Abstract
Although the mammalian immune system is generally thought to develop in a linear fashion, findings in avian and murine species argue instead for the developmentally ordered appearance (or “layering”) of unique hematopoietic stem cells (HSC) that give rise to distinct lymphocyte lineages at different stages of development. Here, we provide evidence of an analogous “layered” immune system in humans. Our results suggest that fetal and adult T cells are distinct populations that arise from different populations of HSC present at different stages of development. We also provide evidence that the fetal T cell lineage is biased towards immune tolerance. These observations offer a mechanistic explanation for the tolerogenic properties of the developing fetus and for variable degrees of immune responsiveness at birth.
The developing fetal immune system is generally believed to induce immune tolerance after exposure to foreign antigens (1). In the mouse, this tolerogenic tendency has been attributed to the absence of a “mature” adaptive immune system prior to birth (2). In the human, however, fetal exposure to foreign antigens, notably maternal alloantigens, can lead to the generation of immune tolerance (4–6), even though the immune system develops at a substantially earlier developmental stage (1, 3). Recently, we reported that tolerance induction in the human fetus is in part mediated by an abundant population of fetal regulatory T cells (Tregs) (7), a cell population comprising a significantly greater percentage (~15%) of total peripheral CD4+ T cells in the developing human fetus than is found in healthy infants and adults (~5%) (8). Unlike adult T cells, we also observed that fetal T cells exhibit enhanced proliferation after exposure to alloantigens and are poised to become Tregs upon stimulation, a process dependent upon transforming growth factor-β (TGF- β) (6). Given these observations, we hypothesized that the human fetal T cell compartment may not simply be an “immature” version of the adult T cell compartment but, instead, one derived from a wholly unique lineage of T cells that is poised to deliver a tolerogenic response to all antigens encountered in utero.
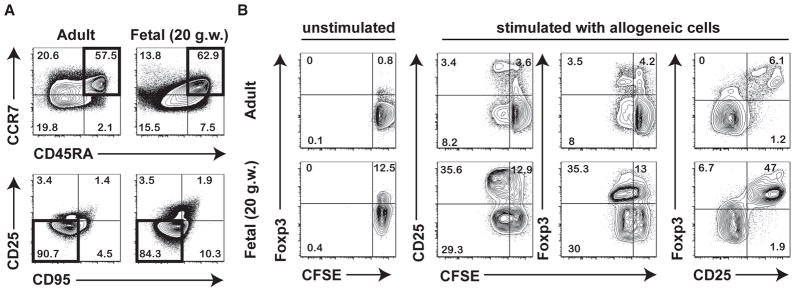
Although the human fetal T cell compartment begins to develop at approximately 10 gestational weeks (g.w.) (9), much of what we know about it arises from studies of cord blood obtained at birth. A few reports indicate that the majority of fetal T cells found at mid-gestation (~16–24 g.w.) have a surface phenotype comparable to that of conventional naïve T cells found in neonates and in adults (10–12). To characterize such cells more completely, we analyzed 18–22 g.w. fetal CD4+ T cells obtained from mesenteric lymph nodes (mLN) for the expression of a panel of known surface antigens specific for naïve CD4+ T cells in adults, to find that many have a phenotype (CD45RA+CCR7+CD95−CD25−) similar to that of naïve adult CD4+ T cells (Fig. 1A). Next, we assessed the proliferative response of sort-purified fetal and adult naïve T cells to stimulation with allogeneic peripheral blood mononuclear cells (PBMCs) in a primary mixed lymphocyte reaction (MLR). Fetal naïve CD4+ T cells were much more highly responsive to stimulation with allogeneic cells: after six days of stimulation, more than 50% had divided as compared to only ~10% of adult naïve CD4+ T cells. Activated fetal naïve CD4+ T cells were also more likely to adopt a Treg fate, as measured by upregulation of Foxp3 and CD25 (Fig. 1B). Although Foxp3 and CD25 can be transiently expressed by some T cells as a consequence of activation, our previous results indicate that activated fetal T cells exhibit sustained expression of Foxp3 and, unlike adult T cells, are prone to upregulate Foxp3 even as a result of “spontaneous” activation in tissue culture (6, 7). We have also demonstrated that fetal Treg cells are capable of suppressing both proliferation and cytokine production, suggesting that their function is similar to that of adult Treg cells (6, 7).
Fig. 1.
Fetal naïve CD4+ T cells display functional differences compared to adult naïve CD4+ T cells. (A) Flow cytometric analysis of the phenotype of naïve CD4+ T cells isolated from fetal mLNs (18–22 g.w.) and adult peripheral blood mononuclear cells (PBMC) (25–35 y.o.). Panels labeled (i) depict initial gating on CD3+CD4+ T cells showing CD45RA vs. CCR7 staining and those labeled (ii) depict CD45RA+CCR7+ cells (highlighted in black in panel i) subsequently gated on CD25 CD95− cells that are considered “naïve” CD4+ T cells (highlighted in black in panel ii). Data are representative of at least 3 independent donors. (B) Sorted naïve CD4+ T cells were either left unstimulated, or cultured for 6 days in the presence of irradiated allogeneic PBMC. Proliferation of fetal and adult naïve CD4+ T cells was measured by CFSE dilution and analyzed by flow cytometry. Tregs were phenotypically identified by upregulation of CD25 and Foxp3.
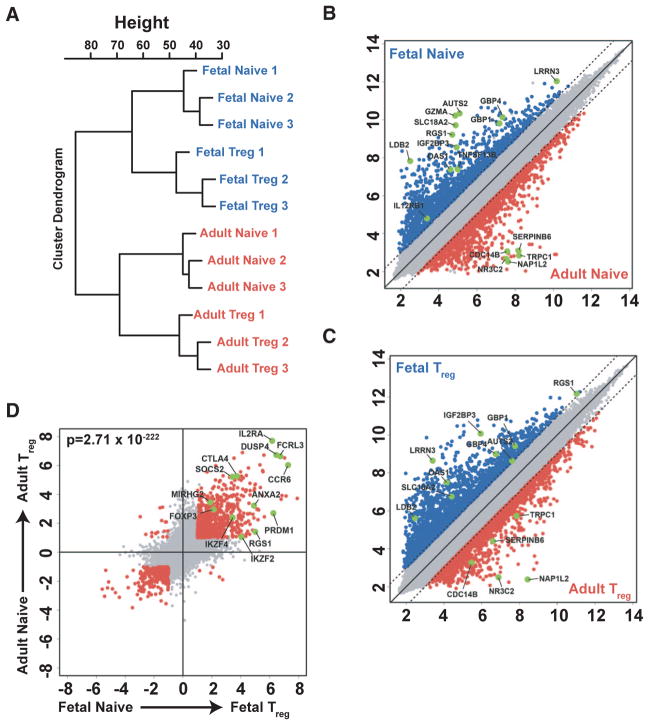
These findings suggested that fetal and adult naïve CD4+ T cells may be phenotypically similar but functionally distinct. To better define the differences between the two populations, we performed gene expression analysis by microarray on sorted populations of fetal and adult naïve CD4+ T cells (Fig. S1) and found substantial differences (Fig. 2A, B, Table S1). A parallel analysis revealed that many of the same differences that were identified for fetal and adult naïve CD4+ T cells were similar to those found for fetal and adult Tregs (Figs. 2, A–C, Table S2). We also noted a substantial overlap in genes that were enriched in both fetal and adult Tregs, when compared to fetal or adult naïve T cells, that was consistent with Treg genes identified in previous studies (13, 14) (Fig. 2D, Tables S3 and S4). To understand the relative contributions of age and functional phenotype, principal component (PC) analysis of the different CD4+ T cells was performed. Age (adult vs. fetal origin) was the largest PC, accounting for 36.1% of the observed variance, whereas a second PC (functional subtype, naïve vs. Treg) accounted for 25.9% (Fig. S2). The consistent observation of differential gene expression in fetal and adult T cells (both naïve and Treg) suggests that they represent different lymphocyte populations. As is individually highlighted in each scatterplot (Fig. 2B, C), this point is illustrated by selected genes that were found to be highly expressed within fetal or adult T cell populations (Fig. S3).
Fig. 2.
Fetal and adult naïve CD4+ T cells show significant differences in gene signature. (A) Unbiased cluster analysis on the basis of gene expression for fetal (mLN) and adult (PBMC) naïve CD4+ T cells and CD4+CD25+ Tregs. (B–D) Scatterplots of pairwise global gene expression comparisons comparing (B) fetal naïve CD4+ T cells and adult naïve CD4+ T cells, (C) fetal and adult CD4+CD25+ Tregs, and (D) CD4+CD25+ Tregs and naïve CD4+ T cells (in both fetal and adult). Gene expression values are plotted on a log scale. Genes that were differentially expressed between groups (determined using a 5% false-discovery rate and fold-change >= 2) are indicated in red and blue. Specific genes that were differentially expressed based on age or subset are highlighted in green.
We hypothesized that adult and fetal human T cells are different because they are derived from distinct populations of multilineage hematopoietic stem cells (HSCs). Thus, previous work in avian and murine models has demonstrated that fetal and adult HSC differ in phenotype (15), tissue localization (16), functional properties (e.g., regarding turnover) (15, 17), and developmental potential (18–20). By example, different populations of HSC seed the murine and avian thymus during fetal development (20, 21), giving rise to successive waves of thymocytes. In the mouse, the earliest to be found are dendritic epidermal T cells (DETC), identified by their expression of a specific γδ T cell receptor (TCR) (Vγ3Vδ1) (18, 20). These cells are replaced by a second wave of thymocytes with a diverse array of both TCRγδ and TCRαβ cells, which are then replaced by a third wave of thymocytes at around the time of birth, of which αβ T cells are the primary population.
In similar fashion, human fetal αβ CD4+ T cells may differ from their adult counterparts because they are derived from distinct populations of HSC. During the course of fetal development in humans, the location of the HSC pool switches from the aorta gonad mesonephros region to the fetal liver and, finally, to the fetal bone marrow (BM), where the majority of HSC are thought to reside throughout adulthood (23). The first wave of thymocytes is derived primarily from HSC present in the fetal liver, whereas thymocytes present at later stages of fetal development and in adults are likely to develop predominantly from BM-derived HSC (23). Thus, besides intrinsic differences between fetal and adult HSC populations, there are also differences in tissue distribution that could contribute to different developmental potential (24).
To determine whether distinct HSC populations give rise to distinct lineages of T cells in the human fetus, we isolated total CD34+ hematopoietic stem/progenitor cells (HSPC) (including primitive CD34+CD38− HSC and CD34+CD38+ progenitors with high proliferative potential) from fetal liver (18–22 g.w.), fetal BM (18–22 g.w.), and adult BM (19–43 years old) (Fig. S4). We used the SCID-hu Thy/Liv model of thymopoiesis to allow these populations of HSPC to mature into single positive (SP) thymocytes in vivo. SCID-hu Thy/Liv mice were generated by engraftment of fetal thymus and liver from an HLA-A2 donor into the immunodeficient C.B17 scid/scid mouse (25). The animals were irradiated and then injected with 500,000 HSPC isolated from HLA-A2-positive fetal liver, fetal BM, adult BM, or vehicle only (n=5 mice/group) (Fig. S4). After 7–8 weeks, mature thymocytes were harvested from the Thy/Liv implant. At this time, we were able to identify donor-derived (HLA-A2+) mature CD3+ thymocytes [CD3+CD4+CD8+ (DP), CD3+CD4−CD8+ (SP8), CD3+CD4+CD8− (SP4)] in each of the groups receiving CD34+ HSPC, but not in animals receiving vehicle alone (Fig. S4–5).
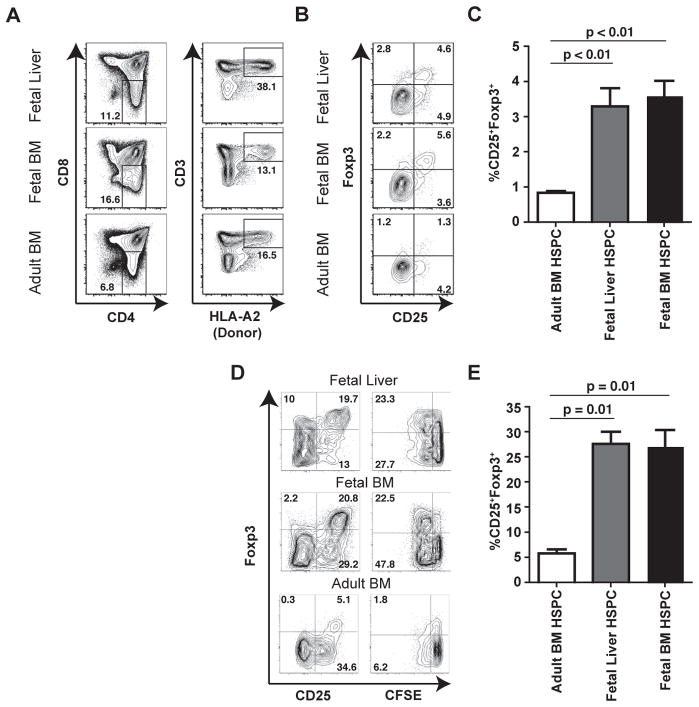
We asked whether fetal and adult HSPC might differ in their capacity to generate Tregs in the thymus because fetal T cells appear to be predisposed to generating Foxp3+ Tregs upon stimulation in vitro and there is an abundance of Tregs in the peripheral tissues of mid-gestation fetuses (7, 8). Both fetal liver and fetal BM-derived HSPC were found to generate populations of CD25+Foxp3+ SP4 thymocytes, whereas adult BM-derived HSPC generated a significantly lower frequency of CD25+Foxp3+ SP4 thymocytes (Figs. 3, A–C, Fig. S6). Thus, it appears that fetal HSPC display a heightened capacity to generate Tregs during thymic maturation.
Fig. 3.
Fetal and adult HSPC give rise to mature SP4 thymocytes that show similar functional differences to fetal and adult peripheral naïve CD4+ T cells. (A) Representative flow cytometry graphs showing thymocyte populations isolated from SCID-hu Thy/Liv implants following transplantation of donor (HLA-A2+) CD34+ HSPC isolated from fetal liver, fetal BM, or adult BM. Panels labeled (i) show initial gating on CD4 vs. CD8 to select for SP4 thymocytes and those labeled (ii) show subsequent gating on donor-derived (HLA-A2+) CD3+ mature SP4 thymocytes. (B) Flow cytometric analysis of the frequencies of CD25+Foxp3+ Tregs dervied from fetal liver, fetal BM or adult BM hematopoietic progenitors. (C) Quantification of CD25+Foxp3+ Treg frequencies observed in 3–5 different thymic implants from each group. (D) Flow cytometric analysis of proliferation and Foxp3 expression by SP4 thymocytes derived from fetal liver, fetal BM, or adult BM HSPC in response to stimulation with irradiated allogeneic PBMCs (7-day stimulation). (E) Quantification of the frequencies of CD25+Foxp3+ Tregs after stimulation with allogeneic PBMCs. Data show at least three separate implants for each group. Data in bar graphs represent mean +/− standard deviation and statistical significance was measured by Student’s t-test.
The initial observation that prompted our comparison between fetal and adult T cells was the elevated proliferation and differentiation of fetal T cells into Tregs after stimulation. To determine whether SP4 thymocytes derived from fetal HSPC were functionally similar to SP4 thymocytes derived from adult HSPC, we stimulated whole thymocyte populations in vitro with allogeneic APCs in a primary MLR (1:3 ratio), and thereafter monitored proliferation and Foxp3 expression. SP4 thymocytes derived from fetal HSPC behaved similarly to peripheral fetal naïve CD4+ T cells, exhibiting robust proliferative responses and a significantly greater expansion of Foxp3+ cells after stimulation as compared to SP4 thymocytes derived from adult HSPC (Fig. 3D and E, compare to Fig. 1B). Thus, we conclude that the functional differences observed between fetal and adult naïve T cells in the periphery can be, in part, accounted for by differences in the progenitor populations from which they arise.
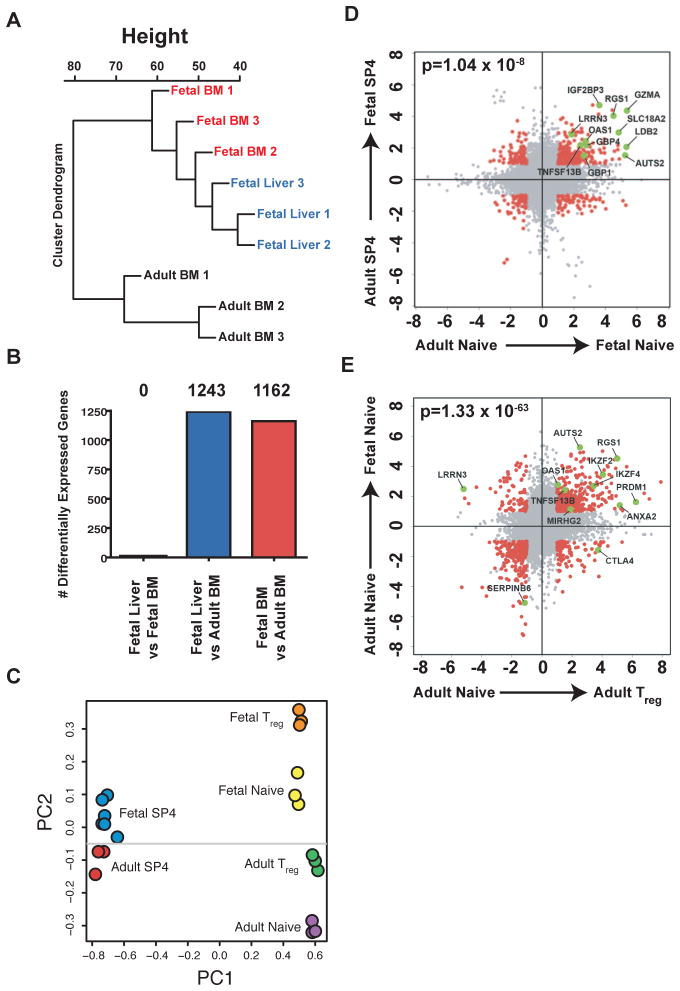
We next sought to determine whether the gene signature of T cells derived from fetal HSPC was different than that of T cells derived from adult HSPC. To make this comparison, HLA-A2+ SP4 thymocytes were sorted from SCID-hu Thy/Liv implants injected with fetal liver, fetal BM, or adult BM HSPC, and microarray analysis was performed using the same platform previously used to test peripheral T cells. This analysis revealed that fetal BM- and fetal liver-derived HSPC gave rise to SP4 thymocytes that were indistinguishable from one another, indicating that the fetal HSPC in each of these tissues have similar developmental potential (Figs. 4, A and B, Table S5). By contrast, the transcriptional profile of SP4 thymocytes matured from adult BM-derived HSPC was vastly different (Figs. 4, A and B, Table S5). Thus, we find that fetal HSPC and adult HSPC give rise to mature SP4 thymocytes with profoundly different functional properties and gene expression patterns. These differences are likely to reflect developmental changes in progenitor populations, rather than differences related to compartmentalization of stem cell subsets.
Fig. 4.
Fetal and adult HSPC give rise to SP4 thymocytes with distinct gene signatures. (A) Unbiased cluster analysis based on gene expression for SP4 thymocytes from fetal BM, fetal liver, and adult BM hematopoietic progenitors.(B) Total number of genes found to be significantly different comparing SP4 thymocytes derived from fetal and adult progenitor populations. (C) Principle component analysis showing clear separations between SP4 thymocyte populations and peripheral T cells (PC1) as well as differences between fetal and adult SP4 and peripheral naïve CD4+ T cell populations (PC2). (D) Scatter plot depicting genes that are differentially expressed between fetal and adult SP4 thymocytes, and between fetal and adult peripheral naïve CD4+ T cells. (E) Scatter plot depicting genes that are differentially expressed between adult and fetal naïve T cells, and between adult naïve and adult Tregs. For both (D) and (E), differential expression was determined using a false discovery rate of ≤ 5% and fold-change of ≥ two-fold. The significance of overlap between groups of genes was determined using a chi-squared test for independence.
Finally, we sought to determine whether the differences observed in the gene expression patterns of peripheral fetal and adult naïve CD4+ T cells could be explained by differences in the fetal and adult stem cell populations. We performed PCA on both peripheral T cells and HSPC-derived thymocytes to identify the sources of variation in gene expression and their relative magnitude. In this analysis, developmental stage (thymocyte vs. mature T cells) was found to be the most prominent source of variance (PC1, 70.4% of total variance), followed by age (PC2, fetal vs. adult, 6.3%) (Fig. 4C). We observed a significant overlap (p= 1.0 × 10−8) between genes that were enriched in both fetal naïve and fetal HSPC-derived SP4 cells (253 probesets) and genes that were enriched in adult naïve and adult HSPC-derived SP4 cells (79 probesets) (Fig. 4D). Because fetal naïve cells displayed a propensity to differentiate into Tregs as compared to adult naïve T cells, we performed an additional correlation to determine whether fetal and adult naïve T cells shared a common gene signature with Tregs. We identified a significant number of genes that were highly expressed in both adult Tregs and fetal naïve T cells, but not in adult naïve T cells, some of which have been previously implicated as playing a specific role in determining Treg fate specification (e.g., IKZ2, IKZ4) (Figs. 4E, fig. S3). This is also reflected in the PCA performed on all T cell subsets (Fig. 4C), which showed that fetal naive cells are more closely related to adult Tregs than to adult naïve T cells along PC2. We can conclude that fetal T cells are a unique population of cells, distinct from those found in adults, and that this in part reflects differences in the populations of stem cells which give rise to the T cell compartment across different stages of development.
In sum, our results support the hypothesis that, as in mice and birds (21,22), so to in humans is development of the immune system “layered.” This hypothesis suggests that hematopoiesis occurs in distinct waves, including an early one that is fetal and a later one that is adult, with each generating a distinct populations of cells that may co-exist (i.e., layer) for a period of time (Fig. S7). The propensity for fetal CD4+ T cells to adopt a Treg fate upon stimulation suggests that the initial wave of T cell differentiation may favor a population of T cells whose role is to promote tolerance to self (and potentially foreign) antigens encountered during development. Whether these cells are enriched for a specific Treg precursor, which has been suggested to exist in the adult thymus and periphery in mice (26, 27), remains to be determined. Although the focus of this study has been CD4+ T cells, the fetal peripheral lymphoid tissues contain a full array of hematopoietic cells that have not been extensively studied and which may also be distinct from their adult counterparts. By example, while our findings are different from those made in the mouse, the results of our study do not rule out the existence of a comparable population of γδ DETC in the human fetus. Our findings have broad implications for the understanding of human hematopoiesis and provide a framework for studying a range of biological processes, from infectious diseases to transplantation strategies and immune tolerance.
Supplementary Material
Acknowledgments
We would like to thank all of the patients who generously donated tissue that was used in these studies. We would also like to thank Drs. S. J. Fisher and D. F. Nixon for valuable discussions, Dr. A. J. Butte, M. E. Moreno, and G. Kosikova for technical support, and the Gladstone Genomics Core for microarray analysis. Support for this work was provided by grants from the National Institutes of Health to J.M.M (OD000329 and AI40312), to K.W. and A.J.B. (CA049605 and AG033314), and from the Harvey V. Berneking Living Trust. This project has been funded in part with Federal funds from BIAID, NIH, under contract no. N01-AI-70002. S.V. is supported by the Deborah Addicott Fellowship for Bioinformatics and Stem Cell Biology. J.M. is supported by the Swedish Research Council. T.D.B. received funding from American Pediatric Society, the American Academy of Pediatrics and the March of Dimes as a fellow of the Pediatric Scientist Development Program. J.M.M. is a recipient of the NIH Director’s Pioneer Award Program, part of the NIH Roadmap for Medical Research, through grant DPI OD00329.
References
- 1.Silverstein AM. Ontogeny of the immune response. Science. 1964;144:1423. doi: 10.1126/science.144.3625.1423. [DOI] [PubMed] [Google Scholar]
- 2.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 3.Brent L, et al. On the feasibility of inducing tolerance in man: a study in the cynomolgus monkey. Immunol Lett. 1989;21:55. doi: 10.1016/0165-2478(89)90012-6. [DOI] [PubMed] [Google Scholar]
- 4.Claas FH, Gijbels Y, van der Velden-de Munck J, van Rood JJ. Induction of B cell unresponsiveness to noninherted maternal HLA antigens during fetal life. Science. 1988;241:1815. doi: 10.1126/science.3051377. [DOI] [PubMed] [Google Scholar]
- 5.Burlingham WJ, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 6.Mold JE, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaëlsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176:5741. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- 8.Takahata Y, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naïve phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32:622. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Haynes BF, Martin ME, Kay HH, Kurtzberg J. Early events in human T cell ontogeny. Phenotypic characterization and immunohistologic localization of T cell precursors in early human fetal tissues. J Exp Med. 1988;168:1061. doi: 10.1084/jem.168.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucivero G, et al. Ontogeny of human lymphocytes. Two-color fluorescence analysis of circulating lymphocyte subsets in fetuses in the second trimester of pregnancy. Fetal Diagn Ther. 1991;6:101. doi: 10.1159/000263632. [DOI] [PubMed] [Google Scholar]
- 11.Byrne JA, Stankovic AK, Cooper MD. A novel subpopulation of primed T cells in the human fetus. J Immunol. 1994;152:3098. [PubMed] [Google Scholar]
- 12.Muench MO, Pott Bartsch EM, Chen JC, Lopoo JB, Barcena A. Ontogenic changes in CD95 expression on human leukocytes: prevalence of T-cells expressing activation markers and identification of CD95-CD45RO+ T-cells in the fetus. Dev Comp Immunol. 2003;27:899. doi: 10.1016/s0145-305x(03)00081-8. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto N, et al. Foxp3-dependent and –independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 14.Feuerer M, et al. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci. 2010;107:5919. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci. 1995;92:10302. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanjani ED, Ascensao JL, Tavassoli M. Liver-derived fetal hematopoietic stem cells selectively and preferentially home to the fetal bone marrow. Blood. 1993;81:399. [PubMed] [Google Scholar]
- 17.Harrison DE, Zhong RK, Jordan CT, Lemischka IR, Astle CM. Relative to adult marrow, fetal liver repopulates nearly five times more effectively long-term than short-term. Exp Hematol. 1997;25:293. [PubMed] [Google Scholar]
- 18.Ikuta K, et al. A developmental switch in thymic lymphocyte maturation occurs at the level of the hematopoietic stem cells. Cell. 1990;62:863. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- 19.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specific progenitor. Nat Immunol. 2006;7:293. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 20.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 21.Coltey M, et al. Analysis of the first two waves of thymus homing stem cells and their T cell progeny in chick-quail chimeras. J Exp Med. 1989;170:543. doi: 10.1084/jem.170.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzenberg LA, Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 23.Tavian M, Péault B. The changing cellular environments of hematopoiesis in human development in utero. Exp Hematol. 2005;33:1062. doi: 10.1016/j.exphem.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Martin MA, Bhatia M. Analysis of the human fetal liver hematopoietic microenvironment. Stem Cells Dev. 2005;14:493. doi: 10.1089/scd.2005.14.493. [DOI] [PubMed] [Google Scholar]
- 25.Namikawa R, et al. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennington DJ, et al. Early events in the thymus affect the balance of effector and regulatory T cells. Nature. 2006;444:1073. doi: 10.1038/nature06051. [DOI] [PubMed] [Google Scholar]
- 27.Schallenberg S, Tsai PY, Riewaldt J, Kretschmer K. Identification of an immediate Foxp3− precursor to Foxp3+ regulatory T cells in peripheral lymphoid organs of nonmanipulated mice. J Exp Med. 2010;207:1393–1407. doi: 10.1084/jem.20100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.