Abstract
The overall objective of this project is to develop a feedback-driven intraspinal microstimulation (ISMS) system. We hypothesize that ISMS will enhance the functionality of stepping by reducing muscle fatigue and producing synergistic movements by activating neural networks in the spinal cord. In the present pilot study, the controller was tested with ISMS and external sensors (force plates, gyroscopes, and accelerometers). Cats were partially supported in a sling and bi-laterally stepped overground on a 4-m instrumented walkway. The walkway had variable friction. Limb angle was controlled to within 10° even in the presence of variable friction. Peak ground reaction forces in each limb were approximately 12% of body weight (12.5% was full load bearing in this experimental setup); rarely, the total supportive force briefly decreased to as low as 4.1%. Magnetic resonance images were acquired of the excised spinal cord and the implanted array. The majority of electrodes (75%) were implanted successfully into their target regions. This represents the first successful application of ISMS for overground walking.
I. Introduction
There are currently about 1.3 million people affected by spinal cord injury in the United States [1]. Of these, 28.2% have complete paraplegia and reduced mobility. Our laboratory’s overall goal is to improve the quality of life of people with paraplegia by increasing their mobility. More specifically, we aim to develop a device which will use functional electrical stimulation (FES) to restore walking. Current FES devices are limited in their ability to restore walking because they generally cannot adapt to muscle fatigue and external perturbations which may lead to falls [2]. As such, the devices have not been widely adopted. We hypothesize that adding sensory feedback will enhance the functionality of stepping by reducing muscle fatigue and adapting to external perturbations.
Our algorithm for controlling locomotion employs intrinsic timing (similar to the central pattern generator for walking, CPG) as well as sensory feedback from external sensors to mimic a physiological walking system. Previous work has shown that such a controller algorithm, called hybrid CPG algorithm, can drive a stimulator to generate stable overground walking when used in conjunction with intramuscular stimulation (IMS) [3, 4]. The controller also adapted the stimulation patterns to environmental perturbations in real time.
During normal walking, a wealth of sensory information is provided to the central nervous system (CNS) from specialized cells. These signals can be accessed in the dorsal root ganglia (DRG) with implantable electrode arrays and provide vital information for the successful coordination of movements. Previously, we have shown that DRG recordings can be decoded to provide prediction of limb position with less than 2 cm error [5].
Our goal is to use feedback to control intraspinal microstimulation (ISMS) based on either neural recordings from dorsal root ganglia (DRG) or external sensors. ISMS is a method of electrically stimulating regions of the spinal cord, called motor pools, to produce coordinated movements with less fatigue than with surface stimulation [6, 7]. Nerve cuff electrodes, while decreasing the current required to activate muscles relative to surface stimulation, also produce fatigable movements and force production that rises steeply with stimulation level [8, 9]. ISMS activates the intact neural networks below a spinal lesion using low amplitudes (100–200 uA) of current. By changing stimulation parameters in response to feedback and timing, it is possible to modify the ISMS-produced stepping in real-time. We have completed experimental trials of 5 cats implanted with ISMS. Data from the cat that has been analyzed most fully are presented in this paper.
II. Materials and methods
A. Animal Preparation
The experiment was conducted according to the University of Alberta Code of Animal Conduct. The cat (female, 3.98 kg) was anesthetized using isoflurane and subsequently switched to sodium pentobarbital. A laminectomy was performed from L4–L6. A pair of fine wire arrays consisting of 12 Pt-Ir electrodes per side (50 µm diameter) was implanted with the electrode tips targeting the motor pools in the ventral horn of the spinal cord. Short bursts of current were passed through each electrode to verify that it elicited the desired movements and the electrode was repositioned if necessary. A custom stimulator developed by Sigenics Inc. (Chicago, IL, USA) used 16 channels of stimulation to produce walking patterns in the hindlimbs. Trains of biphasic charge-balanced pulses (62Hz, up to 250µA, 100µs pulse width, with on and off ramping) were used. Each channel was stimulated sequentially. Amplitudes were chosen to provide adequate forward propulsion. The cat was then transferred to an instrumented walkway and partially suspended in a cart-mounted sling. The cat remained under anesthesia for the duration of the acute experiment. Following the experiment, the animal was euthanized. An acute experiment allowed for faster development of the control algorithm.
B. Sensor Preparation
Three sets of external sensors were used: gyroscopes, accelerometers and force plates. The gyroscope signals were integrated to provide a measure of limb angle. The accelerometers provided information regarding leg orientation with respect to gravity. Both sensor sets were calibrated and fixed to the foot of the hind limb and their signals were used for triggering sensory rule transitions. The 4-m walkway had separate force plates for each leg, which measured ground reaction forces. Sensor data were acquired using a Cerebus analog-to-digital conversion system at a rate of 1000 samples/sec. The data were streamed from the Cerebus into Matlab to be processed in real time by custom written software.
Reflective markers were placed on the right hind limb of the cat to indicate the iliac crest, hip, knee, ankle, and metatarsophalangeal joints. The positions of these markers (using video recording) were used to delineate joint position and leg movement, but were not used in the real time sensory feedback rules.
C. Hybrid CPG Controller
The controller adapted stimulation parameters in real time to create a walking cycle. It contained a state system which divided the walking cycle into four states and restricted the activation of rules to specific states. The states were swing (F), touchdown (E1), stance (E2) and push off (E3). The states of each leg were paired forming 8 different stable walking combinations and a stance combination that produced standing prior to the initiation of walking. The controller was preset to a walking cycle period of 1.5 sec with individual weighting of states at 20%, 20%, 20%, and 40% of the walking period, respectively. There were 13 rules divided into two groups: intrinsic timing rules and sensory feedback rules. The 9 timing rules mimicked the intrinsic timing of the CPG and automatically advanced the 9 states according to preset times. The timing rules prevented the controller from becoming unstable due to inadequate (or inappropriate) sensory information. The four IF-THEN sensory feedback rules were: swing to stance transition, stance to swing transition, fatigue compensation, and a safety rule. The first two transition rules prevented excessive flexion and backward hyperextension, respectively. They immediately changed the walking state if a pre-determined sensory threshold was reached. The next two rules monitored supportive forces and, in the event of a sudden decrease, were designed to restore ground reaction forces to maintain stability. The fatigue compensation rule increased stimulation amplitudes (typically by 30%) in the stance phase to increase load bearing forces. The safety rule held the load bearing leg in stance while the opposite leg cycled through its walking states until it regained sufficient/stable ground reaction force (GRF). If feedback rules did not intervene, the state transitions proceeded according to preset timings.
Operators chose the activation thresholds for the IF-THEN rules after analyzing gyroscope, accelerometer and force records from trials with the feedback disabled (open loop). With feedback engaged, the controller adapted stimulation parameters depending on the current walking state. All sensor data, controller states and kinematics of the leg were recorded.
D. MRI Evaluation of Electrode Placement
The spinal cord with the implanted electrode array was carefully excised post-mortem and placed in formaldehyde until image acquisition. The spinal cord was imaged using a 4.7 T magnet. The images were acquired with a voxel size of 0.25 × 0.25 × 1.0 mm using T2 weighting. A custom sequence was used to minimize the artefacts caused by the Pt-Ir electrodes and optimize the contrast between the gray and white matter.
III. Results
A. Overground Walking
We required 3 trials to set stimulation amplitudes and sensory feedback rule thresholds. A trial was considered successful if the cat began 50 cm from the start of the walkway and walked at least 55% across the length of the walkway (resulting in a total distance of at least 2.5 m). The cat completed 15 of 30 trials successfully (5 of 12 open loop, 10 of 18 Hybrid CPG). The incomplete trials stopped 40 ± 19 cm (mean ± sd) from the end of the walkway. Most often, failure was caused by the operator incorrectly setting thresholds for the IF-THEN rules.
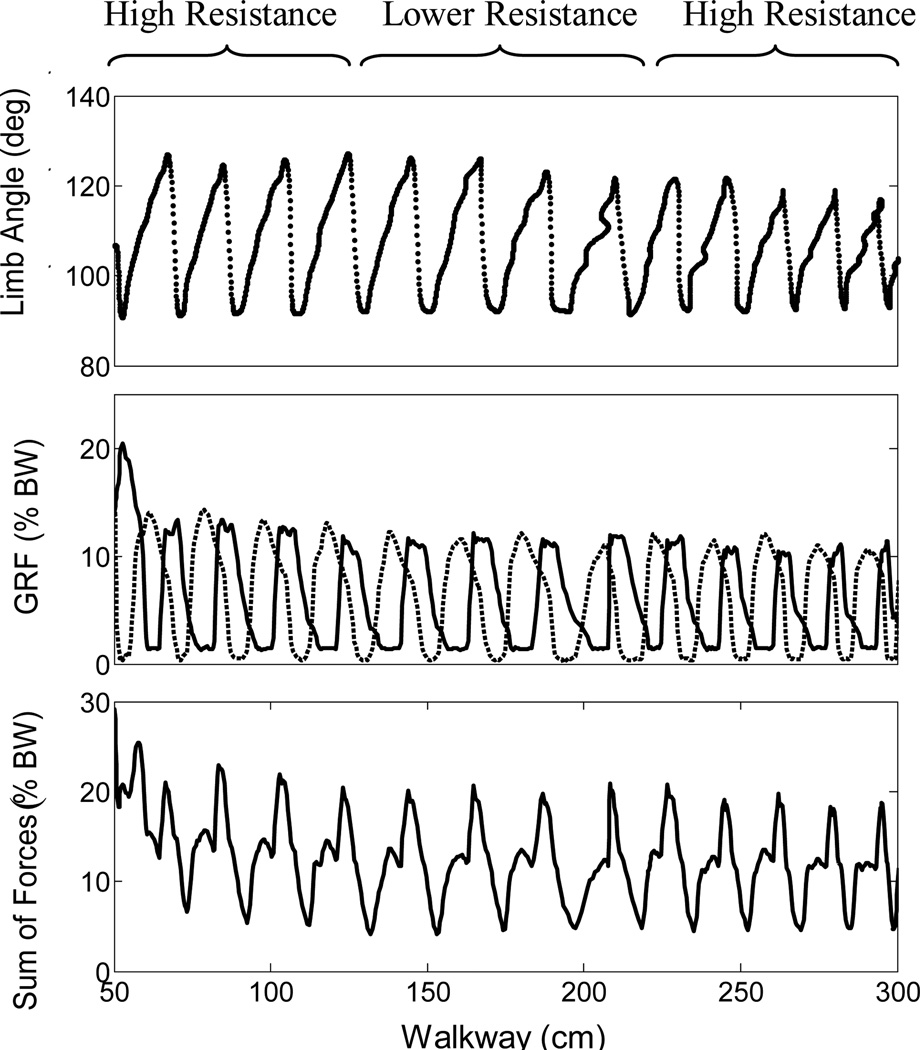
The results for one typical trial using the controller with feedback are shown in Fig. 1. The limb angle remained stable and varied by a maximum of only 10° even in the presence of perturbations from the walkway (the regions of varying resistance, ~Δ15%). When the limb angle reached threshold during backward extension, the remainder of E3 was truncated and the controller transitioned to F. This transition reduced the excessive backward limb extension often observed in trials without sensory feedback due to a longer E3 duration. Due to the experimental setup of our sling, which supported the weight of the head, chest and trunk, 12.5% of body weight was considered full load bearing support for a single hindlimb. The peak ground reaction forces for each leg were constant at around 12% of body weight except for the initial step when GRF peaks at 20% while overcoming static friction of the cart setup. However, there were regions where the total body weight support (sum of GRF’s) dropped to lower than desired levels (4.1% of BW). This would not be adequate for functional walking. These regions occur during transitions between stance and swing phases when one leg has not yet developed enough GRF and the other leg has already begun swinging. Improvements in the feedback control strategy are expected to eliminate such periods of low GRF. Furthermore, stronger movements using ISMS have been observed in different preparations when anesthesia has been removed leading to greater excitability of spinal neural networks [7, 10].
Fig. 1.
Limb angle and ground reaction forces during a trial using the controller with feedback. Regions of lower and higher walkway resistance are indicated above the top panel. Top panel: Video recordings of limb angle (measured from hip to paw) in degrees clockwise from horizontal across the walkway. Middle panel: Left and right ground reaction forces as percent of total body weight. Right leg is shown as dashed line and left leg is shown as solid line. Lower panel: The sum of the ground reaction forces of each leg represents the total load bearing ability of the animal.
Table 1 shows the summary of 15 successful trials of open loop and Hybrid CPG control. By adapting to the varying walkway conditions, the Hybrid CPG controller changed the durations of the walking states according to the IF-THEN rule base. Most often, excess time in E3 was truncated which reduced excess backward extension (P=0.001). This resulted in the cat taking significantly more steps to complete the trial (P=0.009). However, the average walking speed for a trial with open loop control was not different from the Hybrid CPG controller (0.11 ± 0.03 m/s and 0.10 ± 0.03 m/s, P=0.9) in this cat. Summing the total GRF of each leg and averaging over the trial showed a slight increase (but not significant) in supportive force with the Hybrid CPG controller. We anticipate that differences in walking speed and ground reaction forces with Hybrid CPG control will be found when other cats are included in the analysis.
TABLE 1.
Summary of 15 successful trials
| Walking Property | Open Loop Controller (5 trials) |
Hybrid CPG Controller (10 trials) |
|---|---|---|
| Number of Stepsa | 14.4 ± 1.5 | 20 ± 3.9 |
| Stride Length (norm.)ab | 17.7 ± 6.2 | 14.9 ± 5.1 |
| Average Velocity | 0.11 ± 0.03 m/s | 0.10 ± 0.03 m/s |
| Average Sum. GRF (%BW) | 10.2 ± 2.4 | 10.4 ± 1.4 |
Significant difference between trials with intrinsic timing alone and with sensory feedback.
Stride length was normalized against the distance from the ankle to the metacarpophalangeal joint.
B. Electrode Placement
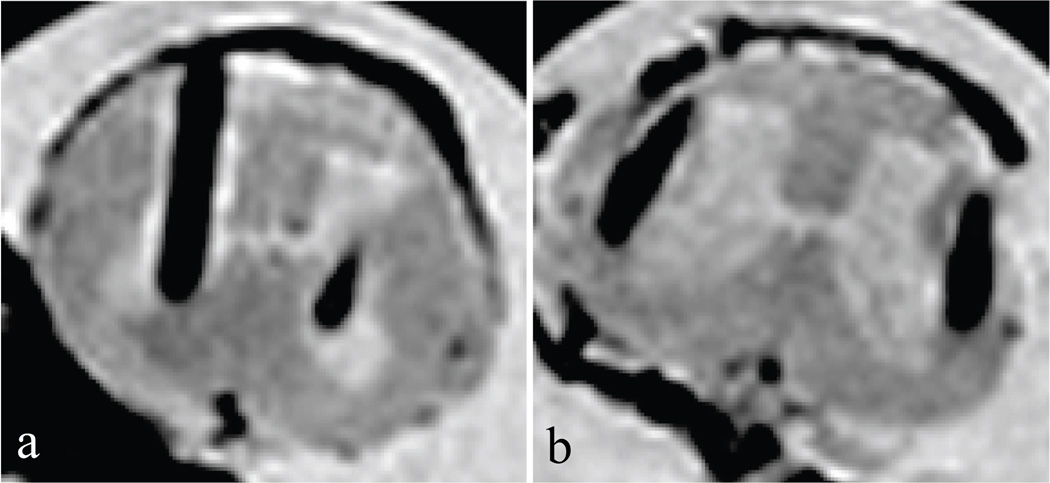
The MR images of the extracted spinal cord are shown in Fig. 2. The artifacts produced by the Pt-Ir electrodes are visible in the images to show placement, but are much thicker than the actual size of the electrodes. Tabulated results are shown in Table 2. The majority of the electrodes (18 of 24, 75%) were correctly implanted into the ventral horn of the spinal cord.
Fig. 2.
MR images showing electrode placement. Both images are transverse slices of the excised spinal cord at the L5 level. Dorsal is upwards in the images. The dark lines running vertical are the artifacts caused by the 50 µm electrodes. a) An acceptable implant with both wire tips located in the ventral gray matter (lighter color in this T2-weighted image). The right electrode is slightly out of plane and only the tip is visible in the image. b) An implantation where both electrodes are too lateral and are implanted in the surrounding white matter.
TABLE 2.
Electrode placement results
| Location | Number of Electrodes |
|---|---|
| Left side | |
| Ventral gray matter | 9 |
| White matter | 2 |
| Not implanted | 1 |
| Right side | |
| Ventral gray matter | 9 |
| White matter | 2 |
| Not implanted | 1 |
Four electrodes missed the gray matter and were found in the surrounding white matter (Fig. 2b). These locations did not produce functionally different movements from ones in the gray matter electrodes during test stimulation but showed much faster force recruitment curve characteristics. Since the array dimensions were preset, in this cat, the most caudal electrodes were outside the functional region of the cord. Two electrodes (1 per side) of the 24 total were cut and not implanted. Although only tip location is functionally important, angles of implantation (both rostral-caudally and medial-laterally) were inconsistent. This is attributed to the manual insertion of each electrode.
IV. Conclusion
The work presented here demonstrates our ability to create a reconfigurable control algorithm for realizing different stimulation paradigms to create walking movements. The Hybrid CPG controller has demonstrated the benefit of sensory feedback in a cat with an ISMS implant. ISMS has shown promise in creating fatigue resistant movements strong enough to be capable of overground locomotion. During successful trials, supportive ground reaction forces appeared promising, falling just short of full weight support. With the removal of anesthesia in a chronic preparation, more excitable neural networks should produce stronger forces. Moreover, improvements in the control strategy would likely eliminate the periods of low GRF altogether.
Due to the manual insertion of electrodes, targeting and alignment of the implanted electrodes requires improvement. A well localized tip results in improved muscle responses and force generation. Currently, we are developing methods to reference our implantation system to the anatomy of the spinal cord in an effort to improve responses. Future work will replace external sensors with DRG recordings to allow the internalization of the prosthetic device. Eventually, we hope to develop this research into a compact and fully implantable walking prosthesis. This study will provide a proof-of-principle for application to humans with paraplegia.
Acknowledgment
The authors thank Dr. Phillip Troyk from Sigenics Inc. for providing the 16-channel stimulator, Mr. Robert Rolf for preparing the gyroscope and accelerometer circuits and Dr. Douglas Weber for the use of his MotionTracker motion capture code.
This work was supported in part by AHFMR, CIHR, NIH and the Christopher and Dana Reeve Foundation.
Contributor Information
Bradley J. Holinski, Department of Biomedical Engineering, University of Alberta, Edmonton, AB Canada (phone: 780-492-3796; bjh2@ualberta.ca).
Kevin A. Mazurek, Department of Electrical and Computer Engineering, Johns Hopkins University, Baltimore, MD USA. (kmazurek@jhu.edu).
Dirk G. Everaert, Department of Physiology, University of Alberta, Edmonton, AB Canada (veraert@ualberta.ca)
Richard B. Stein, Department of Physiology, University of Alberta, Edmonton, AB Canada (Richard.stein@ualberta.ca)
Vivian K. Mushahwar, Department of Cell Biology, University of Alberta, Edmonton, AB Canada (Vivian.mushahwar@ualberta.ca).
REFERENCES
- 1.Christopher and Dana Reeve Foundation. [2010, May, 10]; [Online]. Available: www.christopherreeve.org.
- 2.Brissot R, et al. Clinical experience with functional electrical stimulation-assisted gait with Parastep in spinal cord-injured patients. Spine (Phila Pa 1976) 2000 Feb 15;vol. 25:501–508. doi: 10.1097/00007632-200002150-00018. [DOI] [PubMed] [Google Scholar]
- 3.Guevremont L, et al. Physiologically based controller for generating overground locomotion using functional electrical stimulation. J Neurophysiol. 2007 Mar;vol. 97:2499–2510. doi: 10.1152/jn.01177.2006. [DOI] [PubMed] [Google Scholar]
- 4.Mazurek K, Holinski BJ, Everaert DG, Stein RB, Mushahwar VK. A novel control algorithm with feed forward and feedback control for over-ground locomotion in anesthetized cats. doi: 10.1088/1741-2560/9/2/026003. to be published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber DJ, Stein RB, Evereart DG, Prochazka A. Limb-state feedback from ensembles of simultaneously recorded dorsal root ganglion neurons. J Neural Eng. 2007 Sep;vol. 4:S168–S180. doi: 10.1088/1741-2560/4/3/S04. [DOI] [PubMed] [Google Scholar]
- 6.Lau B, Guevremont L, Mushahwar VK. Strategies for generating prolonged functional standing using intramuscular stimulation or intraspinal microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2007 Jun;vol. 15:273–285. doi: 10.1109/TNSRE.2007.897030. [DOI] [PubMed] [Google Scholar]
- 7.Saigal R, Renzi C, Mushahwar VK. Intraspinal microstimulation generates functional movements after spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004 Dec;vol. 12:430–440. doi: 10.1109/TNSRE.2004.837754. [DOI] [PubMed] [Google Scholar]
- 8.Bamford JA, Putman CT, Mushahwar VK. Intraspinal microstimulation preferentially recruits fatigue-resistant muscle fibres and generates gradual force in rat. J Physiol. 2005 Dec 15;vol. 569:873–884. doi: 10.1113/jphysiol.2005.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snow S, Horch KW, Mushahwar VK. Intraspinal microstimulation using cylindrical multielectrodes. IEEE Trans Biomed Eng. 2006 Feb;vol. 53:311–319. doi: 10.1109/TBME.2005.857638. [DOI] [PubMed] [Google Scholar]
- 10.Stein RB, Aoyagi Y, Mushahwar VK, Prochazka A. Limb movements generated by stimulating muscle, nerve and spinal cord. Arch Ital Biol. 2002 Oct;vol. 140:273–281. [PubMed] [Google Scholar]