein et al. 1993; Ma et al. 1993) and the proneural transcription factor Atonal (Ato) (Jarman et al. 1994; Domínguez 1999). Notch (N)-mediated lateral inhibition then contributes to refining Ato expression into single cells that differentiate as R8 photoreceptors (Cagan and Ready 1989; Dokucu et al. 1996; Baker and Yu 1997). R8 cells secrete Spitz (Spi), a ligand for the epidermal growth factor receptor (EGFR) (Freeman 1994; Tio et al. 1994), which induces the stepwise differentiation of neighboring cells into the seven remaining photoreceptors (Tomlinson and Ready 1987; Freeman 1996; Freeman 1997; Dominguez et al. 1998). The EGFR-dependent ETS transcription factor Pointed (Pnt) directly activates hh expression in these newly recruited photoreceptors (O'Neill et al. 1994; Rogers et al. 2005), creating an indirect autoregulatory loop between Hh and EGFR signaling that drives the anterior propagation of photoreceptor differentiation (Rogers et al. 2005; Roignant and Treisman 2009).
The morphogen Wingless (Wg) promotes head cuticle formation by cells at the margins of the eye disc, excluding retinal differentiation from these regions (Legent and Treisman 2008). Wg prevents ectopic initiation and progression of the MF (Ma and Moses 1995; Treisman and Rubin 1995) by repressing drumstick, which encodes an activator of hh (Bras-Pereira et al. 2006); retinal determination genes such as eyes absent (eya) (Baonza and Freeman 2002; Kenyon et al. 2003); and dpp (Heslip et al. 1997). Mutations in wg or its effector dishevelled (dsh) result in expansion of the retinal field into head regions (Ma and Moses 1995; Heslip et al. 1997). Conversely, loss of negative regulators of the Wg-responsive transcription factor β-Catenin/Armadillo (Arm), such as Axin or glycogen synthase kinase 3 (GSK3) / Shaggy (Sgg), maximally activates the pathway and transforms retinal cells into head cuticle (Heslip et al. 1997; Lee and Treisman 2001; Baonza and Freeman 2002).
The Wg and Hh signaling pathways share several common elements, including members of the Casein kinase 1 (CK1) family of serine/threonine protein kinases. Cell culture and RNAi experiments have shown that CK1γ and CK1ε can phosphorylate the Wg co-receptor LRP6/Arrow (Arr) (Davidson et al. 2005; Swiatek et al. 2006; Zhang et al. 2006; Casagolda et al. 2010) and the downstream component Dsh (Klein et al. 2006; Bernatik et al. 2011). CK1α is thought to behave as a priming kinase for Arm, triggering its proteasomal degradation and inhibiting transcriptional output from the pathway (Liu et al. 2002; Yanagawa et al. 2002; Marin et al. 2003). Similarly, phosphorylation of Cubitus interruptus (Ci), the transcription factor downstream of Hh, promotes its processing into a repressor form; both CK1α and CK1ε have been reported to contribute to this (Jia et al. 2005). CK1 enzymes also phosphorylate and activate Smoothened (Smo) and Fused (Fu), components of the Hh pathway that act upstream of Ci (Jia et al. 2004; Zhou and Kalderon 2011). However, the roles of individual CK1 family members have been difficult to establish with certainty in the absence of specific point mutations (Zhang et al. 2006).
We describe here a mosaic genetic screen for X-linked mutations that affect the pattern of photoreceptor differentiation. We used the Flipase-Flipase recognition target (FLP-FRT) technique (Xu and Rubin 1993) with FLP driven by the eye-specific eyeless (ey) promoter (Newsome et al. 2000), as in our previous screen of the autosomes (Janody et al. 2004). In the screen, we recovered alleles of genes known to influence N, Wg, and EGFR signaling, including the genes that encode CREB binding protein (CBP) and dynamin. In addition, we identified the first mutant alleles of Actin Related Protein 2 (Arp2) and Casein Kinase 1α (CK1α). Our results demonstrate that CK1α is an essential negative regulator of Wg signaling, which cannot be replaced by other CK1 family members. In contrast, our mutation in CK1α has no effect on the transduction of Hh signaling.
Materials and Methods
Fly stocks and genetics
For the screen, isogenic yellow (y) white (w) FRT19A males were mutagenized with 15 mM ethyl methanesulfonate (EMS, Sigma), a concentration determined in a pilot screen to induce one lethal mutation in every five X chromosomes, and mated to ey-FLP1, P[w+, ubiquitin (ubi)-GFP], FRT19A females. F1 females with eye phenotypes were mated to FM6 w/Y males, and stocks were then established over the FM7i pAct-GFP balancer. For each mutant female, at least four F3 stocks were established and retested (Figure 1). In the secondary screen, mutant females were mated to ey-FLP1, P[w+, arm-lacZ], FRT19A males to examine clones in the larval eye disc. Lethal mutations were roughly mapped by crossing to the Bloomington Drosophila Stock Center X chromosome duplication (Dp1) kit (2007), and subsequent fine-scale mapping was achieved by recombination with P[w+] insertions in the region (Figure 4E).
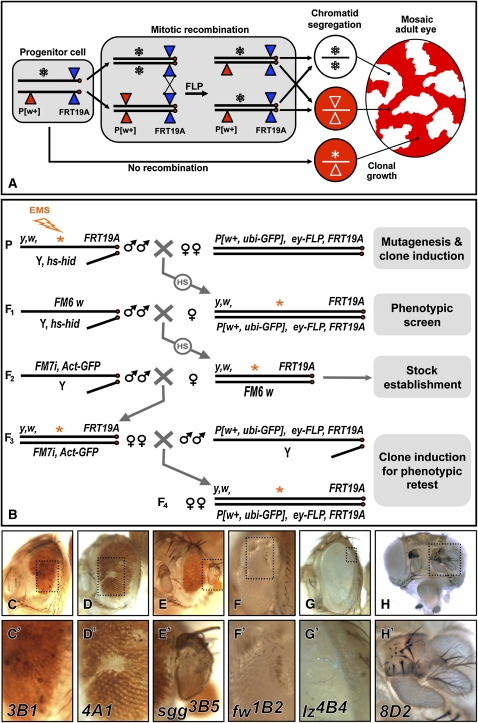
Figure 1 .
Design and results of the screen. (A) During larval development of flies heterozygous for an EMS-induced mutation (*), ey-FLP induces mitotic recombination between FRT sites on duplicated homologous chromosomes. Chromatid segregation at mitosis can produce daughter cells homozygous for the mutation (*/*). In the adult eye, a P(w+) element on the wild-type chromosome allows homozygous mutant clones to be recognized by their lack of red pigment. (B) EMS-mutagenized FRT19A males were crossed to P[w+, ubi-GFP], ey-FLP, FRT19A females. Induction of the cell death gene head involution defective (hid) present on the Y chromosome by heat shock (HS) ensured that the F1 daughters screened were virgins. Selected females were mated to FM6, w, and then FM7i, pAct-GFP marked balancers to establish F3 mutant stocks. Four independent stocks for each mutation were retested by crossing to the ey-FLP line (F4). (C–H) Adult eyes showing mutant phenotypes. Ommatidia fail to differentiate in 3B1 clones (C), and transform into scars in 4A1 clones (D) or cuticle outgrowths in sgg3B5 clones (E). Males hemizygous for fw1B2 display reduced, bumpy eyes (F), while the retina of lz4B4 males is smooth and glossy (G). Ectopic antennae form in 8D2 mutant clones (H).
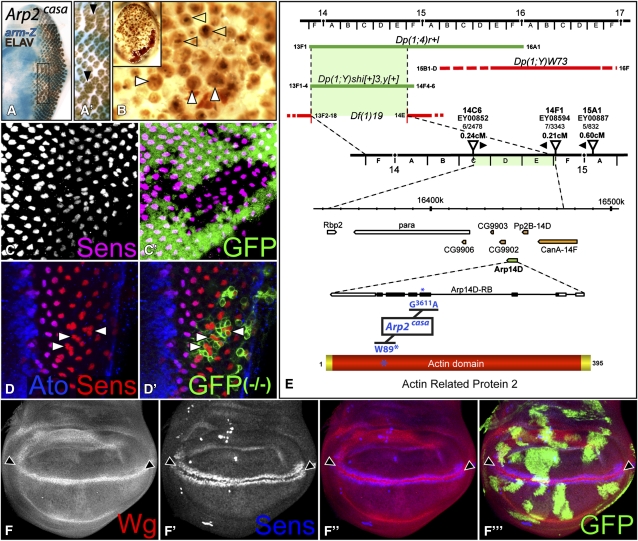
Figure 4 .
Arp2 restricts R8 differentiation. (A) Arp2casa clones, marked by the absence of arm-lacZ (blue), exhibit enlarged ommatidia containing supernumerary photoreceptors (Elav in brown, arrowheads). (B) In adult eyes, large Arp2casa clones in a Minute background exhibit enlarged ommatidia (solid arrowheads) with craters of missing lens material (open arrowheads). A dashed line surrounds w+ heterozygous tissue in the inset. (C) GFP-negative Arp2casa clones contain duplicated Sens-positive R8 cells (magenta). (D) Occasionally, R8 triplets (labeled with Sens in red and Ato in blue, arrowheads) are observed in Arp2casa clones (positively marked by coexpression of GFP in green). (E) Diagram of the mapping of Arp2casa indicating cytogenetic bands and aberration breakpoints. Duplications (Dp) in green rescue hemizygous males, whereas those in red do not. Arp2casa does not complement the deficiency Df(1)19. The map distances and orientations of Arp2casa with respect to three P elements in the region are indicated. The sequence change G3611A in the Arp14D gene (green) and corresponding W89* nonsense mutation in Arp2 are shown in blue. No sequence changes were detected in neighboring genes (orange). (F) In the wing pouch, GFP (green)-negative Arp2casa clones, in a Minute background, show normal expression of the D/V boundary markers Wg (red) and Sens (blue) (arrowheads).
Other stocks used were Df(1)EDF7161 P(w+) [11A1;11B14] / Dp(1;Y)BSC1, y+ [10C1-2;11D3-8], aos-lacZW11, E(spl)m8-lacZ, ds-lacZ05124, Dll-lacZ01092, dpp-lacZBS3.0, ptc-GAL4559.1, ptc-lacZAT90, vgBE-lacZ (Bloomington Drosophila Stock Center), Y hs-hid (a gift from Ruth Lehmann), fz3-RFP (Olson et al. 2011), UAS-dTCFDN (van de Wetering et al. 1997), UAS-CK1α::Flag, UAS-CG2577 (Zhang et al. 2006), UAS-dcoKD (Kinase Domain), dco3 (Cho et al. 2006). Stocks used to generate clones were: (1) y,w, eyFLP1, P[w+, arm-lacZ], FRT19A, (2) w, hsFLP122, P[w+, ubi-GFP], FRT19A, (3) y,w, hsFLP122, P[w+, ubi-RFP], FRT19A, (4) y,w, P[w+, ubi-GFP], M(1) RpS5a2, FRT19A; hsFLP38, (5) w, eyFLP1, tub-GAL80, FRT19A;; tub-GAL4, UAS-CD8::GFP, (6) w, hsFLP122, tub-GAL80, FRT19A;; tub-GAL4, UAS-CD8::GFP.
Immunohistochemistry
Third instar eye and wing imaginal discs were dissected and stained according to (Legent and Treisman 2008). Antibodies used were: rabbit anti-β−galactosidase (1:5000, Cappel), chicken anti-GFP (1:500, Aves), mouse anti-Flag (1:500, Sigma), rabbit anti-Ato (1:5000; Jarman et al. 1994), guinea pig anti-Sens (1:1000; Nolo et al. 2000) mouse anti-NECD (1:10), mouse anti-DlECD (1:10), mouse anti-Arm (1:10), mouse anti-Smo (1:1000), mouse anti-Ptc (1:10), mouse anti-En (1:2), rat anti-Ci (1:2), rat anti-Elav (1:100) (Developmental Studies Hybridoma Bank, University of Iowa), rabbit anti-EGFRCTER (1:500; Rodrigues et al. 2005), guinea pig anti-dMyc (1:100, a gift from Ginés Morata), and mouse anti-Omb (1:100; Shen et al. 2010).
Results
A mosaic screen for X-linked mutations that disrupt photoreceptor differentiation
To identify novel X-linked genes required for the normal pattern of photoreceptor differentiation, regardless of any earlier essential requirement, we conducted a mosaic screen of the X chromosome using the FLP-FRT technique (Figure 1A) (Xu and Rubin 1993). Mutations were induced by EMS treatment in the germline of isogenic males carrying an FRT insertion near the centromere (Figure 1B). In heterozygous daughters, FLP recombinase specifically expressed in the developing eye under the control of the ey promoter (Newsome et al. 2000) catalyzed chromatid exchange between homologous X chromosomes, resulting in clones of cells homozygous for the mutagenized chromosome arm. The presence of a P element marked with white+ (w+) on the wild-type chromosome allowed mutant clones to be recognized in the adult eye by their lack of red pigmentation (Figure 1A). This method enabled us to generate retinal clones homozygous for novel X-linked mutations in the first generation (F1) with high efficiency (Figure 1B).
Adult flies were primarily screened for a lack of white clones in the retina and a reduction in eye size, indicating that mutant clones failed to contribute to the adult retina, but persisted long enough to prevent their replacement by wild-type cells through compensatory proliferation (Ryoo et al. 2004). This phenotype was often accompanied by disruption of the eye shape (Figure 1C), formation of cuticle scars (Figure 1D), or protrusion of cuticle outgrowths from the retina (Figure 1E). Although most such mutations were homozygous lethal, we also recovered a few hemizygous viable mutants. For instance, loss of the selectin encoded by furrowed (fw) (Leshko-Lindsay and Corces 1997) results in a reduced and irregularly shaped eye with depressions in its surface (Figure 1F), while mutants for lozenge (lz), which encodes a transcription factor required for normal differentiation of several retinal cell types (Flores et al. 1998), have smooth, glossy eyes (Figure 1G). In addition to mutations causing eye defects, we also recovered mutants with patterning defects of the head capsule, such as triplication of the antennae (Figure 1H).
Since meiotic recombination in the female germline could result in loss of the mutation from the w chromosome, we established balanced stocks from the progeny of four individual females lacking the P(w+) element and identified those carrying the mutation by crossing them back to the ey–FLP stock (Figure 1B). To select mutations that disrupted photoreceptor differentiation during larval development, we conducted a secondary screen in third instar eye–antennal discs. Mutant clones lacking the marker arm-lacZ (Vincent et al. 1994) were examined for aberrations in the pattern of photoreceptors expressing the neuronal nuclear protein Elav (Robinow and White 1991). We did not pursue mutations such as lz in which photoreceptor differentiation appeared essentially normal at this stage (Figure 2A) despite their severe phenotype in adults (Figure 1G). We also discarded mutations that strongly impaired cell growth or viability, resulting in very small clones.
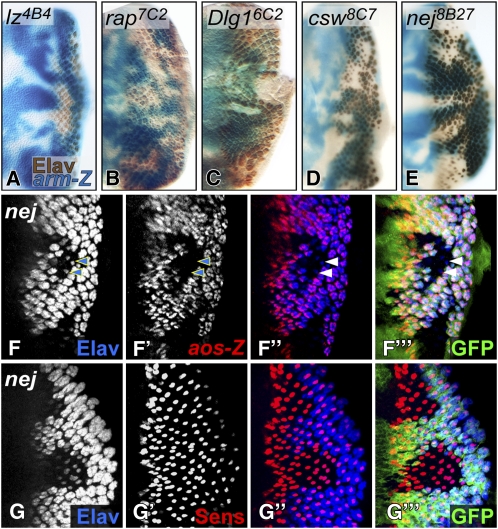
Figure 2 .
Isolation of mutations in genes known to affect photoreceptor differentiation. (A–E) Photoreceptors are stained with anti-Elav (brown) in third instar eye discs carrying mutant clones marked by the absence of arm-lacZ staining (blue). lz4B4 clones (A) show only minor defects in the pattern of photoreceptors; rap7C2 clones (B) show a partial loss of photoreceptors; dlg16C2 clones (C) overgrow and fail to differentiate any photoreceptors; csw8C7 (D) and nej8B27 (E) clones contain many ommatidia consisting of only one Elav-positive cell. (F–G) nej8B27 clones are marked by the absence of GFP (green). (F) aos-lacZ (red), which marks R1–7 photoreceptors, is absent in the few remaining Elav-positive (blue) photoreceptors (arrowheads) in nej8B27 clones. (G) nej8B27 clones still differentiate R8 cells marked by Sens (red).
A total of 43414 F1 flies were screened, allowing us to identify 490 mutants, of which 79% survived and were fertile. Many mutations were not recovered from the four independent lines established, probably due to germline mosaicism in the mutant mother. A total of 139 mutant stocks were established, 119 of these were retained following the secondary screen (Table 1), and 95% of them were hemi-/homozygous lethal. Complementation tests for lethal mutations on the X chromosome require the presence of a duplication that restores male viability. We therefore screened a collection of duplications covering most of the X chromosome for their ability to rescue males hemizygous for each mutation. Sixty-seven mutant stocks were rescued by at least 1 of the 20 duplications of the Bloomington Dp1 kit (2007), allowing for subsequent fine mapping by meiotic recombination with a set of P(w+) markers (Zhai et al. 2003), followed by complementation tests with candidate genes in the region (see Figure 4E). These results are summarized in Table 2.
Table 1 . Outcome of the genetic screen.
| Total | % | |
|---|---|---|
| Flies screened | 43,414 | |
| Mutants identified in primary screen | 490 | 1.13 |
| Viable and fertile mutants | 387 | 79 |
| Recovered mutant stocks | 139 | 28 |
| Mutants kept after secondary screen | 119 | 24 |
Percentages in the right column refer to the 490 mutants identified in our primary screen, except for 1.13%, which refers to the 43,414 flies screened.
Table 2 . Mutations recovered from the screen.
| Function | Fly gene name | Vertebrate homologs | CG no. | Cytology | No. of alleles |
|---|---|---|---|---|---|
| EGFR/MAPK pathway | corkscrew (csw) | SHP-2 | 3954 | 2D1–2 | 7 |
| pole hole (phl) | Raf | 2845 | 3A1 | 6 | |
| Downstream of raf1 (Dsor1) | MEK/MAPKK | 15793 | 8D2–3 | 5 | |
| Wingless pathway | shaggy (sgg) | GSK3β | 2621 | 3A8–3B1 | 9 |
| Casein Kinase 1α (CK1α) | CK1α | 2028 | 11B7 | 1 | |
| Notch pathway | Notch (N) | N | 3936 | 3C7–9 | 1 |
| Endocytosis/cytoskeleton | shibire (shi) | Dynamin | 18102 | 13F18 | 1 |
| Actin-related protein 14D (Arp14D) | Arp2 | 9901 | 14E1 | 1 | |
| Transcriptional regulation | nejire (nej) | CBP | 15319 | 8F7–9 | 2 |
| lozenge (lz) | AML1 | 1689 | 8D5–6 | 3 | |
| Adhesion/polarity | furrowed (fw) | P-selectin | 1500 | 11A1 | 3 |
| discs large 1 (dlg1) | DLG1 | 1725 | 10B6–10 | 3 | |
| Cell cycle regulation | retina aberrant in pattern (rap) | Cdh1/Fzr | 3000 | 4C11–12 | 6 |
| Unidentified genes | Complementation group A | 2D1–2;3C5–6 | 4 | ||
| Complementation group B | 2D1–2;3A1–2 | 2 | |||
| Complementation group C | 5A1–6C | 2 | |||
| Other hits | 63 |
CBP is required for the recruitment of R1–R7
Several of our mutations were in genes already known to act in photoreceptor differentiation. We identified six alleles of retina aberrant in pattern (rap)/Cdh1, which encodes a component of the anaphase promoting complex/cyclosome, a multi-subunit E3 ubiquitin ligase (Sigrist and Lehner 1997), on the basis of a partial lack of photoreceptor differentiation in rap mutant clones (Figure 2B). Clones mutant for discs large (dlg1), which encodes a neoplastic tumor suppressor and component of the Scribble polarity module (Woods and Bryant 1991), showed overgrowth as well as a complete lack of photoreceptors (Figure 2C). Components of the signaling pathways involved in eye patterning and photoreceptor differentiation were also recovered. We isolated alleles of N, sgg, and several positive regulators of EGFR/MAPK signaling: Raf/pole hole, MEK/Downstream of raf1, and SHP2/corkscrew (csw) (Table 2). In clones mutant for these components of the EGFR pathway, R8 differentiated but failed to recruit the full complement of photoreceptors (Figure 2D).
We observed a similar phenotype in clones homozygous for a mutation in nejire (nej); only one Elav-positive photoreceptor was present in most mutant ommatidia (Figure 2E), suggesting a defect in EGFR signaling. We used argos (aos)-lacZ, a direct transcriptional target of the EGFR pathway in R1–R7 (Schweitzer et al. 1995; Golembo et al. 1996), as a reporter for EGFR signaling. aos-lacZ was not expressed in nej8B27 mutant clones (Figure 2F), despite the presence of cells expressing Senseless (Sens), a marker for R8 (Figure 2G). nej encodes the histone acetyltransferase CBP/p300 (Akimaru et al. 1997), a transcriptional coactivator and scaffolding protein that links DNA-binding factors and the basal transcriptional machinery to signaling cascades. Our findings are consistent with genetic interactions previously observed between EGFR components and nej in adult eyes (Anderson et al. 2005), the persistence of Ato expression in nej clones (Kumar et al. 2004), and the requirement for CBP in MAPK-dependent activation of Ets transcription factors in mouse cell culture (Foulds et al. 2004).
Loss of dynamin has opposite effects on Notch and EGFR signaling
We mapped the mutation 7C7 to shibire (shi), which encodes dynamin, a GTPase required to pinch off endocytic vesicles from the plasma membrane (Chen et al. 1991). shi7C7 mutant clones displayed a neurogenic phenotype in which virtually all mutant cells posterior to the furrow differentiated as Elav-positive photoreceptors (Figure 3A). This phenotype is reminiscent of mutations in components of the N pathway that affect its ability to resolve proneural cell clusters into single R8 cells through lateral inhibition (Li and Baker 2001). Several studies have shown that dynamin is required both for endocytosis and activation of the N ligand Delta (Dl) and for N signaling in the receiving cell (Seugnet et al. 1997; Vaccari et al. 2008; Windler and Bilder 2010). Consistent with a defect in N-mediated lateral inhibition, we found that shi7C7 clones downregulated the N transcriptional reporter E(spl)m8-lacZ (Figure 3A’’), differentiated supernumerary Sens-positive R8 cells (Figure 3B), and failed to refine Ato expression to a single R8 cell per ommatidium (Figure 3C). Together with the apical accumulation of Dl and N in shi7C7 mutant clones (Figure 3, D and E), these results confirm that dynamin is required for endocytic trafficking of the ligand and its receptor and subsequent transduction of the signaling pathway. N and Dl themselves also have an earlier role in the activation of ato expression, and clones homozygous for these mutations thus fail to differentiate any photoreceptors (Baker and Yu 1997; Ligoxygakis et al. 1998). Our observations indicate that shi is not required for this proneural function of N.
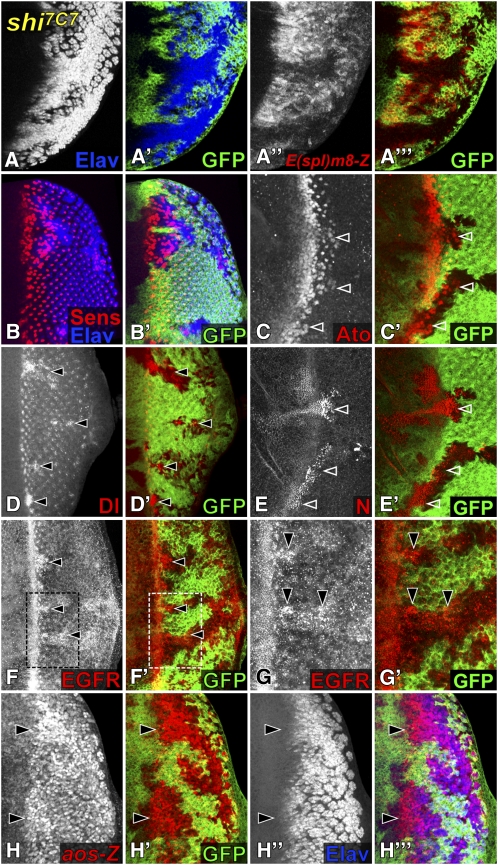
Figure 3 .
shi mutant cells alter N and EGFR signaling to induce excessive photoreceptor differentiation. (A–H) shi7C7 clones in third instar eye discs are marked by the absence of GFP (green). (A) Virtually all shi7C7 cells posterior to the MF differentiate as Elav-positive photoreceptors (blue). The N transcriptional reporter E(spl)m8-lacZ (red) is also downregulated. (B) Many but not all ectopic photoreceptors in shi7C7 clones (stained with Elav in blue) express the R8 marker Sens (red). (C) shi7C7 clones differentiate extra Ato-positive (red) R8 cells just posterior to the MF (arrowheads). In apical regions, shi7C7 clones accumulate increased levels of Dl (red in D), N (red in E), and EGFR (red in F, boxed region enlarged in G), especially just posterior to the MF (arrowheads). (H) shi7C7 clones show increased expression of the EGFR transcriptional reporter aos-lacZ (red), in cells that have not yet differentiated into Elav (blue)-expressing photoreceptors (arrowheads).
Interestingly, not all the ectopic neurons in shi7C7 clones were R8 photoreceptors (Figure 3B). Since EGFR signaling induces the differentiation of R1–R7, the presence of extra R1–R7 cells in shi7C7 mutant clones raised the possibility that dynamin might inhibit EGFR signaling in addition to promoting N activity. Indeed, EGFR protein accumulated in subregions of shi7C7 clones, mostly just posterior to the MF (Figure 3, F and G), suggesting that a failure to internalize the receptor in the absence of dynamin may allow extended EGFR signaling. Consistently, we observed increased expression of aos-lacZ, a transcriptional reporter for EGFR signaling, in shi7C7 clones. This was not a downstream consequence of the increased number of photoreceptors, as aos-lacZ expression preceded Elav expression (Figure 3H). As a whole, these results suggest that shi7C7 cells differentiate as ectopic photoreceptors as a result of both an impairment in N-mediated lateral inhibition and an increase in EGFR signaling.
Arp2 mutations result in supernumerary R8 cells
A different kind of neurogenic phenotype was observed in clones homozygous for a mutation that we named cassandra (casa). Not all casa mutant cells differentiated as neurons, but most ommatidia contained extra photoreceptors (Figure 4A). In adult eyes, large casa clones generated in a Minute background displayed enlarged facets with craters of missing lens material (Figure 4B). Staining for Sens revealed that casa mutant ommatidia often contained two neighboring R8 cells (Figure 4C), or occasionally R8 triplets (Figure 4D). Using rescue by X chromosomal duplications followed by recombination with P-element markers, we mapped casa to the 14C–E region (Figure 4E). Sequencing of candidates in the region revealed a nonsense mutation at position 89 in the gene Actin related protein 14D (Arp14D) (Figure 4E) that encodes the Arp2 subunit of the Arp2/3 complex required for the polymerization of branched actin filaments (Pollard 2007). Mutations in other subunits and regulators of this complex cause a very similar adult eye phenotype (Zallen et al. 2002). The recruitment of multiple R8 cells in Arp2casa mutant ommatidia might be explained by reduced N signaling; in sensory organ precursor lineages, Arp3 has been reported to be required for normal Dl presentation (Rajan et al. 2009). Such an effect of the Arp2/3 complex on the N pathway must be subtle and/or tissue specific, because loss of Arp2 had no visible effect on N-dependent differentiation of the wing margin (Figure 4F).
A novel mutation upregulates Wg target genes
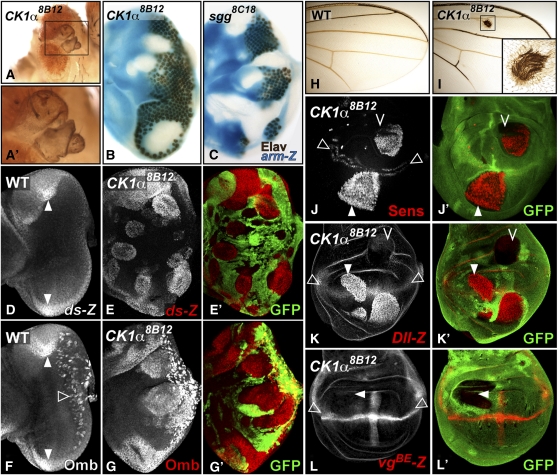
In eye imaginal discs, Wg is expressed at the anterior lateral margins in regions that will differentiate as head cuticle. Activation of Wg signaling within the eye field (e.g., in sgg clones; Figures 1E and 5C) transforms retinal tissue into ectopic head cuticle (Legent and Treisman 2008). Clones homozygous for our mutation 8B12 similarly resulted in cuticle outgrowths protruding from the retina of adult flies (Figure 5A). In third instar eye discs, 8B12 mutant clones completely lacked photoreceptors, had smooth borders, and overgrew (Figure 5B), again resembling sgg mutations (Figure 5C). Given this similarity, we tested whether 8B12 affected the expression of known Wg target genes in the eye disc. The unconventional cadherin Dachsous (Ds) and the transcription factor Optomotor-blind (Omb) are activated by Wg signaling at the anterior lateral margins of the eye disc (Figure 5, D and F) (Zecca et al. 1996). We observed strong autonomous upregulation of Omb and the transcriptional reporter ds-lacZ in 8B12 mutant clones (Figure 5, E and G), consistent with ectopic activation of the Wg pathway.
Figure 5 .
CK1α8B12 clones induce ectopic Wg target gene expression. (A) Adult eyes containing CK1α8B12 clones display cuticle protrusions in the retina (inset in A’). (B and C) CK1α8B12 (B) or sgg8C18 (C) mutant clones in the eye disc marked by the absence of arm-lacZ staining (blue) are overgrown, lack Elav staining (brown), and have smooth boundaries. (D–G) Eye discs stained for ds-lacZ (D,E) or Omb (F and G). ds-lacZ (D) and Omb (F) are normally expressed at the anterior lateral margins of the eye disc (solid arrowheads). Omb also marks retinal glial cells (open arrowhead). (E–G) GFP (green)-negative CK1α8B12 clones ectopically express ds-lacZ (red in E) and Omb (red in G). (H and I) Adult wing blades of wild type (H) or flies carrying CK1α8B12 clones (I). CK1α8B12 clones differentiate ectopic bristles (inset in I). (J–L) Third instar wing discs carrying GFP-negative CK1α8B12 clones. CK1α8B12 clones in the wing pouch (arrowheads) ectopically express high levels of Sens (red in J) and Dll-lacZ (red in K), but do not misexpress vgBE-lacZ (red in L). Cells outside the wing pouch fail to induce ectopic expression (chevrons in J and K). Normal expression of these genes at and/or adjacent to the D/V boundary of the wing pouch is shown by open arrowheads.
To determine whether the 8B12 mutation affects a general regulator of Wg signal transduction, we next investigated its effect on Wg functions in wing development. Wg is normally expressed along the dorsal/ventral (D/V) boundary of the wing pouch, where it induces the differentiation of sensory bristles at the adult wing margin (Figure 5H). Consistent with a gain of Wg function, 8B12 mutant clones within the wing blade differentiated ectopic bristles (Figure 5I). In wing discs, Wg elicits graded responses depending on its concentration. Sens is a high-threshold target expressed in cells abutting the source of Wg secretion (Figure 5J), while Distalless (Dll) is a mid-threshold target induced in a broader domain (Figure 5K)(Neumann and Cohen 1996; Neumann and Cohen 1997). Both targets were strongly and autonomously induced in 8B12 mutant clones in the wing pouch (Figure 5, J and K). Misexpression of Sens and Dll was confined to the wing pouch, suggesting that additional inputs required for their expression are lacking in other regions of the wing disc (Figure 5, J and K). 8B12 had no effect on the boundary enhancer of the vestigial gene (vgBE), a N target expressed at the D/V boundary of the wing pouch, demonstrating its specificity for the Wg pathway (Figure 5L). Another N target gene, wg itself, also never showed increased expression in 8B12 clones (data not shown).
CK1α is specifically required for Wg signaling
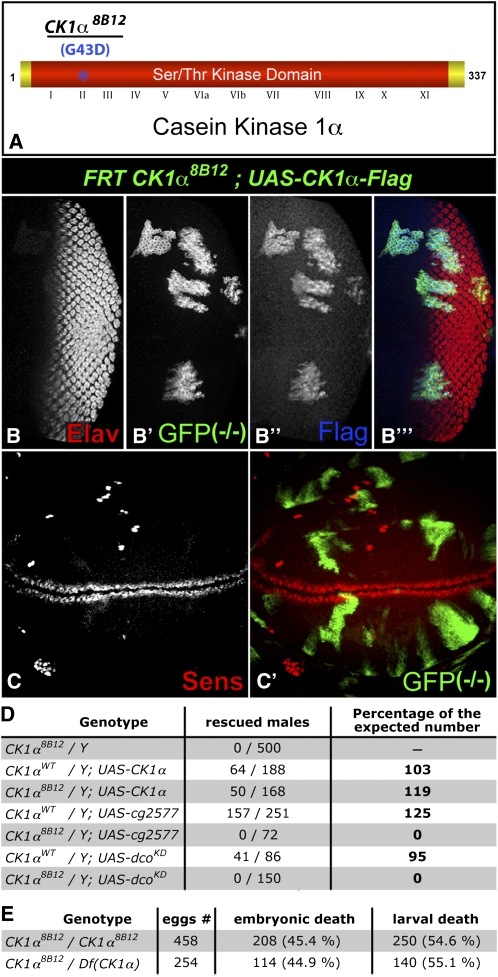
We mapped 8B12 to the cytological region 11A–C and found a missense mutation in the gene CK1α, but no sequence changes in the neighboring CK1 family member CG2577. The mutation transforms the conserved glycine 43 into an aspartic acid (Figure 6A) and falls within subdomain II of the serine/threonine kinase domain of CK1α (Santos et al. 1996). Expression of a FLAG-tagged wild-type CK1α cDNA in 8B12 clones, using the mosaic analysis with a repressible cell marker (MARCM) system (Lee and Luo 2001), rescued both photoreceptor differentiation in the eye and ectopic Sens in the wing (Figure 6, B and C). Additionally, the lethality of hemizygous 8B12 males was rescued by CK1α expression, but not by two closely related CK1 family members, CG2577 or discs overgrown (dco) (Figure 6D). These results confirm that the phenotypes observed are due to the sequence change in CK1α. To our knowledge, 8B12 is the first mutation described in this gene in Drosophila. Homozygous CK1α8B12 animals died primarily during embryogenesis and early larval stages, but showed no obvious cuticle patterning defects (data not shown), perhaps due to a maternal contribution of CK1α. Trans heterozygotes over a deficiency died at the same stages (Figure 6E), suggesting that CK1α8B12 is a strong hypomorph or a null allele.
Figure 6 .
8B12 is a missense mutation in CK1α. (A) Diagram of the CK1α protein showing the G43D mutation in subdomain II of the Ser/Thr kinase domain in CK1α8B12. (B and C) third instar eye (B) and wing (C) discs expressing GFP (green) and FLAG-tagged CK1α (blue) within CK1α8B12 clones. (B) Differentiation of Elav-positive (red) photoreceptors is rescued. (C) Ectopic expression of Sens (red) is abolished. (D) Table showing the survival of hemizygous CK1α8B12 males expressing UAS–CK1α, but not UAS–cg2577 or UAS–dcoKD, driven by tub–GAL4. The number and percentage of males of the expected genotype is displayed. (E) Table showing the rate of embryonic and larval death of CK1α8B12 homozygotes vs. CK1α8B12/Df(CK1α) transheterozygotes.
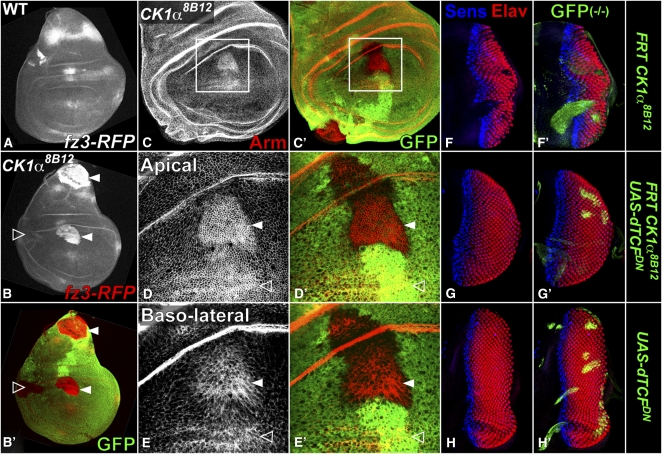
Biochemical and RNAi-based studies have implicated CK1α as a priming kinase for β-Catenin/Arm phosphorylation and subsequent proteolytic processing by the ubiquitin–proteasome pathway (Liu et al. 2002; Yanagawa et al. 2002). However, the presence of eight CK1 family members in Drosophila and the difficulty of achieving both efficient and specific loss of function by RNAi have left open the possibility that other CK1 family members contribute to Arm degradation in vivo (Jia et al. 2005; Zhang et al. 2006). We tested this using an enhancer from the frizzled 3 (fz3) gene that is directly regulated by Wg through TCF binding sites (Olson et al. 2011). fz3–RFP, which is normally expressed in response to high Wg signaling in the notum and to a lesser extent at the D/V boundary in the wing disc (Figure 7A), was strongly induced in CK1α8B12 clones (Figure 7B), indicating robust pathway activation. If phosphorylation of Arm by CK1α is essential for its destruction, Arm levels should increase in the absence of CK1α. Indeed, CK1α8B12 clones in the wing pouch, like cells abutting the source of Wg expression at the D/V boundary, accumulated high levels of Arm (Figure 7C). Arm enrichment was particularly evident at the apical/lateral plasma membrane (Figure 7D), but was also visible more basally (Figure 7E). Thus no other CK1 family member can substitute for CK1α to restrict Arm accumulation.
Figure 7 .
Loss of CK1α activates Wg signaling by promoting Arm accumulation. (A–E) Third instar wing discs; (A) wild type or (B–E) carrying CK1α8B12 clones marked by the absence of GFP (green). (A) fz3–RFP is expressed as a stripe in the notum and more weakly in the central hinge and wing pouch. (B) CK1α8B12 clones induce ectopic fz3–RFP (red) expression in the notum and pouch (arrowheads) but not in the lateral hinge (open arrowhead). (C–E) Arm (red) accumulates close to the D/V boundary (open arrowhead) and in CK1α8B12 clones within the wing pouch (solid arrowhead). Single confocal sections show prominent Arm enrichment at the apical/lateral plasma membrane (D) and also more basally (E). (F–H) Eye discs containing clones marked by coexpression of GFP (green) and stained for Elav (red) and Sens (blue). The lack of photoreceptors in CK1α8B12 clones (F) is rescued by expression of dominant negative dTCF (G). (H) UAS–dTCFDN control clones do not affect photoreceptor differentiation.
To test whether the CK1α eye phenotype results from increased Wg signaling, we impaired signal transduction downstream of Arm accumulation by expressing a dominant negative form of the transcription factor dTCF (van de Wetering et al. 1997). Wg signaling is not required for normal photoreceptor differentiation, and MARCM-induced expression of dTCFDN in the eye field had no effect (Figure 7H). It was nevertheless sufficient to restore photoreceptor differentiation in CK1α8B12 clones (Figure 7, F and G), indicating that this effect of CK1α mutations is due to increased transcriptional activity of Arm. As a whole, these results demonstrate the requirement for CK1α to restrict normal Wg signaling in vivo.
CK1α regulates Myc expression but not Hh signaling
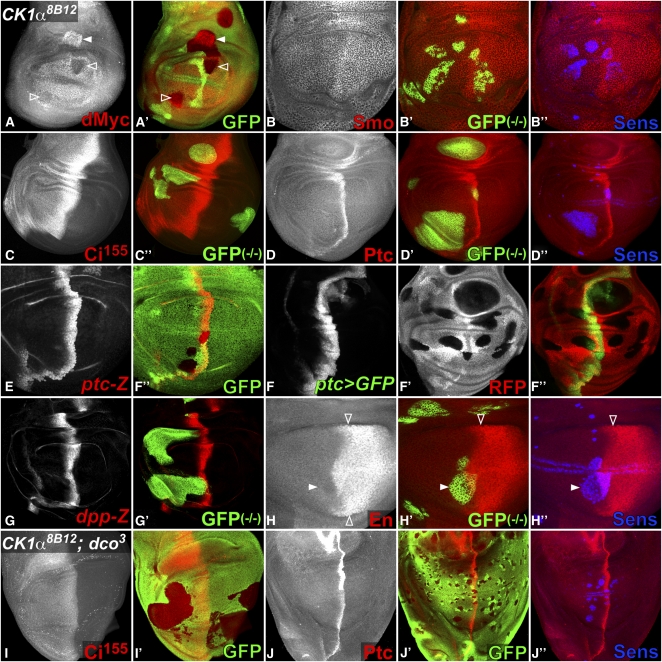
CK1α is a Ser/Thr kinase predicted to phosphorylate multiple substrates. The 8B12 mutation might abolish all CK1α activity, or specifically affect Arm phosphorylation. Another target predicted from biochemical and RNAi studies is the growth regulator dMyc. Direct phosphorylation of dMyc by CK1α is thought to lead to its ubiquitin-mediated degradation (Galletti et al. 2009). In the wing disc, dmyc is expressed in the notum and wing pouch except near the D/V boundary, where its transcription is repressed by N signaling (Herranz et al. 2008). In CK1α8Β12 mutant clones in the wing hinge, dMyc was strongly upregulated (Figure 8A), probably reflecting the absence of CK1α-induced dMyc protein degradation. As reported for CK1α RNAi (Galletti et al. 2009), CK1α8B12 clones in the wing pouch downregulated dMyc, potentially due to increased repression by N. However, we believe it is more likely to be an indirect effect, because loss of CK1α does not increase the ability of N to regulate other target genes (Figure 5L).
Figure 8 .
CK1α8B12 clones do not affect Hh signaling. (A–J) Wing discs carrying CK1α8B12 clones in a dcoWT background (A–H) or in dco3 homozygotes (I–J). Anterior is to the left and dorsal up. Mutant clones are marked by the absence of GFP (green in A, E, I, J) or RFP (red in F) or by coexpression of GFP (green in B–D and G–H). (B’’, D’’,H’’, J’’) CK1α8B12 clones in the wing pouch upregulate the Wg target Sens (blue). (A) dMyc (red) is upregulated in CK1α8B12 clones in the wing hinge (solid arrowhead) but downregulated in the wing pouch (open arrowheads). (B) Expression of the Hh effector Smo (red) in the posterior compartment and just anterior to the A/P boundary is not affected in CK1α8B12 clones. CK1α8B12 clones also do not alter the expression of Ci155, normally observed in the A compartment with elevated levels along the A/P boundary (red in C), or the Hh targets Ptc (red in D), ptc-lacZ (red in E), ptc-GAL4, UAS–GFP (green in F), or dpp-lacZ (red in G). Amplification of the signal in ptc-GAL4, UAS–GFP should make it especially sensitive to changes in ptc transcription. (H) En (red) is expressed in the posterior compartment and activated by high levels of Hh in anterior cells abutting the A/P boundary (open arrowheads). CK1α8B12 clones do not affect En expression (solid arrowhead). CK1α8B12 clones in a homozygous dco3 background do not affect Ci155 (red in I) or Ptc (red in J).
CK1α has also been predicted to affect components of the Hh pathway. In the wing disc, Hh is secreted by posterior (P) cells and diffuses into the anterior (A) compartment to elicit concentration-dependent responses. The seven-transmembrane domain protein Smo contains CK1 phosphorylation sites that are required for Hh-driven Smo accumulation at the cell surface and subsequent signal transduction (Jia et al. 2004). Smo is downregulated in clones lacking the catalytic subunit of Protein Kinase A (PKAc), which primes Smo for CK1 phosphorylation (Jia et al. 2004). In contrast, CK1α8B12 clones left Smo levels and localization unaffected (Figure 8B), suggesting that CK1α is not essential for Smo phosphorylation. The downstream transcription factor Ci also contains CK1 phosphorylation sites, which are required for proteolytic processing of the full-length activator form of Ci (Ci155) into a C-terminally truncated repressor form (Ci75). While CK1δ/ε/Dco is clearly required for the downregulation of Ci, CK1α was also thought to contribute (Jia et al. 2005). We found that Ci155 was unaffected in CK1α8B12 clones (Figure 8C). Consistently, neither the low-threshold transcriptional target decapentaplegic (dpp) (Figure 7G) nor the high-threshold targets patched (ptc) (Figure 8, D–F) and Engrailed (En) (Figure 8H) were modified by CK1α8B12, although the same clones upregulated the Wg target Sens (Figure 8, B, D, H, and J). CK1α8B12 clones in a hypomorphic dco3 background similarly failed to affect the levels of Ci155 or its target Ptc (Figure 8, I and J), suggesting that a low level of CK1δ/ε activity can prevent ectopic Hh signaling in the absence of CK1α. In contrast to the strong upregulation of Ci155, ptc and dpp observed in mutants for sgg/GSK3, a kinase required to phosphorylate and negatively regulate both Arm and Ci (Zecca et al. 1996; Jia et al. 2002; Price and Kalderon 2002), these results do not support an essential role for CK1α in either positive or negative regulation of Hh signal transduction.
Discussion
This study completes our genome-wide screen for mutations that affect photoreceptor differentiation in Drosophila by identifying mutations on the X chromosome. Using EMS as a mutagen allowed us to identify alleles of genes for which a recent screen of lethal transposon insertions on the X chromosome (Call et al. 2007) failed to detect eye phenotypes, such as fw, nej, shi, and CK1α. However, mapping lethal point mutations is much slower than mapping transposon insertion sites, and some of the mutations we generated are still unidentified. Saturation is difficult to achieve by either method. Insertional bias makes transposable elements inefficient at mutating many genes, while the probability of isolating mutations in a gene by chemical mutagenesis is proportional to the size of the essential protein domains. The mosaic screening strategy imposes further restrictions: pericentromeric regions proximal to the FRT19A insertion cannot be screened by this method, and genes may not be identified if they act nonautonomously and/or only in a small region of the eye disc.
Eye phenotypes reveal specific functions of general regulators
Several of the mutations we identified showed specific effects on eye development despite a predicted general role of the protein affected. For example, our mutation in nej specifically disrupts R1–R7 differentiation, which depends on EGFR signaling. CBP has been shown to potentiate MAPK-enhanced transcriptional activation by the Ets-2 transcription factor (Foulds et al. 2004), but it is also important for Dpp, Hh, and Wg signaling in some contexts (Akimaru et al. 1997; Waltzer and Bienz 1998; Waltzer and Bienz 1999; Li et al. 2007). CBP functions as a histone acetyltransferase that acts on H3K27, H3K56, and trimethylated H3K4 (Das et al. 2009; Tie et al. 2009; Crump et al. 2011). The specific effects of nej8B27 suggest that acetylation of these histones is most critical for EGFR signaling during eye disc development.
Similarly, dynamin plays a general role in endocytosis, but shi7C7 mutant clones show defects characteristic of the N and EGFR signaling pathways. The neurogenic phenotype observed appears to result from both loss of N-mediated lateral inhibition, giving rise to extra R8 cells, and increased EGFR-mediated recruitment of non-R8 photoreceptors. In the Drosophila ovary, dynamin-dependent endocytosis is required for the activity of both the ligand Dl in the germline cells and its receptor N in the follicle cells (Vaccari et al. 2008; Windler and Bilder 2010). shi7C7 retinal clones likewise show apical accumulation of N and Dl, but reduced expression of a transcriptional target of N. Interestingly, the ability of N and Dl to induce the early expression of ato (Baker and Yu 1997; Ligoxygakis et al. 1998) is only slightly affected in shi clones. This proneural function of N uses a distinct transcriptional mechanism from its role in lateral inhibition (Li and Baker 2001), and our results suggest that it also differs in its requirement for dynamin-dependent endocytosis of N and Dl. EGFR also accumulates apically in shi mutant cells, but shows an increased ability to activate its transcriptional target aos. Dynamin may reduce EGFR signaling by internalizing it from the plasma membrane (Vieira et al. 1996) or may act in late endosomes to promote EGFR degradation (Schroeder et al. 2010). Our observations highlight the distinct effects of endocytic trafficking on signaling through the N and EGFR pathways.
The seven-protein Arp2/3 complex that regulates actin branching has been reported to be required in the Drosophila sensory organ precursor lineage for trafficking of endocytosed Dl-positive vesicles to the actin-rich apical microvilli of the signal-sending cell. Arp3 mutants show impaired N signaling and fail to differentiate external sensory organs (Rajan et al. 2009). Our screen isolated the first reported Arp2 mutation in Drosophila. The formation of extra R8 cells in Arp2casa clones could similarly result from reduced N signaling, although we have not been able to detect defects in Dl trafficking in these mutant cells at the light microscope level. The requirement for Arp2 in N signaling may be specific for its function in lateral inhibition, since specification of the wing margin by N-mediated inductive signaling proceeds normally in Arp2 mutant cells.
A CK1α mutation affects signaling by Wg, but not Hh
Our screen also identified the first reported allele of Drosophila CK1α. Although RNAi experiments had implicated CK1α in the control of both Wg and Hh signaling, our mutation specifically affects the Wg pathway. Biochemical experiments have shown that in the absence of Wg, cytoplasmic Arm is destabilized by a “destruction complex” in which Axin and adenomatous polyposis coli facilitate Arm phosphorylation by the kinases CK1α and Sgg/GSK3. Phosphorylation of β-Catenin by CK1α is essential to priming it for GSK3 phosphorylation and subsequent degradation (Amit et al. 2002; Liu et al. 2002; Yanagawa et al. 2002). Our finding that in vivo, loss of CK1α strongly increases Arm levels and upregulates Wg target genes supports this model. Since sgg mutants also show Arm accumulation and increased Wg signaling (Peifer et al. 1994; Siegfried et al. 1994), phosphorylation of Arm by both CK1α and GSK3 is necessary to keep Wg signaling in check.
In Drosophila, the contribution of other CK1 family members (CK1ε/dco, CK1γ/Gish, CG7094, CG2577, CG12147, CG9962, and Asator) to Wg signaling in vivo has been difficult to assess because the only tools available for many of them were RNAi transgenes, which face a tradeoff between knock-down efficiency and specificity. CK1δ and CK1ε can interact with Axin and phosphorylate β-Catenin in vitro (Amit et al. 2002; Sakanaka 2002), and genetic evidence suggested that they played both positive and negative roles in Wg signaling (Klein et al. 2006; Zhang et al. 2006; Bernatik et al. 2011). However, our results demonstrate that no other paralog can compensate for CK1α to negatively regulate Wg signaling. Consistently, an RNAi screen in Drosophila cultured cells identified CK1α, but not CK1ε, as a negative regulator of Wg reporter activity (Dasgupta et al. 2005). The conditional ablation of mouse CK1α from intestinal epithelia similarly induces β-Catenin accumulation accompanied by robust activation of many Wnt target genes (Elyada et al. 2011). The role of CK1α as the primary family member that negatively regulates Arm/β-catenin thus seems to be conserved across species.
Interestingly, many studies in different organisms indicate that upstream components of the pathway are also CK1-regulated. Phosphorylation of Dsh by CK1ε is a crucial step in the initiation and subsequent termination of signal transduction (Peters et al. 1999; Yanagawa et al. 2002; Matsubayashi et al. 2004; Klein et al. 2006; Bernatik et al. 2011; Del Valle-Perez et al. 2011). Phosphorylation of the Wg coreceptor Arr/LRP6 by the membrane associated CK1γ/Gish (Davidson et al. 2005; Zhang et al. 2006) or by CK1δ/α (Zeng et al. 2005; Zhang et al. 2006) promotes Wg signaling, while LRP6 phosphorylation by CK1ε may have both positive and negative effects (Swiatek et al. 2006; Casagolda et al. 2010), Although we have not assessed the effect of CK1α8B12 on either Arr or Dsh, our results establish that in vivo the negative regulation of Arm stability is epistatic to any positive contribution of CK1α in the Wg pathway.
In addition to Wnt transduction, the CK1 family has also been shown to regulate Hh signaling in Drosophila. In the absence of Hh, the transcription factor Ci is ubiquitinated following PKA-primed phosphorylation by GSK3 and CK1. Subsequent proteasomal processing cleaves full-length Ci155 into a repressor form, Ci75 (Jia et al. 2002; Price and Kalderon 2002; Jia et al. 2005; Smelkinson and Kalderon 2006). RNAi and overexpression experiments in vivo and in cultured cells suggested that both CK1α and CK1ε contribute to Ci phosphorylation and cleavage (Price and Kalderon 2002; Lum et al. 2003; Jia et al. 2005). Surprisingly, we found that Ci155, as well as the Hh targets ptc, en, and dpp, were unaffected by CK1α8B12 clones in wing discs. In contrast, GSK3/sgg mutant clones strongly upregulate the three Hh readouts (Jia et al. 2002; Price and Kalderon 2002). Since cells double mutant for CK1α and the hypomorphic allele dco3 also did not upregulate Hh signaling, a low level of CK1ε activity may be sufficient to phosphorylate Ci in vivo.
CK1 enzymes have also been implicated in the positive regulation of Hh transduction at the level of the transmembrane protein Smo. Hh reception promotes PKA-primed phosphorylation of Smo by CK1, inducing its cell-surface accumulation and signaling activity (Jia et al. 2004; Apionishev et al. 2005). The kinase Gprk2 further phosphorylates Smo and promotes its optimal conformation, allowing for maximal signaling (Chen et al. 2010; Chen et al. 2011). CK1 also contributes to Hh signaling by phosphorylating the downstream kinase Fu (Zhou and Kalderon 2011). Although CK1α RNAi can attenuate Smo levels and reduce the expression of the Hh target genes collier, ptc, and en (Jia et al. 2004; Apionishev et al. 2005; Zhou and Kalderon 2011), we did not detect any change in Smo levels or Hh target gene expression in CK1α8B12 clones in the wing disc. We cannot rule out the possibility that CK1α8B12 is a hypomorph that retains sufficient activity to sustain normal Hh signaling. However, we note that it activated maximal levels of Wg signaling, comparable to mutations in sgg or axin (Heslip et al. 1997; Lee and Treisman 2001). In addition, the missense mutation is not specific for Arm phosphorylation, as another phosphorylation substrate, dMyc, was also upregulated. It seems likely that CK1α acts redundantly with other CK1 family members to phosphorylate targets in the Hh pathway.
The role of CK1α in Wnt signaling and growth regulation is conserved across species and relevant to human cancer. Conditional ablation of CK1α in the mouse intestine triggers massive Wnt activation associated with anti-tumorigenic activation of the p53 pathway. Knocking down both CK1α and p53 allows for extensive proliferation of invasive carcinomas, establishing CK1α as a tumor suppressor (Elyada et al. 2011). Indeed, CK1α expression is reduced during tumor progression of human melanoma cell lines (Sinnberg et al. 2010). The generation of CK1α mutations in both flies and mice has clarified which of the previously predicted functions of CK1α do in fact specifically require this enzyme in vivo.
Acknowledgments
We thank Yashi Ahmed, Hugo Bellen, Ram Dasgupta, Robert Holmgren, Kenneth Irvine, Andrew Jarman, Jin Jiang, Ruth Lehmann, Ginés Morata, Kevin Moses, Gerd Pflugfelder, Gary Struhl, Claudio Sunkel, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank for fly stocks and reagents. We are grateful to Linda Liu for efficient lab management. The manuscript was improved by the critical comments of Sergio Astigarraga, Ram Dasgupta, Jean-Yves Roignant, Hyung Don Ryoo, and Annabelle Suisse. This work was supported by the National Institutes of Health (grant EY013777 to J.E.T.).
Footnotes
Communicating editor: T. C. Kaufman
Literature Cited
- Akimaru H., Chen Y., Dai P., Hou D. X., Nonaka M., et al. , 1997. Drosophila CBP is a co-activator of Cubitus interruptus in Hedgehog signalling. Nature 386: 735–738 [DOI] [PubMed] [Google Scholar]
- Amit S., Hatzubai A., Birman Y., Andersen J. S., Ben-Shushan E., et al. , 2002. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16: 1066–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Bhandari R., Kumar J. P., 2005. A genetic screen identifies putative targets and binding partners of CREB-binding protein in the developing Drosophila eye. Genetics 171: 1655–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apionishev S., Katanayeva N. M., Marks S. A., Kalderon D., Tomlinson A., 2005. Drosophila Smoothened phosphorylation sites essential for Hedgehog signal transduction. Nat. Cell Biol. 7: 86–92 [DOI] [PubMed] [Google Scholar]
- Baker N. E., Yu S. Y., 1997. Proneural function of neurogenic genes in the developing Drosophila eye. Curr. Biol. 7: 122–132 [DOI] [PubMed] [Google Scholar]
- Baonza A., Freeman M., 2002. Control of Drosophila eye specification by Wingless signalling. Development 129: 5313–5322 [DOI] [PubMed] [Google Scholar]
- Bernatik O., Ganji R. S., Dijksterhuis J. P., Konik P., Cervenka I., et al. , 2011. Sequential activation and inactivation of Dishevelled in the Wnt/beta-catenin pathway by casein kinases. J. Biol. Chem. 286: 10396–10410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras-Pereira C., Bessa J., Casares F., 2006. odd-skipped genes specify the signaling center that triggers retinogenesis in Drosophila. Development 133: 4145–4149 [DOI] [PubMed] [Google Scholar]
- Cagan R. L., Ready D. F., 1989. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 3: 1099–1112 [DOI] [PubMed] [Google Scholar]
- Call G. B., Olson J. M., Chen J., Villarasa N., Ngo K. T., et al. , 2007. Genomewide clonal analysis of lethal mutations in the Drosophila melanogaster eye: comparison of the X chromosome and autosomes. Genetics 177: 689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagolda D., Del Valle-Perez B., Valls G., Lugilde E., Vinyoles M., et al. , 2010. A p120-catenin-CK1epsilon complex regulates Wnt signaling. J. Cell Sci. 123: 2621–2631 [DOI] [PubMed] [Google Scholar]
- Chen M. S., Obar R. A., Schroeder C. C., Austin T. W., Poodry C. A., et al. , 1991. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature 351: 583–586 [DOI] [PubMed] [Google Scholar]
- Chen Y., Li S., Tong C., Zhao Y., Wang B., et al. , 2010. G protein-coupled receptor kinase 2 promotes high-level Hedgehog signaling by regulating the active state of Smo through kinase-dependent and kinase-independent mechanisms in Drosophila. Genes Dev. 24: 2054–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Sasai N., Ma G., Yue T., Jia J., et al. , 2011. Sonic Hedgehog dependent phosphorylation by CK1alpha and GRK2 is required for ciliary accumulation and activation of Smoothened. PLoS Biol. 9: e1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E., Feng Y., Rauskolb C., Maitra S., Fehon R., et al. , 2006. Delineation of a Fat tumor suppressor pathway. Nat. Genet. 38: 1142–1150 [DOI] [PubMed] [Google Scholar]
- Corrigall D., Walther R. F., Rodriguez L., Fichelson P., Pichaud F., 2007. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Dev. Cell 13: 730–742 [DOI] [PubMed] [Google Scholar]
- Crump N. T., Hazzalin C. A., Bowers E. M., Alani R. M., Cole P. A., et al. , 2011. Dynamic acetylation of all lysine-4 trimethylated histone H3 is evolutionarily conserved and mediated by p300/CBP. Proc. Natl. Acad. Sci. USA 108: 7814–7819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das C., Lucia M. S., Hansen K. C., Tyler J. K., 2009. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 459: 113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R., Kaykas A., Moon R. T., Perrimon N., 2005. Functional genomic analysis of the Wnt-wingless signaling pathway. Science 308: 826–833 [DOI] [PubMed] [Google Scholar]
- Davidson G., Wu W., Shen J., Bilic J., Fenger U., et al. , 2005. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438: 867–872 [DOI] [PubMed] [Google Scholar]
- Del Valle-Perez B., Arques O., Vinyoles M., de Herreros A. G., Dunach M., 2011. Coordinated action of CK1 isoforms in canonical Wnt signaling. Mol. Cell. Biol. 31: 2877–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K.-C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156 [DOI] [PubMed] [Google Scholar]
- Dokucu M. E., Zipursky S. L., Cagan R. L., 1996. atonal, rough and the resolution of proneural clusters in the developing Drosophila retina. Development 122: 4139–4147 [DOI] [PubMed] [Google Scholar]
- Domínguez M., 1999. Dual role for Hedgehog in the regulation of the proneural gene atonal during ommatidia development. Development 126: 2345–2353 [DOI] [PubMed] [Google Scholar]
- Domínguez M., Wasserman J. D., Freeman M., 1998. Multiple functions of the EGF receptor in Drosophila eye development. Curr. Biol. 8: 1039–1048 [DOI] [PubMed] [Google Scholar]
- Doroquez D. B., Rebay I., 2006. Signal integration during development: mechanisms of EGFR and Notch pathway function and cross-talk. Crit. Rev. Biochem. Mol. Biol. 41: 339–385 [DOI] [PubMed] [Google Scholar]
- Elyada E., Pribluda A., Goldstein R. E., Morgenstern Y., Brachya G., et al. , 2011. CKIalpha ablation highlights a critical role for p53 in invasiveness control. Nature 470: 409–413 [DOI] [PubMed] [Google Scholar]
- Escudero L. M., Bischoff M., Freeman M., 2007. Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev. Cell 13: 717–729 [DOI] [PubMed] [Google Scholar]
- Flores G. V., Daga A., Kalhor H. R., Banerjee U., 1998. Lozenge is expressed in pluripotent precursor cells and patterns multiple cell types in the Drosophila eye through the control of cell-specific transcription factors. Development 125: 3681–3687 [DOI] [PubMed] [Google Scholar]
- Foulds C. E., Nelson M. L., Blaszczak A. G., Graves B. J., 2004. Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Mol. Cell. Biol. 24: 10954–10964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, M., 1994 The spitz gene is required for photoreceptor determination in the Drosophila eye where it interacts with the EGF receptor. Mech. Dev. 48: 25–33. [DOI] [PubMed]
- Freeman M., 1996. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87: 651–660 [DOI] [PubMed] [Google Scholar]
- Freeman M., 1997. Cell determination strategies in the Drosophila eye. Development 124: 261–270 [DOI] [PubMed] [Google Scholar]
- Galletti M., Riccardo S., Parisi F., Lora C., Saqcena M. K., et al. , 2009. Identification of domains responsible for ubiquitin-dependent degradation of dMyc by glycogen synthase kinase 3beta and casein kinase 1 kinases. Mol. Cell. Biol. 29: 3424–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golembo M., Schweitzer R., Freeman M., Shilo B. Z., 1996. argos transcription is induced by the Drosophila EGF receptor pathway to form an inhibitory feedback loop. Development 122: 223–230 [DOI] [PubMed] [Google Scholar]
- Heberlein U., Wolff T., Rubin G. M., 1993. The TGF beta homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell 75: 913–926 [DOI] [PubMed] [Google Scholar]
- Herranz H., Perez L., Martin F. A., Milan M., 2008. A Wingless and Notch double-repression mechanism regulates G1-S transition in the Drosophila wing. EMBO J. 27: 1633–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslip T. R., Theisen H., Walker H., Marsh J. L., 1997. Shaggy and dishevelled exert opposite effects on Wingless and Decapentaplegic expression and on positional identity in imaginal discs. Development 124: 1069–1078 [DOI] [PubMed] [Google Scholar]
- Janody F., Lee J. D., Jahren N., Hazelett D. J., Benlali A., et al. , 2004. A mosaic genetic screen reveals distinct roles for trithorax and Polycomb group genes in Drosophila eye development. Genetics 166: 187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman A., Grell E., Ackerman L., Jan L., Jan Y., 1994. atonal is the proneural gene for Drosophila photoreceptors. Nature 369: 398–400 [DOI] [PubMed] [Google Scholar]
- Jia J., Amanai K., Wang G., Tang J., Wang B., et al. , 2002. Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature 416: 548–552 [DOI] [PubMed] [Google Scholar]
- Jia J., Tong C., Wang B., Luo L., Jiang J., 2004. Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase a and casein kinase I. Nature 432: 1045–1050 [DOI] [PubMed] [Google Scholar]
- Jia J., Zhang L., Zhang Q., Tong C., Wang B., et al. , 2005. Phosphorylation by double-time/CKIepsilon and CKIalpha targets Cubitus interruptus for Slimb/beta-TRCP-mediated proteolytic processing. Dev. Cell 9: 819–830 [DOI] [PubMed] [Google Scholar]
- Kenyon K. L., Ranade S. S., Curtiss J., Mlodzik M., Pignoni F., 2003. Coordinating proliferation and tissue specification to promote regional identity in the Drosophila head. Dev. Cell 5: 403–414 [DOI] [PubMed] [Google Scholar]
- Klein T. J., Jenny A., Djiane A., Mlodzik M., 2006. CKIepsilon/discs overgrown promotes both Wnt-Fz/beta-catenin and Fz/PCP signaling in Drosophila. Curr. Biol. 16: 1337–1343 [DOI] [PubMed] [Google Scholar]
- Kumar J. P., Jamal T., Doetsch A., Turner F. R., Duffy J. B., 2004. CREB binding protein functions during successive stages of eye development in Drosophila. Genetics 168: 877–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Treisman J. E., 2001. The role of Wingless signaling in establishing the anteroposterior and dorsoventral axes of the eye disc. Development 128: 1519–1529 [DOI] [PubMed] [Google Scholar]
- Lee T., Luo L., 2001. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24: 251–254 [DOI] [PubMed] [Google Scholar]
- Legent K., Treisman J. E., 2008. Wingless signaling in Drosophila eye development. Methods Mol. Biol. 469: 141–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshko-Lindsay L. A., Corces V. G., 1997. The role of selectins in Drosophila eye and bristle development. Development 124: 169–180 [DOI] [PubMed] [Google Scholar]
- Li J., Sutter C., Parker D. S., Blauwkamp T., Fang M., et al. , 2007. CBP/p300 are bimodal regulators of Wnt signaling. EMBO J. 26: 2284–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Baker N. E., 2001. Proneural enhancement by Notch overcomes Suppressor-of-Hairless repressor function in the developing Drosophila eye. Curr. Biol. 11: 330–338 [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P., Yu S. Y., Delidakis C., Baker N. E., 1998. A subset of Notch functions during Drosophila eye development require Su(H) and the E(spl) gene complex. Development 125: 2893–2900 [DOI] [PubMed] [Google Scholar]
- Liu C., Li Y., Semenov M., Han C., Baeg G. H., et al. , 2002. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847 [DOI] [PubMed] [Google Scholar]
- Lum L., Yao S., Mozer B., Rovescalli A., Von Kessler D., et al. , 2003. Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science 299: 2039–2045 [DOI] [PubMed] [Google Scholar]
- Ma C., Moses K., 1995. Wingless and Patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development 121: 2279–2289 [DOI] [PubMed] [Google Scholar]
- Ma C., Zhou Y., Beachy P., Moses K., 1993. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell 75: 927–938 [DOI] [PubMed] [Google Scholar]
- Marin O., Bustos V. H., Cesaro L., Meggio F., Pagano M. A., et al. , 2003. A noncanonical sequence phosphorylated by casein kinase 1 in beta-catenin may play a role in casein kinase 1 targeting of important signaling proteins. Proc. Natl. Acad. Sci. USA 100: 10193–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi H., Sese S., Lee J. S., Shirakawa T., Iwatsubo T., et al. , 2004. Biochemical characterization of the Drosophila Wingless signaling pathway based on RNA interference. Mol. Cell. Biol. 24: 2012–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C., Cohen S., 1996. A hierarchy of cross-regulation involving Notch, wingless, vestigial and cut organizes the dorsal/ventral axis of the Drosophila wing. Development 122: 3477–3485 [DOI] [PubMed] [Google Scholar]
- Neumann C. J., Cohen S. M., 1997. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 124: 871–880 [DOI] [PubMed] [Google Scholar]
- Newsome T., Asling B., Dickson B., 2000. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127: 851–860 [DOI] [PubMed] [Google Scholar]
- Ni J.-Q., Markstein M., Binari R., Pfeiffer B., Liu L.-P., et al. , 2008. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat. Methods 5: 49–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J.-Q., Zhou R., Czech B., Liu L.-P., Holderbaum L., et al. , 2011. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8: 405–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolo R., Abbott L. A., Bellen H. J., 2000. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102: 349–362 [DOI] [PubMed] [Google Scholar]
- Olson E. R., Pancratov R., Chatterjee S. S., Changkakoty B., Pervaiz Z., et al. , 2011. Yan, an ETS-domain transcription factor, negatively modulates the Wingless pathway in the Drosophila eye. EMBO Rep. 12: 1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill E. M., Rebay I., Tjian R., Rubin G. M., 1994. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell 78: 137–147 [DOI] [PubMed] [Google Scholar]
- Peifer M., Sweeton D., Casey M., Wieschaus E., 1994. Wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development 120: 369–380 [DOI] [PubMed] [Google Scholar]
- Peters J. M., McKay R. M., McKay J. P., Graff J. M., 1999. Casein kinase I transduces Wnt signals. Nature 401: 345–350 [DOI] [PubMed] [Google Scholar]
- Pollard T. D., 2007. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 36: 451–477 [DOI] [PubMed] [Google Scholar]
- Price M. A., Kalderon D., 2002. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell 108: 823–835 [DOI] [PubMed] [Google Scholar]
- Rajan A., Tien A. C., Haueter C. M., Schulze K. L., Bellen H. J., 2009. The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors. Nat. Cell Biol. 11: 815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready D., Hanson T., Benzer S., 1976. Development of the Drosophila retina, a neurocrystalline lattice. Dev. Biol. 53: 217–240 [DOI] [PubMed] [Google Scholar]
- Robinow S., White K., 1991. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J. Neurobiol. 22: 443–461 [DOI] [PubMed] [Google Scholar]
- Rodrigues A. B., Werner E., Moses K., 2005. Genetic and biochemical analysis of the role of Egfr in the morphogenetic furrow of the developing Drosophila eye. Development 132: 4697–4707 [DOI] [PubMed] [Google Scholar]
- Rogers E. M., Brennan C. A., Mortimer N. T., Cook S., Morris A. R., et al. , 2005. Pointed regulates an eye-specific transcriptional enhancer in the Drosophila hedgehog gene, which is required for the movement of the morphogenetic furrow. Development 132: 4833–4843 [DOI] [PubMed] [Google Scholar]
- Roignant J.-Y., Treisman J. E., 2009. Pattern formation in the Drosophila eye disc. Int. J. Dev. Biol. 53: 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo H. D., Gorenc T., Steller H., 2004. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev. Cell 7: 491–501 [DOI] [PubMed] [Google Scholar]
- Sakanaka C., 2002. Phosphorylation and regulation of beta-catenin by casein kinase I epsilon. J. Biochem. 132: 697–703 [DOI] [PubMed] [Google Scholar]
- Santos J. A., Logarinho E., Tapia C., Allende C. C., Allende J. E., et al. , 1996. The casein kinase 1 alpha gene of Drosophila melanogaster is developmentally regulated and the kinase activity of the protein induced by DNA damage. J. Cell Sci. 109: 1847–1856 [DOI] [PubMed] [Google Scholar]
- Schroeder B., Weller S. G., Chen J., Billadeau D., McNiven M. A., 2010. A Dyn2–CIN85 complex mediates degradative traffic of the EGFR by regulation of late endosomal budding. EMBO J. 29: 3039–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R., Howes R., Smith R., Shilo B. Z., Freeman M., 1995. Inhibition of Drosophila EGF receptor activation by the secreted protein Argos. Nature 376: 699–702 [DOI] [PubMed] [Google Scholar]
- Seugnet L., Simpson P., Haenlin M., 1997. Requirement for Dynamin during Notch signaling in Drosophila neurogenesis. Dev. Biol. 192: 585–598 [DOI] [PubMed] [Google Scholar]
- Shen J., Dahmann C., Pflugfelder G. O., 2010. Spatial discontinuity of optomotor-blind expression in the Drosophila wing imaginal disc disrupts epithelial architecture and promotes cell sorting. BMC Dev. Biol. 10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried E., Wilder E. L., Perrimon N., 1994. Components of Wingless signalling in Drosophila. Nature 367: 76–80 [DOI] [PubMed] [Google Scholar]
- Sigrist S. J., Lehner C. F., 1997. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90: 671–681 [DOI] [PubMed] [Google Scholar]
- Sinnberg T., Menzel M., Kaesler S., Biedermann T., Sauer B., et al. , 2010. Suppression of casein kinase 1alpha in melanoma cells induces a switch in beta-catenin signaling to promote metastasis. Cancer Res. 70: 6999–7009 [DOI] [PubMed] [Google Scholar]
- Smelkinson M. G., Kalderon D., 2006. Processing of the Drosophila Hedgehog signaling effector Ci-155 to the repressor Ci-75 is mediated by direct binding to the SCF component Slimb. Curr. Biol. 16: 110–116 [DOI] [PubMed] [Google Scholar]
- Swiatek W., Kang H., Garcia B. A., Shabanowitz J., Coombs G. S., et al. , 2006. Negative regulation of LRP6 function by Casein kinase I epsilon phosphorylation. J. Biol. Chem. 281: 12233–12241 [DOI] [PubMed] [Google Scholar]
- Tie F., Banerjee R., Stratton C. A., Prasad-Sinha J., Stepanik V., et al. , 2009. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development 136: 3131–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tio, M., C. Ma, and K. Moses, 1994 Spitz, a Drosophila homolog of Transforming growth factor-alpha, is required in the founding photoreceptor cells of the compound eye facets. Mech. Dev. 48: 13–23. [DOI] [PubMed]
- Tomlinson A., Ready D. F., 1987. Neuronal differentiation in the Drosophila ommatidium. Dev. Biol. 120: 366–376 [DOI] [PubMed] [Google Scholar]
- Treisman J. E., Rubin G. M., 1995. wingless inhibits morphogenetic furrow movement in the Drosophila eye disc. Development 121: 3519–3527 [DOI] [PubMed] [Google Scholar]
- Vaccari T., Lu H., Kanwar R., Fortini M. E., Bilder D., 2008. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J. Cell Biol. 180: 755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M., Cavallo R., Dooijes D., van Beest M., van Es J., et al. , 1997. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799 [DOI] [PubMed] [Google Scholar]
- Vieira A. V., Lamaze C., Schmid S. L., 1996. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274: 2086–2089 [DOI] [PubMed] [Google Scholar]
- Vincent J., Girdham C., O’Farrell P., 1994. A cell-autonomous, ubiquitous marker for the analysis of Drosophila genetic mosaics. Dev. Biol. 164: 328–331 [DOI] [PubMed] [Google Scholar]
- Waltzer L., Bienz M., 1998. Drosophila CBP represses the transcription factor TCF to antagonize Wingless signalling. Nature 395: 521–525 [DOI] [PubMed] [Google Scholar]
- Waltzer L., Bienz M., 1999. A function of CBP as a transcriptional co-activator during Dpp signalling. EMBO J. 18: 1630–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windler S. L., Bilder D., 2010. Endocytic internalization routes required for Delta/Notch signaling. Curr. Biol. 20: 538–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. F., Bryant P. J., 1991. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 66: 451–464 [DOI] [PubMed] [Google Scholar]
- Xu T., Rubin G., 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223–1237 [DOI] [PubMed] [Google Scholar]
- Yanagawa S.-i., Matsuda Y., Lee J.-S., Matsubayashi H., Sese S., et al. , 2002. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 21: 1733–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J. A., Cohen Y., Hudson A. M., Cooley L., Wieschaus E., et al. , 2002. SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J. Cell Biol. 156: 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M., Basler K., Struhl G., 1996. Direct and long-range action of a Wingless morphogen gradient. Cell 87: 833–844 [DOI] [PubMed] [Google Scholar]
- Zeng X., Tamai K., Doble B., Li S., Huang H., et al. , 2005. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438: 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai R., Hiesinger P., Koh T., Verstreken P., Schulze K., et al. , 2003. Mapping Drosophila mutations with molecularly defined P element insertions. Proc. Natl. Acad. Sci. USA 100: 10860–10865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Jia J., Wang B., Amanai K., Wharton K. A., et al. , 2006. Regulation of Wingless signaling by the CKI family in Drosophila limb development. Dev. Biol. 299: 221–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Kalderon D., 2011. Hedgehog activates fused through phosphorylation to elicit a full spectrum of pathway responses. Dev. Cell 20: 802–814 [DOI] [PMC free article] [PubMed] [Google Scholar]