Abstract
To determine whether calcineurin inhibitor (CNI) blood concentrations within the first month after allogeneic hematopoietic cell transplantation (HCT) correlated with the incidence of graft-versus-host disease (GVHD) and other outcomes, we retrospectively analyzed data from 1181 patients with hematologic malignancies who had HCT from HLA-matched related (n=634) or unrelated (n=547) donors at a single institution between 2001 and 2009. After myeloablative HCT (n=774), higher CNI concentrations were not associated with lower risks of acute or chronic GVHD. After nonmyeloablative HCT (n=407), higher cyclosporine concentrations were associated with decreased risks of grade 2–4 and 3–4 acute GVHD (hazard ratio [HR] per 100 ng/ml change in cyclosporine concentrations, 0.7; 95% confidence interval [CI], 0.6–0.82; and HR, 0.66, 95%CI, 0.49–0.9, respectively), non-relapse mortality (HR, 0.6, 95% CI, 0.41–0.88), and overall mortality (HR, 0.83, 95%CI, 0.71–0.99). Cyclosporine concentrations were not associated with risks of chronic GVHD and recurrent malignancy after nonmyeloablative HCT. Among patients given tacrolimus after nonmyeloablative HCT, a similar trend of CNI-associated GVHD-protection was observed. Higher CNI concentrations were not associated with apparent renal toxicity. We conclude that higher cyclosporine concentrations relatively early after nonmyeloablative HCT confer protection against acute GVHD that translates into reduced risks of non-relapse and overall mortality.
Introduction
Graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT) is associated with morbidity and mortality [1,2]. The most commonly used regimens for prevention of acute GVHD consist of a combination of a calcineurin inhibitor (CNI), either cyclosporine (CSP) or tacrolimus (TAC), and an anti-metabolite. In patients receiving myeloablative conditioning, CSP and methotrexate (MTX) have been clinically used for almost three decades [3,4]. Subsequently, the combination of TAC and MTX has been shown to be at least as effective as CSP and MTX [5,6]. For patients receiving a nonmyeloablative preparative regimen, GVHD prophylaxis is frequently based on a CNI and mycophenolate mofetil (MMF) [7,8]. While both MTX and MMF are usually given at fixed doses, CNI dosing is typically adjusted both to avoid toxicities, especially renal, and to maintain whole blood concentrations within a therapeutic range [9–11].
Among patients given myeloablative HCT, attempts aimed at decreasing administered CSP doses during the first 10 days irrespective of concentrations showed that, while incidence and severity of acute GVHD remained unaffected, lower CSP doses were associated with reduced adverse events [12]. Conversely, when CSP concentrations were targeted instead of adhering to an absolute administered dose, lower CSP concentrations were associated with increased grades 2–4 acute GVHD [13]. Four other small cohort studies showed that in patients given myeloablative conditioning, lower CSP concentrations before engraftment were associated with significantly increased risks of grade 2–4 [14–17] and grade 3–4 [17,18] acute GVHD. A large prospective trial, however, showed only a trend toward an association between low CSP concentrations and increased risk of GVHD, while no correlation was found between TAC concentrations and risk of GVHD [10]. Data for patients receiving nonmyeloablative or reduced-intensity conditioning regimens are even scarcer, with only one publication reporting data on a mixed group of patients given either myeloablative or reduced-intensity conditioning [17].
In the present study, we retrospectively analyzed data from two cohorts of patients who received HCT from HLA-matched related or unrelated donors with either myeloablative or nonmyeloablative conditioning and who were treated with either CSP or TAC-based immunosuppression. Each cohort was analyzed for possible associations between CNI concentrations and incidence and severity of acute GVHD among other transplantation outcomes.
Patients and Methods
Patients
Patients who received allogeneic HCT between 01/2001 and 06/2009 at the Fred Hutchinson Cancer Research Center were identified from a computerized database. Patients had signed forms approved by the institutional review board documenting informed consent to participate in clinical trials and to allow the use of medical information for research. To be included in the study, patients had to be at least 18 years old and receive their first allogeneic transplant for hematological malignancy from HLA-identical sibling or 10 of 10 HLA-antigen-matched unrelated donors. HLA typing was performed with intermediate- or high-resolution molecular matching for HLA-A, -B, -C, -DRB1, and -DQB1. Only patients who received a CNI and MTX after myeloablative conditioning or a CNI and MMF after nonmyeloablative conditioning were included. Patients receiving second allogeneic HCT were excluded. Patient characteristics are listed in Table 1.
Table 1.
Patients’ characteristics
| Datum | Conditioning | All Patients (N=1181) | |||
|---|---|---|---|---|---|
| Myeloablative (N=774) | Nonmyeloablative (N=407) | ||||
| Cyclosporine (N=451) | Tacrolimus (N=323) | Cyclosporine (N=280) | Tacrolimus (N=127) | ||
| Sex (Female/male, %) | 45/55 | 42/58 | 40/60 | 46/54 | 43/57 |
| Female donor to male recipient (%) | 24 | 26 | 26 | 22 | 25 |
| Patients’ age, years (median, range) | 45 (19–66) | 45 (18–67) | 56 (18–79) | 57 (20–74) | 49 (18–79) |
| Donors’ age, years (median, range) | 40 (10–74) | 38 (15–80) | 43 (18–83) | 46 (20–77) | 41 (10–83) |
| Donor type (Related/unrelated, %) | 58/42 | 49/51 | 52/48 | 53/47 | 54/46 |
| PBSC/marrow, % | 85/15 | 86/14 | 99/1 | 100/0 | 91/9 |
| Disease risk (low/high, %) | 66/34 | 72/18 | 38/62 | 45/55 | 59/41 |
| Year of transplantation (N patients) | |||||
| 2001 | 117 | 1 | 51 | -- | 169 |
| 2002 | 112 | 1 | 49 | -- | 162 |
| 2003 | 114 | 3 | 52 | 1 | 170 |
| 2004 | 84 | 21 | 38 | 9 | 152 |
| 2005 | 18 | 100 | 21 | 30 | 169 |
| 2006 | 3 | 79 | 33 | 18 | 133 |
| 2007 | 1 | 47 | 24 | 27 | 99 |
| 2008 | 0 | 50 | 5 | 29 | 84 |
| 1–6/2009 | 2 | 21 | 7 | 13 | 43 |
| High-dose Regimen (N patients) | |||||
| TBI-based | 152 | 114 | -- | -- | 266 |
| Non TBI-based | 299 | 117 | -- | -- | 416 |
| Low-dose Regimen (N patients) | |||||
| Flu-2 (3) Gy TBI | -- | -- | 232 | 100 | 332 |
| 2 Gy TBI | -- | -- | 48 | 27 | 75 |
Flu – fludarabine; HD – high dose; LD- low dose; PBSC – G-mobilized peripheral blood stem cells; TBI-total body irradiation.
Preparative Regimen and Immunosuppression Treatment
Myeloablative conditioning regimens were either TBI-based (primarily intravenous cyclophosphamide; 60 mg/kg per day for 2 consecutive days followed by fractionated TBI, 12 Gy) or non-TBI-based (primarily oral busulfan; 4 mg/kg per day for 4 consecutive days) and intravenous cyclophosphamide (60 mg/kg per day for 2 consecutive days). Nonmyeloablative conditioning regimens included low-dose TBI (2–3 Gy) with or without fludarabine (30 mg/m2 body surface area/day, for 3 consecutive days) [19].
For patients receiving myeloablative conditioning, the intravenous CNI was started on day -1 before HCT. Intravenous CSP was administered at 3 mg/kg/day given in two divided doses every 12 hours at (1.5 mg/kg each dose) and infused over a period of 1 hour. TAC was given at 0.03 mg/kg/day (1.25 mcg/kg/hour) by continuous intravenous infusion. Intravenous medications were usually discontinued once patients started to eat, and drugs were switched to oral formulations. For patients receiving nonmyeloablative conditioning, the CNI was started on day -3 before HCT. CSP was given either orally at 5–6.25 mg/kg twice daily or intravenously at 2.5 mg/kg twice daily. TAC was given orally at a dose of 0.06 mg/kg twice daily. All initial dose calculations were based on adjusted body weights.
CNI levels were based on trough whole blood concentrations. As a general rule, if patients received CNIs intravenously, trough concentrations were obtained daily before the next dose was administered. If patients were treated with an oral formulation, trough concentrations were obtained in the morning, twice weekly, before the next dose.
Measurements of whole blood TAC levels were performed using the IMx assay [20]. During the initial study period, CSP concentrations were measured by monoclonal or polyclonal antibody assay. Starting on July, 2007, CSP concentrations were measured by high-pressure liquid chromatography (LCMSMS) [21]. For the purpose of a uniform statistical analysis, a correction factor of 0.8 has been applied to all CSP concentrations measured by monoclonal or polyclonal antibody assay to adjust the antibody assay values to the LCNSMS values. In general, for the first 28 days, CNI concentrations in nonmyeloablative patients were targeted higher than in myeloablative patients and tacrolimus and CSP doses were adjusted to maintain whole concentrations of 5–20 ng/mL and 200–500 ng/mL, respectively.
In addition to CNIs, patients receiving myeloablative conditioning were treated with intravenous MTX (15 mg/m2 on day 1; 10 mg/m2 on days +3, +6 and +11). For patients receiving nonmyeloablative conditioning, administration of MMF was started 4–6 hours after HCT at a dose of 15 mg/kg given two or three times daily for recipients of related or unrelated grafts, respectively.
If necessitated by the onset of GVHD, toxicity or management of persistent or recurrent malignancy, doses and tapering schedules of immunosuppressive agents were modified at the discretion of the attending physicians. However, withdrawal of immunosuppressive medications was rarely initiated before day 28.
GVHD evaluation and treatment
Acute GVHD was diagnosed and graded according to the current grading system [22]. For patients who developed acute GVHD during the taper of immunosuppressive medications, the CNI taper was held and a therapeutic CNI dose was reinstituted. For most patients, systemic glucocorticoids were used for initial treatment of GVHD. After 7–10 days, if GVHD symptoms had resolved, a glucocorticoid taper was initiated [23].
For the purpose of evaluating CNI-associated renal toxicity, the day-14 and 28 glomerular filtration rate (GFR) was calculated using the Cockcroft and Gault formula CCr=((140-age) × weight)/(72 SCr) × 0.85 if female [24].
Statistical analysis
Cumulative incidence curves for acute GVHD were estimated using methods previously described [25]. Cox regression analysis was used to model the impact of CNI levels on time-to-event endpoints. Death and relapse were treated as competing risks for analysis of acute and chronic GVHD. Relapse was treated as a competing risk for the analysis of NRM. The effect of CNI levels on hazard ratios were expressed as per 100 ng/ml increase of CSP and 5 ng/mL increase of TAC (both were approximately the interquartile ranges for both myeloablative and nonmyeloablative patients). Mean CNI levels were calculated for the first and second week and were treated as fixed covariates. Cumulative mean CNI up to day +30 was treated as a time-dependent covariate, i.e., at each time the covariate represented the mean of all prior levels up to the onset of GVHD or day +30, whichever occurred first. We decided to use the week two time point because it was after initial dose adjustments had been made, but before most GVHD occurred. Overall, results were similar for the 3 different time points. Simple linear regression was used to correlate mean CNI levels with maximum bilirubin and ALT level during the first month and the maximal change in creatinine and GFR through day 14 and day 28. All P-values are 2-sided and are not adjusted for multiple comparisons.
Results
Between January 1, 2001 and June 30, 2009, 1181 patients given allogeneic HCT fulfilled study inclusion criteria (Table 1). Preparative regimens were myeloablative or nonmyeloablative for 774 (66%) and 407 (34%) patients, respectively. CSP was given to 58% of patients in the myeloablative group and to 69% of those in the nonmyeloablative group. Most patients (86% and 99.5% in the myeloablative and in the nonmyeloablative groups, respectively) received G-CSF-mobilized peripheral blood stem cell grafts. The proportion of patients given TAC-based GVHD prophylaxis increased during the study period from 0.6% in 2001 to 87% in 2008. The median follow up was 5.1 (range, 0.6–9) years. CNI concentrations were monitored at least twice weekly and the mean numbers of concentrations assessed per patient during the first month were 10 and 11 for CSP and TAC-treated patients, respectively. Correlation between mean CNI and median CNI in our cohort was around 0.98 for each group. Among myeloablative patients, week-2 mean CSP and TAC concentrations were 261 ng/ml and 11.7 ng/ml, respectively. Among nonmyeloablative patients, week-2 mean CSP and TAC concentrations were 409 ng/ml and 13.7 ng/ml, respectively.
There was some trend of change in the mean CNI levels over years of treatment (2001–2009), but the effect size was only 1–2% per year relative to the overall mean levels. For patients given myeloablative conditioning with either CSP or TAC, there were 5.1 ng/ml (p=0.1) and −0.31 ng/ml (p=0.01) changes in the CNI blood concentration, respectively. For patients given nonmyeloablative conditioning with either CSP or TAC, there were −4.4 ng/ml (p=0.1) and −0.28 ng/ml (p=0.2) changes in the CNI blood concentration, respectively.
Acute GVHD
Incidence of grade 2–4 GVHD decreased over the years of treatment (2001–2009), but the effect size was small. For patients given myeloablative and nonmyeloablative conditioning, hazard ratios for grade 2–4 acute GVHD were 0.94 (p=0.002) and 0.94 (p=0.02) for each year. For patients given myeloablative conditioning, median time to acute GVHD onset was 24 (3–119) days, while for patients given nonmyeloablative conditioning the median was 34 (5–151) days, p<0.0001.
Myeloablative conditioning
Of the 774 patients given myeloablative conditioning, 451 (58%) were treated with CSP and 323 (42%) with TAC (Table 2). Grade 2–4 acute GVHD was documented in 310 (69%) and 228 (71%) patients, respectively. Grade 3–4 acute GVHD was documented in 56 (12%) and 33 (10%) patients, respectively. We found no correlations between week-2 mean CNI concentrations and risk of grade 2–4 acute GVHD either in patients given CSP or TAC (HR 0.99, 95% CI 0.87–1.13, p=0.88; and HR 1.02, 95% CI 0.82–1.26, p=0.88, respectively). We also found no correlation between week-2 mean CSP concentrations and the risk of grade 3–4 acute GVHD (HR 0.98, 95% CI 0.71–1.34, p=0.88). Unexpectedly, higher week-2 mean TAC concentrations were correlated with a higher risk of grade 3–4 acute GVHD (HR 1.79, 95% CI 1.12–2.86, p=0.02). When the risk of grade 3–4 acute GVHD was analyzed according to week-1 mean TAC concentrations and cumulative mean to GVHD onset, no correlation was demonstrated (0.98, 95% CI 0.88–1.08, p=0.63; and 0.99, 95% CI 0.93–1.05, p=0.65, respectively).
Table 2.
Effects of increasing week 2 mean calcineurin inhibitors blood concentrations levels as fixed covariates on acute GVHD, chronic GVHD, relapse rate, NRM and OM. Hazard ratios (HR) are given per 100 ng/ml (CSP) or 5 ng/ml (TAC)
| Datum | Myeloablative conditioning (n=774) | Nonmyeloablative conditioning (N=407) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cyclosporine (n=451) | Tacrolimus (n=323) | Cyclosporine(n=280) | Tacrolimus (n=127) | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Acute GVHD 2–4 | 0.99 (0.87–1.13) | 0.88 | 1.02 (0.82–1.26) | 0.88 | 0.70 (0.60–0.82) | <0.0001 | 0.77 (0.53–1.13) | 0.18 |
| Acute GVHD 3–4 | 0.98 (0.71–1.34) | 0.88 | 1.79 (1.12–2.86) | 0.02 | 0.66 (0.49–0.90) | 0.008 | 0.31 (0.11–0.86) | 0.02 |
| Chronic GVHD | 1.03 (0.88–1.19) | 0.74 | 0.93 (0.70–1.24) | 0.61 | 1.00 (0.83–1.21) | 0.99 | 1.25 (0.86–1.81) | 0.25 |
| Relapse | 0.82 (0.58–1.16) | 0.26 | 0.91 (0.55–1.51) | 0.71 | 0.96 (0.72–1.28) | 0.78 | 1.25 (0.80–1.96) | 0.33 |
| NRM | 0.99 (0.76–1.30) | 0.94 | 1.75 (1.17–2.61) | 0.007 | 0.60 (0.41–0.88) | 0.008 | 0.55 (0.19–1.53) | 0.25 |
| OM | 0.98 (0.84–1.15) | 0.80 | 1.18 (0.90–1.54) | 0.22 | 0.83 (0.71–0.99) | 0.03 | 0.80 (0.56–1.14) | 0.21 |
GVHD – graft vs. host disease, NRM – non relapse mortality, OM – overall mortality
Nonmyeloablative conditioning
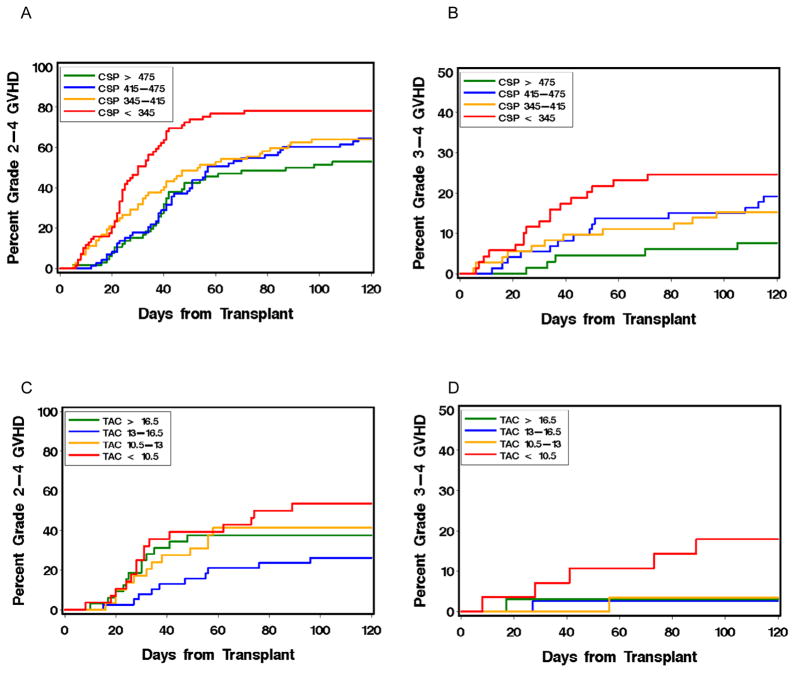
Of the 407 patients given nonmyeloablative conditioning, 280 (69%) were treated with CSP and 127 (31%) with TAC (Table 2). Grade 2–4 acute GVHD was documented in 184 (66%) and 49 (39%) patients, respectively. Grade 3–4 acute GVHD was documented in 47 (17%) and 8 (6%) patients, respectively. Among patients who were treated with CSP, a higher week-2 mean concentration was associated with a decreased risk of both grades 2–4 and 3–4 acute GVHD (HR 0.7, 95% CI 0.6–0.82, p<0.0001; and HR=0.66, 95% 0.49–0.9, p=0.008, respectively). Thus, for each 100 ng/ml increase in the CSP concentration, we observed a 30% and 34% decrease in the risks of grades 2–4 and 3–4 acute GVHD, respectively. Analysis of GVHD incidence in quartiles of patients grouped according to week-2 mean CSP concentrations showed inferior control of grades 2–4 and 3–4 acute GVHD in the lower quartiles (Figure 1a–b).
Figure 1.
Association of mean week 2 CSP (A and B) and TAC (C and D) concentrations with the cumulative incidence of grade 2–4 (A and C) and grade 3–4 (B and D) acute GVHD after HCT with nonmyeloablative conditioning. CNI concentrations were divided according to quartiles.
When recipients of grafts from related and unrelated donors were analyzed separately, we did not observe a statistically significant difference in the correlation between CSP concentration and acute GVHD (grade 2–4, p=0.19; grade 3–4, p=0.21).
We found no correlation between higher week-2 mean TAC concentrations and the risk of grade 2–4 acute GVHD (HR=0.77, 95% CI 0.53–1.13, p=0.18) (Table 2). Nonetheless, higher mean week-2 TAC concentrations were associated with a decreased risk of grade 3–4 acute GVHD (HR=0.31, 95% CI 0.11–0.86, p=0.02). Analysis of GVHD incidence in quartiles of patients grouped according to week-2 mean TAC concentrations suggested inferior control of grade 3–4 acute GVHD in the lowest quartile (<10.5 ng/ml) (Figure 1c–d).
Other Transplantation Outcomes (Table 2)
Chronic GVHD
Among recipients of myeloablative HCT given either CSP or TAC, we found no correlations between week-2 mean CNI concentrations and risk of chronic GVHD (HR 1.03, 95% CI 0.88–1.19, p=0.74 and HR 0.93, 95% CI 0.7–1.24, p=0.61, respectively) (Table 2). This was also true for patients receiving nonmyeloablative conditioning (HR 1.00, 95% CI 0.83–1.21, p=0.99 and HR 1.25, 95% CI 0.86–1.81, p=0.25, respectively).
Relapse
Among recipients of myeloablative HCT given either CSP or TAC, we found no correlation between week-2 mean CNI concentrations and the risk of relapse (HR 0.82, 95% CI 0.58–1.16, p=0.26 and HR 0.91, 95% CI 0.55–1.51, p=0.71, respectively) (Table 2). Similarly, for recipients of nonmyeloablative HCT, we found no correlation between week-2 mean CNI concentrations and risk of relapse (HR 0.96, 95% CI 0.72–1.28, p=0.78 and HR 1.25, 95% CI 0.80–1.96, p=0.33, respectively).
There was no correlation between mean week 2 CNI levels with day 28 CD3 chimerism (−0.7% per 100 ng/ml, p=0.67 in patients given CSP and +0.9% per 5 ng/ml, p=0.71 in patients given TAC). This fact might suggest an explanation for the lack of association between the CNI blood concentrations and the overall risk of relapse.
Non-relapse mortality
Among recipients of myeloablative HCT given CSP, we found no correlation between week-2 mean CSP concentrations and risk of NRM (HR 0.99, 95% CI 0.76–1.35, p=0.03) (Table 2). Among recipients of myeloablative HCT given TAC, higher mean week-2 concentrations were correlated with a higher risk of NRM (HR 1.79, 95% CI 1.17–2. 61, p=0.007). When NRM was analyzed according to week-1 mean TAC concentrations and the cumulative mean to GVHD onset, however, no correlation was demonstrated (HR 0.94, 95% CI 0.86–1.02, p=0.12 and HR 0.97, 95% CI 0.84–1.11, p=0.62, respectively). For recipients of nonmyeloablative HCT given CSP, higher week-2 mean concentrations were correlated with a lower risk of NRM (HR 0.60, 95% CI 0.41–0.88, p=0.008). Conversely, for recipients of nonmyeloablative HCT given TAC, no such correlation was observed (HR 0.55, 95% CI 0.19–1.53, p=0.25).
Overall mortality
Among recipients of myeloablative HCT given either CSP or TAC, we found no correlation between week-2 mean concentrations and risk of OM (HR 0.98, 95% CI 0.84–1.15, p=0.80 and 1.18, 95% CI 0.9–1.54, p=0.22, respectively) (Table 2). For recipients of nonmyeloablative HCT given CSP, we found a correlation between higher week-2 mean CSP concentrations and lower OM (HR 0.83, 95% CI 0.71–0.88, p=0.008). Conversely, for recipients of nonmyeloablative HCT given TAC, no such correlation was observed (HR 0.80, 95% CI 0.56–1.14, p=0.21).
Renal toxicity
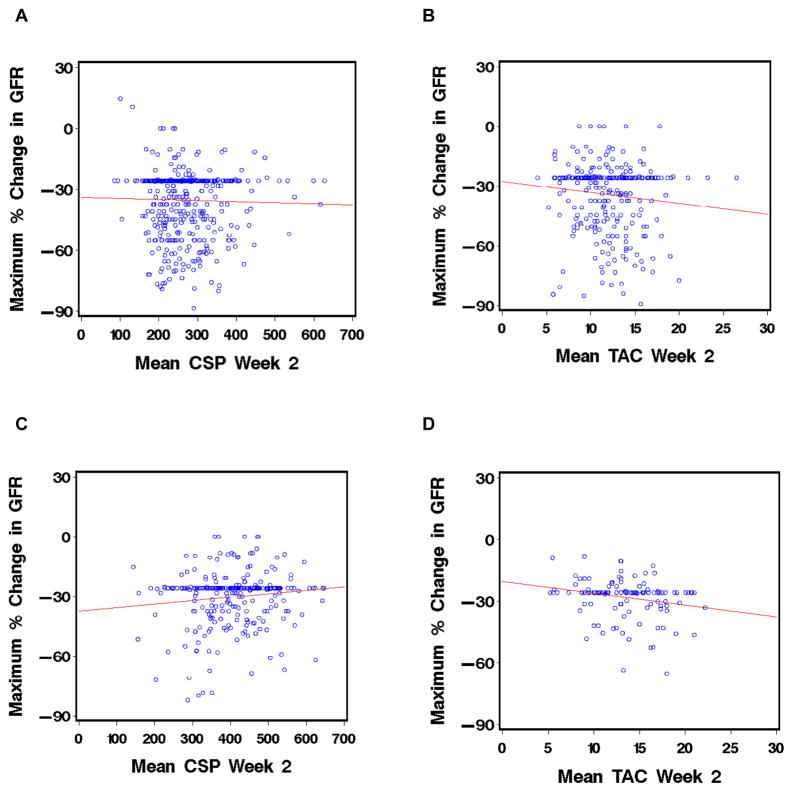
In both recipients of myeloablative and nonmyeloablative HCT, higher week-2 mean CSP concentrations were not significantly correlated with the maximal increase in creatinine concentration or with the maximal decrease in GFR during the first 4 weeks after HCT (Figure 2).
Figure 2.
Correlation of mean week 2 CNI concentrations in patients treated with CSP (A and C) or TAC (B and D) with the maximal change in GFR during the first 4 weeks after HCT with myeloablative conditioning (A and B) or nonmyeloablative conditioning (C and D).
For patients given myeloablative conditioning and TAC, higher week-2 mean concentrations were significantly correlated with both the maximal increase in creatinine concentration and the maximal decrease in GFR during the first 4 weeks after HCT, although the effect was small (estimated effect 0.16 mg/dL, standard error 0.06, p=0.004 and −3.1 ml/min, standard error 1.5, p=0.03). For patients conditioned with nonmyeloablative regimens, higher week-2 mean TAC concentrations were significantly correlated only with the maximal decrease in GFR during the first 4 weeks, although the effect was small (estimated effect −2/9 mg/dL, standard error 1.1, p=0.01).
Results were similar for the subgroup of patients who received HCT with nonmyeloablative conditioning for treatment of multiple myeloma, who were presumed to be at a higher risk of renal toxicity.
Liver toxicity
In both recipients of myeloablative and nonmyeloablative HCT, higher week-2 mean CSP concentrations were not significantly correlated with increases in liver bilirubin or ALT during the first 4 weeks after HCT.
For patients given myeloablative conditioning with CSP or TAC, concentration increases of 100 ng/ml and 5 ng/ml, respectively, were associated with changes in bilirubin of 0.6 units (p=0.52) and 1.4 units (p=0.16), respectively, and changes in ALT of 1.4 units (p=0.18) and −0.2 units (p=0.86).
For patients given nonmyeloablative conditioning with CSP or TAC, increases in 100 ng/ml and 5 ng/ml, respectively, were associated with changes in bilirubin of −2.5 units (p=0.01) and −0.3 units (p=0.77), respectively, and changes in ALT of −1.8 units (p=0.08) and −0.7 units (p=0.50).
Discussion
This retrospective study that included data from 1181 patients given hematopoietic cell grafts from HLA-matched related and unrelated donors showed that among patients given myeloablative conditioning, higher week-2 mean whole blood concentrations of CNIs did not lead to decreased incidence or severity of acute GVHD. Among patients given nonmyeloablative HCT, in contrast, relatively high CSP levels early after HCT predicted both a lower incidence and lessened severity of acute GVHD. For patients given TAC, higher levels were only correlated with protection against grade 3–4 acute GVHD. Consistent with the findings by others, we did not find associations between high CNI concentrations and chronic GVHD [17].
Other investigators have analyzed relationships between CSP concentrations and incidence and severity of acute GVHD after myeloablative HCT [10,13–18]. All studies except one [10] were small and retrospective, and GVHD prophylaxis, sometimes with addition of ATG, was not always uniform [13,16–18]. Most of these studies showed that low CSP concentrations early after myeloablative HCT were associated with higher risk of acute GVHD [13–16,18].
Conversely, a large prospective randomized trial comparing CSP-MTX with TAC-MTX showed only a trend between low CSP concentrations and high risk of GVHD, but no such correlation in patients given TAC [10]. Among current recipients of myeloablative HCT, we found no correlation between CSP concentrations and risk of acute GVHD. Unexpectedly, higher week-2 mean TAC concentrations were associated with higher risks of both grade 3–4 acute GVHD and NRM, but this counter-intuitive correlation disappeared when TAC concentrations were analyzed at other time points. We speculate that this association was probably related to undefined confounders and not to an inherent effect of early TAC concentrations.
Data on possible correlations between CNI concentrations and outcomes after nonmyeloablative HCT are scarce. To minimize the risk of graft rejection after nonmyeloablative HCT, CNI concentrations have typically been targeted to higher levels than after myeloablative HCT. Here we showed that for patients given CSP, higher week-2 mean concentrations were correlated with lower incidence and severity of acute GVHD. In patients given TAC, higher week-2 mean levels were correlated only with the risk of grade 3–4 acute GVHD, while no correlation was observed with grade 2–4 acute GVHD, possibly reflecting the smaller sample size (only 31% of nonmyeloablative patients received TAC) and the small number of acute GVHD cases.
Reasons for the differential impact of CNI blood level variations on outcomes after HCT with myeloablative and nonmyeloablative conditioning are unclear. One could speculate that (I) the protective effect associated with higher CNI concentrations could not be fully assessed in myeloablative patients where CNI levels are maintained in relatively low therapeutic ranges, and (ii) the benefit associated with higher CNI concentrations after nonmyeloablative HCT might be related to the use of MMF instead of MTX.
It is also recognized that the clinical and immunobiological characteristics of acute GVHD differ according to conditioning intensity [26]. Compared to myeloablative HCT, nonmyeloablative HCT is associated with only minimal tissue injury, initial persistence of host antigen presenting cells and a transient state of mixed chimerism [27,28]. Given that the tissue milieu after nonmyeloablative conditioning appears to be overall less “inflammatory” than after myeloablative conditioning, increased CNI-levels may have GVHD-protective effects in the former setting while not providing an incremental therapeutic benefit in the myeloablative setting.
After nonmyeloablative HCT, protection against acute GVHD associated with higher CSP concentrations early after transplant translated into decreased risks of NRM and OM. Importantly, the benefit of GVHD-protection in this setting was not outweighed by an increased risk of recurrent malignancy. Similar trends, although not statistically significant, were observed in patients given TAC. After myeloablative HCT, no correlations between CNI concentrations and long-term outcomes (relapse, NRM and OM) were observed.
Based on our observations, we recommend that patients given nonmyeloablative conditioning and CSP- or TAC-based postgrafting immunosuppression in combination with MMF should have their CNI doses targeted to relatively high CNI concentrations early after transplant. At a minimum, concentrations should be targeted above the lowest quartile range in our study, which is equivalent to 345 ng/mL for CSP and 10.5 ng/mL for TAC. Our results further suggest that CSP concentrations above 345 ng/mL provide incremental protection against grades 2–4 and grades 3–4 GVHD, whereas TAC concentrations above 10.5 ng/mL did not appear to provide any incremental GVHD-protection. The absence of clinically significant renal and liver impairment associated with higher CNI concentrations early after HCT further supports our recommendation, even in patients with multiple myeloma. Non-renal or liver CNI-associated toxicities, however, were not analyzed in this retrospective study. Nevertheless, these recommendations apply only to patients given truly nonmyeloablative conditioning and can not be extrapolated to patients given other reduced intensity regimens.
Our finding that high CSP levels translate into improved survival are remarkable in light of the fact that modifications of prophylactic regimens against acute GVHD, with only few exceptions, rarely translated into reduced risks in mortality [3,4,29,30]. Our group reported recently, for example, that even though donor statin treatment was associated with a profoundly decreased risk of grades 3–4 acute GVHD, this benefit did not translate into improved overall survival [31]. The statistical power in the current study was not sufficient to determine whether the same observation applies for TAC, but it is noteworthy that point estimates for the association of higher CNI concentrations with decreased risks of mortality are similar for the two drugs.
In conclusion, within the limits of a retrospective study, we provide evidence that after HCT with nonmyeloablative conditioning, higher CNI concentrations in conjunction with MMF are associated with decreased risks of acute GVHD, NRM and OM. The benefit and risks of targeting high CNI concentrations early after nonmyeloablative HCT should be confirmed in prospective clinical trials.
Acknowledgments
We thank the research nurses and data manager Gresford Thomas for their invaluable help in this study; Helen Crawford, Bonnie Larson, and Sue Carbonneau for manuscript preparation; and especially the patients and their families, the transplantation teams, physicians, nurses, long-term follow-up team and support personnel for their dedicated care of patients on this study.
The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD grants CA78902, CA18029, CA15704, and HL36444. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers. RR was a recipient of a fellowship award from the Davidoff Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cutler C, Antin JH. Manifestations and treatment of acute graft-versus-host disease. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. Oxford, UK: Wiley-Blackwell; 2009. pp. 1287–1303. [Google Scholar]
- 2.Gratwohl A, Hermans J, Apperley J, et al. Acute graft-versus-host disease: grade and outcome in patients with chronic myelogenous leukemia (Review) Blood. 1995;86:813–818. [PubMed] [Google Scholar]
- 3.Storb R, Deeg HJ, Farewell V, et al. Marrow transplantation for severe aplastic anemia: Methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986;68:119–125. [PubMed] [Google Scholar]
- 4.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 5.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (Prograf, FK506) with methotrexate and cyclosporine for graft-versus-host-disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 6.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 7.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 8.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 9.Kershner RP, Fitzsimmons WE. Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation. 1996;62:920–926. doi: 10.1097/00007890-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Wingard JR, Nash RA, Przepiorka D, et al. Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA-identical sibling bone marrow transplantation. Biol Blood Marrow Transplant. 1998;4:157–163. doi: 10.1053/bbmt.1998.v4.pm9923414. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong VW, Oellerich M. New developments in the immunosuppressive drug monitoring of cyclosporine, tacrolimus, and azathioprine (Review) Clin Biochem. 2001;34:9–16. doi: 10.1016/s0009-9120(00)00175-2. [DOI] [PubMed] [Google Scholar]
- 12.Stockschlaeder M, Storb R, Pepe M, et al. A pilot study of low-dose cyclosporin for graft-versus-host prophylaxis in marrow transplantation. Br J Haematol. 1992;80:49–54. doi: 10.1111/j.1365-2141.1992.tb06399.x. [DOI] [PubMed] [Google Scholar]
- 13.Yee GC, Self SG, McGuire TR, Carlin J, Sanders JE, Deeg HJ. Serum cyclosporine concentration and risk of acute graft-versus-host disease after allogeneic marrow transplantation. N Engl J Med. 1988;319:65–70. doi: 10.1056/NEJM198807143190201. [DOI] [PubMed] [Google Scholar]
- 14.Przepiorka D, Shapiro S, Schwinghammer TL, et al. Cyclosporine and methylprednisolone after allogeneic marrow transplantation: association between low cyclosporine concentration and risk of acute graft-versus-host disease. Bone Marrow Transplant. 1991;7:461–465. [PubMed] [Google Scholar]
- 15.Ghalie R, Fitzsimmons WE, Weinstein A, Manson S, Kaizer H. Cyclosporine monitoring improves graft-versus-host disease prophylaxis after bone marrow transplantation. Annals of Pharmacotherapy. 1994;28:379–383. doi: 10.1177/106002809402800315. [DOI] [PubMed] [Google Scholar]
- 16.Martin P, Bleyzac N, Souillet G, et al. Relationship between CsA trough blood concentration and severity of acute graft-versus-host disease after paediatric stem cell transplantation from matched-sibling or unrelated donors. Bone Marrow Transplant. 2003;32:777–784. doi: 10.1038/sj.bmt.1704213. [DOI] [PubMed] [Google Scholar]
- 17.Malard F, Szydlo RM, Brissot E, et al. Impact of cyclosporine-A concentration on the incidence of severe acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:28–34. doi: 10.1016/j.bbmt.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Punnett A, Sung L, Price V, et al. Achievement of target cyclosporine concentrations as a predictor of severe acute graft versus host disease in children undergoing hematopoietic stem cell transplantation and receiving cyclosporine and methotrexate prophylaxis. Therapeutic Drug Monitoring. 2007;29:750–757. doi: 10.1097/FTD.0b013e31815c12ca. [DOI] [PubMed] [Google Scholar]
- 19.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 20.Zucchelli GC, Pilo A, Chiesa MR, Scarlattini M, Bizollon CA. Progress report of an external quality assessment scheme for cyclosporine assay. Therapeutic Drug Monitoring. 1996;18:273–279. doi: 10.1097/00007691-199606000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Andrews DJ, Cramb R. Cyclosporin: revisions in monitoring guidelines and review of current analytical methods (Review) Annals of Clinical Biochemistry. 2002;39:424–435. doi: 10.1258/000456302320314430. [DOI] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 23.Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557–4563. doi: 10.1182/blood-2006-05-021139. [DOI] [PubMed] [Google Scholar]
- 24.Gault MH, Longerich LL, Harnett JD, Wesolowski C. Predicting glomerular function from adjusted serum creatinine. Neph. 1992;62:249–256. doi: 10.1159/000187054. [DOI] [PubMed] [Google Scholar]
- 25.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 27.Sandmaier BM, Maris M, Storer B, et al. A randomized 3-arm phase II study to determine the most promising postgrafting immunosuppression for prevention of acute graft-versus-host disease (GVHD) after unrelated donor hematopoietic cell transplantation (HCT) using nonmyeloablative conditioning for patients with hematologic malignancies: a multi-center trial. Blood. 2009;114:147. #348[abstr.] [Google Scholar]
- 28.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 29.Martin PJ, Bachier CR, Klingemann H-G, et al. Endpoints for clinical trials testing treatment of acute graft-versus-host disease: a joint statement. Biol Blood Marrow Transplant. 2009;15:777–784. doi: 10.1016/j.bbmt.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burroughs L, Mielcarek M, Leisenring W, et al. Extending postgrafting cyclosporine decreases the risk of severe graft-versus-host disease after nonmyeloablative hematopoietic cell transplantation. Transplantation. 2006;81:818–825. doi: 10.1097/01.tp.0000203556.06145.5b. [DOI] [PubMed] [Google Scholar]
- 31.Rotta M, Storer BE, Storb RF, et al. Donor statin treatment protects against severe acute graft-versus-host disease after related allogeneic hematopoietic cell transplantation. Blood. 2010;115:1288–1295. doi: 10.1182/blood-2009-08-240358. [DOI] [PMC free article] [PubMed] [Google Scholar]