Abstract
The intrinsic mitochondrial apoptotic pathway acts through two core pro-apoptotic proteins Bax and Bak. While Bax and Bak seem to play redundant roles in apoptosis, accumulating evidence also suggests that they might not be interchangeable under certain conditions, at least in some human cell lines. Here we report the generation of Bak knockout as well as BaxBak double knockout HCT116 human colon carcinoma cells. We show that Bak is dispensable for apoptosis induced by a variety of stimuli including ABT-737 but not for 5-FU-induced apoptosis. In addition, Bax deficiency only provides partial protection against camptothecin and cisplatin-induced apoptosis and no protection against killing by Puma or ABT-737 plus Noxa overexpression. Moreover, Bak is activated normally in response to many chemotherapeutic drugs in the presence of Bax but remains kept in check by Mcl-1 in the absence of Bax. Our data suggest that Bax and Bak are functionally redundant but they are counteracted by distinct anti-apopotic Bcl-2 family proteins in different species.
Keywords: HCT116, Bax, Bak, DKO, BH3-only, apoptosis
Introduction
The essential roles of Bax and Bak in apoptosis were demonstrated by the complete resistance of BaxBak double knockout (DKO) mouse embryonic fibroblast (MEF) cells to a variety of apoptotic stimuli (Wei et al 2001) and the finding that pro-apoptotic BH3-only proteins such as Bim and Puma act through Bax/Bak (Zong et al 2001). Bax and Bak appear functionally redundant since loss of Bax or Bak alone does not provide significant protection against apoptosis (Lindsten et al 2000, Wei et al 2001). However, growing evidence suggests that Bax and Bak play non-redundant roles in apoptosis induced by a certain specific stimuli, at least in human cells. For example, Bak or Bax single knockdown renders HeLa cells resistant to Ngo or cisplatin-induced apoptosis (Kepp et al 2007). Bax deficient or Bak knockdown human glioblastoma multiforme (GBM) tumor cells are also refractory to UV, staurosporine (STS) and doxorubicin (Cartron et al 2003). The BH3-only protein natural born killer (NBK)/Bcl-2-interacting killer (Bik) has been shown to act entirely through Bax in human cells (Gillissen et al 2003) but solely dependent on Bak in mouse cells (Shimazu et al 2007).
Unlike Bax deficient MEF cells, Bax−/− HCT116 cells are extremely resistant to nonsteroidal anti-inflammatory drugs (NSAIDs) (Zhang et al 2000) and TRAIL (Deng et al 2002, von Haefen et al 2004). Since Bak is expressed at similar levels in Bax−/− as in the wild type HCT116 cells and is functional (Theodorakis et al 2002), it suggests that Bax is predominantly required for a large variety of apoptotic stimuli in certain cell lineages. Another possibility is that Bak is completely suppressed by some inhibitors, such as Bcl-xL and Mcl-1 (Willis et al 2005). Although Bak activation is absent in Bax−/− HCT116 cells upon cisplatin exposure (Kepp et al 2007) or NBK overexpression (Gillissen et al 2007), it has never been tested whether or not Bak activation occurs in wild type HCT116 cells in response to those particular stimuli. Hence, it is not clear whether Bax is required for Bak activation (directly or indirectly) or certain apoptotic stimuli only specifically activate Bax but not Bak. The roles of Bax and Bak can only be explicitly dissected by establishing Bak−/− and Bax−/−Bak−/− DKO HCT116 cell lines.
Here we report the generation of Bak single and BaxBak double knockout HCT116 cell lines. We show that Bax−/−Bak−/− DKO cells are more resistant to various apoptotic agents than Bax−/− cells, indicating that Bak is still involved in many apoptotic responses. In cases where Bax−/− cells are as resistant as Bax−/−Bak−/− DKO cells, for instance, to ABT-737 treatment, Bak is activated in the wild type cells but becomes restrained by Mcl-1 in Bax−/− cells, highlighting the important role of Bax in removing Mcl-1’s grip on Bak. Our data also suggest that Bax is not restrained by Mcl-1 in human cells and the differential expression levels of Mcl-1 in MEFs and HCT116 cells might underlie the distinct phenotypes of Bax deficiency in these two species.
Results
Generation of Bak−/− and Bax−/−Bak−/− DKO cells
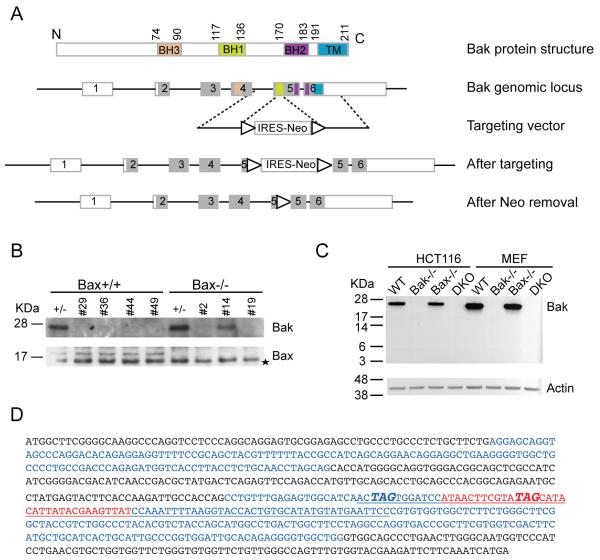
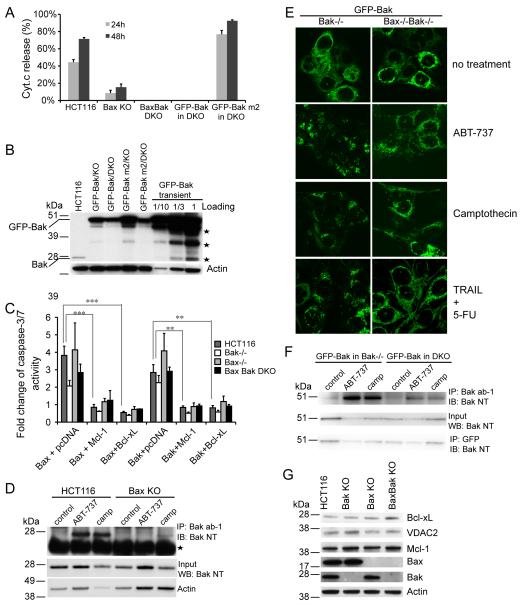
Bax seems to be the key pro-apoptotic factor in human cells since Bax−/− HCT116 cells are extremely resistant to apoptosis (Zhang et al 2000) and Bak appears to be dispensable for apoptosis based on gene knockdown studies (Cartron et al 2003, Chandra et al 2005, Gillissen et al 2007, Gillissen et al 2010). In order to explicitly dissect the role of Bak in apoptosis, we sought to knock out Bak by homologous recombination in HCT116 cells. We targeted exon 5 of the Bak gene. Exon 5 encodes the BH1 domain and part of the BH2 domain whereas the BH3 domain is located on exon 4 and the transmembrane domain (TM) resides on exon 6 (Figure 1A). Targeting exon 5 would result in a C-terminal truncated protein lacking the TM domain (due to the premature stop codon introduced by the targeting vector, Figure 1D) or an N-terminal truncated protein lacking the BH3 domain. Since the transmembrane domain is essential for mitochondrial localization of Bak and the BH3 domain is central to its apoptotic activity, either of these truncated proteins would be inactive. A promoter-less gene-targeting vector (pSEPT) was employed (Topaloglu et al 2005). In combination with the effective rAAV transduction system (Kohli et al 2004), the pSEPT vector exhibits high recombination efficiency. 12 out of 36 clones were Bak+/- and 5 out of 24 clones of Bax−/− cells were Bak+/-, about 30% and 20% targeting efficiency, respectively. A similar targeting frequency was achieved in the second round targeting. Western blotting confirmed the successful abolishment of Bak expression in clones #29, 36, 44 and 49 yielding Bak−/− and clones # 2 and 19 yielding Bax−/−Bak−/− DKO (Figure 1B). The premature stop codon introduced by the targeting vector (Figure 1D) likely triggers mRNA decay and aberrant splicing does not occur as no truncated Bak is produced in Bak−/− and Bax−/−Bak−/− DKO cells (Figure 1C), indicating that our Bak−/− cells are truly null.
Figure 1.
Generation of Bak KO and BaxBak DKO HCT116 cells. (A) Schematic diagram of gene targeting of Bak locus. The top panel shows BH3, BH1, BH2 and the transmembrane (TM) domains of Bak protein in orange, green, pink and blue. The exons encoding the corresponding domains are labeled in the same color in the Bak genomic locus and are shown as rectangles. Shaded regions of rectangles represent coding exons. Triangles indicate LoxP sites. (B) Confirmation of Bak knockout by Western blot. #29, 36, 44 and 49 are homozygous Bak KO and #2 and #19 are homozgyous BaxBak DKO clones. Asterisk indicates a non-specific band detected by anti-Bax antibody. (C). No truncated or aberrant Bak was produced in Bak KO and BaxBak DKO HCT116 cells. Bak KO and BaxBak DKO MEFs were used as a control. anti-Bak NT recognizes the N-terminal region of Bak. (D) Final Bak ORF sequence after gene targeting. Sequence underlined is introduced by the targeting vector. LoxP site is shown in red. The two premature stop codons are shown as bold italic. Alternating coding exons are shown in black or blue color. Note that exon 1 is a non-coding exon.
Bak is dispensable for apoptosis induced by a variety of stimuli when Bax is present
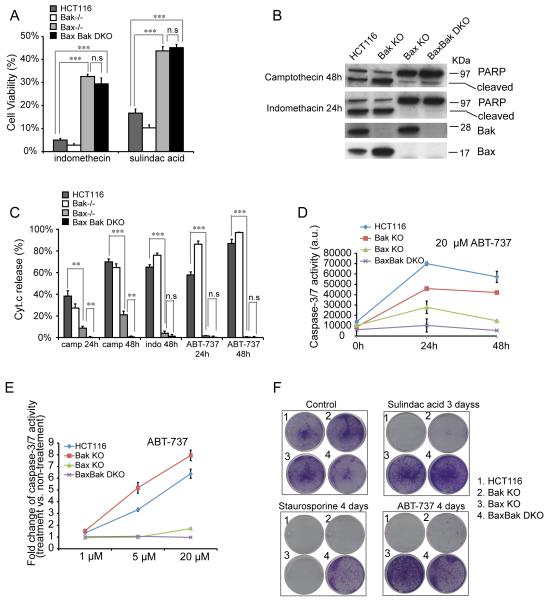
We first sought to test whether Bak deficiency also leads to protection against NSAIDs. As shown in Figure 2A, Bax−/−Bak−/− DKO cells are no more resistant to either indomethacin or sulindac acid treatment than Bax−/− cells. Bak−/− cells display no resistance and are even more sensitive to both NSAID agents than the wild type cells. This is confirmed by analysis of indomethacin-induced PARP cleavage (Figure 2B), an indicator of caspase activity. Consistently, upon indomethacin treatment, over 60% of wild type and Bak−/− cells exhibited Cyt.c release whereas less than 4% of Bax−/− and 2% of Bax−/− Bak−/− DKO cells displayed Cyt.c release (Figure 2C). Similarly, ABT-737, an inhibitor of anti-apoptotic Bcl-2 family proteins, induced a similar level of Cyt.c release in wild type and Bak−/− cells at 48h treatment (Figure 2C). In contrast, Bax−/− cells exhibited as strong resistance as Bax−/−Bak−/− KO cells to ABT-737. This is further confirmed by caspase-3/7 activity assay shown in Figure 2D. ABT-737-induced apoptosis in HCT116 cells is dosage dependent (Figure 2E). Bax−/− and Bax−/−Bak−/− DKO cells remain resistant to treatment of increasing ABT-737 concentrations whereas Bak−/− cells exhibit similar sensitivity as the wild type cells (Figure 2E).
Figure 2.
Bak deficiency provides no protection against many apoptotic stimuli. (A) Cell viability assay in wild type, Bak KO, Bax KO and BaxBak DKO cells with 500 μM indomethacin and 120 μM sulindac acid treatment for 48h. Cell viability was measured with Promega’s CellTiter-Glo luminescent cell viability assay and normalized to non-treatment controls. The experiment was repeated three times and representative data were shown as average±SD. Since non-treated cells proliferate at a greater rate than the treated cells, this assay underestimates the survival rate of treated cells. (B) PARP cleavage in indicated cell lines with 1 μM camptothecin and 500 μM indomethacin treatment. (C) Cyt. c release in indicated cell lines with 1 μM camptothecin (camp), 500 μM indomethacin (indo) and 20 μM ABT-737. The experiment was repeated three times and representative data were shown as average±SD. (D) Caspase-3/7 activity in indicated cell lines with 20 μM ABT-737 treatment. The experiment was repeated twice and representative data were shown as average±SD. (E) Caspase-3/7 activity in indicated cell lines with different concentrations of ABT-737 for 24h. Representative data were shown as average±SD. (F) Clonogenic assay of tested cell lines with 120 μM sulindac acid, 0.2 μM staurosporine and 20 μM ABT-737 treatment. The experiment was repeated three times and a representative image was shown. The p-value was obtained using the Student’s t-test. *, p<0.05; **, p<0.01; ***, p<0.001.
Finally, we conducted clonogenic assays. Again, while no colony survival was detected in the wild type and Bak−/− cells upon sulindac acid or ABT-737 treatment, similar numbers of colonies were observed in Bax−/− and Bax−/−Bak−/− DKO cells (Figure 2F). Altogether, these data strongly suggest that Bax, not Bak, plays a major role in apoptosis in HCT116 cells and Bak seems to be dispensable. However, Bax−/− cells are significantly less resistant to camptothecin treatment (either 24 h or 48 h) than Bax−/−Bak−/− DKO cells (Figure 2C), indicating that Bak does play a role in apoptosis against certain stimuli. In support of this, no colony survival can be detected in either Bax−/− or Bak−/− cells after 4 days of staurosporine treatment whereas a substantial number of colonies survived with Bax−/−Bak−/− DKO cells (Figure 2F). Therefore, Bak can be activated to mediate apoptosis under certain circumstances or extended treatment but remains inactive when Bax is not present under most conditions.
Bak is involved in 5-FU induced apoptosis even when Bax is present
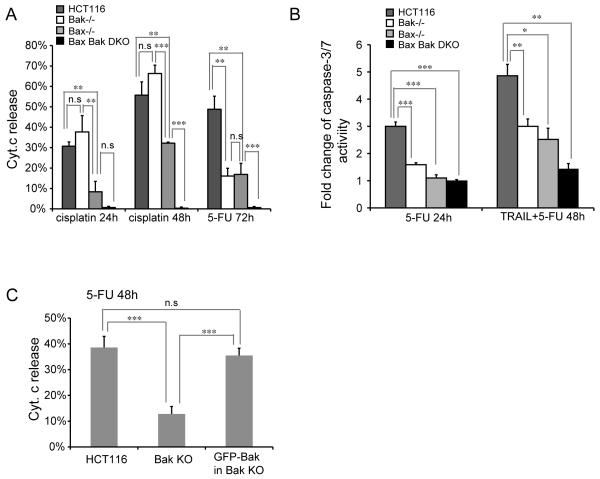
Bax−/− HCT116 cells display moderate resistance to 5-FU as reported previously (Zhang et al 2000), suggesting that Bak might be involved in 5-FU induced apoptosis. Indeed, Bak−/− cells are as resistant to 5-FU as Bax−/− cells as shown by reduced Cyt.c release (Figure 3A) up to 72 h treatment and display lower caspase-3/7 activity than wild type cells at 24h (Figure 3B). Loss of both Bax and Bak led to complete protection against 5-FU (Figure 3A), indicating that both Bax and Bak mediate 5-FU-induced apoptosis. In contrast to a previous report that rules out a role of Bak in synergistic induction of apoptosis by TRAIL and 5-FU (von Haefen et al 2004), Bak−/−cells exhibited a significant reduction in caspase-3/7 activity after TRAIL and 5-FU for 48h, to a similar extent as Bax−/− cells (Figure 3B). Corroborating this, re-introduction of Bak expression in Bak−/− cells sensitizes them to the 5-FU insult (Figure 3C). Bak is also shown to be required for cisplatin-induced apoptosis (Kepp et al 2007) using Bak siRNA. In contrast, we found that loss of Bax but not Bak provides strong protection whereas the loss of both Bax and Bak leads to complete resistance to cisplatin (Figure 3A). This indicates that Bak is dispensable for cisplatin-induced cell death when Bax is present but becomes essential for apoptosis when Bax is absent.
Figure 3.
Bak deficiency leads to protection against 5-FU. (A) Cyt. c release in tested cell lines with 100 μM cisplatin or 375 μM 5-FU treatment. The experiment was repeated twice and representative data were shown as average±SD. (B) Caspase-3/7 activity in tested cell lines with 375 μM 5-FU or 10 ng/ml TRAIL plus 50 uM 5-FU treatment. The experiment was repeated twice and representative data were shown as average±SD. (C) Re-introduction of Bak in Bak KO cells restores the sensitivity to 5-FU (375 μM for 48h) indicated by Cyt.c release. The experiment was repeated twice and representative data were shown as average±SD. The p-value was obtained using the Student’s t-test.*, p<0.05; **, p<0.01; ***, p<0.001.
Bak is responsive to Puma activation in the absence of Bax
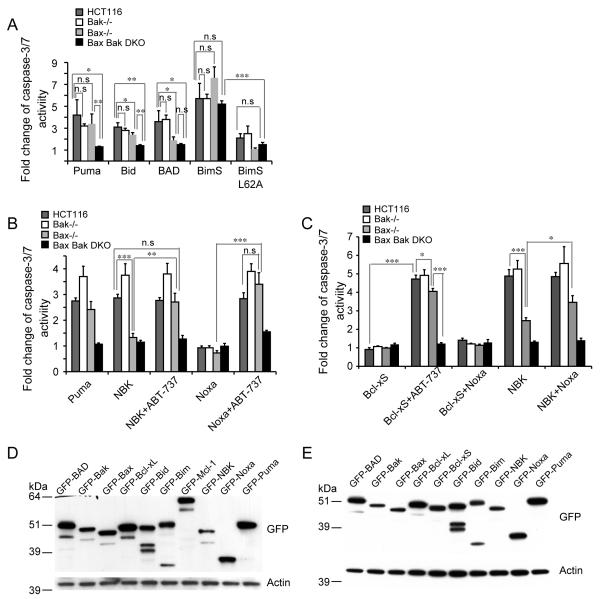
Is Bax/Bak differentially activated by various apoptotic stimuli as suggested by previous reports (Cartron et al 2003, Deng et al 2002, Gillissen et al 2003, Kepp et al 2007, Lindenboim et al 2005, Neise et al 2008) or is Bak activation dependent on Bax? To shed some insight into this question, we first tested if Bax and Bak respond to pro-apoptotic BH3 proteins in different manners. Overexpression of Noxa did not kill wild type cells but co-treatment with ABT-737 allowed Noxa to kill both Bak−/− and Bax−/− cells as previously reported (Figure 4B). BAD, binding to all anti-Bcl-2 members except Mcl-1 (Chen et al 2005), can kill wild type cells and Bak−/−cells but not Bax−/− cells (Figure 4A).
Figure 4.
Bak is kept in check by Mcl-1 in Bax KO cells. (A) BH3-only proteins display different killing profiles to Bax KO cells shown by caspase-3/7 activity normalized to GFP-C1 vector control. BH3-only proteins were transiently expressed in the indicated cell lines and caspase-3/7 activity was measured 18 h after transfection. The experiment was repeated three times and representative data were shown as average±SD. (B) Noxa and NBK sensitize Bax KO cells to ABT-737. Puma, NBK or Noxa was transiently transfected and 20 μMABT-737 was added to the cells during transfection. Caspase-3/7 activity was measured 24 h after transfection and normalized to GFP-C1 vector control. The experiment was repeated twice and representative data were shown as average±SD. (C) Bcl-xS sensitizes cells to ABT-737 and Noxa sensitizes Bax KO cells to NBK. Untagged Bcl-xS, GFP-NBK and GFP-Noxa were transiently transfected and 20 μM ABT-737 was added during transfection. The experiment was repeated twice and representative data were shown as average±SD. (D-E) Protein levels of transiently expressed pro-and anti-apoptotic genes detected by Western blotting in HCT116 WT cells (E) and BaxBak DKO HCT116 cells (E). The p-value was obtained using the Student’s t-test.*, p<0.05; **, p<0.01; ***, p<0.001.
The three Bax/Bak direct activators (Kim et al 2006), Bim, Bid and Puma exhibited differential killing ability in Bax−/− and Bax−/−Bak−/− DKO cells. Both Bax−/− and Bax−/−Bak−/− DKO cells are resistant to Bid killing whereas Bim kills Bax−/− cells and Bax−/−Bak−/− DKO cells effectively to similar extent (Figure 4A), suggesting that Bim could exert its killing ability independent of Bax/Bak. Nonetheless, a point mutation in Bim’s BH3 domain (BimS L62A), which disrupts its binding to Bcl-2 and Bcl-xL (Lee et al 2008), abolishes Bim’s killing ability (Figure 4A). In contrast, Puma kills Bak−/− and Bax−/− cells as effectively as the wild type cells and exhibited no killing in Bax−/−Bak−/− DKO cells (Figure 4A-B), indicating that Bak can be activated by Puma in the absence of Bax. While all pro-and anti-apoptotic genes used in this study are expressed at a comparable level (Figure 4D-E), transient expression can lead to more than a 10-fold greater expression level than the stable expression level as shown for example with Bak expression (Figure 5B).
Figure 5.
Interdependence of Bak and Bax activation. (A) A Bak mutant (m2, I85A/N86A) that fails to be bound by Bcl-xL and Mcl-1 sensitizes BaxBak DKO cells to camptothecin. GFP-Bak or GFP-Bak m2 was stably expressed in BaxBak DKO cells. Cells were treated with 1 μM camptothecin and Cyt.c release was examined. The experiment was repeated twice and representative data were shown as average±SD. (B) Bak expression levels in transiently expressed or stably expressed cells shown by Western blotting. Asteries indicate degraded Bak bands. (C) Caspase-3/7 acitivity was measured in indicated cell lines transiently transfected with GFP-Bax, GFP-Bak or in combination with GFP-Mcl-1 or GFP-Bcl-xL (1:3 ratio of DNA amount). Fold change was normalized with cells transfected with GFP-C1 vector control. The experiment was repeated twice and representative data were shown as average±SD. (D) Bak activation occurs normally in wild type cells in response to camptothecin and ABT-737 treatment detected by immunoprecipitation with anti-Bak (ab-1) antibody. Asteria indicates the light chain of Ig. (E) Bak activation indicated by Bak foci. GFP-Bak was stably expressed in Bak KO and BaxBak DKO cells. Cells were treated with 20 μM ABT-737, 1 μM camptothecin or 50 ng/ml TRAIL plus 375 μM 5-FU in the presence of 10 μM Q-VD for 24 h. (F) Bak activation in GFP-Bak stably expressing cells. Cells were treated and immnuoprecipitated as in (D). (G) Bcl-xL, VDAC2 and Mcl-1 express normally in Bax KO and Bak KO cells. The p-value was obtained using the Student’s t-test.*, p<0.05; **, p<0.01; ***, p<0.001.
Since Bak is mainly sequestered by Bcl-xL and Mcl-1 whereas Bax could be bound by Bcl-2, Bcl-w, Bcl-xL and Mcl-1 (Willis et al 2005, Willis et al 2007), it is not surprising that NBK, which specifically binds Bcl-xL and Mcl-1, is completely dependent on Bak for killing activity in murine iBMK cells (Shimazu et al 2007). However, NBK is reported to be solely dependent on Bax in HCT116 cells (Gillissen et al 2007). To address this discrepancy, we tested NBK’s killing in our Bak−/− and Bax−/− Bak−/− DKO cells. As shown in Figure 4B-C, NBK killed Bak−/− HCT116 cells as effectively as wild type cells but exhibited limited killing of Bax−/− cells. Co-treatment with ABT-737 exposes Bax−/− cells to NBK-mediated killing (Figure 4B). NBK displays low affinity to Mcl-1 (Chen et al 2005), but can Co-IP with Mcl-1 in vivo (Chen et al 2005, Shimazu et al 2007). However, NBK/Mcl-1 interaction is not detected in HCT116 cells (Gillissen et al 2007). The fact that both ABT-737 and Noxa sensitize Bax−/− cells to NBK (Figure 4B-C) suggests that NBK might be able to sequester Mcl-1 in human cells, but not as efficiently as in murine cells. Bcl-xS has also been shown to be exclusively dependent on Bak in MEFs as it kills both wild type and Bax−/− MEFs very effectively (Lindenboim et al 2005). However, untagged Bcl-xS does not even kill wild type HCT116 cells (Figure 4C) although it does sensitize Bax−/− HCT116 cells to ABT-737 treatment, acting like Noxa (Figure 4C).
Bak activation is dependent on Bax indirectly
It has been reported that Mcl-1 has low binding affinity to Bax and overexpression of Mcl-1 does not block overexpression of Bax-induced cell death (Zhai et al 2008). This is consistent with the observation here that Bak−/− HCT116 cells are sensitive but Bax−/− cells are refractory to ABT-737 killing since ABT-737 is a specific inhibitor for Bcl-2, Bcl-w and Bcl-xL but not Mcl-1. It is further supported by the fact that Noxa can sensitize Bax−/− HCT116 cells to ABT-737 (Figure 4B). Corroborating this, when a Bak mutant (Bak m2, I85A/N86A) that fails to be bound by Bcl-xL and Mcl-1 (Kim et al 2006) is stably expressed in Bax−/−Bak−/− DKO cells (that should behave like Bax−/− cells), they are even more sensitive to camptothecin treatment than are wild type cells (Figure 5A). In contrast, Bax−/−Bak−/− DKO cells expressing wild type Bak remain resistant to camptothecin treatment (Figure 5A). The wild type and mutant Bak are expressed at similar levels in those stable cell lines (Figure 5B). It is noteworthy that when highly expressed (such as transient overexpression), Bak can kill wild type and Bax−/−Bak−/− DKO HCT116 cells (Figure 5C). The killing ability of Bak overexpressioin is again blocked by overexpression of Bcl-xL or Mcl-1 (Figure 5C). Like overexpression of Bak, overexpression of Bax also kills all four types of cell lines (wild type, Bak−/−, Bax−/− and Bax−/−Bak−/− DKO) (Figure 5C). In contrast to a previous report (Zhai et al 2008), overexpression of either Bcl-xL or Mcl-1 blocks the killing ability of Bax overexpression (Figure 5C). This is consistent with previous observations that overexpression of Bcl-xL or Mcl-1 can still protect Bak m2 mutant from apoptosis (Kim et al 2006).
Given that Bax−/− HCT116 cells are resistant to a variety of apoptotic agents, it is not unexpected that Bak activation is not observed in Bax−/− cells. But is Bax required for Bak activation? To test this, we performed immunoprecipitation with antibodies that can detect activated Bak (ab-1). Whereas activated Bak is readily detected in wild type cells with either camptothecin or ABT-737 treatment, there is no Bak activation in the Bax−/− cells under these conditions (Figure 5D). This is further confirmed by confocal imaging of Bak−/− cells and Bax−/−Bak−/− DKO cells. We used cells stably expressing GFP-Bak (Figure 5E) since anti-Bak (ab-1) antibody does not work well in HCT116 cells for immunofluoresecence staining. ABT-737, camptothecin and the combination of TRAIL plus 5-FU all induce Bak activation indicated by the foci formation in GFP-Bak expressing Bak−/− cells, but much less in GFP-Bak expressing Bax−/−Bak−/− DKO cells (Figure 5E, supplementary Figure 1). Corroborating this, immunoprecipitation with anti-Bak ab-1 antibody in GFP-Bak stably expressing cells also clearly showed that Bak activation occurs normally in response to ABT-737 or camptothecin treatment but is largely reduced when Bax is absent (Figure 5F). These data suggest that Bak can be activated during many death stimuli-induced apoptosis when Bak seems to be dispensable, which is likely indirectly dependent on Bax. It has also been shown that Bak can be activated by Actinomycin D and staurosporine in MCF-7 cells (Neise et al 2008). Three common Bak antagonists: Bcl-xL, Mcl-1 and VDAC2 are expressed at similar levels in the wild type, Bax−/−, Bak−/− and Bax−/−Bak−/− DKO cells (Figure 5G).
Mcl-1 determines the resistance of Bax KO cells
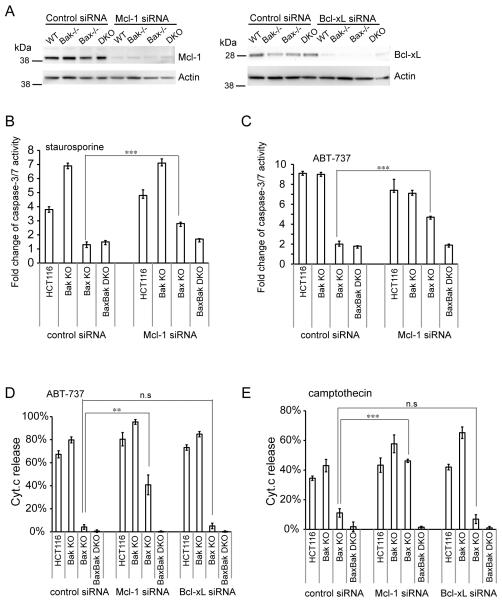
The fact that Noxa sensitizes Bax−/− cells to ABT-737 suggests that Bak is inhibited by Mcl-1 in the absence of Bax. If this is indeed the case, then silencing Mcl-1 in Bax−/− cells should sensitize them to apoptosis. As shown in Figure 6A, Mcl-1 and Bcl-xL were effectively knocked down by corresponding siRNAs. Whereas control siRNA has no effect on Bax−/− cells as they remain resistant to apoptosis, Mcl-1 siRNA sensitizes Bax−/− cells to staurosporine (Figure 6B) or ABT-737 (Figure 6C) indicated by caspase-3/7 activity (Figure 6B-C) or Cyt. c release (Figure 6D-E). In contrast, Bax−/−Bak−/− DKO Mcl-1 siRNA cells remain completely resistant to all treatment (Figure 6B-E). Unlike Mcl-1 siRNA, Bxl-xL siRNA has no effect on the resistance of Bax−/− cells to ABT-737 (Figure 6D) or camptothecin (Figure 6E), suggesting that Mcl-1 but not Bcl-xL keeps Bak in check in Bax−/− cells.
Figure 6.
Mcl-1 determines the resistance of Bax KO cells. (A) Knockdown level of Mcl-1 siRNA and Bcl-xL siRNA shown by Western blotting. Caspase-3/7 activity in control and Mcl-1 siRNA cells treated with 0.2 μM staurosporine (B) and 20 μM ABT-737 (C) for 24h was measured and normalized to untreated controls. Data were shown as average±SD. (D-E) Cyt.c release in control, Mcl-1 siRNA and Bcl-xL siRNA cells treated with 20 μM ABT-737 (D) and 1 μM camptothecin (E) for 24h. Data were shown as average±SD. The p-value was obtained using the Student’s t-test.*, p<0.05; **, p<0.01; ***, p<0.001.
Discrepancy of Bax/Bak mediated apoptosis in HCT116 and MEF cells
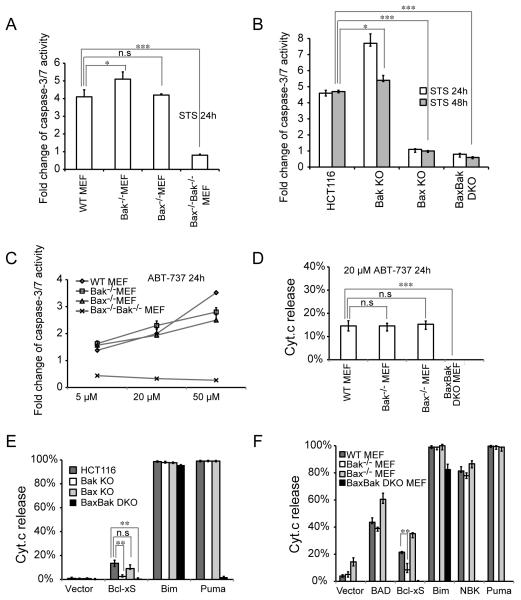
The discrepancies on Bax versus Bak-dependent apoptotic responses reported in published studies could be due to different experimental settings, apoptotic treatments and cell lines (see Table 1). In order to gain insight into this discrepancy, we sought to compare Bax/Bak-mediated apoptosis in HCT16 and MEF cells directly under the same experimental settings. First, as previously reported (Wei et al 2001), Bak−/− MEF and Bax−/− MEF cells are similarly sensitive to staurosporine as wild type MEF cells whereas Bax−/− Bak−/− DKO MEFs remains resistant (Figure 7A), suggesting that Bax and Bak are redundant in mediating staurosporine-induced apoptosis in MEF cells. In contrast, Bax−/− HCT116 cells are nearly as resistant to staurosporine as Bax−/−Bak−/− DKO cells (Figure 7B). Second, as previously reported (van Delft et al 2006), even wild type MEFs seem to be resistant to ABT-737 as 20 μM ABT-737 induces very limited apoptosis indicated by both caspase-3/7 activity (Figure 7C) and Cyt.c release (Figure 7D). Bak−/− MEF and Bax−/− MEF seem to be as sensitive to ABT-737 as to WT MEF cells (Figure 7C-D). Third, unlike untagged Bcl-xS (Figure 4D), GFP-tagged Bcl-xS does induce cell death in HCT116 cells (Figure 7E), although it is much less potent than in MEF cells (Figure 7F). Even in MEF cells, GFP-Bcl-xS triggers moderate cell death comparing to Bim, NBK or Puma (Figure 7F). Nonetheless, in both species Bcl-xS seems to preferentially trigger Bak-mediated cell death, consistent with a previous report (Lindenboim et al 2005). Fourth, in both species, Puma-induced apoptosis is completely dependent on Bax and Bak (Figure 7E-F). Moreover, whereas NBK-induced apoptosis seems to be dependent on Bax (Figure 4C-D) in human cells, Bax and Bak are equally involved in NBK-induced cell death in murine cells (Figure 7F). In addition, Bim is able to induce Bax/Bak-independent apoptosis in both HCT116 and MEF cells (Figure 7E-F). However, it does require much higher expression level of Bim to kill BaxBak DKO cells than the wild type cells (data not shown).
Table 1.
Comparison of Bax/Bak dependence in human and murine cells in response to apoptotic stimuli
| HCT116 | MEF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Stimuli | Bak KO | Bax KO | DKO | Condition | Reference | Bak KO | Bax KO | DKO | Condition | Reference |
| 5-FU | p.r | 375 μM for 24, 48, 72hr |
Zhang et al, 2000 | |||||||
| p.r | p.r | v.r | 375 μM 24h, 48h |
This study | ||||||
| 5-FU+TRAIL | v.r | 3.125 μg/ml 5- FU+ 10 ng/ml TRAIL for 72h |
von Haefen et al, 2004 | |||||||
| ABT-737 | s | v.r | v.r | 10-20 μM for 24, 48, 72h |
This study | v.r | v.r | v.r | 10 μM for 48h | van Delft et al, 2006 |
| v.r | 10 μM for 48h | Konopleva et al, 2006 | v.r | v.r | v.r | 100 nM for 72h | Konopleva et al, 2006 | |||
| s | s | v.r | 20, 50 μM for 24h |
This study | ||||||
| ABT-737+ Noxa |
s | s | v.r | Noxa + 20 μM ABT-737 for 24h |
This study | v.r | p.r | v.r | 0.625 μM ABT- 737 + Noxa for 8h |
van Delft et al, 2006 |
| p.r | s | v.r | Noxa+ 10 uM ABT-737 for 8h |
van Delft et al, 2006 | ||||||
| Apo2L/TRAIL | v.r | 1 μg/ml for 24h | LeBlanc et al, 2002 | |||||||
| BAD | s | v.r | v.r | overexpression | This study | n.a | v.r | v.r | overexpression | Willis et al, 2005 |
| Bcl-xS | p.r | s | v.r | overexpression | This study | v.r | s | v.r | overexpression | Lindenboim et al 2005 |
| Bid | v.r | overexpression | Theodorakis et al, 2002 | |||||||
| s | s | v.r | overexpression | This study | ||||||
| Bik/NBK | v.r | overexpression | Theodorakis et al, 2002 | s | s | v.r | overexpression | This study | ||
| s | v.r | v.r | overexpression | This study | ||||||
| v.r | overexpression | Gillissen et al, 2003 | ||||||||
| sa | v.r | v.ra | overexpression | Gillissen et al, 2007 | ||||||
| BimS | sa | s | s | overexpression | This study | s | s | v.r | overexpression | Zong et al, 2001 |
| cisplatin | p.ra | p.ra | 60 μM for 15h | Kepp et al, 2007 | p.rb | p.rb | v.rb | 100 μM for 8h | Jiang et al, 2009 | |
| s | p.r | v.r | 100 μM for 24, 48h |
This study | ||||||
| cycloheximide | v.rc | sc | v.rc | 1-10 μg/ml | Shimazu et al, 2007 | |||||
| etoposide | sd | sd | v.rd | 10 or 100 μM for 24 hr |
Lindsten et al 2000 | |||||
| p.r | p.r | v.r | 100 μM, 48h | Wei et al, 2001 | ||||||
| s | s | v.r | 100 μM for 24h | Willis et al, 2005 | ||||||
| s | s | v.r | 10 μM for 48h | van Delft et al, 2006 | ||||||
| etoposide+ Apo2L/TRAIL |
s | 50 μg/ml for 17 h + 1 μg/ml Apo2L/TRAIL 3h |
LeBlanc et al, 2002 | |||||||
| indomethacin | v.r | 500 μM, 24, 48, 72h |
Zhang et al, 2000 | |||||||
| s | v.r | v.r | 500 μM for 24, 48h |
This study | ||||||
| Noxa m3 | v.r | s | overexpression | Willis et al, 2007 | ||||||
| Puma | s | s | v.r | overexpression | This study | s | s | v.r | overexpression | This study |
| serum withdrawal |
s | s | v.r | 1, 2, 3 days | Wei et al, 2001 | |||||
| staurosorine | v.r | 50 μM for 48h | Theodorakis et al, 2002 | s | s | v.r | 1 μM for 48h | Wei et al, 2001 | ||
| sa | s | v.ra | 1-20 μM for 24h | Cartron et al, 2003 | ||||||
| sa | p.r | v.ra | 0.5 μM for 24h | Chandra et al, 2005 | ||||||
| s | v.r | v.r | 0.2 μM for 24, 48h |
This study | s | s | v.r | 0.5 μM for 24h | This study | |
| sulindac acid | v.r | 120 μM for 24, 48, 72h |
Zhang et al, 2000 | |||||||
| s | v.r | v.r | 120 μM for 24, 48, 72h |
This study | ||||||
| thapsigargin | v.r | 0.5 μM for 48h | Theodorakis et al, 2002 | p.r | s | v.r | 2 μM for 48h | Wei et al, 2001 | ||
| thapsigargin | sa | p.r | v.ra | 5 μM for 24h | Chandra et al, 2005 | |||||
| TNF/CHX | sb | sb | rb | 100 μg/ml TNF+50 ng/ml CHX for 16h |
Degenhardt et al, 2002 | |||||
| TNF+CHX | v.r | 1 ng/ml TNF + 25 μg/ml cycloheximide for 24h |
Theodorakis et al, 2002 | |||||||
| TRAIL | v.r | 100 ng/ml for 6h | Deng et al, 2002 | |||||||
| v.r | not indicated | Theodorakis et al, 2002 | ||||||||
| sa | v.r | v.ra | 50 ng/ml for 48h |
Gillissen et al, 2010 | ||||||
| s | v.r | v.r | 50 ng/ml for 48h |
This study | ||||||
| tunicamycin | p.r | s | v.r | 1 μg/ml for 48h | Wei et al, 2001 | |||||
| UVC | p.ra,e | p.re | v.re | Cartron et al, 2003 | p.r | s | v.r | 60 k/m2 for 48h | Wei et al, 2001 | |
| UVC | p.r | s | v.r | 110-200 J/m2 for 24h |
Willis et al, 2005 | |||||
Note: p.r, partial resistant; s, sensitive; v.r, very resistant; n.a, not assayed
siRNA or shRNA knockdown
BMK( baby mouse kedne)y cells
iBMK cells
thymocytes
GBM (glioblastoma multiforme tumor) cells
Figure 7.
Comparison of Bax/Bak-mediated cell death in HCT116 and MEF cells. (A-B) Caspase-3/7 activity in indicated MEF (A) or HCT116 (B) cell lines treated with 0.2 μM staurosporine. Fold change of caspase activity was normalized to untreated controls. The experiment was repeated twice and representative data were shown as average±SD. (C-D) Caspase-3/7 activity (C) or Cyt.c release (D) in indicated MEF cell lines treated with ABT-737. The experiment was repeated twice and representative data were shown as average±SD. (E-F) Cyt.c release in HCT116 (E) or MEF (F) cells transfected with GFP-tagged BH-3 only proteins and GFP-Bcl-xS for 24 hrs. GFP-C1 vector was used as a control. The experiment was repeated twice and representative data were shown as average±SD. The p-value was obtained using the Student’s t-test. *, p<0.05; **, p<0.01; ***, p<0.001.
Discussion
Although some members of the intrinsic cell death pathway are conserved between nematodes and mammals, the two essential core pro-apoptotic proteins, Bax and Bak, are missing from the C.elegans genome. Bcl-2 like proteins in Drosophila are more closely related to Bok. Nonetheless, Bax and Bak orthologs have been recently found in many ancient species, such as Hydra (Dunn et al 2006) and sea anenome (Zmasek et al 2007). Bax and Bak appear to have been lost several times during evolution since the zebrafish genome contains only Bax orthologs but not Bak (Kratz et al 2006), and sea urchin retains Bak but not Bax orthologs (Dunn et al 2006). However, zBax2, although more closely related to human Bax than hBak, behaves functionally like hBak since it cannot be antagonized by the Bcl-2 ortholog in zebrafish (Kratz et al 2006). It is not clear whether the evolution of Bax and Bak in mammals reflects the regulatory complexity of these species or acts as a surveillance mechanism so that proper cell death proceeds in case the other has been shut off.
Bax and Bak have been long believed to be redundant to one another since Bax−/− and Bak−/− MEFs or BMK cells are both sensitive to various apoptotic stimuli such as staurosporine, etoposide, UV-C and serum withdraw (Degenhardt et al 2002, Lindsten et al 2000, Wei et al 2001). However, they can be counteracted by distinct anti-apoptotic Bcl-2 family proteins. Bak is specifically bound by Bcl-xL and Mcl-1 whereas Bax can be inhibited by Bcl-2, Bcl-w, Bcl-xL and Mcl-1 (Willis et al 2005, Willis et al 2007). Therefore, Bak seems to be less restrained than Bax. Consistently, loss of Bak in general provides better protection than the loss of Bax against apoptosis when certain stimuli only remove the inhibitory effects of a subset of Bcl-2 family proteins (Table 1). For instance, Bak−/− MEF cells are reported to be more resistant to UV-C treatment than Bax−/− MEFs (Willis et al 2005). Bax−/− MEFs are more sensitive than Bak−/− MEFs to the killing of a Noxa m3 mutant, which can inhibit Bcl-xL, Bcl-w and Mcl-1(Willis et al 2007). Corroborating this, both Bak−/− MEFs and Bax−/− MEFs are refractory to ABT-737 (Konopleva et al 2006), which can only inhibit Bcl-2, Bcl-w and Bcl-xL but not Mcl-1. If the inhibitory effect of Mcl-1 is removed by stably expressing human Noxa, Bak−/− MEFs are still more resistant to ABT-737 than Bax−/− MEFs (van Delft et al 2006). As an extreme example, Bak−/− iBMK (immortalized baby mouse kidney) cells are as resistant as Bax−/−Bak−/− iBMK cells to NBK, which only binds Bcl-xL and Mcl-1(Shimazu et al 2007).
However, this does not appear to be the case in human colon cancer cells. First, it has been shown that Bax−/− HCT116 cells are resistant to many apoptotic insults such as indomethacin, sulindac acid, 5-FU, TRAIL and ABT-737 (Deng et al 2002, Gillissen et al 2010, Zhang et al 2000). We also found that Bax−/− HCT116 cells are as resistant to ABT-737 as Bax−/−Bak−/− DKO HCT116 cells whereas Bak−/− HCT116 cells are as sensitive as wild type cells. Given that ABT-737 sequesters Bcl-2, Bcl-w and Bcl-xL but not Mcl-1 and that Bak−/− HCT116 cells are as sensitive as wild type HCT116 cells to ABT-737, our data strongly suggest that Bax is not restrained by Mcl-1 in human cells. Consistently, no Bax-Mcl-1 interaction was detected in OCI-AML3 cells (Konopleva et al 2006) or HEK293 cells (Zhai et al 2008). In addition, when endogenous Mcl-1 is inhibited by stably expressing Noxa or siRNA silencing, Bax−/− HCT116 cells become as sensitive to ABT-737, camptothecin, or TRAIL as wild type cells (Figure 4B and Figure 6) (Gillissen et al 2010), indicating that Bak, on the other hand, is still kept in check by Mcl-1 in the absence of Bax in HCT116 cells. Although VDAC2 was reported to be the main antagonist to keep Bak in check (Kim et al 2006), our data show that it is Mcl-1 that keeps Bak in check in Bax−/− HCT116 cells. However, in wild type cells, once Bax is activated and oligomerizes, the Mcl-1 inhibitory effect on Bak is removed indirectly. Second, NBK exclusively kills Bax−/− iBMK not Bak−/− iBMK cells (Shimazu et al 2007), but kills both Bax−/− MEF and Bak−/− MEF as effectively as wild type MEF cells (Figure 7F). In contrast, NBK kills Bak−/− HCT116 but not Bax−/− HCT116 cells (Figure 4B-C) (Gillissen et al 2007). It has also been shown that NBK/Bik does not bind Mcl-1 in HCT116 cells (Gillissen et al 2007). However, we found that like Noxa, NBK also sensitizes Bax−/− HCT116 cells to ABT-737 and partially kill Bax−/− HCT116 cells when highly expressed (Figure 4B-C), indicating that NBK might be able to sequester Mcl-1 directly or indirectly. In addition, the relatively high expression level of endogenous Mcl-1 in human cells also contributes to the resistance to apoptosis of Bax KO HCT116 cells.
In summary, our data strongly suggest that Bax and Bak are functional redundant although they appeared to play non-redundant roles due to the extreme resistance of Bax KO HCT116 cells to apoptosis (Cartron et al 2003, Gillissen et al 2003, Gillissen et al 2007, Kepp et al 2007, LeBlanc et al 2002). Once the inhibitory effect of Mcl-1 on Bak is removed by Noxa overexpression (Figure 4B) (Konopleva et al 2006), Mcl-1 knockdown (Figure 6) (Gillissen et al 2010) or increased Bak expression (Figure 5C) (LeBlanc et al 2002), Bax−/− HCT116 cells become as sensitive as wild type cells. In addition, significant differences on the interaction specificity between anti-and pro-apoptotic proteins might exist between human and mice (such as the effect of NBK). Since HCT116 cells are less refractory to lipid-based transfection than MEF cells, the Bak−/− and Bax−/−Bak−/− DKO HCT116 cells provide a complete set complementary to the widely used Bax-/- HCT116 cells and shall be a valuable tool for more relevant studies on chemotherapeutic drugs and cancer.
Materials and methods
Cell culture and chemicals
HCT116 cells were purchased from ATCC (Manassas, VA) and AAV-293 cells from Stratagene (Cedar Creek, TX). Bax−/− HCT116 cells were provided by Bert Vogelstein (Zhang et al 2000). WT, Bax−/−, Bak−/− and Bax−/−Bak−/− DKO MEF cells were provided by Stanley Korsmeyer (Wei et al 2001). Cells were cultured as described (Cleland et al 2011). 5-FU (fluorouracil), camptothecin, sulindac sulfate, cisplatin, TRAIL, indomethacin and staurosporine were purchased from Sigma (St. Louis, MO) and dissolved in DMSO for stock preparation. ABT-737 was purchased from Selleck Chemicals LLC (Houston, Texas).
Plasmids
GFP-Puma was gifted from Karen Vousden (National Cancer Institute at Frederick, MD). Bid, BAD, Bax, Bak, Noxa, Bcl-xL, Mcl-1 and BimS ORF were cloned into pEGFP-C1 vector (Clontech,) to make corresponding EGFP fusion proteins. BimS L62A, Bak m2 (I85A/N86A) mutations were created by site-directed mutagenesis. GFP-NBK/Bik was obtained from Addgene (Addgene plasmid 10952) (Kagawa et al 2001). GFP-Bak was also cloned into pBabe-puro (Addgene plasmid 1764) for stable expression. Bcl-xS cloned in pCMV6-XL5 vector was purchased from Origene (Rockville, MD). GFP-Bcl-xS was gifted by Dr. Reuven Stein (Lindenboim et al 2005).
Gene Targeting
To make gene-targeting construct (KO), two 1-kb sequences flanking exon 5 of the human Bak gene were PCR amplified from HCT116 genomic DNA and cloned into targeting vector as described, so is the rest of gene targeting procedures (Otera et al).
Gene Silencing
ON-TARGETplus SMARTpool siRNA for Mcl-1 and Bcl-xL as well as the non-targeting control siRNA were purchased from Dharmacon (Lafayette, CO). 2-3×105 cells were seeded in 6-well plates and 50 nM siRNA was transfected with 2 μl DharmaFECT (Dharmacon). 2 days later, cells were harvested and re-seeded in 2-well chamber slides for treatment.
Immunofluorescence and Microscopy
Cells were fixed, permeabilized, imunostained as described (Cleland et al 2011). Anti-Cyt.c was purchased from BD Biosciences (San Diego, CA) and anti-Tom20 from Santa Cruz Biotechnology (Santa Cruz, CA). Cells were imaged with Zeiss 510 microscope. Alexa Fluor 488 conjugated goat anti-mouse IgG (H+L) was from Invitrogen.
Cytochrome c release assay
1.5×105 HCT16 cells or 5 ×104 MEF cells were seeded in 2-well Lab-Tek chamber slides. After treatment or transfection for certain time, cells were immunostained with anti-cyt.c antibody and more than 300 cells were counted in three different view fields.
Immunoprecipitation
Cells were lysized in 1% Chaps buffer (10 mM HEPES, pH7.4, 1% Chaps, 150 mM NaCl, 1× complete protease inhibitor cocktail (Roche) and 1 mM PMSF) and 0.5 mg whole cell lysate was used for immunoprecipitation with anti-Bak ab-1 and Protein A/G plus agarose (Santa Cruz) or chromoteck GFP-trap magnetic beads (Allele Biotech).
Immunoblot
Proteins were run on NuPAGE 4-12% Bis-Tris gel and blotted onto nitrocellulose membranes. Anti-Bax NT and anti-Bak NT were from Upstate (Waltham, MA), anti-Bak ab-1 from Calbiochem (San Diego, CA), anti-PARP from Biomol (Plymouth, PA), anti-VADC2 from Abcam (Cambridge, MA), anti-Mcl-1 from Santa Cruz Biotechnology or Abcam (Cambridge, MA) and anti-Actin from Sigma. ECL™ sheep anti-mouse or anti-rabbit IgG, HRP-linked F(ab’)2 fragment was from GE Healthcare (Piscataway, NJ).
Cell Viability and Caspse-3/7 Activity Assay
1×104 HCT116 cells or 4 ×103 MEF cells were seeded in triplicate in 96-well assay plates from Corning (Corning, NY). For transfection, 2.5×104 cells were seeded and transfected with Lipofectamine LTX (Invitrogen). Cell viability and caspse-3/7 activity were measured with CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, Madison, WI) and Caspase-Glo 3/7 Assay kit (Promega), respectively.
Supplementary Material
Figure S1: Clustering of GFP-Bak on mitochondria during apoptosis induced by TRAIL plus 5-FU in Bak−/− and Bax−/−Bak−/− HCT116 cells stablying expressing GFP-Bak. The experiment was performed as in Figure 5E and cells were immunostained with anti-Tom20 antibody. Arrows indicate Bak clustering.
Acknowledgement
We thank Bert Vogelstein for Bax KO HCT116 cells, Fred Bunz for pSEPT vector and advice on gene targeting, Jean-Claude Martinou for comments on the manuscript, NINDS DNA sequencing facility, NINDS imaging facility and NINDS FACS facility. This work was supported in part by the Intramural Research Program, NINDS, NIH.
This study is supported in part by the Intramural Research Program of the National Institutes of Neurological Disorders and Stroke, National Institutes of Health
Footnotes
Supplementary information
The supplementary information includes one figure about the clustering of Bak on mitochondria during apoptosis in HCT116 cells.
Supplementary information is available at Oncogene’s website.
Conflict of interest
The authors declare no conflict of interest.
References
- Cartron P-F, Juin P, Oliver L, Martin S, Meflah K, Vallette FM. Nonredundant role of Bax and Bak in Bid-mediated apoptosis. Molecular and cellular Biology. 2003;23:4701–4712. doi: 10.1128/MCB.23.13.4701-4712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Choy G, Daniel P, Tang D. Bax-dependent Regulation of Bak by Voltage-dependent Anion Channel 2. Journal of Biological Chemistry. 2005;280:19051. doi: 10.1074/jbc.M501391200. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cleland MM, Norris KL, Karbowski M, Wang C, Suen DF, Jiao S, et al. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ. 2011;18:235–247. doi: 10.1038/cdd.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt K, Sundararajan R, Lindsten T, Thompson C, White E. Bax and Bak independently promote cytochrome C release from mitochondria. The Journal of biological chemistry. 2002;277:14127–14134. doi: 10.1074/jbc.M109939200. [DOI] [PubMed] [Google Scholar]
- Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SR, Phillips WS, Spatafora JW, Green DR, Weis VM. Highly conserved caspase and Bcl-2 homologues from the sea anemone Aiptasia pallida: lower metazoans as models for the study of apoptosis evolution. J Mol Evol. 2006;63:95–107. doi: 10.1007/s00239-005-0236-7. [DOI] [PubMed] [Google Scholar]
- Gillissen B, Essmann F, Graupner V, Stärck L, Radetzki S, Dörken B, et al. Induction of cell death by the BH3-only Bcl-2 homolog Nbk/Bik is mediated by an entirely Bax-dependent mitochondrial pathway. The EMBO journal. 2003;22:3580–3590. doi: 10.1093/emboj/cdg343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillissen B, Essmann F, Hemmati P, Richter A, Richter A, Oztop I, et al. Mcl-1 determines the Bax dependency of Nbk/Bik-induced apoptosis. The Journal of cell biology. 2007;179:701. doi: 10.1083/jcb.200703040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillissen B, Wendt J, Richter A, Richter A, Müer A, Overkamp T, et al. Endogenous Bak inhibitors Mcl-1 and Bcl-xL: differential impact on TRAIL resistance in Bax-deficient carcinoma. The Journal of cell biology. 2010;188:851–862. doi: 10.1083/jcb.200912070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA, et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61:3330–3338. [PubMed] [Google Scholar]
- Kepp O, Rajalingam K, Kimmig S, Rudel T. Bak and Bax are non-redundant during infection- and DNA damage-induced apoptosis. The EMBO journal. 2007;26:825–834. doi: 10.1038/sj.emboj.7601533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu H-C, Jeffers JR, Zambetti GP, Hsieh JJ-D, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nature cell biology. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Kohli M, Rago C, Lengauer C, Kinzler KW, Vogelstein B. Facile methods for generating human somatic cell gene knockouts using recombinant adeno-associated viruses. Nucleic Acids Res. 2004;32:e3. doi: 10.1093/nar/gnh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kratz E, Eimon PM, Mukhyala K, Stern H, Zha J, Strasser A, et al. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ. 2006;13:1631–1640. doi: 10.1038/sj.cdd.4402016. [DOI] [PubMed] [Google Scholar]
- LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, et al. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- Lee EF, Czabotar PE, van Delft MF, Michalak EM, Boyle MJ, Willis SN, et al. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol. 2008;180:341–355. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenboim L, Kringel S, Braun T, Borner C, Stein R. Bak but not Bax is essential for Bcl-xS-induced apoptosis. Cell Death Differ. 2005;12:713–723. doi: 10.1038/sj.cdd.4401638. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neise D, Graupner V, Gillissen BF, Daniel PT, Schulze-Osthoff K, Jänicke RU, et al. Activation of the mitochondrial death pathway is commonly mediated by a preferential engagement of Bak. Oncogene. 2008;27:1387–1396. doi: 10.1038/sj.onc.1210773. [DOI] [PubMed] [Google Scholar]
- Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, Degenhardt K, Kamal A, Zhang J, Yoshida T, Zhang Y, et al. NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated apoptosis in response to protein synthesis inhibition. Genes & development. 2007;21:929–941. doi: 10.1101/gad.1522007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorakis P, Lomonosova E, Chinnadurai G. Critical requirement of BAX for manifestation of apoptosis induced by multiple stimuli in human epithelial cancer cells. Cancer Res. 2002;62:3373–3376. [PubMed] [Google Scholar]
- Topaloglu O, Hurley PJ, Yildirim O, Civin CI, Bunz F. Improved methods for the generation of human gene knockout and knockin cell lines. Nucleic Acids Res. 2005;33:e158. doi: 10.1093/nar/gni160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Haefen C, Gillissen B, Hemmati PG, Wendt J, Güner D, Mrozek A, et al. Multidomain Bcl-2 homolog Bax but not Bak mediates synergistic induction of apoptosis by TRAIL and 5-FU through the mitochondrial apoptosis pathway. Oncogene. 2004;23:8320–8332. doi: 10.1038/sj.onc.1207971. [DOI] [PubMed] [Google Scholar]
- Wei M, Zong W, Cheng E, Lindsten T, Panoutsakopoulou V, Ross A, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science (New York, NY) 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes & development. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Zhai D, Jin C, Huang Z, Satterthwait AC, Reed JC. Differential regulation of Bax and Bak by anti-apoptotic Bcl-2 family proteins Bcl-B and Mcl-1. J Biol Chem. 2008;283:9580–9586. doi: 10.1074/jbc.M708426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- Zmasek CM, Zhang Q, Ye Y, Godzik A. Surprising complexity of the ancestral apoptosis network. Genome Biol. 2007;8:R226. doi: 10.1186/gb-2007-8-10-r226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Clustering of GFP-Bak on mitochondria during apoptosis induced by TRAIL plus 5-FU in Bak−/− and Bax−/−Bak−/− HCT116 cells stablying expressing GFP-Bak. The experiment was performed as in Figure 5E and cells were immunostained with anti-Tom20 antibody. Arrows indicate Bak clustering.