Abstract
Tylosis esophageal cancer (TOC) is an autosomal-dominant syndrome characterized by palmoplantar keratoderma, oral precursor lesions, and a high lifetime risk of esophageal cancer. We have previously localized the TOC locus to a small genomic interval within chromosomal region 17q25. Using a targeted capture array and next-generation sequencing, we have now identified missense mutations (c.557T>C [p.Ile186Thr] and c.566C>T [p.Pro189Leu] in RHBDF2, which encodes the inactive rhomboid protease RHBDF2 (also known as iRhom2), as the underlying cause of TOC. We show that the distribution of RHBDF2 in tylotic skin is altered in comparison with that in normal skin, and immortalized tylotic keratinocytes have decreased levels of total epidermal growth factor receptor (EGFR) and display an increased proliferative and migratory potential relative to normal cells, even when normal cells are stimulated with exogenous epidermal growth factor. It would thus appear that EGFR signaling is dysregulated in tylotic cells. Furthermore, we also show an altered localization of RHBDF2 in both tylotic and sporadic squamous esophageal tumors. The elucidation of a role of RHBDF2 in growth-factor signaling in esophageal cancer will help to determine whether targeting this pathway in chemotherapy for this and other squamous cell carcinomas will be effective.
Main Text
Esophageal cancer (predominantly squamous esophageal cancer) is the sixth leading cause of cancer-related deaths worldwide.1 Pathogenesis of esophageal squamous carcinoma is still poorly understood, and there are few targeted drugs to effectively treat the condition. Type A tylosis (focal nonepidermolytic palmoplantar keratoderma; Figure 1A) is associated with a high risk of squamous cell esophageal cancer (up to 95% by age 65) in three extensive pedigrees (an example of the UK pedigree is shown in Figure S1, available online).2–4 The incidence of other cancers in these families is not altered.2,4 Tylosis esophageal cancer (TOC [OMIM 148500])5 is inherited as an autosomal-dominant trait, and the cutaneous phenotype is fully penetrant by the onset of puberty. Oral leukokeratosis (Figure 1B) and follicular hyperkeratosis are also features of the syndrome.6 We have previously mapped the TOC locus to a small section of chromosomal region 17q25 by using linkage and haplotype analysis in three extensive pedigrees from the UK, the USA, and Germany.7–10
Figure 1.
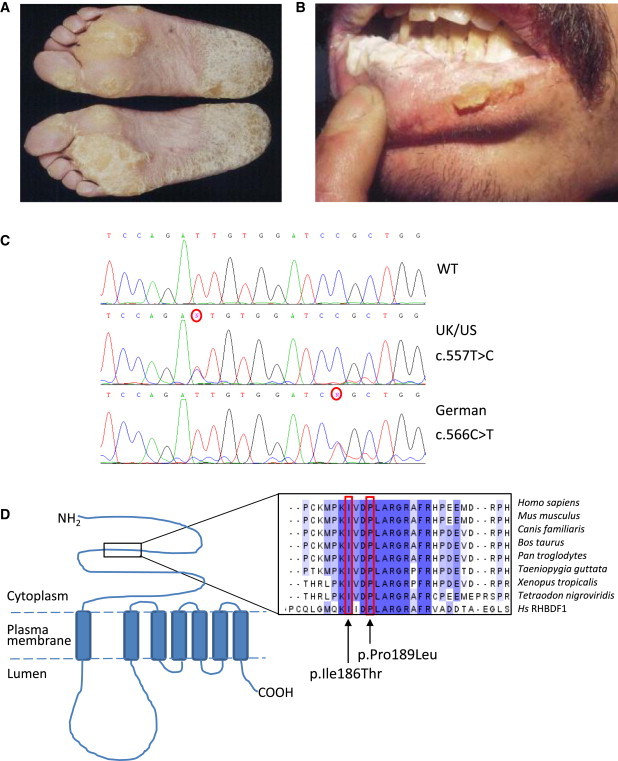
Mutations in RHBDF2 Underlie Tylosis
Clinical images of a tylosis patient showing the focal nonepidermolytic palmoplantar keratoderma (A) and oral leukokeratosis (B). Sanger sequence traces (C) displaying the c.557T>C mutation identified in the UK and USA families and the c.566C>T mutation identified in the German family.
(D) Schematic illustrating of the structure of RHBDF2, a seven-transmembrane-domain protein, and the approximate location of the alterations identified in tylosis patients. Protein alignment with ClustalW illustrates that the amino acid residues mutated in the tylosis patients (in p.Ile186Thr and p.Pro189Leu) are highly conserved across a wide range of eukaryotic species as well as in RHBDF1, a closely related member of the iRhom family.
With the development of high-throughput sequencing platforms, we have revisited the TOC locus. This included extending the minimal region (new distal recombination event between D17S1839 and D17S1603) after reassessing the recombination and affected status of a family member. The study was approved by National Health Services research ethics committees, and all patients enrolled gave their informed consent. Coordinates for all the exons from this revised minimal region, chr17q25.1–q25.2 (Figure S2), as well as some noncoding sequence, were extracted from the UCSC Genome Browser database, and probes were designed and included on a custom capture microarray (Roche NimbleGen, Madison, WI, USA). DNA from an affected individual from the UK family was randomly fragmented with a Bioruptor sonicator (Diagenode, Denville, NJ, USA), and the library of adaptor-ligated DNA fragments was prepared according to the Illumina protocol. The DNA library was then hybridized to the custom-designed microarray from NimbleGen for 72 hr, after which time any unbound DNA was washed off and the captured DNA was eluted with sodium hydroxide and amplified in a PCR with primers against the common adaptor sequences. The captured amplified DNA fragments were then sequenced as paired-end reads on the Illumina GAIIx (Illumina, San Diego, CA, USA). Raw 76 bp paired-end reads were aligned to the UCSC human genome reference sequence hg18 with novoalign, including the soft clipping, adaptor-trimming, and base-call quality calibration options. We filtered clonal reads, generated pileup, and called SNPs on the basis of allele counts and read depth by using custom Perl/C++ scripts. Exclusion of previously reported SNPs (by accessing dbSNP and 1000 Genomes Browser via the Seattle SNP website) revealed a novel isoleucine-to-threonine substitution (c.557T>C [p.Ile186Thr]) in exon 6 of the RHBDF2 (NM_024599.5 [UCSC hg19 assembly]; Figure 1C). Using a pyrosequencing assay to screen other members of the UK and US families, we found that this mutation segregated with the disease in both extensive tylosis-affected families. In brief, PCR primers and a pyrosequencing primer were designed (Table S1) to cover the c.557T>C mutation site. Hot-start PCR was carried out with 50 ng/μl of DNA template in each reaction (PCR conditions: 94°C for 5 min; 35 cycles of 94°C for 30 s, 65°C for 40 s, and 72°C for 30 s; and a final extension of 72°C for 10 min). Confirmation of PCR product quality and freedom from contamination was established on 2% agarose gels with ethidium bromide staining. Pyrosequencing was carried out with the PSQ 96MA System (Biotage), including Single-Stranded Binding Protein (PyroGold reagents), according to the manufacturers' protocol. The c.557T>C mutation was heterozygous in all affected family members genotyped (n = 34) and absent in 16 unaffected family members. Previously, haplotype data showed the segregation of two distinct disease haplotypes between these two families;6 thus, the c.557T>C mutations are likely to have arisen independently in the two families. Standard PCR and Sanger sequence analysis of the German tylosis-affected family revealed that RHBDF2 contained a distinct missense substitution, c.566C>T (p.Pro189Leu), which was 3 amino acids downstream of the c.557T>C mutation and segregated with the disease in this family (Figure 1C). Analysis revealed that both amino acids are highly conserved within the iRhom (inactive rhomboid) family in the N-terminal portion of the protein (Figure 1D). These mutations are not present in dbSNP or the 1000 Genomes database. These data strongly support RHBDF2 as the TOC-associated gene.
RHBDF2 (also known as RHBDL6 or iRhom2) belongs to a family of seven transmembrane-spanning proteins called rhomboids, which were first identified in Drosophila and were shown to be serine intramembrane proteases linked with epidermal growth factor receptor (EGFR) signaling and mitochondrial remodeling.11–14 Rhomboid proteins are relatively poorly understood proteases that are involved in the preparation of proteins, including epidermal growth factor (EGF), for export. Whereas RHBDF2 is an iRhom lacking the necessary serine721 residue for protease activity, iRhoms have been shown to regulate rhomboid-dependent proteolysis (and thus EGF signaling) by targeting specific client proteins like EGF for proteosomal removal by ER-associated degradation (ERAD).15 There is little published data on RHBDF2, although in vitro studies have shown that a closely related family member, RHBDF1, can regulate epithelial cancer cell growth and survival, possibly via GPCR-mediated EGFR signaling and/or by interacting with TGFα ligands.16,17
We performed immunohistochemistry to compare the localization of RHBDF2 between control and tylotic skin sections. We air dried frozen skin sections for 30–60 min before staining, and we fixed sections by applying ice-cold methanol-acetone (50:50) and left them to air dry for 20 min. Sections were then permeabilized for 10 min in 0.1% Triton X-100 and blocked for 20 min in 3% bovine serum albumin (BSA). Rabbit-anti-RHBDF2 (Sigma, HPA018080) was diluted 1:100 in 3% BSA and incubated overnight at 4°C. The following day, sections were washed in PBS, incubated for 1 hr at room temperature with Alexafluor-488 goat-anti-rabbit secondary antibody, and diluted 1:800 in PBS. Sections were counterstained with 100 ng/ml 4′,6-diamidino-2-phenylindole (DAPI) and mounted with Shandon Immu-Mount mounting medium (Thermo Fisher Scientific, Waltham, MA, USA). Images were taken with the Zeiss Meta-510 LSM confocal microscope. In control skin, RHBDF2 appeared throughout the epidermis with strong localization to the cell membrane (Figure 2A, see Figure S3 for antibody specificity). This was also the case in the normal esophagus epithelium. In contrast, tylotic skin (biopsied from individuals from the UK family, n = 2) showed a reduction in RHBDF2 in the cell membrane (Figure 2B). Plasma-membrane staining with an antibody against the desmosomal cadherin proteins desmogleins 1 and 2 revealed that the membrane integrity was maintained in the tylotic sections as seen in the normal skin (Figure 2C and 2D).
Figure 2.
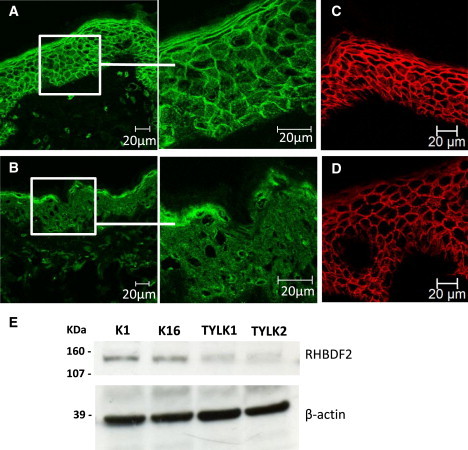
RHBDF2 Localization is Altered in Tylotic Skin
Immunofluorescence staining of frozen sections from normal skin (A) and tylotic skin (B). RHBDF2 appears to localize predominantly to the cell membrane in sections from normal skin, whereas the localization is mostly cytoplasmic in skin sections from patients with tylosis. Plasma-membrane staining with an antibody against the desmosomal cadherin proteins desmogleins 1 and 2 is shown in normal skin (C) and tylotic skin (D) and reveals that the localization of at least one other plasma-membrane protein remains the same in the tylotic-skin sections as it is in the normal-skin sections. Scale bars represent 20 μm.
(E) Immunoblotting of lysates from control keratinocyte cell lines K1 and K16 and tylotic cell lines TYLK1 and TYLK2 cultured in the presence of exogenous EGF with anti-RHBDF2 shows a reduction in RHBDF2 levels in tylotic keratinocytes. The use of anti-actin demonstrated equal loading.
RHBDF2 was also analyzed in cultured keratinocytes. After gaining appropriate consent, we obtained biopsy samples from affected (papular) skin from male and female tylosis patients (TYLK1 and TYLK2, respectively), breast skin from a 31-year-old healthy female (K1), and leg skin from a 45-year-old healthy female (K16). Primary keratinocytes were isolated and grown in the presence of a γ-irradiated 3T3 feeder layer.18 Cells were immortalized with HPV16 (E6/E7) as described previously.19 All experiments were carried out on cells that had been passaged between 10 and 40 times postimmortalization. Control cells were immortalized in the same way and have been described previously.20 Cells were cultured in DMEM:Ham's F12 medium (Invitrogen Ltd, Paisley, UK) at a 3:1 ratio with 10% fetal calf serum (ICN Biomedicals, Costa Mesa, CA) and were supplemented with 0.4 μg/ml hydrocortisone (Sigma-Aldrich), 10−10 M cholera toxin (Sigma-Aldrich, St Louis, MO, USA), and 5 μg/ml insulin (Sigma-Aldrich) at 37°C in 10% CO2. Cells were routinely cultured in media containing 10 ng/ml recombinant EGF (AbD Serotec Raleigh, NC, USA), which was omitted where indicated. We determined cell numbers by using a CASY Model TT cell counter (Roche Diagnostics, West Sussex, UK). Total protein was extracted, resolved under reducing conditions on a 4%–15% Mini-PROTEAN TGX precast gel (BioRad), transferred to Hybond ECL (GE Healthcare), incubated with the appropriate antibodies, and detected with the ECL Advance Western Blotting Detection Kit (GE Healthcare Life Sciences, Buckinghamshire, UK) according to the manufacturers' directions. Immunoblotting of lysates from immortalized tylotic keratinocytes confirmed a reduction of RHBDF2 compared to control keratinocytes (Figure 2E).
Because EGFR activation can be regulated indirectly by iRhoms, EGFR activity was investigated. Total EGFR levels were lower in tylotic keratinocytes in control cells (Figure 3A; quantification shown in Figure S4). This could be consistent with postactivation EGFR downregulation, which can occur via a number of mechanisms (reviewed in Segatto et al., 2011).21 EGFR dysregulation is seen in many tumor types, including those of the esophagous.22 In addition, when we cultured the tylotic keratinocytes in the absence of exogenous EGF, we observed that their behavior was quite different from that of control keratinocytes (n = 2, K1 and K16), which were cultured in medium supplemented with EGF. The behavior of tylotic keratinocytes suggests that exogenous EGF had little effect on them. To investigate this further, we measured the proliferation rates of normal and tylotic keratinocytes with an assay of mitochondrial dehydrogenase activity (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium, inner salt [MTS]) when they were cultured in the presence and absence, respectively, of exogenous EGF. In contrast to control keratinocytes, tylotic cells did not show any increased proliferation when they were switched to an EGF-containing medium, indicating that they were not responsive to EGF (Figure 3B).
Figure 3.
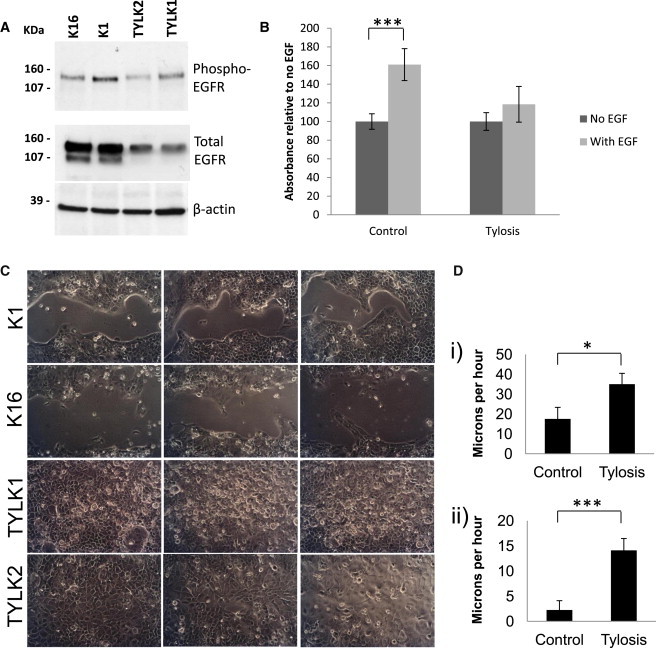
Tylosis Cell Lines Show Increased Migration and Are Less Responsive to Exogenous EGF
(A) Immunoblotting of lysates from control cells K1 and K16 and tylotic cells TYLK1 and TYLK2 blotted with anti-phospho-EGFR and anti-EGFR (total) shows a reduction in the levels of total EGFR in the lysates from tylotic cells. The use of anti-β-actin demonstrated equal loading. Cells were grown in the absence of exogenous EGF.
(B) An MTS proliferation assay shows proliferation levels in two tylotic cell lines, TYLK1 and TYLK2, and two control cell lines, K1 and K16, after a 72 hr culture in the presence and absence of exogenous EGF. The assay was carried out in quadruplicate (p = 0.00035).
(C) Light-microscopy images of cells 2 days after we scraped them in the absence of exogenous EGF. The experiment was carried out in triplicate, and a representative image is shown from each experiment for control cell lines K1 and K16 and for tylotic cell lines TYLK1 and TYLK2.
(D) Quantification of migration of control cells and tylotic cells: (1) after 1 day in the presence of EGF (p = 0.025, experiments in duplicate, control cells n = 2, tylosis cells n = 1) and (2) after 3 days in the absence of EGF (p = 2.13 × 10−6, experiments in triplicate, n = 2 in each case).
Because EGF signaling is strongly implicated in cell adhesion and migration, we performed scratch assays to imitate trauma and wounding in keratinocytes in order to assess the effect of mutant RHBDF2 on these cellular processes. Cells were seeded at equal density (3 × 105/well in a 24-well plate) and grown for 16–48 hr in media containing EGF. We wounded the resulting confluent cultures by scraping a 1 ml micro-Gilson pipette tip across the center of the well to remove cells in a linear fashion, leaving a width of 0.5–1.5 mm. Cultures were washed three times before they were incubated in media with or without EGF as indicated. Media were changed each day, and cells were photographed consecutively for 3 days or until the wounds had closed. The wounded areas were measured with Adobe Photoshop CS5 (Adobe). After calibration, we calculated the migrated distances by subtracting the area at each time point from the area immediately after we scraped the wells (time zero). Both tylotic cell lines migrated much more quickly than the control cell lines and did so in an exogenous EGF-independent manner (Figures 3C and 3D). The migration of control keratinocytes was greatly enhanced with EGF in the media (Figure 3D) but was still less than that of tylotic cells.
To investigate RHBDF2 localization in tylotic and sporadic squamous esophageal tumors, we cut 3 μm formalin-fixed, paraffin-embedded wax blocks with a rotary microtome (Thermo Fisher Scientific, Waltham, MA, USA) and applied them to 3-aminopropyltrioxysilane-coated slides. The sections were dried overnight at 37°C. The slides were de-waxed and dehydrated in two changes of Xylene and Industrial Methylated Spirit (IMS) for 3 min each. Heat-mediated antigen retrieval was carried out, and endogenous peroxidase was blocked with 0.3% hydrogen peroxide for 10 min. Staining was carried out with the RTU kit (Vector Laboratories Ltd., Peterborough, UK) and two-component 3,3′-diaminobenzidine (Launch Diagnostics Ltd., Kent, UK). Light microscopy was carried out with the Leica DM5000B epi-fluorescence microscope. Squamous esophageal tumors from both tylotic (n = 2) and sporadic (n = 4) cases also show cytoplasmic localization of RHBDF2 (Figure 4).
Figure 4.
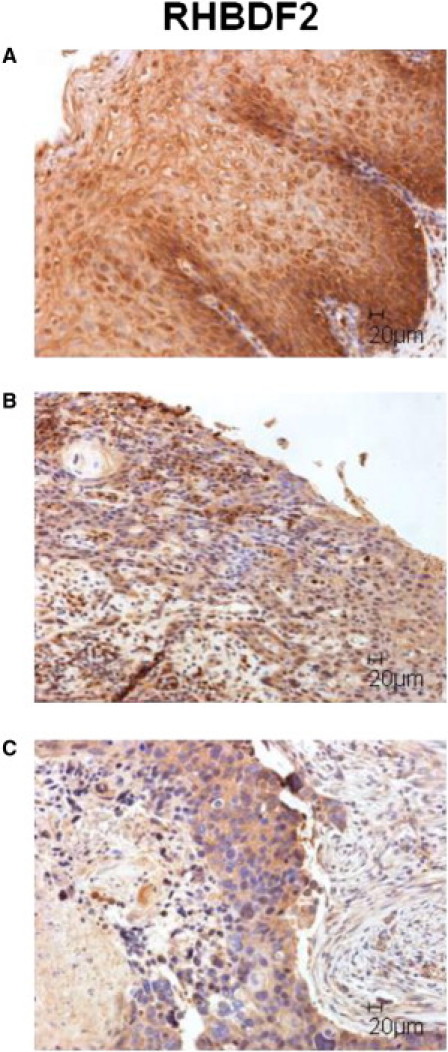
Squamous Esophageal Tumors Show Cytoplasmic Localization of RHBDF2
Immunohistochemical staining of formalin-fixed, paraffin-embedded sections from a control esophagus with esophagitis (A), tylotic (B), and sporadic (C) squamous cell tumors. The localization of RHBDF2 in both tumor types is strongly cytoplasmic compared to the control tissue. Scale bars represent 20 μm.
The high risk of developing squamous cell esophageal carcinoma in the tylosis-affected families indicates the importance of the TOC-associated gene, RHBDF2, in the molecular pathogenesis of this malignancy. Its importance is substantiated by findings of chromosomal deletions around the TOC locus in region 17q25 in the sporadic form of esophageal cancer23–26 as well as by our data showing dysregulated RHBDF2 localization in sporadic cases of TOC. It is of note that a similarly located minimal region of deletion at 17q25 has been described in ovarian cancer, and RHBDF2 was postulated as being the strongest candidate for containing a causative mutation in this region.27 Our functional data suggest that the altered RHBDF2 represents a gain-of-function allele that results in sustained EGFR signaling within the cells; this sustained EGFR signaling, in turn, leads to a hyperproliferative phenotype. Our results support the hypothesis that tylosis results from dysregulated wound repair that leads to precancerous lesions in the esophagus and other nonkeratinized epithelium of the upper gastrointestinal tract.
In summary, we have identified RHBDF2 missense mutations as the underlying cause of a highly penetrant form of inherited esophageal cancer. Furthermore, our preliminary immunohistochemical data suggest that RHBDF2 might also be dysregulated in a similar manner in sporadic esophageal squamous cell carcinomas, in which case these tumors might be resistant to treatment with EGFR inhibitors. Therefore, developing chemotherapies targeted to RHBDF2-regulated pathways could be more effective in reducing the hyperproliferative and invasive aspects of esophageal cancers. Characterizing the TOC-associated gene will contribute to understanding the more common, sporadically occurring esophageal cancer, a major cause of mortality in both developing and developed countries.
Acknowledgments
This work was funded by the Queen Mary Innovation Fund (to D.P.K.) and, in part, by Cancer Research UK (to J.M.R. [C7738/A10476] and I.M.L. [C5314/A6695]). We thank the Barts and the London Genome Centre and the Blizard Advanced Light Microscopy core facilities. We also thank Rebecca Harrison for helpful comments.
Supplemental Data
Web Resources
The URLs for data presented herein are as follows:
1000 Genomes, http://www.1000genomes.org/
Novoalign, http://www.novocraft.com
Online Mendelian Inheritance in Man (OMIM), http://www.omim.org
Seattle SNP, http://pga.gs.washington.edu/
UCSC Genome Browser database, http://genome.ucsc.edu/
References
- 1.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Ellis A., Field J.K., Field E.A., Friedmann P.S., Fryer A., Howard P., Leigh I.M., Risk J., Shaw J.M., Whittaker J. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family—a review of six generations. Eur. J. Cancer B Oral Oncol. 1994;30B:102–112. doi: 10.1016/0964-1955(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 3.Hennies H.C., Hagedorn M., Reis A. Palmoplantar keratoderma in association with carcinoma of the esophagus maps to chromosome 17q distal to the keratin gene cluster. Genomics. 1995;29:537–540. doi: 10.1006/geno.1995.9971. [DOI] [PubMed] [Google Scholar]
- 4.Stevens H.P., Kelsell D.P., Bryant S.P., Bishop D.T., Spurr N.K., Weissenbach J., Marger D., Marger R.S., Leigh I.M. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. Literature survey and proposed updated classification of the keratodermas. Arch. Dermatol. 1996;132:640–651. [PubMed] [Google Scholar]
- 5.Risk J.M., Field E.A., Field J.K., Whittaker J., Fryer A., Ellis A., Shaw J.M., Friedmann P.S., Bishop D.T., Bodmer J. Tylosis oesophageal cancer mapped. Nat. Genet. 1994;8:319–321. doi: 10.1038/ng1294-319. [DOI] [PubMed] [Google Scholar]
- 6.Field E.A., Ellis A., Friedmann P.S., Leigh I.M., Field J.K. Oral tylosis: A re-appraisal. Oral Oncol. 1997;33:55–57. doi: 10.1016/s0964-1955(96)00052-8. [DOI] [PubMed] [Google Scholar]
- 7.Kelsell D.P., Risk J.M., Leigh I.M., Stevens H.P., Ellis A., Hennies H.C., Reis A., Weissenbach J., Bishop D.T., Spurr N.K., Field J.K. Close mapping of the focal non-epidermolytic palmoplantar keratoderma (PPK) locus associated with oesophageal cancer (TOC) Hum. Mol. Genet. 1996;5:857–860. doi: 10.1093/hmg/5.6.857. [DOI] [PubMed] [Google Scholar]
- 8.Langan J.E., Cole C.G., Huckle E.J., Byrne S., McRonald F.E., Rowbottom L., Ellis A., Shaw J.M., Leigh I.M., Kelsell D.P. Novel microsatellite markers and single nucleotide polymorphisms refine the tylosis with oesophageal cancer (TOC) minimal region on 17q25 to 42.5 kb: Sequencing does not identify the causative gene. Hum. Genet. 2004;114:534–540. doi: 10.1007/s00439-004-1100-3. [DOI] [PubMed] [Google Scholar]
- 9.Risk J.M., Evans K.E., Jones J., Langan J.E., Rowbottom L., McRonald F.E., Mills H.S., Ellis A., Shaw J.M., Leigh I.M. Characterization of a 500 kb region on 17q25 and the exclusion of candidate genes as the familial Tylosis Oesophageal Cancer (TOC) locus. Oncogene. 2002;21:6395–6402. doi: 10.1038/sj.onc.1205768. [DOI] [PubMed] [Google Scholar]
- 10.Risk J.M., Ruhrberg C., Hennies H., Mills H.S., Di Colandrea T., Evans K.E., Ellis A., Watt F.M., Bishop D.T., Spurr N.K. Envoplakin, a possible candidate gene for focal NEPPK/esophageal cancer (TOC): The integration of genetic and physical maps of the TOC region on 17q25. Genomics. 1999;59:234–242. doi: 10.1006/geno.1999.5857. [DOI] [PubMed] [Google Scholar]
- 11.McQuibban G.A., Saurya S., Freeman M. Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature. 2003;423:537–541. doi: 10.1038/nature01633. [DOI] [PubMed] [Google Scholar]
- 12.Urban S., Lee J.R., Freeman M. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell. 2001;107:173–182. doi: 10.1016/s0092-8674(01)00525-6. [DOI] [PubMed] [Google Scholar]
- 13.Dimmer K.S., Fritz S., Fuchs F., Messerschmitt M., Weinbach N., Neupert W., Westermann B. Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol. Biol. Cell. 2002;13:847–853. doi: 10.1091/mbc.01-12-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman M., Kimmel B.E., Rubin G.M. Identifying targets of the rough homeobox gene of Drosophila: evidence that rhomboid functions in eye development. Development. 1992;116:335–346. doi: 10.1242/dev.116.2.335. [DOI] [PubMed] [Google Scholar]
- 15.Zettl M., Adrain C., Strisovsky K., Lastun V., Freeman M. Rhomboid family pseudoproteases use the ER quality control machinery to regulate intercellular signaling. Cell. 2011;145:79–91. doi: 10.1016/j.cell.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagawa T., Guichard A., Castro C.P., Xiao Y., Rizen M., Zhang H.-Z., Hu D., Bang A., Helms J., Bier E., Derynck R. Characterization of a human rhomboid homolog, p100hRho/RHBDF1, which interacts with TGF-α family ligands. Dev. Dyn. 2005;233:1315–1331. doi: 10.1002/dvdy.20450. [DOI] [PubMed] [Google Scholar]
- 17.Zou H., Thomas S.M., Yan Z.W., Grandis J.R., Vogt A., Li L.Y. Human rhomboid family-1 gene RHBDF1 participates in GPCR-mediated transactivation of EGFR growth signals in head and neck squamous cancer cells. FASEB J. 2009;23:425–432. doi: 10.1096/fj.08-112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rheinwald J.G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 19.Storey A., Pim D., Murray A., Osborn K., Banks L., Crawford L. Comparison of the in vitro transforming activities of human papillomavirus types. EMBO J. 1988;7:1815–1820. doi: 10.1002/j.1460-2075.1988.tb03013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morley S.M., Dundas S.R., James J.L., Gupta T., Brown R.A., Sexton C.J., Navsaria H.A., Leigh I.M., Lane E.B. Temperature sensitivity of the keratin cytoskeleton and delayed spreading of keratinocyte lines derived from EBS patients. J. Cell Sci. 1995;108:3463–3471. doi: 10.1242/jcs.108.11.3463. [DOI] [PubMed] [Google Scholar]
- 21.Segatto O., Anastasi S., Alemà S. Regulation of epidermal growth factor receptor signalling by inducible feedback inhibitors. J. Cell Sci. 2011;124:1785–1793. doi: 10.1242/jcs.083303. [DOI] [PubMed] [Google Scholar]
- 22.Morgan S., Grandis J.R. ErbB receptors in the biology and pathology of the aerodigestive tract. Exp. Cell Res. 2009;315:572–582. doi: 10.1016/j.yexcr.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwaya T., Maesawa C., Ogasawara S., Tamura G. Tylosis esophageal cancer locus on chromosome 17q25.1 is commonly deleted in sporadic human esophageal cancer. Gastroenterology. 1998;114:1206–1210. doi: 10.1016/s0016-5085(98)70426-3. [DOI] [PubMed] [Google Scholar]
- 24.Shahabi M., Noori Daloii M.R., Langan J.E., Rowbottom L., Jahanzad E., Khoshbin E., Taghikhani M., Field J.K., Risk J.M. An investigation of the tylosis with oesophageal cancer (TOC) locus in Iranian patients with oesophageal squamous cell carcinoma. Int. J. Oncol. 2004;25:389–395. doi: 10.3892/ijo.25.2.389. [DOI] [PubMed] [Google Scholar]
- 25.von Brevern M., Hollstein M.C., Risk J.M., Garde J., Bennett W.P., Harris C.C., Muehlbauer K.R., Field J.K. Loss of heterozygosity in sporadic oesophageal tumors in the tylosis oesophageal cancer (TOC) gene region of chromosome 17q. Oncogene. 1998;17:2101–2105. doi: 10.1038/sj.onc.1202139. [DOI] [PubMed] [Google Scholar]
- 26.Rumiato E., Pasello G., Montagna M., Scaini M.C., De Salvo G.L., Parenti A., Cagol M., Ruol A., Ancona E., Amadori A., Saggioro D. DNA copy number profile discriminates between esophageal adenocarcinoma and squamous cell carcinoma and represents an independent prognostic parameter in esophageal adenocarcinoma. Cancer Lett. 2011;310:84–93. doi: 10.1016/j.canlet.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Presneau N., Dewar K., Forgetta V., Provencher D., Mes-Masson A.M., Tonin P.N. Loss of heterozygosity and transcriptome analyses of a 1.2 Mb candidate ovarian cancer tumor suppressor locus region at 17q25.1-q25.2. Mol. Carcinog. 2005;43:141–154. doi: 10.1002/mc.20096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


