Abstract
Background
NMDA receptor (NMDA-R) hypofunction plays an important role in cognitive impairment in schizophrenia. NMDA-R antagonists elicit psychotic symptoms in human and schizophrenia-relevant signs in rodents, including a strong increase in cortical gamma activity. NMDA-Rs are composed of different subunits and accumulating evidence indicates that neuronal damage due to NMDA-R antagonists depends on their action on a specific type of the receptor containing the NR2A subunit. In human schizophrenics, NR2A is selectively reduced in fast firing interneurons. These neurons are critical for gamma oscillations indicating that pathological changes in gamma activity may depend on subunit-specific NMDA-R deficit. The present study tested this hypothesis.
Methods
Cortical electroencephalograms were recorded in freely moving rats and the changes in gamma power were measured after administration of NMDA-R antagonists with different subunit selectivity, including NR2A-preferring (PEAQX, n=5; NVP-AAM077, n=18), NR2B-selective (ifenprodil, n=6; threo-ifenprodil, n=4; Ro25–6985, n=13), and NR2C/D-selective (PPDA, n=8) antagonists, along with vehicle and non-selective NMDA-R antagonists (ketamine, n=10, MK801, n=12). Changes in prepulse inhibition of startle was tested after MK-801(n=6), NVP-AAM077, and Ro-6891 (n=5) injection.
Results
Strong increase in gamma power was induced by non-selective NMDA-R antagonists and by blockade of NMDA-Rs containing the NR2A subunit, with co-occurring gating deficits and diminished low frequency modulation of gamma oscillations. In contrast, selective blockade of NR2B, C, or D subunit-containing receptors had minor effects.
Conclusions
Major subtype-specific differences in the role of NMDA-Rs in cortical gamma oscillation may have implications for the pathomechanism and treatment of cognitive impairment in schizophrenia.
Keywords: Schizophrenia, NMDA hypofunction, MK801, ketamine, neural network, glutamate
Introduction
NMDA receptor (NMDA-R) hypofunction has been strongly implicated in the pathomechanism of schizophrenia (1–4). NMDA-Rs are involved in various aspects of cortical information processing and their dysfunction leads to cognitive deficits. Gamma oscillation is a key mechanism of cognitive processes and it’s been proposed that, in schizophrenia, insufficient NMDA-R mediated drive of fast firing, parvalbumin (PV) expressing interneurons (4) lead to disturbances in gamma oscillations thus preventing normal neuronal synchrony necessary for cognitive functions (5). NMDA-R antagonists, e.g. the abused drug ketamine, recapitulate most clinical symptoms of schizophrenia (1–3). In addition to their detrimental effect on cognitive performance, they elicit psychotic symptoms in human (3) and schizophrenia-relevant acute signs in rodents, including a strong increase in gamma activity in different cortical areas (6–8).
The NMDA-R is a hetero-oligomeric complex consisting primarily of two NR1 and two of several types of NR2 subunits. NMDA-Rs in the cortex are expressed in both pyramidal cells and interneurons but the subunit-composition of the receptor differs between cell types; a disproportional distribution was reported of NR2A-containing receptors on fast firing interneurons expressing PV (9–10). This group of interneurons is essential for oscillatory synchronization of pyramidal cells (11–12), and show characteristic deficits in GAD67 and PV expression both in animal models of schizophrenia and in human postmortem material (13). There are major functional differences between NMDA-Rs containing the NR2A and NR2B subunits, including a dominant role of NR2A in phencyclidine-induced apoptosis (14) and in maintenance of PV and GAD67 immunoreactivity (9). These properties may be relevant for the pathomechanism of schizophrenia, as a selective decrease of interneurons co-expressing NR2A and PV was found in post-mortem studies in schizophrenic patients (15). The known developmental switch from NR2B- to NR2A-containing receptors (16–18) provides further support for such hypothesis.
There has been considerable progress in understanding the differences in development, regulation, trafficking, and subcellular signaling of NMDA-R subtypes but less is known about the implications of these differences on network level neural activity. Differences in the distribution of the two receptor subtypes and in their dynamical properties (19–20) indicate that the two receptors may indeed play different roles in network activity, and that hypofunction of these receptors may differently affect gamma oscillations. To test this hypothesis, the changes in cortical gamma oscillation were studied in the present study in freely behaving rats before and after administration of NMDA-R antagonists with different subunit selectivity.
Methods and Materials
A more detailed description of Methods and Materials is provided in Supplement 1.
Experimental procedures
Cortical EEG over the frontal and occipital corticies were recorded in 33 rats along with EMG was in the neck muscles. In two rats, gross movements were also monitored using accelerometers. Electrophysiological recordings started after a 7–10 day recovery period. Experiments with drug injections started after several daily control recordings. For recording sessions, the rats were placed in a recording box and connected to a slip-ring commutator or had the telemetric transmitter mounted on the head connector. The recordings started early morning and lasted 10–24 hours; the drugs were administered after 4 hr control recording. Other than the drug injection, the rats were left undisturbed. Each rat received 1–5 injections (in 1ml/kg volume, intraperitoneal or subcutenoaus injections), separated by at least 4 days to allow time for washout.
Prepulse inhibition of startle
(PPI) was tested in a subset of animals (n=11) on three occasions separated by 4–5 days. Six rats were taken to the PPI apparatus once before an injection, once 60–90 min after MK801 injection on the height of the induced gamma oscillation, and once 5–6 hours after the injection i.e. after the large on-going gamma subsided and the normal sleep-wake cycles returned, verified by electrophysiological monitoring. In another 5 rats, PPI was tested in control, after injection of Ro--25–6891 and after injection of NVP-AAM077. Cortical EEG was monitored before and after the PPI test to ensure timing at the height of the changes in EEG gamma power. PPI measures the gating of the startle response induced by a 120 dB auditory stimulus by a preceding subthreshold stimulus (21–22).
Drugs
The list of test compounds and their origin and principal features of NMDA-R subunit selectivity is as follows. Non-selective NMDA-R antagonists ketamine (10 mg/kg, n=10, Fort Dodge Animal Health, USA) and MK801 (0.2 mg/kg, n=12, Tocris). NR2A-preferring antagonists NVP-AAM077 (10 and 20 mg/kg s/c, n=12 or i/p n=8, Novartis, 12-120 fold IC50 differences for NR2A vs. NR2B) and PEAQX (10 mg/kg, n=5, SIGMA, it is the racemic version of NVP-AAM077, i.e. the PEAQX dose used in this study was equivalent to 5 mg/kg of this NVP-AAM077). NR2B-selective antagonists, ifenprodil (5 and 10 mg/kg, n=6, Tocris; NMDA antagonist acting on at the polyamine site), threo-ifenprodil (10 mg/kg, n=4, Tocris; IC50 values are 0.22 and 324 µM at NR2B and NR2A, respectively), and Ro25–6985 (5, 10, 20, and 30 mg s/c, n=13, Tocris; IC50 values are 0.009 and 52 µM for cloned receptor subunit combinations NR1C/NR2B and NR1C/NR2A, respectively). Selective antagonist of NR2C/D receptors (PPDA 10 and 20 mg/kg, n=8, Tocris; Ki values are 0.096, 0.125, 0.31 and 0.55 µM for NR2C, NR2D, NR2B and NR2A subunits, respectively). Vehicle: saline.
Electrophysiology and data analysis
EEG signals were subjected to Fast Fourier transform to generate power spectra for consecutive 16 s windows. Gamma oscillations were assessed using the average spectral power in the gamma band surrounding 40 Hz (+/−10Hz) and in the higher gamma band (65–90 Hz). For detailed examination of the features of spectral components, average power spectra of two hours of control recording and two hours at the height of the drug effect were generated and the difference between the two was used. For comparison between rats and different compounds, including all drugs at all doses, the time course of the changes in gamma band power was built for each recording session and the values for gamma power were normalized using the average of gamma power during the first hr of control recording, i.e. the changes in this case were expressed as after vs. before injection ratio. Statistical analysis was based on pair-wise comparison between each individual drug with saline using Student’s t-test and on ANOVA and post-hoc Bonferroni for testing dose-dependence.
Intrastructural theta-gamma cross-frequency coupling was calculated using the algorithm of Canolty et al. (23–24). Briefly, the instantaneous theta phase and gamma amplitude were determined and used to construct a continuous, complex-valued signal. The modulus length of this signal was then compared with the distribution of surrogate lengths to obtain a measure of coupling strength. The resulting modulation index (M) is a normalized z-score value which can directly be used to determine the probability that the results would be due to chance. When it is calculated using recording boxes of the same length, the comparison of this metric between different signals and between signals recorded under different conditions are valid (see (23–24) for more details and discussions). The intrastructural M index was calculated on consecutive 5 min segments and were averaged over 1hr periods for the frontal and occipital cortex EEGs, separately, in 8 rats before and after MK801 (0.2 mg/kg) injection and in 6 of these rats also before and after administration of NVP-AAM077 (20 mg/kg) and Ro-25 6891 (10 or 20 mg/kg).
Neck muscle EMG and the accelerometer signals were processed by calculating the root-mean square (RMS) values in the same time windows as used for the EEG signals. EMG/movement signals were correlated with gamma band power using average gamma power vs. RMS over 5 min segments. Thus, these correlations detect co-occurrence of fluctuations rather than correlation on the short time scale (or interference). Besides a certain steady tone present when the animal is awake, necks muscle activity fluctuates with motor activity as most movements of the rat, confined to a limited space (e.g. digging, turning, rearing, grooming, wiskering, sniffing, etc.), involve head movement.
Results
NMDA-R antagonists induce high power gamma oscillations coincident with lasting motor activity and PPI deficit
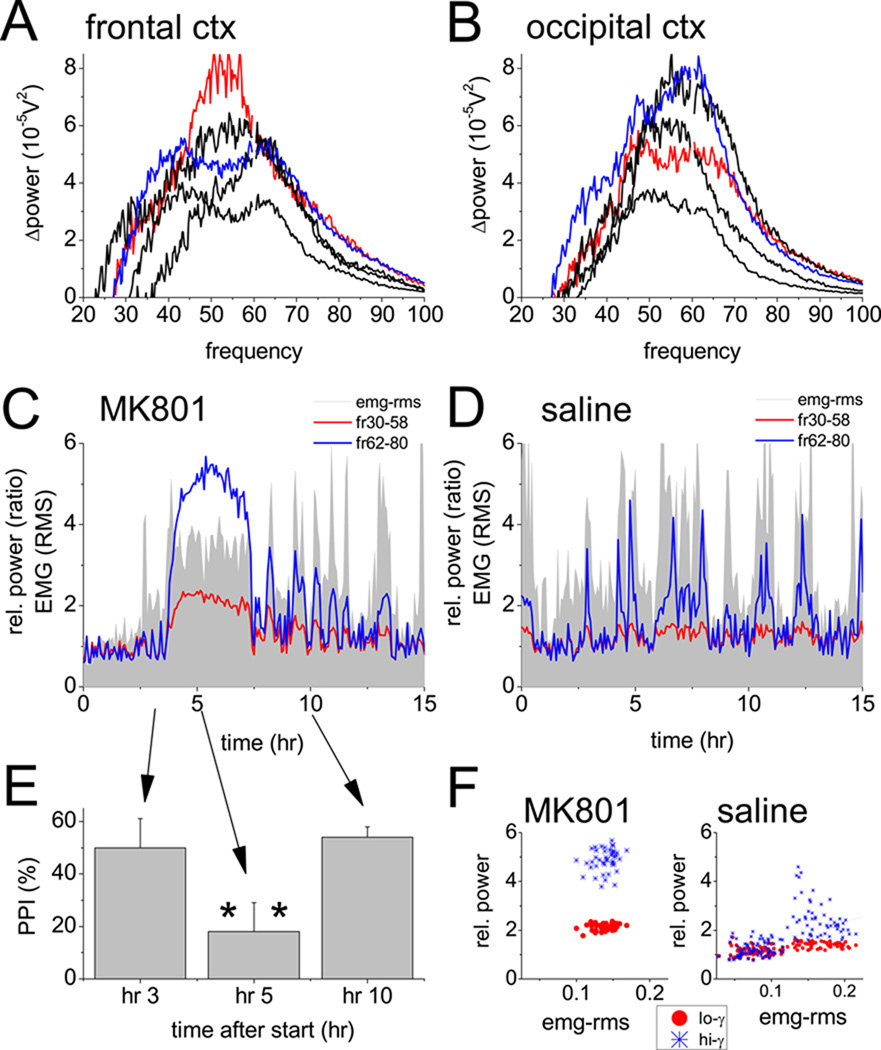
Non-selective NMDA-R antagonists ketamine and MK801 increased spectral power in a wide range between 30 and 90 Hz (Fig. 1A, 3A, 3B) at short latencies (<5 min and <20 min, for ketamine and MK801). The effect lasted for ~1 hr and 3–4 hrs, respectively, after ketamine and MK-801 (Figs. 1B and 3C). During this period, the animals showed characteristic behavior, i.e. increased motor activity (Fig. 3C), stereotypic head movements (21), and impaired performance on the schizophrenia-relevant cognitive test of prepulse inhibition of startle (PPI; Figs. 2A, 3E, decrease of gating from 60±3.4% to 27±6.1%, t-test, n=6, p=0.03) as shown previously (21–22).
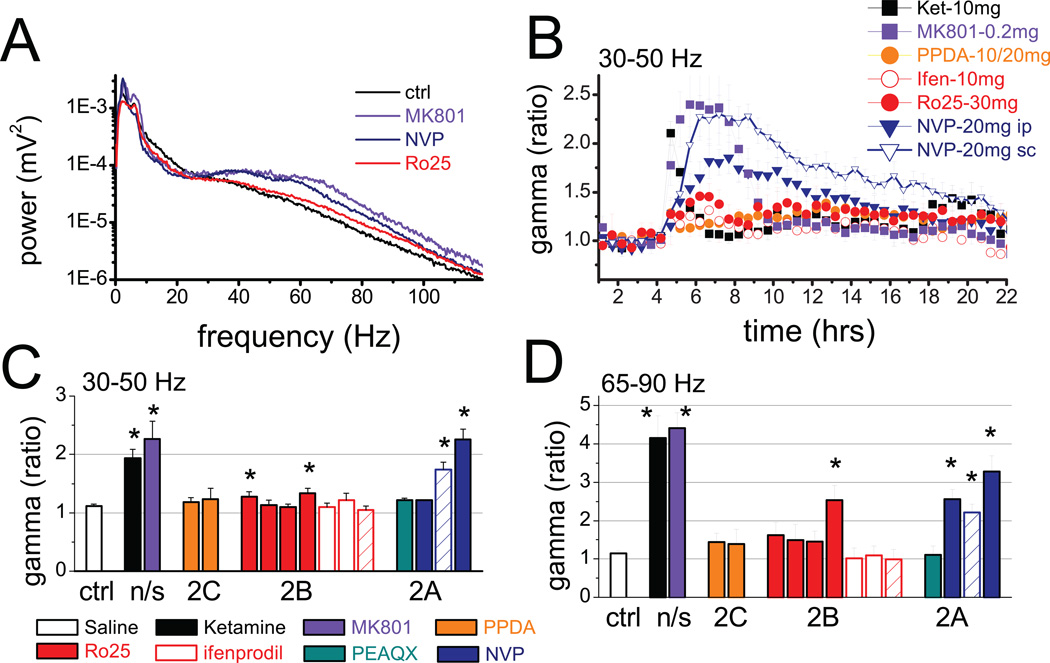
Figure 1.
Changes in cortical gamma oscillations after injection of NMDA antagonists acting on different subunit-containing receptors. A. Comparison of power spectra (shown on semi-logarithmic scale) of frontal cortex EEG after different NMDA antagonists with control recording in a representative experiment (the spectra were made using 2 hr segments on the top of the reactions). B. Time course of integrated gamma power (30–50 Hz, group averages) over the frontal cortex in consecutive 30 min segments for 3 hrs before and 18 hrs after injections. C and D. Group averages and SEM of relative gamma power (post/pre-injection ratio) in the 30–50 Hz (C) and 65–90 Hz range (D) calculated in a 30 min (ketamine) or a 2 hr (all other compounds) segment at the peak of the reaction after injection of saline, non-selective NMDA antagonists ketamine (10mg/kg, n=10) and MK801 (0.2mg/kg, n=12), the NR2C/D-selective PPDA (10mg/kg and 20 mg/kg, n8), NR2B-selective Ro25–6985 (5, 10, 20, and 30 mg/kg, n=13), ifenprodil (5 and 10mg/kg, n=6), and threo-ifenprodil (10mg/kg, n=4), and NR2A-selective PEAQX (10mg/kg), and NVP-AAM077 (10, 20mg/kg i/p, and 20 mg/kg s/c, n=18). Different compounds are shown in different colors, and increasing doses are represented by columns from left to right, for each compound.
Figure 3.
Cortical gamma activity, muscle activity, and prepulse inhibition of startle after blockade of NMDA receptors using the non-selective antagonist, MK-801. A and B. Changes in spectral power in frontal (A) and occipital (B) cortex recordings at different frequencies in the 30–100 Hz range (post- vs. pre-injection difference) after injection of 0.2 mg/kg MK801 in 5 individual experiments (blue and red traces in A and B show spectra in the same rat). Note two components of the elevated gamma activity. C and D. Time course of integrated gamma power in the low (30–58 Hz, red) and high (62–90 Hz, blue) gamma bands, along with neck muscle activity (EMG root mean square, grey), in one experiment, for 3 hrs before and 12 hrs after injections of MK-801 (C) or saline (D). EEG power spectra and EMG RMS were calculated in consecutive 4 min segments and expressed as change (ratio) relative to a 1 hr average before injection. E. Changes in prepulse inhibition of the startle response (PPI) elicited by 120 dB auditory stimulation, expressed as percent decrease of the reaction to stimuli preceded by a subthreshold (80 dB) stimulus measured on different days before MK801 injection, at the top of the gamma increase, and after the return to control level of gamma power, i.e. 3, 5, and 10 hours after the start of recording. F. Scatter plot of EMG-RMS vs. low and high frequency gamma power averaged over consecutive in 5 min segments for 4 hours after injection of MK-801 or saline.
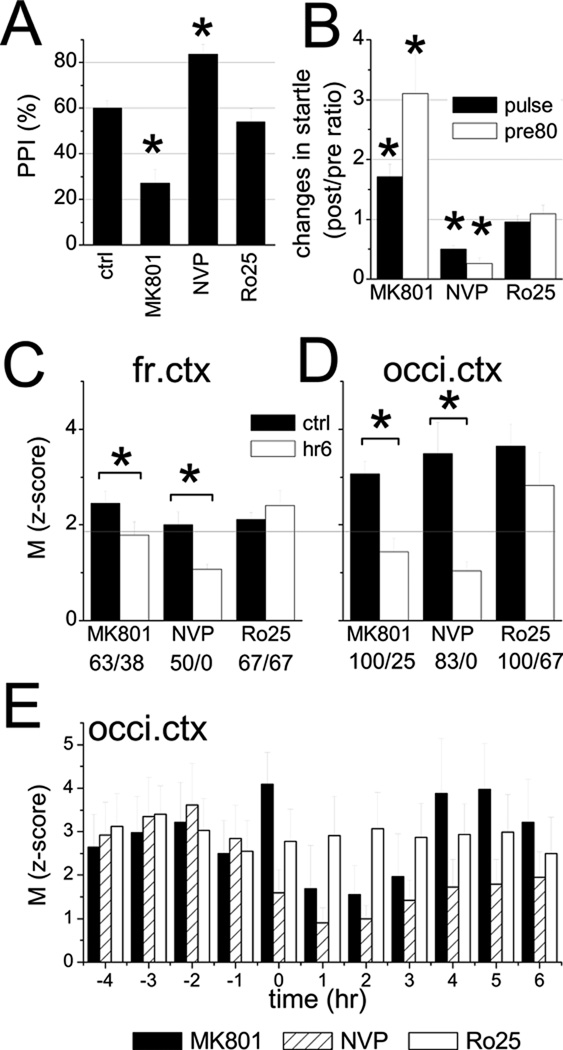
Figure 2.
Effect of subunit specific blockade of NMDA receptors on prepulse inhibition (PPI) on startle and on phasic modulation of gamma oscillations by theta rhythm. A. Gating in control, and after injection of MK-801, NVP-AAM077, or Ro25–689. B. Changes in the startle response elicited by 120 dB auditory stimulus alone (black) or preceded by 80 dB prepulse (white) compared with pre-injection control (post/pre ratio). C and D. Average modulation index (M) determined for EEG recordings over the frontal (C) and occipital cortex EEGs (D) during 1 hr periods before drug injection (ctrl) and 2 hours after injection (hr 6). Horizontal line shows the p=0.05 probability for the results to occur by chance (24). The numbers below the graph indicate the percent of rats showing significant modulation of gamma oscillations before and after drug injections. E. Temporal evolution of the M index in the occipital cortex (hourly averages in 6 rats) before and after injection of MK801, NVP-AAM077, and Ro25–6891. Time of injection: at the beginning of 0th hr.
The peak frequency of the increased gamma activity varied between experiments and between simultaneously recorded signals in different areas of the cortex. As shown in the examples in Figs. 3A and 3B, gamma enhancement was not limited to the frequencies surrounding 40 Hz, but covered higher frequencies as well, often generating a separate or even dominant peak around 65 Hz. The large fluctuations associated with behavioral activity vs. immobility reported earlier (6), mostly appeared in this band (see example of control recording after saline injection in Fig. 3D). After ketamine or MK-801, both the low and high frequency gamma power showed steady elevation, accompanied by continuously increased muscle activity. On average, the gamma increase in the frontal cortex reached 93±15% (n=10, p<0.001, t-test comparison with saline) after ketamine and 166±30% (n=12, p=0.008) after MK801 in the 30–50 Hz band and 316±57% (p<0.001) and 341±40% (p<0.001) for ketamine and MK801, respectively, in the 62–90Hz band (Fig. 1C and 1D). The changes were similar in the occipital cortex (Figure S1 in the Supplement).
The functional characteristics of the elevated gamma activity were further tested using theta-gamma cross-frequency coupling which is important for cognitive processing in rodents (25–26) as well as in human (23) and which was shown to be impaired in mice with genetically induced NMDA-R hypofunction (24). In pre-injection control recordings, the average modulation index was 2.45±0.26 in the frontal cortical EEG, i.e. above the 5% significance level (Fig 2C), and was even higher in the occipital cortex (M=3.07±1.43, Fig 2D). After MK-801 injection, there was a transient increase in cross frequency coupling (M=2.89±0.92 in frontal and 4.09±0.74 in occipital cortex during the first hour) followed by a significant decrease (M=1.79± 0.28, p=0.04, and 1.43±0.30, p=0.009, Figs 2C, D) which lasted for several hours, i.e. following the time course of the gamma elevation (Fig 2E).
NMDA-R antagonism with NR2A subunit preference induces lasting high power gamma oscillations and severe impairment of auditory startle response
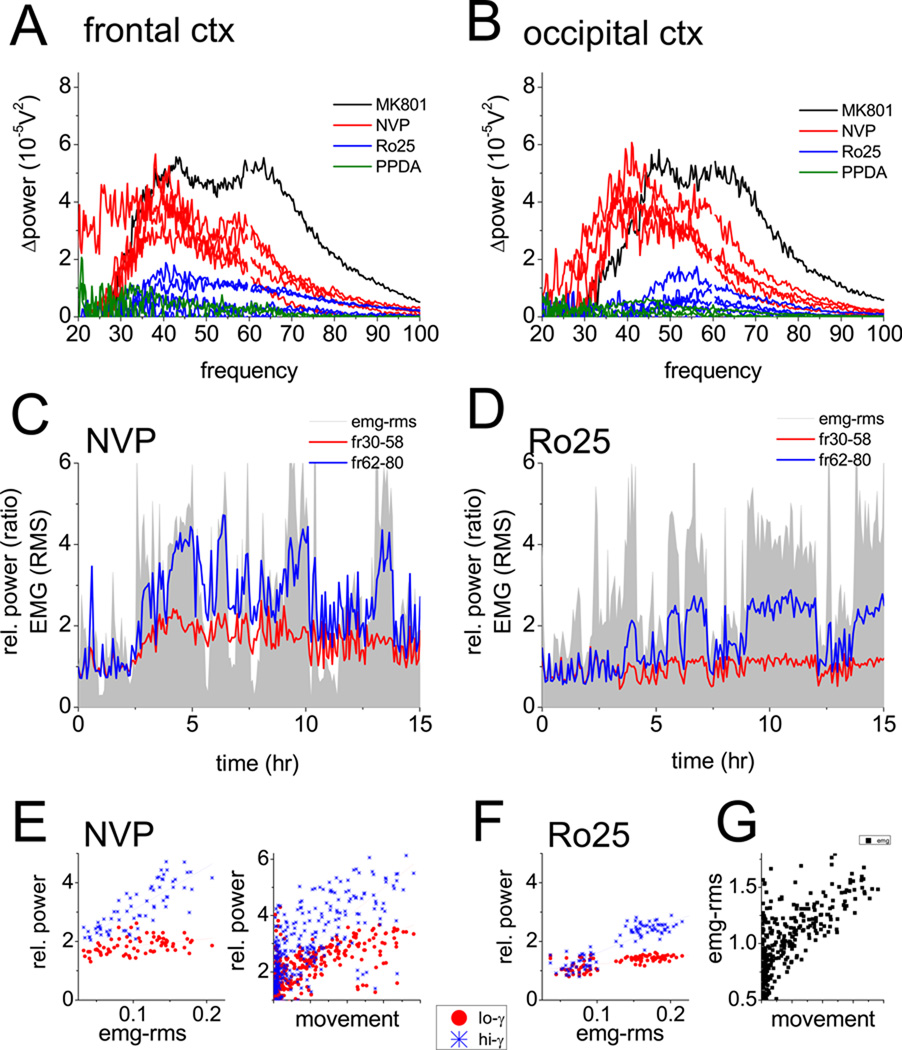
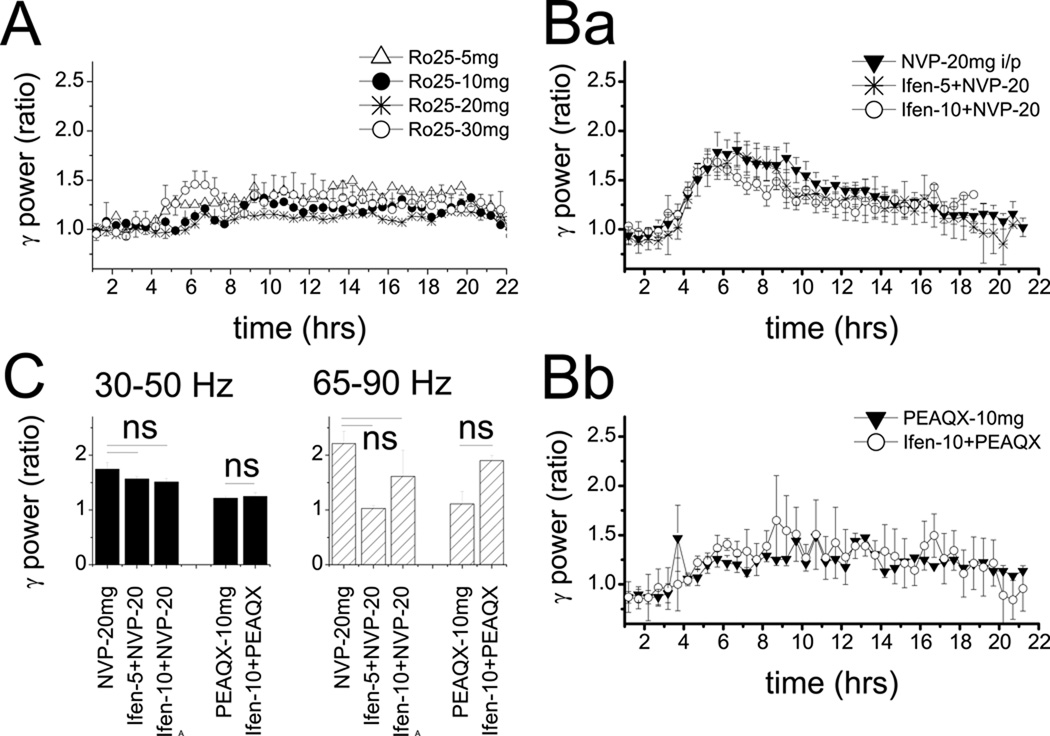
The NR2A-preferring antagonist NVP-AAM077 (27–28) also increased gamma power (see examples in Fig. 1A, 4A, 4B) but the reaction developed slower and lasted longer (8–10 hrs) (Figs. 1B and 4C). The spectral composition of the EEG signals differed from that after non-selective NMDA-R blockade; the NVP-AAM077 induced gamma enhancement was biased toward the low-frequency gamma band in both frontal and occipital cortex (see examples in Figs. 4A and B). Furthermore, in these experiments, the 62–90 Hz gamma not only preferentially appeared during motor activity but also correlated with the level of muscle activity (R2=0.61±0.09), whereas gamma in the 30–50 Hz range did not (R2=0.26±0.1) (see example in Fig 4E). In two rats, high frequency gamma power was also shown to correlate with locomotion recorded using an accelerometer in three spatial dimensions (Fig 4E). On average, gamma increase in frontal cortex reached a level of 126±17% (n=11, p<0.001) in the 30–50 Hz band (Fig. 1C) and 228±42% (p<0.001) in the 65–90 Hz band (Fig. 1D), at the highest dose of the antagonist (20 mg/kg s/c). The effect was dose-dependent (ANOVA F[37,4]=15.89, p<0.001); the compound was ineffective at 10 mg/kg (n=4, p=0.18) and 10 mg/kg PEAQX, equivalent to 5 mg/kg NVP-AAM077, was also ineffective (n=5, p=0.13,) (Fig. 1). NVP-AAM077 injected i/p was less effective than s/c injection (t-test comparing 20 mg/kg s/c vs. i/p, p=0.026). NR2A subunit preferring NMDA-R antagonists also dose-dependently increased gamma power in the occipital cortex in both frequency bands (Figure S1 in the Supplement); statistical comparison of the changes in frontal and occipital cortices revealed no significant differences at any dose of the compounds.
Figure 4.
Cortical gamma activity, prepulse inhibition of startle, locomotion and muscle activity after blockade of NMDA receptors using antagonist preferentially blocking NR2A or NR2B subunit containing receptors. A and B. Changes in spectral power in frontal (A) and occipital (B) cortex recordings at different frequencies in the 30–100 Hz range (post-vs. pre-injection difference) after injection of NVP-AAM077 (red, 20 mg/kg, n=5), Ro25–6891 (blue, 20 mg/kg, n=5), or PPDA (green, 20 mg/kg, n=3). Except for PPDA, all traces (including those in Fig 2A, B) are from the same rats. Note stronger increase in the low frequency range after NVP-AAM077 and minor changes after NR2B or NR2C/D blockade (one MK-801 experiment, black, is repeated from Fig 1 for comparison). C and D. Time course of integrated gamma power in the low (30–58 Hz, red) and high (62–90 Hz, blue) gamma bands, along with neck muscle activity (EMG root mean square), in one experiment, for 3 hrs before and 12 hrs after injections of NVP-AAM077 (C) or Ro25–6891 (D). All signals were processed as in Fig. 1. E. Scatter plot of EMG-RMS vs. low and high frequency gamma power averaged over consecutive in 5 min segments for 4 hours and gamma vs. motor activity recorded by an accelerometer after injection of NVP-AAM077 F. EMG-RMS vs. low and high frequency gamma power after injection of Ro25–6891. G. Comparison of the signals from EMG and the accelerometer.
Theta-gamma cross coupling significant decreased, from M=2.00±0.27 to 1.07±0.12 (p=0.016) in frontal and from 3.49±0.65 to 1.04±0.19 (p=0.006) in the occipital cortex (Fig 2C,D). Compared with MK801, the decrease after NVP-AAM077 injection was more severe, started right after the injection, i.e. without an initial rise, and lasted for at least 6 hours (Fig. 2E). The modulation index was below significance level in all rats without exception.
PPI was tested in 5 rats before injection and 4 hours after s/c injection of 20 mg/kg NVP-AAM077 and showed increased gating (84±4, p=0.026, Fig. 2A), on the background of drastic suppression of the startle reaction elicited by the 120 dB stimulus either delivered alone (50±6% decrease, p=0.005) or preceded by a 80 dB pre-pulse (74±10% decrease, p=0.009)(Fig. 2B).
Selective blockade of NR2B or NR2C/D subunit containing NMDA-R has minor effect on gamma oscillations and PPI
Three NR2B-selective antagonists, Ro25–6985 (n=13) (29), ifenprodil (n=6), and threo-ifenprodil (n=4) were tested in different doses. Injection of 10 mg/kg (n=5, p=0.13), and 20 mg/kg (n=7, p=0.68) Ro25–6985 had no effect on gamma power in the 30–50 Hz, but relatively small increases were observed at the 5 mg/kg (27±8%, n=4, p=0.019) and 30 mg/kg doses (33±9%, n=3, p=0.029)(Figs. 1A, 1B and 4A). This compound also increased gamma at higher frequencies in a dose dependent manner (153±38%, p<0.001 at the largest dose), although still less than NR2A- and non-selective antagonists (Fig. 1D). Theta-gamma cross-frequency coupling did not change (p=0.20 and p=0.19 in frontal and occipital cortex, respectively, Fig. 2C–E). Ifenprodil and threo-ifenprodil had no effect on gamma oscillations in either frequency band (Fig 1C and 1D). In 6 rats, ifenprodil was also co-administered with NVP-AAM077 in several combination of various doses for both compounds, but the effect was not different from that after injection of NVP-AAM077, alone (Fig 4B and 4C). Selective antagonism of NR2C/D receptors (PPDA 10 mg/kg, n=4, and 20 mg/kg, n=4) had no effect on gamma activity, either. PPI tested in a subset of 5 rats was 54±6% after injection of 20mg/kg Ro25–6891 which was not significantly different from the 60±3% gating in these rats before injection (p=0.90)(Fig. 3E).
Discussion
This study provided evidence for subunit-specificity of cortical gamma activity induced by NMDA-R blockade. The massive increase in aberrant gamma activity associated with schizophrenia-relevant stereotypic behavior and PPI deficit was primarily mediated by NMDA-Rs which contained the NR2A subunit whereas blockade of NR2B, C, or D subunit-containing receptors did not elicit this reaction. Accumulating evidence indicate that both chronic and acute damage due to NMDA-R antagonists may depend on the same type of the receptor containing the NR2A subunit. NR2A receptors were implicated in delayed behavioral deficits in several chronic animal models, such as in rats reared in isolation (30) or subjected to neonatal treatment with phencyclidine (14) or ventral hippocampal lesion (31) and now the results of this study show their preferential involvement in acute aberrant gamma activation after NMDA-R blockade, as well.
It should be noted that pharmacological manipulation of a very complex receptor is not without limitations due to imperfect selectivity of the available compounds. In particular, the selectivity of PEAQX and NVP-AAM077 is lower compared with NR2B antagonists (12–120 vs. >400 fold IC50 differences, respectively (32)). Nevertheless, clear subunit specific NMDA-R actions were reported using these compounds in previous investigations into the molecular aspects of the pathology of schizophrenia (9; 33) which are compatible with the findings of this study. In the present study, data obtained with all compounds taken together strongly suggest that the massive gamma activation by NVP-AAM077 was primarily mediated by NR2A rather than NR2B-type receptors, since the highly selective NR2B antagonists given in a wide range of doses had minor effects, at best. This range included doses shown in previous studies to be effective in other measures as well as substantially higher doses for both ifenprodil (14) and Ro25–6891 (34). Furthermore, co-administration of NR2A and NR2B subunit selective antagonists, attempting to mimic the combined effect of non-selective antagonists, did not replicate their effect any better than NVP-AAM077 alone, i.e. adding ifenprodil to ineffective doses of PEAQX did not generate increased gamma activity and adding ifenprodil to the effective dose of NVP-AAM077 did not enhance its effect. Yet, given the limitations of the existing NR2A-preferring antagonists, we can not exclude the possibility that for eliciting gamma increase it is necessary to antagonize both types of receptors even if the blockade is disproportionally favoring NR2A receptors.
The nature of subunit specificity has been extensively studied on the cellular level, primarily in the context of neurotoxicity due to increased Ca influx through NMDA-Rs. In particular, functional differences were identified between synaptic vs. extrasynaptic NMDA-Rs and were traced back to differences in intracellular signaling to pro-survival or pro-apoptotic pathways, respectively (35). This may parallel subunit specificity, owing to the developmental shift during the first weeks after birth (17) when NR2A expression sharply increases in the synapse and NR2Bs move to extrasynaptic receptors, resulting in preferential synaptic enrichment of NR2A (36) and extrasynaptic abundance of NR2B (37) in the adult brain. Activation of pro-apoptotic pathways by shifting the balance from NR2A to NR2B associated mechanisms was demonstrated in a model of schizophrenia in which subchronic blockade of NR2A, rather than NR2B, was found responsible for phencyclidine-induced apoptosis in the cortex (14). In contrast, shifting the balance in the other direction, by blocking NR2B receptors, appears beneficial for the neuron’s survival and is explored as prospective treatment for a number of diseases, including depression (38–39), Alzheimer’s (40), stroke (41), and neuropathic pain (42).
PV positive (PV+) interneurons, which are essential for gamma rhythmogenesis (11–12), are specifically vulnerable to NR2A selective NMDA-R blockade. NR2A subunit-containing NMDA-Rs play a specific role in the maintenance of the phenotype of PV+ interneurons. Exposure of cultured PV+ neurons to ketamine or NVP-AAM077 induced time and dose-dependent decrease in PV and GAD67 whereas Ro-25–6985 had no effect on PV and only partially reduced GAD67 (9). This type of interneurons, i.e. co-expressing NR2A and PV, was shown to be selectively lost in schizophrenic patients (15). Thus, NMDA-R subunit-specific activation of genomic programs and intracellular signaling pathways (9; 14; 33; 35) may as well contribute to impairment of neuronal synchronization in schizophrenia in human (43) and in NMDA-R hypofunction-based chronic animal models (8; 44–45). The deficits due to such mechanisms, however, may only be relevant for the chronic or subchronic condition but are most likely too slow to explain the effect of NMDA-R blockade at short latencies.
The short latency of the effect of NMDA-R antagonists indicates that gamma activation is the consequence of rapid alterations in the activity of neural networks responsible for rhythmogenesis. NMDA-R antagonists produce opposite changes in pyramidal cells and interneurons in the rat (at <30 min latency) (4) suggesting that the primary effect is a direct disfacilitation of GABA interneurons which then leads to increase in the firing rate in the majority of pyramidal cells (4). This interpretation is in agreement with prior observations of increased metabolic activity within an hour after taking ketamine by human volunteers (46) and with in vitro demonstration of high sensitivity of PV+ basket cells to NMDA-R antagonists (47). The results of the present study, i.e. that blockade of NR2A rather than NR2B subunit-containing NMDA-Rs reproduce the effect of non-selective NMDA-R antagonists, lends further support to this mechanism. Since NR2A expression ratio in PV+ interneurons exceeds 4–5 times that in pyramidal cells (9–10; 18), a decrease in the NMDA drive to these neurons is most likely a major factor leading to augmentation of gamma activity.
The characteristics of the chronic gamma deficit are different from that after acute NMDA-R blockade also in human schizophrenia. Gamma power is generally decreased in schizophrenics in various task-related EEG measurements (43) as opposed to its acute increase after administration of ketamine (48–49). Gamma power is also elevated during psychotic episodes (50), and hallucinations also correlate with increased propensity to ~40 Hz oscillations (51). Our observations in rodents (see also (6–7)) are analogous to those in human after administration of ketamine (48–49) and may be relevant for understanding the changes in cortical networks associated with positive symptoms. Reports on gamma increase in human associated with positive symptoms are available and included both recordings of spontaneous activity (50) and induced oscillations (48–49; 51).
Despite opposite changes in gamma power in the chronic state vs. during psychosis, in human schizophrenia, both conditions are associated with impaired cognitive performance. In animal models, too, schizophrenia-relevant cognitive deficits were shown after subchronic (52–54) as well as after acute NMDA-R blockade (21–22). Gamma rhythm is essential for a variety of cognitive processes. Yet, data from both human schizophrenics and animal models indicate that gamma activity induced by NMDA-R antagonists is an “aberrant” pattern of cortical activity (6; 55) which is in some way disadvantageous for cognitive processes and might instead contribute to psychotic symptoms. This is supported by the findings of this study that the increase in gamma power was accompanied by a disruption of the oscillatory hierarchy which normally operates across multiple spatial and temporal scales (26; 56) and plays a critical role in various cognitive processes (23; 57). Theta-gamma cross-frequency coupling was recently found impaired in mice with genetically induced chronic NMDA-R hypofunction (24), similar to the present findings after acute NMDA-R blockade. Furthermore, this effect also showed subunit-specificity; i.e. elevated gamma oscillation induced by NR2A preferring antagonists suffered severe impairment of low-frequency modulation whereas gamma after NR2B receptor blockade appeared normal according to this metric.
In the present study, the electrophysiological findings also correlated with abnormal behavior commonly described in most rodent models of schizophrenia. Motor activity, estimated here by neck muscle EMG and backed up in 2 rats with direct activity recordings, confirmed previous findings of increased motor activity after NMDA-R blockade and showed, not surprisingly, that gamma increase accompanied this hyperactivity. Fluctuations of muscle activity correlated with the high frequency component of normal gamma activity after saline and of enhanced gamma after subunit-selective antagonists. The correlation was not that obvious for the more steadily enhanced 30–50 Hz component which was activated by NVP-AAM077 injection.
Deficits in the test of PPI on startle induced by non-selective or NR2A subunit-preferring NMDA-R antagonists also followed the time course of gamma activation, verified by electrophysiology in each experiment. The deficit differed, however, between non-selective and subunit-selective antagonists. MK-801 drastically reduced gating, whereas after administration of NVP-AAM077 a decrease in the startle response was the leading symptom and PPI actually increased due to uneven drop in the first and second startle reactions. Selective blockade of NR2B subunit containing NMDA receptors did not alter PPI ((58) and this study). In a previous study, NR2A knock out mice also showed normal gating but administration of selective NR2B antagonists to these mice significantly disrupted PPI (58). Furthermore, impaired sensory gating may accompany not only elevated gamma, observed in this study, but also a drastic decrease in background gamma activity, as e.g. after activation of cannabinoid-1 receptors (59). Thus, oscillatory network activity and sensory-motor gating may not be causally related.
Differences in the cellular mechanisms, in particular in the role of PV interneurons, of the impairment of gamma oscillation and sensory-motor gating was also demonstrated in a recent study (60) in which mice lacking NMDA receptors on PV+ cells showed enhanced background gamma activity but normal PPI. These mice, however showed significant cognitive deficits in other dimensions, e.g. acoustic startle habituation, associative learning, working memory, but not in spatial reference memory in the water maze (60). Deficits in PPI represent an endophenotype of schizophrenia and strong validity of its animal equivalent has been demonstrated (61). Schizophrenia is, however, a heterogeneous neuropsychiatric illness characterized by deficits in many domains of cognition including attention, motivation, sensory gating, working memory, affective regulation, and social behavior. Many of these functions depend on the performance of the network generating gamma oscillations. Thus, future investigation of the possible role of subunit-specific alteration of the NMDA-R function in schizophrenia-relevant cognitive deficits will have to include an extended battery of cognitive tests.
Supplementary Material
Figure 5.
Minor effect t of NR2B subunit-selective NMDA-R antagonists administered in different doses and together with NVP-AAM077. A. Time course of integrated gamma power (30–50 Hz, group averages) in consecutive 30 min segments for 3 hrs before and 18 hrs after injection of Ro25–6891 in 5, 10, 20, and 30 mg/kg doses (n=4, 5, 7, 3). Ba. comparison of the effect of 20 mg/kg NVP-AAM077 injected alone (n=7) or co-administered with Ifenprodil in doses of 5 mg/kg (n=2) or 10 mg/kg (n=3). Bb. Comparison of the ineffective dose of NR2A subunit preferring antagonist (10 mg/kg PEAQX) injected alone or co-administered with high dose of ifenprodil (10 mg/kg, n=2). C. Group averages of the effect (2 hr average power at the peak of the reaction) of the drug administration shown in Ba and Bb.
Acknowledgement
This study was supported by National Institute of Health Grants MH83199 and MH87777. NVP-AAM077 was provided by Dr. Yves P. Auberson (Novartis Institute of BioMedical Research, Basel, Switzerland). I also thank Dr K. Dzirasa of Duke University for advice regarding signal analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr. Kocsis reported no biomedical financial interests or potential conflicts of interest
References
- 1.Jentsch JD, Redmond DE, Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- 2.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 3.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 4.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Ma J, Leung LS. The supramammillo-septal-hippocampal pathway mediates sensorimotor gating impairment and hyperlocomotion induced by MK-801 and ketamine in rats. Psychopharmacology (Berl) 2007;191:961–974. doi: 10.1007/s00213-006-0667-x. [DOI] [PubMed] [Google Scholar]
- 8.Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations. Brain Struct Funct. 2011 doi: 10.1007/s00429-011-0351-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi D, Keeler B, Zhang W, Houle JD, Gao WJ. NMDA receptor subunit expression in GABAergic interneurons in the prefrontal cortex: application of laser microdissection technique. J Neurosci Methods. 2009;176:172–181. doi: 10.1016/j.jneumeth.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buzsaki G. Rhythms of the Brain. Oxford: Oxford University Press; 2006. [Google Scholar]
- 13.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 14.Anastasio NC, Xia Y, O'Connor ZR, Johnson KM. Differential role of N-methyl-D-aspartate receptor subunits 2A and 2B in mediating phencyclidine-induced perinatal neuronal apoptosis and behavioral deficits. Neuroscience. 2009;163:1181–1191. doi: 10.1016/j.neuroscience.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Archives of General Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 16.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 17.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 18.Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, et al. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- 20.Gielen M, Siegler Retchless B, Mony L, Johnson JW, Paoletti P. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 2009;459:703–707. doi: 10.1038/nature07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125–134. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]
- 22.Mansbach RS, Geyer MA. Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neuropsychopharmacology. 1989;2:299–308. doi: 10.1016/0893-133x(89)90035-3. [DOI] [PubMed] [Google Scholar]
- 23.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dzirasa K, Ramsey AJ, Takahashi DY, Stapleton J, Potes JM, Williams JK, et al. Hyperdopaminergia and NMDA receptor hypofunction disrupt neural phase signaling. J Neurosci. 2009;29:8215–8224. doi: 10.1523/JNEUROSCI.1773-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 26.Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. Journal of Neuroscience. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 29.Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, et al. Ro 25–6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- 30.Turnock-Jones JJ, Jennings CA, Robbins MJ, Cluderay JE, Cilia J, Reid JL, et al. Increased expression of the NR2A NMDA receptor subunit in the prefrontal cortex of rats reared in isolation. Synapse. 2009;63:836–846. doi: 10.1002/syn.20665. [DOI] [PubMed] [Google Scholar]
- 31.El-Rawas R, Saade NE, Thiriet N, Atweh S, Jaber M, Al-Amin HA. Developmental changes in the mRNA expression of neuropeptides and dopamine and glutamate receptors in neonates and adult rats after ventral hippocampal lesion. Schizophr Res. 2009;113:298–307. doi: 10.1016/j.schres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anastasio NC, Johnson KM. Differential regulation of the NMDA receptor by acute and sub-chronic phencyclidine administration in the developing rat. J Neurochem. 2008;104:1210–1218. doi: 10.1111/j.1471-4159.2007.05047.x. [DOI] [PubMed] [Google Scholar]
- 34.Hu NW, Klyubin I, Anwyl R, Rowan MJ. GluN2B subunit-containing NMDA receptor antagonists prevent Abeta-mediated synaptic plasticity disruption in vivo. Proc Natl Acad Sci U S A. 2009;106:20504–20509. doi: 10.1073/pnas.0908083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, et al. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 41.Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM, et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell. 2010;140:222–234. doi: 10.1016/j.cell.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan PH, Yang LC, Shih HC, Lan KC, Cheng JT. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Ther. 2005;12:59–66. doi: 10.1038/sj.gt.3302376. [DOI] [PubMed] [Google Scholar]
- 43.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 44.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- 47.Buhl EH, Szilagyi T, Halasy K, Somogyi P. Physiological properties of anatomically identified basket and bistratified cells in the CA1 area of the rat hippocampus in vitro. Hippocampus. 1996;6:294–305. doi: 10.1002/(SICI)1098-1063(1996)6:3<294::AID-HIPO7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 48.Plourde G, Baribeau J, Bonhomme V. Ketamine increases the amplitude of the 40-Hz auditory steady-state response in humans. Br J Anaesth. 1997;78:524–529. doi: 10.1093/bja/78.5.524. [DOI] [PubMed] [Google Scholar]
- 49.Hong LE, Summerfelt A, Buchanan RW, O'Donnell P, Thaker GK, Weiler MA, et al. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 2010;35:632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldeweg T, Spence S, Hirsch SR, Gruzelier J. Gamma-band electroencephalographic oscillations in a patient with somatic hallucinations. Lancet. 1998;352:620–621. doi: 10.1016/S0140-6736(05)79575-1. [DOI] [PubMed] [Google Scholar]
- 51.Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder HA, et al. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 53.Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G. Ketamine-induced changes in rat behaviour: A possible animal model of schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 54.Stefani MR, Moghaddam B. Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biol Psychiatry. 2005;57:433–436. doi: 10.1016/j.biopsych.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 55.Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, et al. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One. 2009;4:e6755. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- 57.Lisman J, Buzsaki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull. 2008;34:974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spooren W, Mombereau C, Maco M, Gill R, Kemp JA, Ozmen L, et al. Pharmacological and genetic evidence indicates that combined inhibition of NR2A and NR2B subunit containing NMDA receptors is required to disrupt prepulse inhibition. Psychopharmacology (Berl) 2004;175:99–105. doi: 10.1007/s00213-004-1785-y. [DOI] [PubMed] [Google Scholar]
- 59.Hajos M, Hoffman WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillations: Relevance to schizophrenia. Biological Psychiatry. 2008;63:1075–1083. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.