Abstract
Background
The enteric nervous system (ENS) possesses extensive synaptic connections which integrate information and provide appropriate outputs to coordinate the activity of the gastrointestinal (GI) tract. The regulation of enteric synapses is not well understood. Cannabinoid (CB)1 receptors inhibit the release of acetylcholine (ACh) in the ENS, but their role in the synapse is not understood. We tested the hypothesis that enteric CB1 receptors provide inhibitory control of excitatory neurotransmission in the ENS.
Methods
Intracellular microelectrode recordings were obtained from mouse myenteric plexus neurons. Interganglionic fibres were stimulated with a concentric stimulating electrode to elicit synaptic events on to the recorded neuron. Differences between spontaneous and evoked fast synaptic transmission was examined within preparations from CB1 deficient mice (CB1−/−) and wild-type (WT) littermate controls.
Key Results
CB1 receptors were colocalized on terminals expressing the vesicular Ach transporter and the synaptic protein synaptotagmin. A greater proportion of CB1−/− neurons received spontaneous fast excitatory post-synaptic potentials than neurons from WT preparations. The CB1 agonist WIN55,212 depressed WT synapses without any effect on CB1−/− synapses. Synaptic activity in response to depolarization was markedly enhanced at CB1−/− synapses and after treatment with a CB1 antagonist in WT preparations. Activity dependent liberation of a retrograde purine messenger was demonstrated to facilitate synaptic transmission in CB1−/− mice.
Conclusions & inferences
CB1 receptors inhibit transmitter release at enteric synapses and depress synaptic strength basally and in an activity-dependent manner. These actions help explain accelerated intestinal transit observed in the absence of CB1 receptors.
Keywords: Enteric nervous system, plasticity, retrograde transmission, purine, cannabinoid
The myenteric and submucosal plexuses of the enteric nervous system (ENS) exist as interconnected integrative nerve networks within the wall of the gastrointestinal (GI) tract. Activity of the ENS is responsible for the control of the digestive and protective functions of the gut.1 Synaptic transmission between enteric neurons propagates information from intrinsic afferent neurons to interneurons, and then from interneurons to motor neurons that control final effectors such as smooth muscle and the secretory epithelium. Acetylcholine (ACh) is the major excitatory neurotransmitter in the myenteric plexus, acting on nicotinic receptors at synapses between neurons and on muscarinic receptors at neuromuscular junctions.1,2 Unlike the central nervous system (CNS), fast inhibitory neurotransmission is not commonly observed in the myenteric plexus. Norepinephrine released from extrinsic sympathetic terminals generates inhibitory post-synaptic potentials of an intermediate duration in the guinea pig. But the role of norepinephrine in regulating the moment-to-moment function of the GI tract is unclear, since under physiological conditions sympathetic denervation has little effect.3 Thus, other mechanisms are likely to exist that could more specifically control synaptic strength in the ENS, through the graded control of ACh release from the presynaptic neuron.
In the CNS, the discovery of retrograde endocannabinoid signalling provided an explanation for some forms of postsynaptic activity-dependent changes in presynaptic neurotransmitter release.4,5 Presynaptic activation of the cannabinoid (CB)1 receptor causes both transient and sustained inhibition of neurotransmitter release, allowing for several forms of synaptic plasticity.4,6,7 Furthermore, CB1 receptor-mediated plasticity at the synapse can itself be regulated, a phenomenon known as metaplasticity.8,9 If a similar system is functioning at enteric synapses, then activity-dependent endocannabinoid signalling could exert homeostatic inhibitory control of excitatory neurotransmission in the ENS.
CB1 receptors are expressed in the ENS, and in this system, exogenous CB1 receptor agonists inhibit ACh release and reduce the amplitude of fast excitatory post-synaptic potentials (fast EPSPs).10–12 The key elements of the endocannabinoid signalling system are present in the ENS, including receptors13–15 and putative transporters,16 as well as the enzymes responsible for the synthesis and degradation of endocannabinoids.13,17,18 Through the use of drugs that target individual components of this system it is apparent that cannabinoid signalling influences motor and other gut functions,13 but its role in enteric neurotransmission remains to be determined. In the gut, there also appears to be a basal level of endocannabinoid tone.12,13,19 We tested the hypothesis that enteric CB1 receptors provide inhibitory control of excitatory neurotransmission in the ENS. Our data support this hypothesis, and we have also discovered a new form of metaplasticity in the ENS, that has not been previously been reported in the peripheral or central nervous system.
METHODS
Animals
CB1 receptor-deficient mice (CB1−/−) or littermate wild type controls (WT) on a predominant C57BL/6N background (6 generations of backcrossing) of either sex were used and genotyped as previously described.20 Mice were housed under specified pathogen free conditions with free access to food and water and at a constant temperature (22°C) under a 12:12-hour light-dark cycle. Animal use for this study was approved by the University of Calgary Animal Care Committee under the guidelines of the Canadian Council on Animal Care.
Reagents
AM251 and WIN55,212-2 were purchased from Tocris (Ellisville, MO, USA). 1,2-bis(o-Aminophenoxy)ethane-N,N,N′,N′-tetraacetic Acid, tetrasodium salt (BAPTA) was purchased from EMD Chemicals (Gibbstown, NJ, USA). Unless otherwise noted, all other compounds were purchased from Sigma (St Louis, MO, USA).
Tissue Preparation for Neurophysiology
Animals were sacrificed by cervical dislocation. Segments of ileum, 1 cm from the ileocecal junction, from WT and CB1−/− animals were rapidly placed in a Sylgard-lined dissecting dish, opened along the mesenteric border, and pinned tautly with the mucosa facing up. The oral end of the ileum was marked and the tissue was maintained in ice-cold oxygenated Krebs solution (in mM: 117 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, and 11 D-glucose; aerated with 95% O2-5% CO2) containing nicardipine (3 µM) and scopolamine (1 µM). The myenteric plexus was dissected from a segment of ileum. Briefly, the mucosa and submucosa were dissected off the underlying myenteric plexus/longitudinal muscle. Circular muscle was dissected leaving a myenteric plexus/longitudinal muscle preparation that was transferred to a Silastic elastomer-lined recording chamber and superfused with oxygenated Krebs preheated to 36–37°C.
Neurophysiological Characterization of Myenteric Neurons
Neurons were impaled with microelectrodes fabricated from 1-mm-outer-diameter borosilicate glass (World Precision Instruments, Sarasota, FL, USA) and filled with 1% biocytin in 1 M KCl. Electrode resistances were 90–150 mΩ. Recordings were made with a Multiclamp 700A amplifier in current-clamp mode (Axon Instruments, Molecular Devices, Sunnyvale, CA, USA). Recordings were digitized at 5–50 kHz and stored and analyzed by using PC-based data acquisition and analysis software (pClamp 9.2 suite, Axon Instruments). Following a 10-min stabilization period after impalement, passive and active electrical properties were evaluated, as well as synaptic events evoked by focal stimulation of adjacent ganglia. Only neurons that fired an action potential and had a resting membrane potential more negative than −30 mV, were included in the electrophysiological analyses. Neurons were classified as AH or S according to well-established criteria.21 Because AH neurons typically do not consistently exhibit fast EPSPs they were excluded from this study.
Neuronal excitability was assessed as previously described.22 Synaptic inputs were stimulated by positioning a concentric bipolar stimulating electrode (World Precision Instruments) on interganglionic nerve bundles. Stimulus pulses of 0.5 ms duration and 5–15 V intensity were delivered via a Grass SD9 (Grass Medical Instruments, Quincy, MA, USA) stimulator. For all experiments the final stimulus intensity was chosen as the minimum stimulus capable of evoking a fast EPSP plus 10%. Paired pulse responses were obtained by applying a pair of synaptic stimuli 30 mSec apart at 0.2 Hz. To measure fast EPSPs, cells were hyperpolarized to −80 mV to prevent action potential firing. Fast EPSPs were identified using threshold detection set at 2 mV above the background noise. All results were confirmed by visual inspection. Peak amplitude of the fast EPSP was measured from the membrane potential immediately preceding the stimulus. To determine the magnitude of the EPSPs, the area under the curve (AUC) of the EPSPs, was calculated in comparison to the membrane potential immediately preceding the fast EPSP. We therefore use “magnitude” in conjunction with amplitude in our studies to express both the intensity and the duration of the postsynaptic depolarization.
To elicit slow excitatory post-synaptic potentials (slow EPSPs), 1 s trains of 20 Hz 5–15 V stimulation (intensity determined as above) were applied to interganglionic nerve bundles. Slow EPSPs were evoked from the resting membrane potential.
Visualization of Recorded Neurons
Once the electrophysiological data were obtained, depolarizing pulses were delivered to the recording electrode to iontophoretically fill the neuron with biocytin. At the end of each experiment the tissue was fixed in modified Zamboni's fixative (0.1 M PBS containing 2% formaldehyde plus 0.2% picric acid) overnight at 4°C then washed in PBS. For biocytin visualization, the tissue was washed three times in PBS containing 0.1% Triton X-100 (3 × 10 min) followed by incubation with FITC-conjugated avidin for 2 h at room temperature (1:100). Neurons were imaged on an Olympus FluoView FV1000 confocal microscope (Olympus America Inc., Melville, NY, USA) using a 60X (PlanApo N, 1.42 n.a.) oil immersion lens. Optical sections (0.5 µm) were acquired through each field of view and then compiled into a z-stack.
Examination of Synaptic Plasticity
To examine possible retrograde transmission, a protocol of depolarization of the impaled postsynaptic neurons was utilized.23 Current injections were used to depolarize the neuron to 0mV. The depolarizing current injections were 2ms in duration at 20Hz for a period of 1min. In some experiments this protocol was combined with presynaptic fibre tract stimulation at the start of the depolarization to induce synaptic events at the same frequency for the first 30sec of the depolarization protocol. This was employed to examine coincidence detection. Presynaptic stimulation only occurred for half the protocol (30 sec of the 1 min stimulation time) in order to avoid synaptic run down persisting into the post-stimulation period.24 When examining change in fast EPSP amplitude and area following stimulation, data are presented normalized to a 5 min pre-recording period where fast EPSPs were evoked at an identical rate. The normalized amplitudes of the fast EPSPs were graphed over a 200 second time course following the initiation of the postsynaptic neuron depolarization protocol, and the AUC was determined. Area under the curve data were then compared between mouse strains and represent normalized amplitudes · s. which are given as arbitrary units.
Postsynaptic sensitivity to 1 mM ACh dissolved in normal Krebs’ solution was determined using a 500 ms pressure microejection at 15 psi. Substitution of normal Krebs’ solution into the picospritz pipette elicited no response from recorded neurons.
Immunohistochemistry
Segments of ileum, 1 cm from the ileocecal junction, from WT and CB1−/− animals, were placed in a Sylgard-lined dissecting dish, opened along the mesenteric border, and pinned tautly with the mucosa facing up. Tissue was fixed overnight in Zamboni's fixative at 4°C. Fixative was removed by three 10-min washes in PBS. Ileal segments were dissected to yield preparations of myenteric plexus. Preparations were incubated in the primary antisera for 48 h at 4°C.
Localization of the CB1 receptor was accomplished using a well characterized N terminal CB1 receptor antibody (1:500).25 A goat polyclonal antibody specific for the vesicular acetylcholine transporter (VAChT, 1:400) was purchased from Phoenix Pharmaceuticals (Belmont, CA, USA; H-V007). A mouse monoclonal antibody specific for synaptotagmin (1:500) was purchased from Stressgen Biotechnologies (Victoria, BC, Canada; SYA-148). Following incubation in primary antiserum, immunoreactivity was detected following incubation of preparations for 2 h at room temperature in donkey anti-rabbit IgG-CY3 (1:100; Jackson, PA, USA) anti-goat IgG-FITC (1:50; Jackson), as well as goat anti-mouse IgG-CY5 (1:100; Jackson) and goat anti-mouse IgG-FITC (1:50; Biocan Scientific, Mississauga, ON, Canada). Following washes in PBS, the preparations were mounted on glass slides in bicarbonate-buffered glycerol at pH 8.6. Immunofluorescence was imaged on an Olympus FluoView FV1000 confocal microscope (Olympus America Inc., Melville, NY, USA) using a 60X oil immersion lens. Optical sections (0.5 µm) were acquired through each field of view and then compiled into a z-stack. Sequential scanning of the 2 separate fluorescence channels avoided “bleed through” of signal from the inappropriate channel.
Upper Gastrointestinal Transit
Mice were fasted for 12 hrs with free access to tap water and upper GI transit experiments were started between 8 and 9 am. 0.2 ml of 5% Evans blue suspension in 5% gum Arabic was given by gastric gavage using an animal feeding needle. 15 min later animals were euthanized and the total intestine was immediately removed. The distance travelled by the leading edge of the marker was measured and then expressed as percentage of the distance from the pylorus to the cecum.
Statistics
Statistical analyses and data plotting were carried out by use of Prism 4 (Graphpad Software, San Diego, CA, USA). Differences between data sets were compared by using unpaired two-tailed Student's t-tests or one-way anaylsis of variance (ANOVA) with Dunnett’s post-test. Chi squared tests were employed to determine significant differences in population sizes. A P value of <0.05 was considered statistically significant. In all experiments results were obtained from a minimum of 3 preparations from 3 individual animals.
RESULTS
Synaptic Localization of the CB1 Receptor
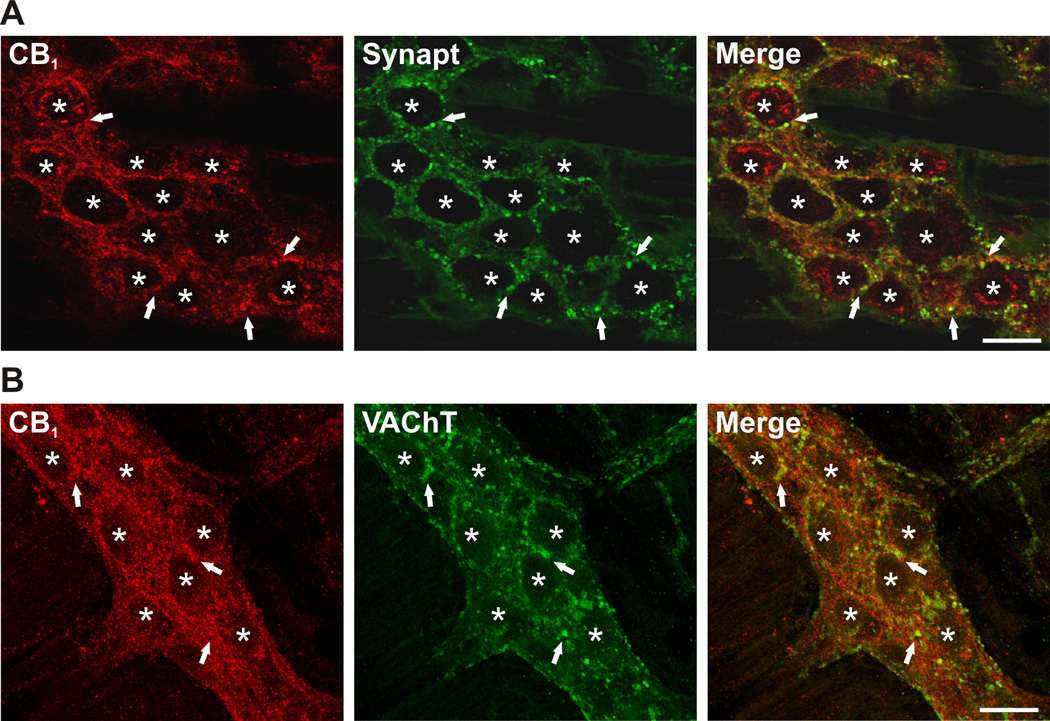
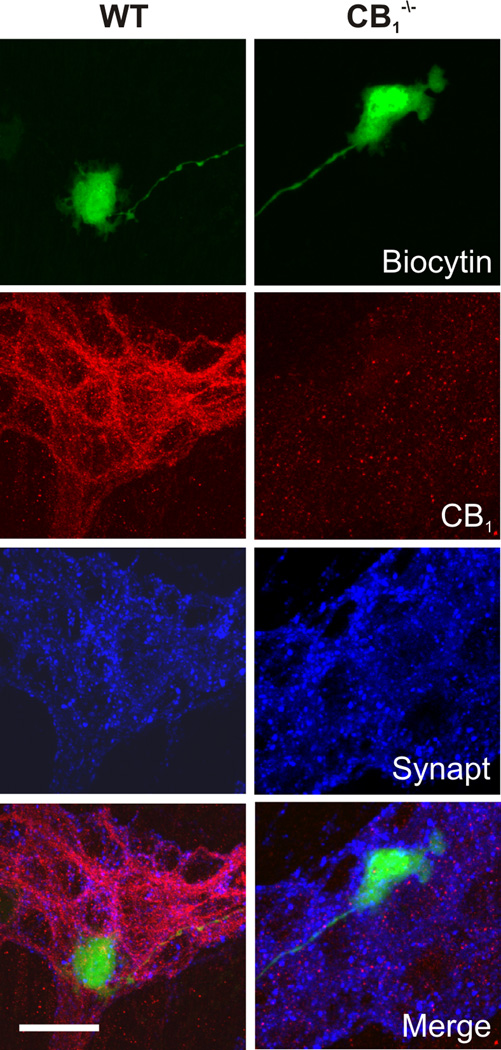
Intense CB1 immunoreactivity was observed throughout the myenteric ganglia in structures identified as synaptic varicosities, in close proximity to the majority of neurons, and in interganglionic nerve fibre tracts (Fig. 1). A subset of neuronal cell bodies exhibited intense intracellular immunoreactivity, indicating an intracellular store of receptor. This pattern of labelling is similar to that shown by others.26 Synaptic release sites were labelled for synaptotagmin, a well-characterized calcium sensitive protein found on synaptic vesicles. The resulting punctate staining extensively overlapped with CB1 receptor staining, indicative of synaptic localization of the receptor (Fig. 1A). CB1 receptors also co-localized with VAChT, a marker of cholinergic synaptic terminals (Fig. 1B). There was a high degree of overlap between CB1 and VACht strongly suggesting coexpression. The extent of this was not quantified in the present study. CB1 receptor immunoreactivity was not detected in the myenteric plexus of CB1−/− mice (Fig. 2).
Figure 1.
Colocalization of the CB1 receptor with synaptotagmin (Synapt) and the vesicular ACh transporter (VAChT) in WT mouse ileal myenteric plexus. (A) Apparent colocalization of CB1 (red) occurs with Synapt (green) in the myenteric plexus. (B) A subpopulation of synapses are VAChT immunoreactive (green) and these synaptic terminals also appear to colocalize with the CB1 receptor (red). Representative confocal photomicrographs of localization studies performed in myenteric plexus preparations from 3 WT animals. Asterisks indicate the position of cell bodies within the enteric ganglion; arrows indicate areas of significant colocalization. Scale bars: 20 µm.
Figure 2.
Spatial relationship between recorded neurons, CB1 receptor immunoreactivity and synaptic densities in myenteric S neurons. Colocalization of recorded neurons previously filled with biocytin (conjugated to FITC labelled avidin, green), with CB1 receptor (red) and synaptotagmin (Synapt, blue) immunoreactivity in WT and CB1−/− neurons. Scale bar: 20 µm.
Electrophysiology of the Soma, Spontaneous Synaptic Events and GI Transit
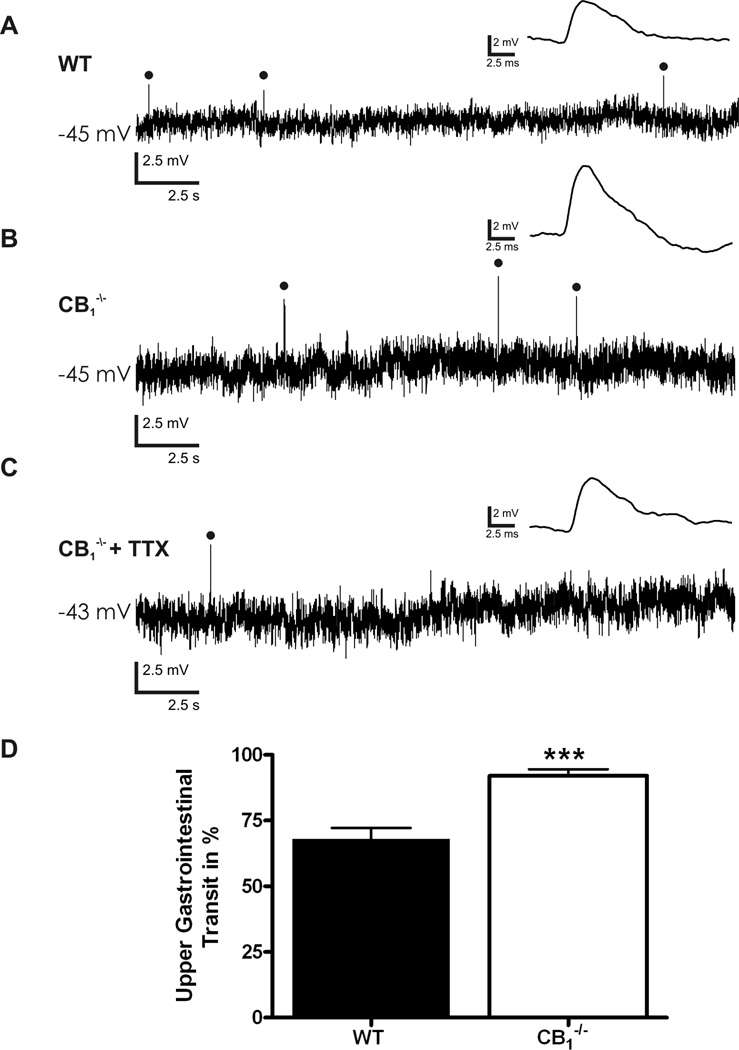
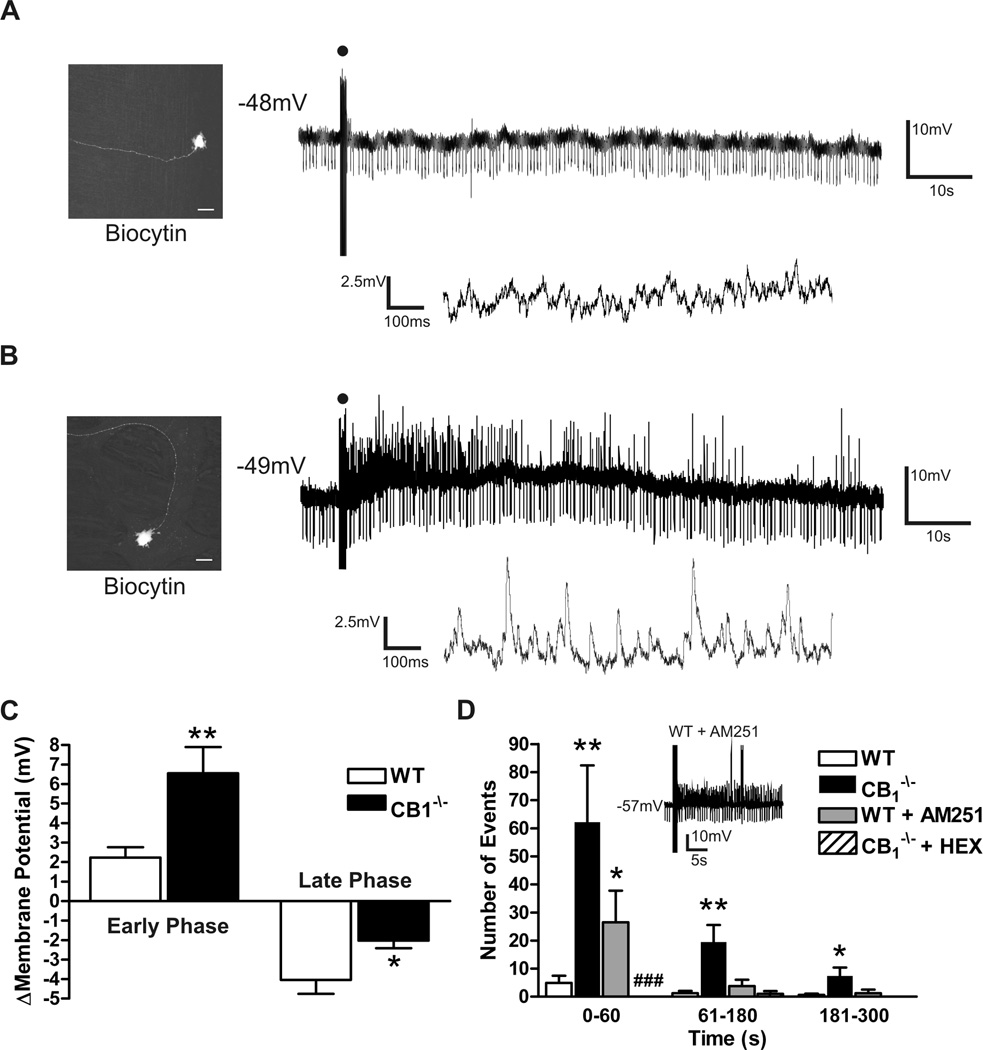
Intracellular transmembrane voltage recordings were obtained from 80 neurons from WT mice and 80 from CB1−/− animals. All of these neurons received evoked fast synaptic inputs and had a uniaxonal Dogiel type I morphology, consistent with a classification as synaptic (S) neurons (Fig. 2).27,28 Basal neuronal electrical properties were comparable between the groups (Table 1). At resting membrane potential, and prior to any stimulation, spontaneous fast EPSPs were frequently seen in S neurons from CB1−/− mice (Fig. 3), but were significantly less common in WT S neurons, (39/66 CB1−/−, vs. 32/80 WT, Chi squared test, p<0.01). The amplitude of spontaneous fast EPSPs was not different between the groups (5.0 ± 0.1 mV, CB1−/− [n = 302] vs. 5.0 ± 0.2 mV, WT [n = 64]). The frequency of spontaneous fEPSPs did not differ between neurons that received multiple spontaneous fast EPSPs (0.08 ± 0.03 Hz, CB1−/− [n = 6] vs. 0.04 ± 0.007 Hz, WT [n = 7]). Analysis of the frequency distribution and cumulative distribution curves of fast EPSP amplitude indicate no difference in spontaneous fast EPSPs received by WT and CB1−/− S neurons and no subpopulation of larger/smaller fast EPSPs (Supplemental Fig. 1). The generation of spontaneous EPSPs was not due to propagating action potentials as spontaneous EPSPs persisted following application of tetrodotoxin (TTX, 300 nM, Fig. 3C). These findings suggests that endocannabinoids may tonically inhibit synaptic release at cholinergic synapses in the ENS, consistent with observations that CB1−/− mice have enhanced intestinal motility (Fig. 3D),13 and that cannabinoid receptor agonists inhibit GI motility in mice.29
Table 1.
Summary of basal and stimulated electrophysiological characteristics of S neurons
| Rin (mΩ) |
RMP (mV) |
Rheobase (pA) |
APs at Rheobase |
|
|---|---|---|---|---|
| WT (n = 80) |
187 ± 14 | −51 ± 1.4 | 177 ± 23 | 1 ± 0 |
| CB1−/− (n = 66) |
221 ± 14 | −49 ± 1.2 | 168 ± 20 | 1 ± 0 |
Rin - Input resistance, RMP - Resting membrane potential, Rheobase - Minimum current injection required to elicit a action potential, APs - Action potential.
Figure 3.
Spontaneous synaptic events in CB1−/− and WT myenteric plexus preparations. (A) Spontaneous fast excitatory post-synaptic potentials (EPSPs) occurring in WT S neurons at resting membrane potential. (B) Spontaneous fast EPSPs occurring in CB1−/− S neurons at resting membrane potential. (C) Spontaneous fast EPSPs remain following incubation of CB1−/− preparations in 300nM tetrodotoxin (TTX) to block action potential conduction. (D) Significant increase in upper gastrointestinal transit in CB1−/− animals as indicated by an increased distance travelled by Evans blue given by gastric gavage 15min previously. Individual fast EPSPs within traces A-C are expanded and shown above the original recording. Spontaneously occurring fast EPSPs are indicated with closed circles. Resting membrane potential is indicated to the left of each recording. *** = P < 0.0001.
CB1 Mediated Inhibition of Fast Synaptic Transmission
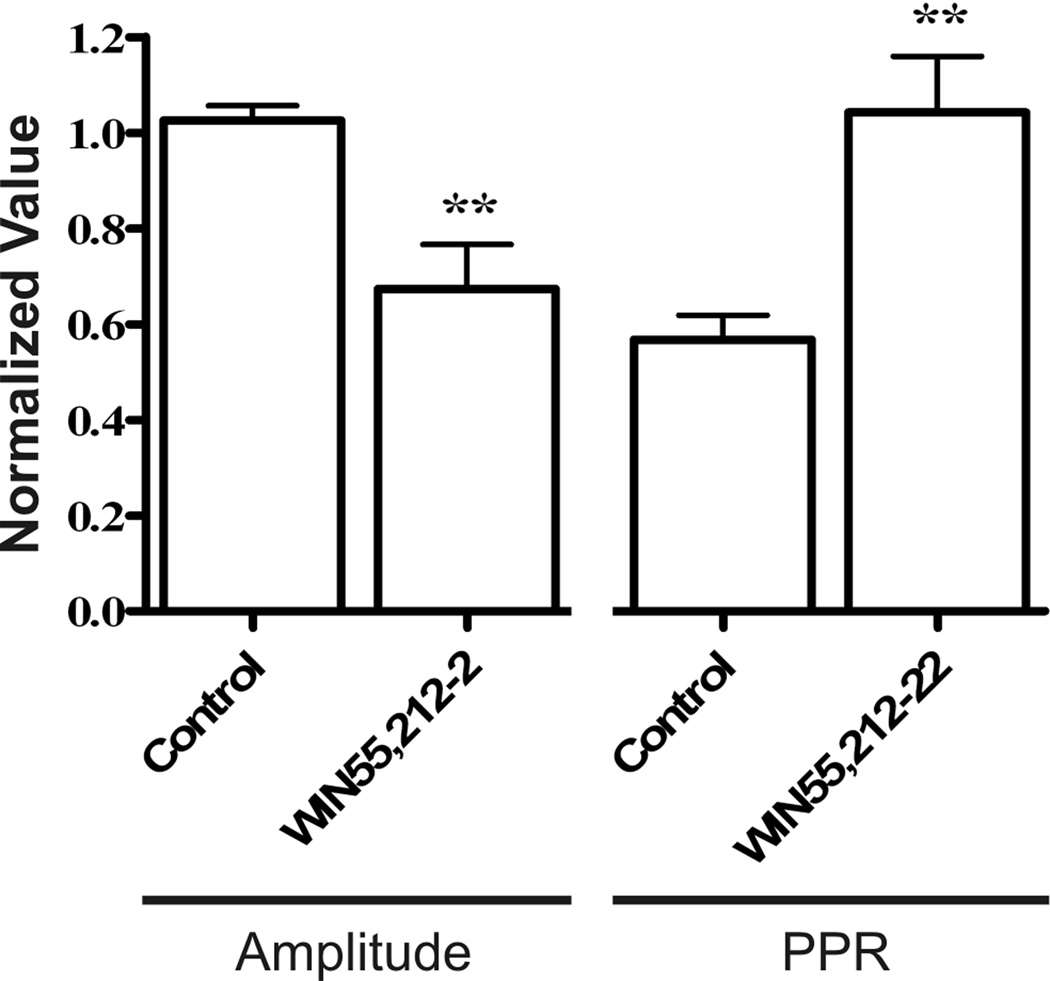
Stimulation of circumferential interganglionic nerve fiber bundles evoked single or compound fast EPSPs in all S neurons that were studied from CB1−/− and WT mice. Fast EPSPs in the CB1−/− S neurons were comparable in amplitude (15.2 ± 1.4 mV, CB1−/− [n = 23] vs. 11.9 ± 1.1 mV, WT [n = 16], Student’s t-test, ns) and in magnitude (188.1 ± 19 mV·s, CB1−/− [n=23] vs. 162.9 ± 16 mV·s, WT [n=16], Student’s t-test, ns) to those of WT mice. In WT preparations, application of the CB1 receptor agonist WIN55,212-2 (100 nM) caused a significant depression in the amplitude of evoked EPSPs (67 ± 18% of baseline EPSP amplitude, Student’s t-test, P < 0.01, Fig. 4). The paired-pulse ratio (PPR), was equivalent in at CB1−/− and WT synapses (0.76 ± 0.05 CB1−/− [n = 7] vs. 0.73 ± 0.06 WT [n = 8], ns), but was increased in WT synapses following perfusion with WIN55,212-2 (Fig. 4). This is consistent with presynaptic CB1 receptors inhibiting vesicular release. In CB1−/− mice, WIN55,212-2 did not significantly reduce EPSP amplitude (minimum value 84.4 ± 13.1% of baseline EPSP amplitude, [n = 4]) nor did it increase the PPR (maximum value 0.73 ± 0.14, [n = 4]).
Figure 4.
Demonstration of presynaptic action of CB1 receptors. Normalized first EPSP amplitude and the paired-pulse ratio PPR (30 ms interval) before and 4.5 minutes after the perfusion of 100 nM WIN55,212-2 on to WT neurons. In the presence of WIN55,212-2 the PPR was increased and first EPSP amplitude was reduced. ** P < 0.01.
CB1 Mediated Inhibition of Vesicular Release and Slow Synaptic Transmission
In WT mice, brief trains (1sec, 20 Hz) of stimuli induced neurotransmitter release after the end of the stimulus train (Fig. 5A). WT S neurons manifest a slowly developing hyperpolarization (~4mV) following this stimulation which returned to the resting membrane potential (Fig. 5C). The source of the late phase hyperpolarization is unknown. In preparations from CB1−/− mice, the same stimulation resulted in a significant greater depolarization (~6mV; Fig. 5B & 5C), and a dramatic increase in the occurrence of fast synaptic events following the stimulus (Fig. 5D). Occasionally, the depolarization and superimposed fast EPSPs were sufficient to elicit action potentials in otherwise quiescent neurons. These action potentials were included in the synaptic event totals. These synaptic events involve a cholinergic nicotinic synapse as they were abolished in the presence of hexamethonium (100µM, Fig 5D).
Figure 5.
Slow EPSPs evoked in WT and CB1−/− S neurons. (A) Lack of Slow EPSP or fast EPSPs in a WT myenteric S neuron. Lower trace indicates membrane potential activity 20 sec following the slow EPSP stimulus with increased time resolution. Slow membrane hyperpolarization typically occurs at a later time point, outside the sample traces. (B) Slow EPSP evoked in a CB1−/− S neuron demonstrating increased fast synaptic events as shown in the lower trace. (C) Maximum depolarization occurring within the first minute of the response (early phase) and maximum hyperpolarization during 5 min of recorded membrane potential dynamics (late phase) following slow EPSP stimulus. (D) Significant increase in fast synaptic events in CB1−/− S neurons can be mimicked in WT neurons with incubation of the tissue with the CB1 receptor antagonist AM251 (100 nM, sample trace d inset. Sample trace includes fast EPSP and APs). The fast synaptic events are entirely cholinergic as they are abolished by the addition of hexamethonium (HEX, 100µM). The proportion of neurons which received fast synaptic events during slow EPSP stimulus is significantly reduced with HEX, ### P < 0.0001 Chi squared test compared to control). * P < 0.05, ** P < 0.01 compared to control, Student’s unpaired T test. Micrographs in A and B demonstrate representative S neuron morphology of recorded neurons from WT and CB1−/− myenteric plexus. Scale bar in A and B: 20 µm. Downward deflections on membrane traces reflect current injections utilized to monitor input resistance.
When recordings from WT tissue were made in the presence of CB1 receptor antagonist AM251 (100nM), the number of fast synaptic events was also significantly increased above untreated controls during the first minute following stimulation (26.5 ± 11.2 events WT+AM251 [n = 4] vs. 7.2 ± 3.3 events WT-AM251 [n = 14], Student’s t-test, P< 0.05, Fig 5D inset). A significant depolarization did not occur in the presence of AM251 (2.9 ± 1.1 mV [n = 5]) and the subsequent late hyperpolarization remained (−7.2 ± 1.6 mV [n = 5]).
Retrograde Messengers at Enteric Synapses Regulate Synaptic Strength
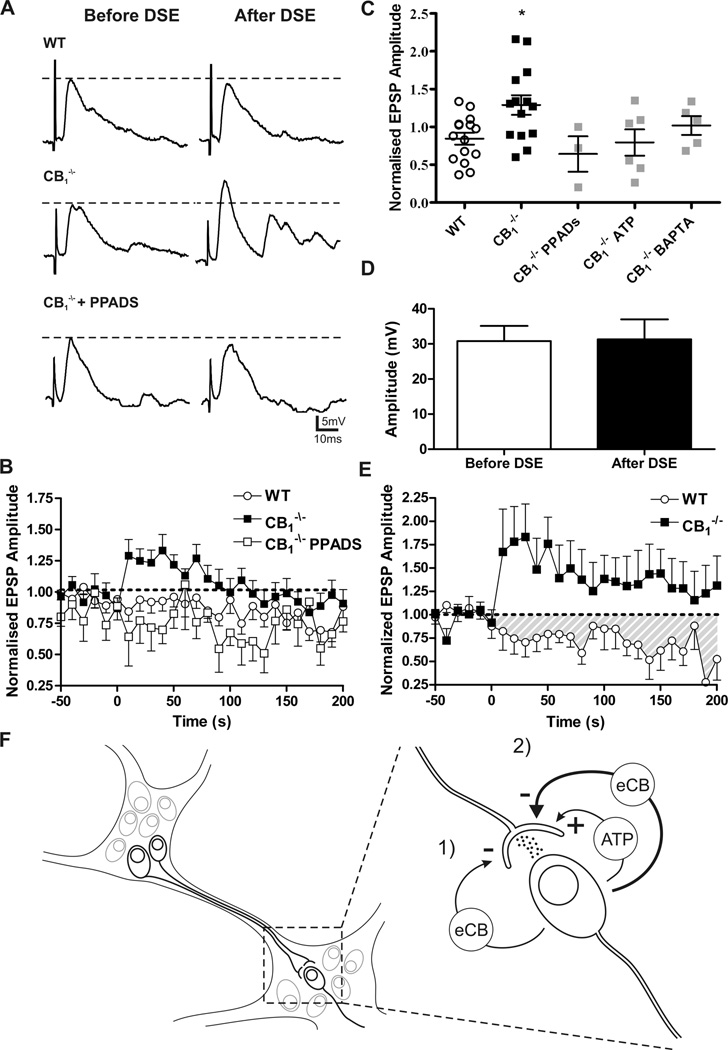
We hypothesized that depolarization of the postsynaptic neuron (1 min train of 20Hz, 2ms current injections to achieve 0mV) would result in retrograde neurotransmitter release, resulting in actions on presynaptic terminals to alter neurotransmitter release as has been shown elsewhere for endocannabinoids.4,5,30 Following this postsynaptic stimulation protocol in WT S neurons there was no change in the amplitude or area of evoked fast EPSPs indicating no alteration in vesicular release from the presynaptic terminal. However, in S neurons from CB1−/− mice the same protocol resulted in a significant potentiation of fast EPSPs (area under the curve [AUC] 14.6 ± 7.8 arbitrary units, CB1−/− [n = 14] vs. −7.3 ± 5.4 arbitrary units, WT [n = 16], Student’s t-test, p < 0.01, Fig. 6A–C). Importantly, this potentiation occurred in the absence of any change in postsynaptic sensitivity to exogenously applied ACh (30.8 ± 4.3 mV depolarization before protocol vs. 31.1 ± 5.7 mV after protocol, n = 4, ns) or change in the input resistance of the postsynaptic neuron (409 ± 50 mΩ before protocol vs. 489 ± 56 mΩ after protocol, n = 4, ns). This result suggested that in the absence of CB1 receptors, a previously unrecognized chemical signal causes short-term synaptic potentiation in the myenteric plexus.
Figure 6.
Depolarization induced facilitation of fast EPSPs in CB1−/− S neurons. (A) Representative traces of evoked fast EPSP before and immediately after the postsynaptic depolarization protocol. There is no change in fast EPSP amplitude in WT neurons (upper), but there is a significant potentiation in CB1−/− (middle) neurons that is abolished in the presence of PPADs (lower). (B) Time course of the fast EPSP amplitude facilitation observed following the postsynaptic depolarization protocol. (C) Degree of fast EPSP facilitation following the postsynaptic stimulation protocol. Fast EPSPs are significantly potentiated in CB1−/− S neurons. This facilitation is blocked by PPADs, purine receptor desensitization, with 1 mM ATP (ATP) preincubation, and by intracellular calcium chelation of the postsynaptic neuron (BAPTA, 10mM). Groups were compared using one-way ANOVA with Dunnett’s post-test. (D) No change to postsynaptic sensitivity to ACh as demonstrated by the depolarization caused by picospritz application of 1mM ACh before and following the postsynaptic depolarization protocol. (E) Synaptic depression after coincidence stimulation in WT but not CB1−/− S neurons. The same experiment in CB1−/− S neurons caused significant facilitation. (F) Schematic representation of the organization and interconnectivity of ENS as well as synaptic regulation. 1) There is a tonic depression of synapses caused by endocannabinoids (eCB) acting through CB1. 2) High frequency postsynaptic depolarization induces the release of a purine (here ATP is shown) which potentiates further synaptic transmission. When this postsynaptic activity is paired with presynaptic activity there is a potentiation of eCB production and synaptic depression. * P < 0.05.
Adenosine triphosphate (ATP) has been suggested to facilitate ACh release at autonomic synapses31–33 as well as at enteric synapses,34 through an unknown mechanism. Baldassano et al.35 demonstrated in C57BL/10SnJ mice that purines acting at P2X receptors antagonize the suppression of spontaneous contractions of longitudinal muscle by CB1 receptor activation. We therefore applied the P2 purinergic receptor antagonist pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) (PPADs, 30µM) during the stimulation protocol. PPADs had no effect on the WT S neuron fast EPSPs following repeated depolarizations, nor did PPADS change the basal fast EPSP amplitude (13.7 ± 5.1mV, CB1−/− + PPADS [n = 3]; 13.1 ± 2.8mV,WT + PPADS [n=5]) or fast EPSP magnitude (143 ±34.2mV·s, CB1−/− + PPADS[n=3]; 222.9 ± 73.6mV·s, WT + PPADS [n=5]) in comparison to untreated controls (see above). PPADs abolished the facilitation in S neurons from CB1−/− mice following the postsynaptic stimulation protocol (Fig. 6A–C). In this setting it seems that purinergic signals acting at P2 receptors induce synaptic facilitation. To further test the involvement of purine receptors in the facilitation of neurotransmitter release purine receptor desensitization was induced by incubating the tissue in 1 mM ATP for a minimum of 15 minutes. Following this treatment the synaptic potentiation was eliminated in the CB1−/− preparations consistent with the PPADs results and suggestive of the involvement of a desensitizing P2 receptor in enteric synaptic potentiation (Fig. 6C).
To validate our hypothesis that the postsynaptic neuron is the source of the retrograde purine signal the fast calcium chelator BAPTA was infused into the impaled neuron via the recording pipette. The ability of BAPTA to enter the impaled neuron and chelate intracellular calcium was confirmed in AH neurons. These neurons possess well-characterized calcium dependent afterhyperpolarizations (AHP) following an action potential. Within ten minutes of establishing an impalement of a AH neuron the AHP was eliminated with 10 mM BAPTA (unpublished observations). When BAPTA was perfused into enteric S neurons and the postsynaptic stimulation protocol was applied as previously the potentiation was eliminated (Fig. 6C).
When the same depolarization protocol was combined with coincident presynaptic stimulation (fibre tract stimulation at 20 Hz for 30 sec with 30 sec recovery, 5–10V) to evoke synaptic events, a significant depression of fast EPSPs was seen in WT neurons (Fig. 6E). In the first 5 min following this stimulation protocol, fast EPSPs were significantly depressed (AUC, −62.2 ± 20.14 arbitrary units [n = 9]) in comparison to fast EPSPs in WT S neurons following only a postsynaptic stimulation (AUC 0.1 ± 0.1 arbitrary units [n = 19] postsynaptic stimulation, Student’s t-test, P< 0.05 Fig. 6B). In S neurons from CB1−/− animals, the same protocol induced a dramatic potentiation of fast EPSPs that was significantly greater than following the identical protocol in WT S neurons (Fig. 6E). The enhanced facilitation could be the result of greater liberation of a purine though coincidence stimulation, though other mechanisms are possible. Taken together, we propose a model of action of endocannabinoids acting as inhibitory retrograde neurotransmitters together with purines acting as facilitatory retrograde neurotransmitters (Fig. 6F).
DISCUSSION
Regulation of synaptic activity in the ENS is a rapidly evolving area of study in enteric neurobiology. Here we tested the hypothesis that enteric CB1 receptors provide a mechanism of dampening excitatory neurotransmission and maintaining network stability through the regulation of synaptic strength in the myenteric plexus. Collectively, our results indicate that endocannabinoids acting via the CB1 receptor modulate cholinergic transmission in myenteric neurons through presynaptic mechanisms. This modulation includes activity-independent inhibition of synapses as well as activity-dependent inhibition. This finding provides an explanation for previous reports that endocannabinoid tone inhibits neurogenic spontaneous contractility in the mouse ileum.36
The increased spontaneous and fast EPSPs evoked by prolonged presynaptic stimulation in CB1−/− mice, combined with the CB1 mediated depression of fast EPSPs and paired-pulse facilitation in WT mice, implicate a presynaptic site of action of endocannabinoids. This conclusion is further supported by the observation in CB1−/− mice that postsynaptic depolarization to applied ACh is unchanged. In the cerebellum, CB1 coupling via Gβγ decreases Ca2+ entry into the presynaptic bouton, and hyperpolarizes the bouton by increasing K+ conductances.5 Both actions decrease quantal release and thus reduce the fast EPSP amplitude and increase the paired-pulse ratio of fast postsynaptic currents.37 A similar mechanism may be active at the enteric synapse to inhibit vesicular neurotransmitter release. It should be noted that others have demonstrated the opposite effect of CB1 receptor agonists on vesicular release;19 however, as those authors state, prolonged incubation with CB1 compounds (48 h) may have led to CB1 receptor desensitization. In our study we have demonstrated CB1 receptor mediated inhibition of vesicular release on a shorter time scale minimizing the confound of receptor desensitization.
Other possible sites of action for endocannabinoid mediated inhibition of vesicular release include blockade of axonal conductance, as well as interference with the vesicular release machinery.37 Both could account for the observed effects. However, uniform Ca2+ waves were seen in climbing fibres to Purkinje cells following a protocol to elicit depolarization suppression of excitation,5 arguing against axonal conductance failure as a mechanism. CB1 receptor activity and cannabinoids may also interfere with the proteins responsible for vesicular fusion and neurotransmitter release. However, the fact that the frequency of spontaneous events was unchanged between WT and CB1−/− S neurons suggests that sensitivity of the vesicular release machinery is the same in the presence of an intact CB1/endocannabinoid system. Thus, direct interference with the vesicular release machinery by endocannabinoids acting at CB1 may not be involved. Indeed, this mechanism of inhibition has been ruled out in other regions such as the hippocampus.38
Another possible explanation is the direct inhibition of the firing of interneurons within the preparation. However, the S neurons investigated in this study will have included a population of interneurons, and spontaneous action potential firing was never detected. Furthermore, spontaneous release of neurotransmitter was not affected by the application of TTX to block interneuron action potentials. Boesmans et al.19 demonstrated in enteric neurons that CB1 antagonism increased the percentage of neurons with spontaneous Ca2+ waves within the soma. This result parallels well our observation of an increased percentage of neurons receiving spontaneous EPSPs in CB1−/− myenteric plexus. It seems most likely that the CB1 mediated inhibition of synaptic transmission is due to changes in Ca2+ influx to the presynaptic neuron. Additionally, prolonged synaptic stimulation, such as occurs during the slow EPSP stimulus, would lead to a large increase in presynaptic Ca2+, possibly further potentiated by calcium-induced calcium release. The occurrence of a prolonged train of fast EPSPs superimposed on the slow EPSP of CB1−/− S neurons (Fig. 5) may result from the unopposed presynaptic influx of Ca2+ triggering late neurotransmitter release. In the WT mice with an intact inhibitory endocannabinoid signalling system, the Ca2+ influx may be inhibited in a CB1 dependent fashion. Hence, by a process of elimination we propose that at the enteric synapse, CB1 receptors act to inhibit the Ca2+ influx driving vesicular release. It is interesting that the CB1 agonist WIN55,212-2 is able to depress WT synapses globally while spontaneous activity is apparently regulated by CB1 receptors in only a subset of these synapses. It is possible that endocannabinoid signalling is not uniform across all synapses in the ENS. Additionally synapses originating from different subtypes of neurons may be regulated by endocannabinoids to differing degrees.
The absence of the inhibitory influence of the CB1 receptor in CB1−/− animals reveals a potent activity-dependent purinergic facilitation of fast EPSPs. A synergistic postsynaptic relationship between ATP and ACh has been demonstrated in pancreatic islets;39 here we show that a similar relationship exists in the ENS involving a presynaptic mechanism. Our data indicate that retrograde endocannabinoid and purinergic transmitters interact to regulate vesicle release probability and control synaptic communication in the myenteric plexus (Fig. 6F). Removal of endocannabinoid signalling results in unopposed purinergic synaptic facilitation and increased vesicle release, ultimately culminating in the increased excitatory neurotransmission in the ENS, and this likely leads to accelerated GI transit as shown here (Fig. 3D) and by others.13 The P2 receptor isotype that mediates the response described here has not been identified. The rapid onset and transient nature of the observed phenomenon suggests a ligand-gated ion channel, such as the P2X receptors which are ligand-gated non-specific cation channels. When open, these channels would allow for the permeation of calcium into the presynaptic neuron, a mechanism by which a purine messenger could potentiate synaptic transmission. However, the concentration of PPADS used was sufficient to block P2Y1 receptors40 and activation of these receptors can excite a relatively rapid (50 ms rise, 250 ms duration) depolarization in enteric neurons,40,41 albeit in guinea pig. Thus, the data do not exclude the possibility of the involvement of P2Y1 receptors. These receptors are coupled by inositol trisphosphate to intracellular calcium stores, so that they might be expected to enhance transmitter release.
We have established that purinergic-endocannabinoid interactions in the ENS regulate synaptic homeostasis. An intimate link between purine liberation and endocannabinoid action is suggested by our observation that P2 antagonism with PPADs did not reveal potent synaptic inhibition in WT animals. Autocrine signalling by purines may be required to generate a burst of endocannabinoid production. Indeed purines have been shown to stimulate endocannabinoid production in the cerebellar cortex.42 Alternatively, the inability to reveal synaptic depression with a P2 antagonist in the WT preparations may be due to a dosage effect of liberated endocannabinoid. Coincident stimulation would lead to activation of metabotopic receptor activation and potentiation of endocannabinoid release.6 Indeed, using this experimental paradigm of coincident stimulation we revealed synaptic depression (Fig. 6E). Unfortunately, it is difficult to test if P2 antagonism could potentiate this phenomenon as activity of PPADS on postsynaptic P2Y receptors would inhibit part of the postsynaptic metabotopic stimulation that this protocol would elicit.43
Our model does not account for the contribution of other cell types in the enteric nervous system. Enteric glia can participate in the metabolism of neurotransmitters,44 and neuron – glia purinergic communication is a feature of the murine ENS.45 Our model also does not address the possibility of polysynaptic contributions from downstream neurons excited by our depolarization protocol. Myenteric S neurons project to longitudinal or circular smooth muscle, or extend in a oral or aboral direction to distant enteric ganglion.21 The projections are uniaxonal and highly polarized to allow the propagation of peristaltic patterns of motility. We cannot rule out the contribution of feedback mechanisms from downstream enteric neurons, which have been demonstrated in the ENS.46–48 The route travelled by the trans-synaptic signal may be tortuous; however, the ability of postsynaptic calcium chelation to block synaptic potentiation indicates that the signal originates from the postsynaptic neuron. Furthermore, if the retrograde purine signal also originates from the postsynaptic neuron this implicates a calcium sensitive release mechanism for ATP and related purines from the cell soma of neurons within the ENS.
These results are the first to identify presynaptic endocannabinoid modulation of enteric synapses. In the absence of endocannabinoid modulation a purinergic signal is revealed to also be involved in synaptic regulation by facilitating transmitter release in an activity dependent manner. In the postsynaptic depolarization model we employed we are unable to conclusively demonstrate activity and CB1 receptor dependent inhibition of synapses. However, the absence of apparent synaptic plasticity when the CB1 receptor is intact suggests an endocannabinoid effect that opposes activity dependent purinergic facilitation on an identical time course. These findings suggest a novel form of metaplasticity through the balance of endocannabinoid and purinergic signalling at the enteric synapse, and have important implications for our understanding of enteric physiology. These findings demonstrate that the enteric synapse can respond to high levels of network activity and that at least two signalling molecules are involved in manipulating synaptic strength in response to this activity. Clearly computations that occur at the enteric synapse are an important feature in maintaining ENS network homeostasis.
Supplementary Material
Acknowledgements
We thank Winnie Ho and Cathy Keenan for excellent technical support. Keith Sharkey and Quentin Pittman are Alberta Heritage Foundation for Medical Research Scientists. Keith Sharkey holds the CCFC Chair in IBD Research at University of Calgary. Quentin Pittman is a University Professor.
Grant Support:
This work was supported by grants from the Canadian Institutes of Health Research (to KAS and QJP), the Crohn’s and Colitis Foundation of Canada (CCFC, to MS and KAS) and NIH (grants DA11322 and DA21696 to KM; grant DK62267 to GMM).
Abbreviations
- ACh
acetylcholine
- AHP
afterhyperpolarization
- ATP
adenosine triphosphate
- AUC
area under the curve
- BAPTA
1,2-bis(o-Aminophenoxy)ethane-N,N,N′,N′-tetraacetic Acid, tetrasodium salt
- CB1
cannabinoid type 1 receptor
- CB1−/−
CB1 receptor-deficient mice
- CNS
central nervous system
- ENS
enteric nervous system
- EPSP
excitatory postsynaptic potential
- GI
gastrointestinal
- HEX
hexamethonium
- PPADS
pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid)
- PPR
paired-pulse ration
- TTX
tetrodotoxin
- WT
wild type
Footnotes
Author contributions
IMH, MAS, QJP, GMM & KAS designed the studies, IMH & MAS performed research, KM & BL provided unique reagents, IMH, MAS, QJP, GMM & KAS analyzed data and IMH, MAS, KM, BL, QJP, GMM & KAS wrote and revised the paper.
References
- 1.Furness JB. The enteric nervous system. Malden, Mass: Blackwell Pub; 2006. [Google Scholar]
- 2.Galligan JJ, North RA. Pharmacology and function of nicotinic acetylcholine and P2X receptors in the enteric nervous system. Neurogastroenterol Motil. 2004;16 Suppl 1:64–70. doi: 10.1111/j.1743-3150.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- 3.Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7–18. doi: 10.1111/j.1365-2982.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 5.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 6.Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 7.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 8.Kuzmiski JB, Pittman QJ, Bains JS. Metaplasticity of hypothalamic synapses following in vivo challenge. Neuron. 2009;62:839–849. doi: 10.1016/j.neuron.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Redondo F, Lees GM, Pertwee RG. Effects of cannabinoid receptor ligands on electrophysiological properties of myenteric neurones of the guinea-pig ileum. Br J Pharmacol. 1997;122:330–334. doi: 10.1038/sj.bjp.0701393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coutts AA, Pertwee RG. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br J Pharmacol. 1997;121:1557–1566. doi: 10.1038/sj.bjp.0701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010;126:21–38. doi: 10.1016/j.pharmthera.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Duncan M, Mouihate A, Mackie K, et al. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am J Physiol Gastrointest Liver Physiol. 2008;295:G78–G87. doi: 10.1152/ajpgi.90285.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan M, Davison JS, Sharkey KA. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005;22:667–683. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- 16.Pinto L, Izzo AA, Cascio MG, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002;123:227–234. doi: 10.1053/gast.2002.34242. [DOI] [PubMed] [Google Scholar]
- 17.Duncan M, Thomas AD, Cluny NL, et al. Distribution and function of monoacylglycerol lipase in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1255–G1265. doi: 10.1152/ajpgi.90500.2008. [DOI] [PubMed] [Google Scholar]
- 18.Marquez L, Suarez J, Iglesias M, et al. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS One. 2009;4:e6893. doi: 10.1371/journal.pone.0006893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boesmans W, Ameloot K, van den Abbeel V, et al. Cannabinoid receptor 1 signalling dampens activity and mitochondrial transport in networks of enteric neurones. Neurogastroenterol Motil. 2009;21:958–e77. doi: 10.1111/j.1365-2982.2009.01300.x. [DOI] [PubMed] [Google Scholar]
- 20.Marsicano G, Wotjak CT, Azad SC, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 21.Nurgali K, Stebbing MJ, Furness JB. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J Comp Neurol. 2004;468:112–124. doi: 10.1002/cne.10948. [DOI] [PubMed] [Google Scholar]
- 22.Hons IM, Burda JE, Grider JR, et al. Alterations to enteric neural signaling underlie secretory abnormalities of the ileum in experimental colitis in the guinea pig. Am J Physiol Gastrointest Liver Physiol. 2009;296:G717–G726. doi: 10.1152/ajpgi.90472.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krauter EM, Linden DR, Sharkey KA, et al. Synaptic plasticity in myenteric neurons of the guinea-pig distal colon: presynaptic mechanisms of inflammation-induced synaptic facilitation. J Physiol. 2007;581:787–800. doi: 10.1113/jphysiol.2007.128082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tappe-Theodor A, Agarwal N, Katona I, et al. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci. 2007;27:4165–4177. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coutts AA, Irving AJ, Mackie K, et al. Localisation of cannabinoid CB(1) receptor immunoreactivity in the guinea pig and rat myenteric plexus. J Comp Neurol. 2002;448:410–422. doi: 10.1002/cne.10270. [DOI] [PubMed] [Google Scholar]
- 27.Qu ZD, Thacker M, Castelucci P, et al. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 28.Wong V, Blennerhassett M, Vanner S. Electrophysiological and morphological properties of submucosal neurons in the mouse distal colon. Neurogastroenterol Motil. 2008;20:725–734. doi: 10.1111/j.1365-2982.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 29.Casu MA, Porcella A, Ruiu S, et al. Differential distribution of functional cannabinoid CB1 receptors in the mouse gastroenteric tract. Eur J Pharmacol. 2003;459:97–105. doi: 10.1016/s0014-2999(02)02830-3. [DOI] [PubMed] [Google Scholar]
- 30.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 31.Cunha RA, Ribeiro JA. ATP as a presynaptic modulator. Life Sci. 2000;68:119–137. doi: 10.1016/s0024-3205(00)00923-1. [DOI] [PubMed] [Google Scholar]
- 32.Sperlagh B, Vizi ES. Effect of presynaptic P2 receptor stimulation on transmitter release. J Neurochem. 1991;56:1466–1470. doi: 10.1111/j.1471-4159.1991.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 33.Sperlagh B, Heinrich A, Csolle C. P2 receptor-mediated modulation of neurotransmitter release-an update. Purinergic Signal. 2007;3:269–284. doi: 10.1007/s11302-007-9080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duarte-Araujo M, Nascimento C, Timoteo MA, et al. Relative contribution of ecto-ATPase and ecto-ATPDase pathways to the biphasic effect of ATP on acetylcholine release from myenteric motoneurons. Br J Pharmacol. 2009;156:519–533. doi: 10.1111/j.1476-5381.2008.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldassano S, Zizzo MG, Serio R, et al. Interaction between cannabinoid CB1 receptors and endogenous ATP in the control of spontaneous mechanical activity in mouse ileum. Br J Pharmacol. 2009;158:243–251. doi: 10.1111/j.1476-5381.2009.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldassano S, Serio R, Mule' F. Cannabinoid CB(1) receptor activation modulates spontaneous contractile activity in mouse ileal longitudinal muscle. Eur J Pharmacol. 2008;582:132–138. doi: 10.1016/j.ejphar.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 38.Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertrand G, Chapal J, Loubatieres-Mariani MM. Potentiating synergism between adenosine diphosphate or triphosphate and acetylcholine on insulin secretion. Am J Physiol. 1986;251:E416–E421. doi: 10.1152/ajpendo.1986.251.4.E416. [DOI] [PubMed] [Google Scholar]
- 40.Gwynne RM, Bornstein JC. Electrical stimulation of the mucosa evokes slow EPSPs mediated by NK1 tachykinin receptors and by P2Y1 purinoceptors in different myenteric neurons. Am J Physiol Gastrointest Liver Physiol. 2009;297:G179–G186. doi: 10.1152/ajpgi.90700.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monro RL, Bertrand PP, Bornstein JC. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J Physiol. 2004;556:571–584. doi: 10.1113/jphysiol.2004.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovacs FE, Illes P, Szabo B. Purine receptor-mediated endocannabinoid production and retrograde synaptic signalling in the cerebellar cortex. Br J Pharmacol. 2011;162:974–988. doi: 10.1111/j.1476-5381.2010.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood JD, Kirchgessner A. Slow excitatory metabotropic signal transmission in the enteric nervous system. Neurogastroenterol Motil. 2004;16 Suppl 1:71–80. doi: 10.1111/j.1743-3150.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 44.Lavoie EG, Gulbransen BD, Martin-Satue M, et al. Ectonucleotidases in the digestive system: focus on NTPDase3 localization. Am J Physiol Gastrointest Liver Physiol. 2011;300:G608–G620. doi: 10.1152/ajpgi.00207.2010. [DOI] [PubMed] [Google Scholar]
- 45.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 46.Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea pig small intestine. J Neurosci. 1992;12:1502–1510. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neal KB, Bornstein JC. Targets of myenteric interneurons in the guinea-pig small intestine. Neurogastroenterol Motil. 2008;20:566–575. doi: 10.1111/j.1365-2982.2007.01052.x. [DOI] [PubMed] [Google Scholar]
- 48.Neal KB, Bornstein JC. Mapping 5-HT inputs to enteric neurons of the guinea-pig small intestine. Neuroscience. 2007;145:556–567. doi: 10.1016/j.neuroscience.2006.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.