Abstract
Although the central nervous system (CNS) is considered to be an immunoprivileged site, it is susceptible to a host of autoimmune as well as neuroinflammatory disorders owing to recruitment of immune cells across the blood–brain barrier into perivascular and parenchymal spaces. Dendritic cells (DCs), which are involved in both primary and secondary immune responses, are the most potent immune cells in terms of antigen uptake and processing as well as presentation to T cells. In light of the emerging importance of DC traficking into the CNS, these cells represent good candidates for targeted immunotherapy against various neuroinflammatory diseases. This review focuses on potential physiological events and receptor interactions between DCs and the microvascular endothelial cells of the brain as they transmigrate into the CNS during degeneration and injury. A clear understanding of the underlying mechanisms involved in DC migration may advance the development of new therapies that manipulate these mechanistic properties via pharmacologic intervention. Furthermore, therapeutic validation should be in concurrence with the molecular imaging techniques that can detect migration of these cells in vivo. Since the use of noninvasive methods to image migration of DCs into CNS has barely been explored, we highlighted potential molecular imaging techniques to achieve this goal. Overall, information provided will bring this important leukocyte population to the forefront as key players in the immune cascade in the light of the emerging contribution of DCs to CNS health and disease.
Keywords: Dendritic cell trafficking, Lectins and integrins, Blood–brain barrier, Molecular imaging, Neuroinflammation, Microvascular endothelial cells
Introduction
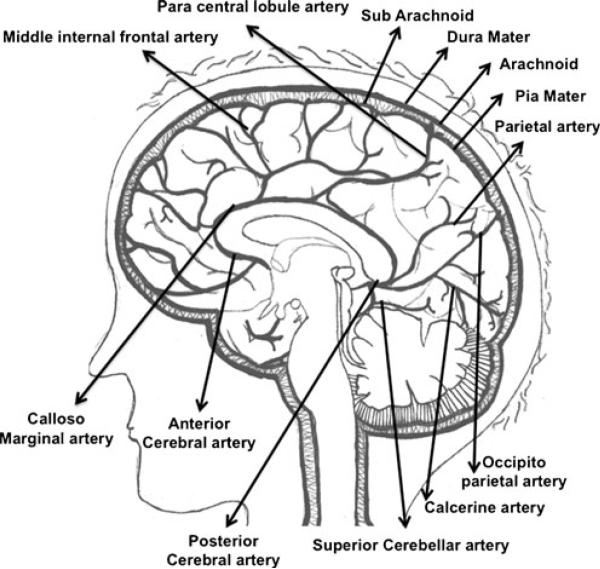
The microvasculature of the normal human brain consists of meningeal, cerebral, and cerebellar arteries. Cerebral and cerebellar arteries can be cortical, subcortical, or medullary depending on the depth of penetration (Nonaka et al. 2002). Thus, the brain consists of a very dense network of blood vessels where every inch of parenchyma is vascularized. The pia mater, the bottom layer of the meninges, is rich in blood vessels and even ensheathes arteries as they enter the cerebral cortex until they begin to disappear in the capillary beds (Patel and Kirmi 2009). This ensheathment is related to the pathways required for the drainage of interstitial fluid, which plays a role in inflammatory responses in the brain. Thus, the entire blood supply of the brain and spinal cord is derived from meningeal arteries as depicted in Fig. 1, and the blood vessels continue to maintain specialized architecture as they branch out and penetrate deeper. This specialization contributes to the immunoprivilege from which the central nervous system (CNS) benefits because it enables physiological functioning of the human brain in a well-controlled environment separate from systemic circulation. The cellular components of these blood vessels are collectively referred to as the blood–brain barrier (BBB) (Abbott et al. 2010; Dallasta et al. 1999). The BBB is a highly resistant barrier consisting of endothelial cells that selectively regulate intracellular and paracellular passage of ions, toxins, cells, water, oxygen, and nonionic molecules including alcohol and certain drugs. During ongoing inflammation in the CNS, BBB permeability is modified and selective passage of these substances is lost. This dysfunction usually occurs through a massive infiltration of immune cells from the blood present in the microvasculature transmigrating into the perivascular and parenchymal spaces of the CNS. Whether this migration is a direct cause or a result of this breach in BBB permeability is still unknown. It is known, however, that these immune cells are able to contribute to progression of disease immunopathogenesis. Hence, targeting the mechanism involved in transmigration of immune cells across the BBB is important from the pharmacologic perspective. Understanding the components of the BBB known to be involved in regulating its properties can help to understand this mechanism.
Fig. 1.
Sagittal section of the human brain revealing midsagittal arterial vasculature. The anterior cerebral artery and middle cerebral arteries (not shown) arise from the internal carotid artery and curve around and above the corpus callosum. The basilar artery (not shown) joins the internal carotids in an arterial ring at the base of the brain called the circle of Willis. The posterior cerebral arteries arise at this confluence. The pia mater is the blood-supply covering of the brain. The pia dips down into the sulci, and send prolongations along with these arteries into the brain (perivascular sheaths). The arteries differentiate into finer capillaries as they occupy deeper subcortical and medullary structures
The neurovascular unit consists of a single microvascular endothelial cell enclosing the circumference of the blood vessel lumen. The unit is also composed of pericytes, found in the perivascular space between the basement membranes that separate the endothelial cell from the astrocytic end feet (Bandopadhyay et al. 2001). Pericytes are able to regulate endothelial proliferation and differentiation, influence capillary blood flow, and synthesize structural constituents of the extracellular matrix (ECM) (Allt and Lawrenson 2001; Balabanov and Dore-Duffy 1998; Thanabalasundaram et al. 2011). Pericytes have recently been implicated as essential for BBB formation and relative vascular permeability during embryogenesis (Daneman et al. 2010). CNS pericytes have been reported to be a source of a number of immunoregulatory cytokines that influence cytokine-mediated endothelial cell activation and leukocyte recruitment (Antonelli-Orlidge et al. 1989; Dore-Duffy et al. 1994; Fabry et al. 1993a, b). Astrocytes are glial cells that are positioned between neurons and pericytes and communicate with these cells via their numerous foot processes. Astrocytes are known to create the necessary brain microenvironment required to induce BBB properties (Janzer and Raff 1987; Schlosshauer 1993). Their contribution to immunopathogenesis via leukocyte recruitment during CNS inflammation is also established (Abbott 2002; Muratori et al. 2010; Prat et al. 2001; Weiss et al. 1998). The astrocyte end feet, along with pericytes and basement membrane, make up the glial limitans perivascularis. Neurons found in proximity to the glial limitans are said to play a role in regulating blood flow as well as BBB permeability (Paemeleire 2002), by signaling through the intervention of the microvascular endothelium and the other components of the unit. The endothelial cells of the unit of the BBB lack fenestrations and are tightly sealed together by complex tight junction (TJ) proteins, which confer high electrical resistance compared with other endothelial cells of the periphery (Crone and Christensen 1981). HIV encephalitis (HIVE), most pronounced in the deep gray matter and cortical white matter, includes alterations in the expression of vascular TJ proteins (Dallasta et al. 1999). Loss of the TJ protein claudin-3 has also been reported in experimental autoimmune encephalomyelitis (EAE), in a mouse model for multiple sclerosis (MS), and in human glioblastoma multiforme tissue (Wolburg et al. 2003). Proinflammatory cytokine-mediated reactive oxygen species production has been shown to downregulate TJ proteins occludin and claudin-5 (Huppert et al. 2010; Schreibelt et al. 2007).
A modification of the BBB that vascularizes the brain ventricular system is the blood-cerebrospinal fluid (BCSF) barrier. The BCSF barrier lies at the choroid plexuses in the lateral, third, and fourth ventricles of the brain where tight junctions are formed between the epithelial cells of the choroid plexus (Abbott 2002; Brown et al. 2004). The endothelial cells of the blood vessel overlying the epithelium of the BCSF barrier are fenestrated, unlike at the BBB. Organs exhibiting such incomplete barriers are also broadly known as circumventricular organs. Other such areas of the brain include the area postrema, pineal gland, subfornical organ, median eminence, and posterior pituitary. The lack of resistance to exchange of substances helps define the role of these organs in functions such as blood flow regulation, easy diffusion of hormones, and detection of toxins and peptides. Both BCSF barriers as well as circumventricular organs have promoted increased immunopathogenesis through immune cell infiltration during neuroinflammation (Schulz and Engelhardt 2005).
Besides endothelial cells of the BBB, another endothelial cell type that has been extensively studied in leukocyte migration is the high endothelial venules (HEVs). These specialized postcapillary venules allow exit of blood cells into secondary lymphoid organs. The role of HEVs with respect to leukocyte trafficking into the CNS has not been well studied. HEV differentiation markers, however, have been shown to be expressed in mice during relapse of EAE (Cannella et al. 1991) at sites away from perivascular spaces that show high inflammatory pathogenesis (Duijvestijn et al. 1988; Raine et al. 1990), indicating their role in immune cell recruitment to the CNS.
The most extensively studied neuroinflammatory model of disease that engages almost all aspects of the BBB that influence immunogenicity in the CNS is EAE. Immune cell migration has proved to be the key to disease pathogenesis in EAE (Alvarez et al. 2011; Jordan et al. 2008; Kielian et al. 2002; Roberts et al. 2010; Wekerle 1993). However, even among immune cells, each subtype contributes toward pathogenesis in varying capacities. DCs have the greatest antigen- presenting capacity among all immune cells. Emerging research from our laboratory has proven DCs as one of the most effective responders to neuroinflammation when compared to other immune cell types, using an in vitro model of the BBB (unpublished observations). Our recent in vivo studies further strengthen this argument (Jain et al. 2010). Hence, it is important to highlight the contribution of DCs to the field of CNS disease. A detailed understanding of DC migration across the transendothelial lumen into the CNS will help focus treatment strategies on established targets and mechanisms. This review provides an up-to-date account of work that has been conducted on the migration of DCs from the endothelial lumen across the BBB into the CNS, highlighting areas that need further understanding.
Dendritic cells and CNS
Under inflammatory conditions of the CNS such as in MS or HIVE, DCs along with other circulating lymphocytes and monocytes/macrophages readily gain access to the CNS, resulting in edema, further inflammation, or demyelination. DCs are the most potent antigen-presenting cells (APCs), leading to the activation of naive Tcells in secondary lymphoid organs (Matsuno et al. 2010; Villablanca et al. 2008). In steady-state conditions, DCs are found in low numbers in the meninges, choroid plexus, and CSF (Pashenkov et al. 2001), possibly because the BBB limits penetration of immune cells into the brain parenchyma. During neuroinflammation, however, these DCs have been shown to infiltrate the CNS in larger numbers to the meninges (pia mater cerebellum and inner brain stem), periventricular (lumen of lateral ventricle, third ventricle), perivascular (close to cuffed blood vessels), and parenchymal (molecular layer of brain stem and cerebellum) spaces (Hatterer et al. 2008). Immunophenotyping of human CSF mononuclear cells has shown that they contain both types of peripheral blood DCs (myeloid and plasmacytoid) (Pashenkov et al. 2001). Thus, emerging research shows the growing importance of infiltration of peripheral blood DCs into the CNS during neuroinflammation. Microglia, the resident immune cells of the CNS, also assume the classic DC-like CD11c+ phenotype and are present in brain parenchyma and juxtavascular regions (Prodinger et al. 2011). These cells are derived from myeloid precursors, which colonize the CNS during embryonic development. While there is some evidence showing that DCs are essential APCs in the context of autoimmune disease progression (Bailey et al. 2007; Greter et al. 2005), the importance of DCs relative to microglia in viral clearance has recently been noted by researchers who ablated peripheral DCs in mice and infected them with a non-fatal encephalitic viral load (Ciavarra et al. 2006; Steel et al. 2009). Ablation of peripheral DCs profoundly inhibited the viral uptake and presentation to induce virus-specific CD8+ T cells needed for viral clearance. Delayed viral clearance in the brain depleted of DCs correlated with decreased survival in these mice. The infiltration of DCs from the periphery during neuroinflammatory autoimmunity has been studied particularly in EAE models of MS. Studies indicate that DCs are capable of perpetuating CNS-targeted autoimmunity when antigens are readily available, but other APCs (B cells, microglia) may be required to efficiently initiate pathogenic CD4 T cell responses (Wu et al. 2011). In studies using transgenic mice in which APC capacity is restricted only to peripheral blood DCs, peripheral DCs seem to be sufficient to reactivate myelin-specific T cells in order to initiate EAE (Greter et al. 2005). The number of DCs infiltrating from the blood increases with increasing clinical severity of EAE. In fact, evidence shows that they interact with naive CD4+ T cells driving Th17 differentiation, a T-cell subset involved in chronic inflammatory disease (Bailey et al. 2007). The most compelling evidence of DC migration into the CNS comes from recent intravital videomicroscopy showing in vivo DC migration across inflamed spinal cord white matter micro-vasculature in EAE mice (Jain et al. 2010). The multi-step sequence of events associated with immune cell transmigration during neuroinflammation, including the role of DCs as antigen presenting cells in autoimmunity, is potrayed in Fig. 2. Although the site for antigen presentation is often debated, owing to the absence of lymphatics in the CNS, there are indications that these DCs are known to both drain into the cervical lymph node (CLN) during onset of inflammation and also stay in situ in brain parenchyma (Bailey et al. 2007; Hatterer et al. 2008). These findings suggests that DCs can indeed operate as major antigen-presenting cells once recruited within the CNS during inflammation and infection.
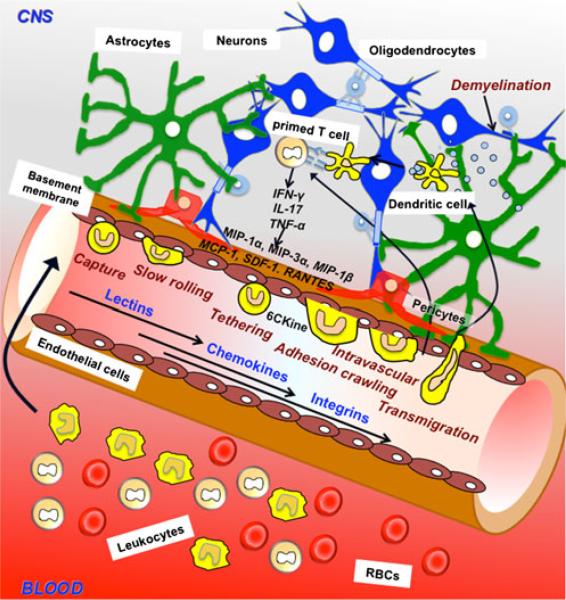
Fig. 2.
Dynamic interaction between the immune system and the blood–brain barrier (BBB). Lymphocytes are able to migrate to the central nervous system through the processes of tethering, rolling, adhesion, and transmigration through the BBB during neuroinflammation. Dendritic cells (DCs) are believed to undergo the same process but the mechanism by which the transmigration happens remains to be investigated. The circumference of the capillary lumen is completely surrounded by a single endothelial cell. Pericytes are attached to the abluminal surface of the endothelial cell, and these two cell types are surrounded by the basal lamina, which is contiguous with the plasma membranes of astrocyte end-feet and endothelial cells. Neurons also populate the area surrounding the unit. In diseases like multiple sclerosis, there is an infiltration of myelin specific T cells from systemic circulation into the CNS resulting in demyelination and increase in proinflammatory cytokines IFN-γ, TNF-α and interleukin-17 (IL-17). The components of the neurovasculature are stimulated by these cytokines and may secrete their own chemokines such as MCP-1, MIP-1, RANTES, SDF-1, CCL19, CCL20, CCL21. DCs bearing receptors for these chemokines become attracted at the vasculature and will follow the same multistep cascade as the T cells in order to transmigrate to sites of lesion. Once in the parenchymal and perivascular spaces, they may be involved in uptake and presentation of myelin peptides to T cells, that secrete more proinflammatory cytokines, thereby augmenting inflammation
Evidence is emerging for the importance of DC migration in CNS infection and inflammation and of the mechanistic interactions of DCs with the microvasculature of the BBB in terms of receptors to which they bind and chemokines to which they respond. Information on the migratory properties of DCs will help us understand their subsequent phenotype and function in the CNS. Moreover, targeting the mechanisms involved in DC trafficking can be useful in selectively exploiting migration in order to potentiate cancer immunotherapeutic strategies such as vaccination or to attenuate autoimmunity and subsequent neuroinflammation.
Chemoattraction of DCs at the BBB
The BBB strictly controls the movement of immune cells into the CNS thereby making the brain an immunoprivileged organ. However, under pathologic conditions such as viral or bacterial infections or during inflammatory disease such as MS, DCs along with other immune cells (T cells, monocytes, macrophages) readily traverse the BBB and subsequently enter the perivascular, CSF and parenchymal regions of the brain. They exist endogenously in both immature as well as mature states. One key difference in DC maturation includes differential chemokine receptor expression and its resulting response to receptor associated chemokines. Certain combinations of chemokines and cytokines, based of the state of DC maturation effectively recruit DCs from the blood to the site of the BBB (Dieu et al. 1998).
Immature DCs
Immature DCs express C-C Chemokine Receptor 2, 3, 5 (CCR2, CCR3 and CCR5) and thereby are shown to migrate in the presence of their respective ligands including C-C Chemokine Ligand 2 [Monocyte Chemotactic Protein-1 (MCP-1, a.k.a. CCL2)], C-C Chemokine Ligand 3 [Macrophage Inflammatory Protein (MIP-1α, a.k.a. CCL3)], and C-C Chemokine Ligand 5 [Regulated Upon Activation, Normal T-cell Expressed and Secreted (RANTES a.k.a. CCL5)] in mice as well as in primates (Barratt-Boyes et al. 2000; Zozulya et al. 2007). In vitro treatment of brain endothelial cells with Tumor Necrosis Factor-alpha (TNF-α), interleukin-1β (IL-1β), lipopolysaccharide (LPS), and a combination of TNF-α and interferon-γ (IFN-γ), but not IFN-γ alone, significantly upregulated the expression and release of CCL2 and CCL3 in a time-dependent manner (Chui and Dorovini-Zis 2010). A substantial level of CCL2 expression was also observed late in acute disease and continued to be evident in the relapsing phase of the disease. CCL2 expression correlated with increasing severity of clinical relapses (Kennedy et al. 1998). When chemokine production during EAE was evaluated, CCL2 and CCL5 levels were found to be high during the onset of EAE induction, peaking at the time of most severe phenotype. Inhibiting production of these chemokines results in inhibited adherence and migration of leukocytes to endothelium as seen by intravital videomicroscopy (dos Santos et al. 2005). C-C chemokine expression in the CNS throughout the entire course of EAE showed that the production of CCL3 correlated with increasing acute disease severity and remained elevated throughout chronic and relapsing disease (Kennedy et al. 1998). Therefore, release and presentation of CCL2, CCL3 and CCL5 surrounding cerebral endothelium suggests an important role for these chemokines in regulating the trafficking of DCs across the BBB in CNS inflammation. Indeed astrocytes, an important component of the neurovascular unit, have been shown (in vitro) to produce CCL2, CCL3, CCL4, CCL5, CCL20, and CXCL12, which are chemoattractive to immature DCs (Ambrosini et al. 2005). A desensitization of immature DC receptor CCR5 to by treatment with high doses of CCL5 showed reduced migration of these cells towards inflamed areas and they do not exhibit considerable APC capacity (Ambrosini et al. 2005).
Mature DCs
In marked contrast to immature DCs, mature DCs lack CCR5 but have upregulated CCR7. CCR7 responds to chemokines C-C Chemokine Ligand 19 (CCL19) and 6Ckine (CCL21). Thus, mature but not immature DCs transmigrate in the presence of these chemokines (Barratt-Boyes et al. 2000; Ricart et al. 2011; Zozulya et al. 2007). Recently, CCL21 by itself has been deemed sufficient to mediate mature DC migration (Britschgi et al. 2010). There is also evidence for CXCR4 upregulation in mature DCs independent of CCR7 receptor presence. This indicates that CXCL12, the endogenous ligand for CXCR4, can direct migration of mature DCs as well (Ricart et al. 2011). Upregulation of chemokines CCL19 and CCL21 have been further implicated in stimulating DCs to produce IL-23. This further facilitates IL-23 dependent differentiation of Th17+ cells that promote EAE induction (Kuwabara et al. 2009). These data suggest that mature DCs contribute to autoimmune disease pathology, with the help of receptors responding to these two chemokines. Table 1 summarizes various chemokines, their receptors, and their implications in CNS disease.
Table 1.
Chemokines, receptors, and their implications in CNS disease
| Chemokine | Receptor | Source (NVU) | CNS disease implications |
|---|---|---|---|
| Immature DCs | |||
| MCP-1 (CCL2) | CCR2, CCR4 | Astrocytes (Conant et al. 1998); MVECs (Chui and Dorovini-Zis 2010); Microglia (Zhou et al. 2008) | MS (Dos Santos et al. 2008); NeuroAIDS (Eugenin et al. 2006); Brain injury (Glabinski et al. 1996); Alzheimer's disease (Jaeger et al. 2009); Parkinson's (Reale et al. 2009); Bacterial meningitis (Pashenkov et al. 2002); LCMV (Zhou et al. 2008); HSV-1 encephalitis (Vilela et al. 2009) |
| MIP-1α (CCL3) | CCR1, CCR5 | Astrocytes (Ambrosini et al. 2005); Neurons (Xia et al. 1998) | Viral infection (Ireland and Reiss 2006); MS (Simpson et al. 1998); HSV-1 encephalitis (Vilela et al. 2009); Alzheimer's (Smits et al. 2002); Ischemic stroke (Kim et al. 1995); Bacterial meningitis (Diab et al. 1999) |
| RANTES (CCL5) | CCR1, CCR3, CCR5 | Astrocytes (Ambrosini et al. 2005); Neurons (Ovanesov et al. 2008); MVECs (Shukaliak and Dorovini-Zis 2000) | Viral infection (Chen et al. 2011); MS (Boven et al. 2000); SCI (Lin et al. 2011); NeuroAIDS (Vago et al. 2001); HSV-1 encephalitis (Vilela et al. 2009); Alzheimer's (Tripathy et al. 2010) |
| MIP-1β (CCL4) | CCR5 | Astrocytes (Ambrosini et al. 2005); MVECs (Shukaliak and Dorovini-Zis 2000) | Viral infection (Ireland and Reiss 2006); Alzheimer's (Smits et al. 2002); Axonal injury (Babcock et al. 2003); MS (Boven et al. 2000); Ischemic stroke (Gourmala et al. 1999); Alzheimer's disease (Xia et al. 1998) |
| MIP-3α (CCL20) | CCR6 | Astrocytes (Ambrosini et al. 2003) | MS (Ambrosini et al. 2003); Ischemic stroke (Terao et al. 2009); Bacterial infection (McKimmie and Graham 2010) |
| SDF-1 (CXCL12) | CXCR4, CXCR7 | Astrocytes (Ambrosini et al. 2005); Pericytes (Seo et al. 2009); MVECs (Liu and Dorovini-Zis 2009) | Ischemic stroke (Hill et al. 2004); MS (Cruz-Orengo et al. 2011); Lymphoma (Venetz et al. 2010); Bacterial meningitis (Pashenkov et al. 2002); Brain tumor (Li and Ransohoff 2009) |
| Mature DCs | |||
| MIP-3β (CCL19) | CCR7, CXCR3 | Astrocytes (Columba-Cabezas et al. 2003) | MS (Alt et al. 2002; Krumbholz et al. 2007) |
| 6Ckine (CCL21) | CCR7, CXCR3 | Neurons (Biber et al. 2001); MVECs (Columba-Cabezas et al. 2003) | MS (Alt et al. 2002); Ischemic stroke (Biber et al. 2001) |
It is being established that immature DCs possess greater migratory potential across the BBB than mature DCs. Possibly mature DCs lose the ability to be chemoattracted by CNS-emitted chemokines that are known to act exclusively on immature DCs, and gain CCR7 receptor that is required to present antigen in areas rich in T cells (Bianchi et al. 2000). Just as DCs mature upon reaching lymph nodes after attraction and emigration of their immature form through lymphatics in the periphery, maturation of DCs and subsequent antigen presentation to T cells might take place in situ in brain tissue after chemotactic attraction of immature DCs across the BBB. For example, DCs may follow the same transmigratory path as monocytes in the presence of the CCL2 ligand at the endothelial vasculature. CCL2 is a chemoattractant to monocytes known to recruit them via CCR2 binding (Rollins 1996). When monocytes were characterized for maturation and differentiation during transmigration across the BBB (Buckner et al. 2011), it was found that only monocytes that have begun the process of maturation bind firmly to endothelium in the presence of CCL2 and are able to transmigrate across the BBB. Once transmigrated, they fully mature into macrophages capable of antigen presentation (Buckner et al. 2011; Geissmann et al. 2010). There is additional evidence that monocytes can differentiate into DCs during transendothelial migration (Geissmann et al. 2010; Ifergan et al. 2008; Randolph et al. 1998). Recent unpublished work from our laboratory has shown that DCs can traffic across the brain endothelium monolayer in vitro at much higher rates than can other immune cell types (including monocytes) in the presence of CCL2, one of the key chemoattractive stimuli released in neuroinflammation.
Adhesion and recruitment of DCs to endothelial basement membrane
DCs interact with blood vessel endothelial cells during recruitment from the blood into the CNS. It has been suggested that DCs may express “homing receptors,” similar to those of T cells, for certain endothelia (Austyn et al. 1988). A multistep paradigm for leukocyte trafficking was elucidated to suggest migration from blood into tissues as tightly regulated and mediated by a multistep process involving four steps: 1) leukocyte tethering and rolling; 2) rapid activation by chemokines; 3) firm adhesion to endothelial proteins; and 4) diapedesis or transmigration (Springer 1994). Immature DCs migrate from the blood into peripheral tissues either to replenish resident DCs or in response to inflammatory signals. As illustrated in Fig. 3, DCs, like other leukocytes, may undergo chemoattraction and activation due to chemokines from the inflamed neurovasculature; tethering and rolling by upregulating selectins and DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3 grabbing non-integrin); firm adhesion by upregulating integrins; enabling eventual transmigration using DC-SIGN. Intercellular adhesion molecules (ICAMs), vascular cell adhesion molecules (VCAMs), platelet-endothelial cell adhesion molecules (PECAMs), and other TJ proteins on endothelial cells facilitate binding of DCs to endothelium. Table 2 summarizes the ligands and receptors involved in DC migration during the multistep cascade.
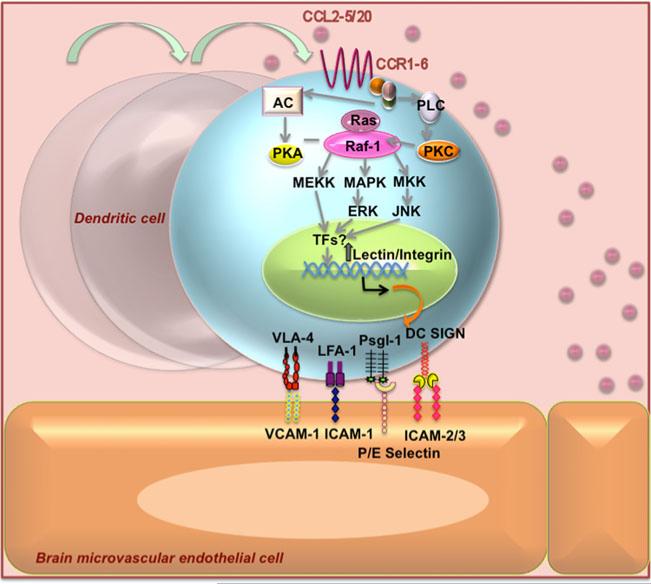
Fig. 3.
Postulated chemokine dependent regulation of lectin and integrin receptor/ligand expression on immature Dendritic Cells and subsequent binding to brain endothelium. Chemokines CCL2-6 secreted from neurovascular unit of the blood brain barrier activate their respective G protein coupled receptors-CCR1-5/20 that can potentially be involved in increased transcription of genes encoding for lectins and integrins that can potentiate the adhesion and transmigration process. The molecules that participate in DC adhesion to endothelial cells are integrins (LFA-1 and VLA-4/5) and lectins (DC-SIGN). DCs interact with intercellular adhesion molecules ICAM-2 and ICAM-3, vascular cell adhesion molecule VCAM-1 and P/E-selectins present in the surface of endothelial cells, allowing and strengthening DC adhesion
Table 2.
Ligands and receptors involved in dendritic cell-endothelial interaction during tethering, rolling and transmigration
| Ligands/receptors |
References | ||
|---|---|---|---|
| Dendritic cell | Endothelium | ||
| Tethering/rolling (lectins) | PSGL-1 | P/E selectin | (Figdor 2003; Geijtenbeek et al. 2000a; Hess et al. 1996; Kanda et al. 1995; Kieffer et al. 2001; Pendl et al. 2002) |
| L-selectin | SGPG | ||
| DC-SIGN | ICAM-2/3 | ||
| Adhesion (integrins) | β1: VLA-4 (CD49d), VLA-5 (CD49e) | VCAM-1 | (Arjmandi et al. 2009; Brown et al. 1997; D'Amico et al. 1998; Jain et al. 2010; Yusuf-Makagiansar et al. 2002) |
| β2: CD11a, CD11b (Mac-1), CD11c, LFA-1 | ICAM-1 | ||
| Transmigration (CAMs and TJs) | CD18 (β2 integrin subunit), JAM-A, L1CAM, ZO-1, L1CAM, claudins | ICAM-1, PECAM, JAM-A, L1CAM, ZO-1, claudins | (Cera et al. 2004; Muller and Randolph 1999; Ogasawara et al. 2009; Wojcikiewicz et al. 2009) |
Tethering and rolling
Tethering and rolling is the first step in the transmigration cascade resulting from recruitment by chemokines and functional immune surveillance.
Receptors and ligands on DCs
PSGL-1
It was shown that in Tcells, Cutaneous Lymphocyte-associated (CLA) Antigen is sometimes present and occurs almost exclusively on the protein backbone of P-Selectin Glycoprotein Ligand-1 (PSGL-1) (Engelhardt et al. 2005). Tcells exhibiting the CLA isoform of PSGL-1 can tether and roll on both E- and P-selectin decorated endothelium, whereas Tcells expressing PSGL-1 without the CLA epitope do not bind E-selectin, though they may bind P-selectin. Circulating neutrophils, monocytes and cultured blood dendritic cells have also been shown to express CLA almost entirely as an isoform of PSGL-1 (Kieffer et al. 2001). These cells tether and roll on both E- and P-selectin. By comparing the selectin-binding capacity of the different DC subpopulations, it was confirmed that only CLA-expressing DCs bind P and E selectin (Schakel et al. 2002). A role in immunosurveillance has also been attributed to CLA/PSGL-1 ligand which was found to roll continuously on noninflamed endothelium in vivo mediated by P- and E-selectin binding (Robert et al. 1999). Conversely, emigration of immature DCs into inflamed tissue was retained in the presence of a PSGL-1 blocking antibody (Pendl et al. 2002), suggesting that other ligands may be involved in homing to P- and E-selectins on endothelium.
L-selectin
A type of cell adhesion molecule belonging to the selectin family known as L-selectin (CD62L) is expressed on all circulating leukocytes, except for a subpopulation of memory T lymphocytes. Increased expression of sulfoglucuronosyl paragloboside (SGPG), a ligand to L-selectin on DCs and other leukocytes, was shown to occur upon treatment of brain microvascular endothelial cells (BMVECs) with interlukin-1 beta (IL-1β) (Kanda et al. 1995), potentially allowing increased DC binding during inflammation. Soluble L-selectin has been found in serum and the CSF of patients with MS, suggesting that L-selectin on leukocytes and possibly DCs adhere to BMVECs via an appropriate ligand, transmigrate, and shed from the cell surface after adhesion as circulating soluble receptors (Mossner et al. 1996).
DC-SIGN
DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin), a C-type lectin, has been found to be exclusively expressed on monocyte-derived DCs and not on monocytes, activated monocytes, monocytic cell lines, granulocytes, Tcells, B cells, activated B and T cells, thymocytes, or CD34+ bone marrow cells. DC-SIGN was first identified as a DC-specific marker that binds with high affinity to ICAM-3 present on resting T cells. DCs generated in vitro abundantly express DC-SIGN at day 7, and flow cytometric analysis with anti-DC-SIGN antibodies demonstrates that DC-SIGN is expressed by both immature and mature DCs (Arjmandi et al. 2009; Geijtenbeek et al. 2000b). Researchers have proposed that DC-SIGN efficiently captures HIV-1 in the periphery and facilitates its transport to secondary lymphoid organs rich in T cells, to enhance infection in trans of these targeted cells (Geijtenbeek et al. 2000a). DC-SIGN also mediates the tethering and rolling of cells along ICAM-2-expressing surfaces. ICAM-2 is expressed constitutively on the endothelium of both blood and lymphatic vessels, as well as on high endothelial vascular cells and leukocytes. DC-SIGN also plays a part in DC trafficking because it binds not only ICAM-2 but also ICAM-3 expressed by many endothelial cells, supporting tethering and rolling of DCs on endothelium as well as chemokine-induced transmigration of DCs across both resting and activated endothelium in vitro (Figdor 2003; Geijtenbeek et al. 2000b). Immature DC adhesion to activated human brain microvascular endothelial cells (HBMECs) was significantly downregulated with primary blocking antibodies against ICAM-2 (down to 60%) and DC-SIGN on immature DCs (down 49%) (Arjmandi et al. 2009). Finally, DC arrest, preceeding transendothelial intravasation, was shown to be mediated by integrin (Leukocyte Function-associated Antigen-1 [LFA-1]) interactions with the endothelium (van Kooyk and Geijtenbeek 2002).
Receptors and ligands on brain endothelium
P- and E-selectins
The selectin family of adhesion molecules has N-terminal domains homologous to calcium-dependent lectins (Bevilacqua and Nelson 1993; Rosen 1993). Selectins are responsible for the initial tethering of a flowing leukocyte to the vessel wall and for labile, rolling adhesions. Circulating DCs first need to tether to endothelial cells through the interaction of E- and P-selectins with their respective ligands. Little is known about DC-brain endothelium in terms of selectin interaction; however, these components have been found to exist on both DCs and brain endothelium. P-selectin was found to be stored in the Weibel-Palade bodies of endothelial cells and the granules of platelets (Bonfanti et al. 1989). P-selectin has been found in BMVECs and is upregulated during focal cerebral ischemia/reperfusion, which may contribute to enhanced leukocyte adherence and persistent activation (Okada et al. 1994).
The domain organization of P-selectin was shown to be strikingly similar to two other cell surface structures: ELAM-1 (E-selectin/CD62E) and LAM-1 (L-selectin/ CD62L) (McEver 1990). These “selectins” constitute a new gene family of receptors with related structure and potentially related function. Exon duplication and rearrangement play a role in generating this family of proteins that facilitate cellular interactions during inflammation (Johnston et al. 1990). E-selectin is induced on vascular endothelial cells by cytokines such as IL-l (Barkalow et al. 1996; Kanda et al. 1995), LPS (Hess et al. 1996; Wong and Dorovini-Zis 1996), or TNF (Barkalow et al. 1996; Hess et al. 1996; Wong and Dorovini-Zis 1996) and requires de novo mRNA and protein synthesis. No basal expression of E-selectin was found in human BMVECs (Hess et al. 1996). Presence of elevated levels of soluble E-selectin has been suggested as an indicator of endothelial cell damage in patients with MS (Tsukada et al. 1995). One study determined that patients with primary progressive MS had significantly increased soluble E-selectin concentrations (shed after leukocyte tethering) compared with patients with relapsing-remitting and secondary progressive disease, who had normal E-selectin concentrations (Giovannoni et al. 1996; McDonnell et al. 1999). Like P-selectin, E-selectin has been shown to upregulate during focal ischemic brain injury (Wang and Feuerstein 1995; Zhang et al. 1996) and also in the presence of LPS-activated HIV-infected monocytes. Other studies showed that microRNA constructs miR-E1 and miR-E2 complementary to the human E-selectin cDNA can be used to suppress E-selectin expression (Nottet et al. 1996; Yoshizaki et al. 2008). Thus, E-selectin can be silenced to inhibit leukocyteendothelial adhesive interactions, during inflammatory conditions.
Little conclusive information exists about ligands on DCs that bind to P- and E-selectins. However, these selectins have been shown to be important retention of immature DCs to sites of inflamed tissue (Pendl et al. 2002). Interestingly, antibodies directed against E- and P-selectin that inhibited the recruitment of TH1 cells into inflamed skin in vivo did not influence recruitment of inflammatory cells into the CNS during EAE (Engelhardt et al. 1997). A role for P and E-selectins in development of EAE in mice has also been ruled out because E/P-selectin-deficient SJL and C57BL/6 mice develop clinical EAE indistinguishable from wild-type mice (Doring et al. 2007). One study showed that blocking E-selectin and its ligand s-Lex did not affect immature DC adhesion to activated MVECs (Arjmandi et al. 2009). P- and E-selectin are probably involved only in immune surveillance (Robert et al. 1999) and very early stages of recruitment (Carrithers et al. 2000; Engelhardt et al. 1997) and may not be necessary or sufficient for all immune cell trafficking. Most studies have been carried out with T cells and macrophages, and it remains to be seen whether blood DCs interact with P/E-selectin on brain endothelium in the same manner.
Firm adhesion
The second and most extensively studied step in the cascade, firm adhesion is essential for homing DCs onto the endothelium to facilitate the process of transmigration.
Receptors and ligands on DCs
Integrins
Chemoattractants bind to transmembrane receptors on the surface of leukocytes. These couple to cytosolic G proteins which transduce signals that activate integrin adhesiveness. The integrins can then bind to immunoglobulin-SF (IgSF) members on the endothelium, increasing adhesiveness and resulting in arrest of the rolling leukocyte. Following directional cues from chemoattractants and using integrins for traction, leukocytes then cross the endothelial lining of the blood vessel and enter the tissue (Springer 1994). Firm adhesion between DCs and endothelial cells is dependent on the engagement of chemotactic receptors and subsequent integrin activation on DCs. Human DCs express the β2 integrins LFA-1, Mac-1 and p150; and the β1 integrins very late antigen VLA-4 and VLA-5, which mediate their binding to both resting and activated endothelial cells and to endothelial cell–derived ECM (D'Amico et al. 1998).
The identification of adhesion molecules involved in the binding to resting endothelial cells was performed by using functional blocking by monoclonal antibodies. Anti-CD11a, anti-CD11b, and anti-CD18 antibodies partially inhibited adhesion (Brown et al. 1997). Anti-VLA-4 alone did not inhibit adhesion but the combination of anti-CD18+ anti-VLA-4 was more inhibitory compared with anti-CD18 alone. These results show the important role of VLA-4 in leukocyte adhesion. Antibody blocking studies demonstrated that DC binding to untreated and TNF-α-treated endothelium was dependent upon the expression of CD11a, CD18, and CD49d on DCs, and the simultaneous application of anti-CD18 and anti-CD49d antibodies produced an approximate 70% inhibition of adhesion. Thus, the expression of both β1 and β2 integrins contributes to the adhesive interaction between DCs and endothelium. When neutralizing antibodies were also applied against both endothelial cell adhesion molecules and their ligands, adhesion of immature DCs was significantly downregulated by blocking their ICAM-1/CD18 and VCAM-1/VLA-4 interactions (Arjmandi et al. 2009).
Blocking α4 integrins interfered with the adhesion but not the rolling or capturing of immature and LPS-matured DCs to the CNS microvascular endothelium, inhibiting their migration across the vascular wall (Jain et al. 2010). Functional absence of β1 integrins but not of β7 integrins or α4β7 integrin similarly reduced the adhesion of immature DCs to the CNS microvascular endothelium, demonstrating that α4β1 but not α4β7 integrin mediates this step of immature DCs’ interaction with the inflamed BBB during EAE.
L1CAM
The adhesion molecule L1, found on glia and extensively characterized in the nervous system (Dahme et al. 1997; Kenwrick et al. 2000), is also expressed in cells of myeloid origin such as DCs (Maddaluno et al. 2009; Pancook et al. 1997), but its function there has remained elusive. To address this issue, L1CAM expression was ablated in DCs of conditional knockout mice. L1CAM-deficient DCs were impaired in adhesion to and transmigration through monolayers of either lymphatic or blood vessel endothelial cells, implicating L1CAM in transendothelial migration of DCs (Maddaluno et al. 2009) The interaction of L1CAM with homophilic and heterophilic receptors on brain microvasculature has yet to be examined.
Receptors and ligands on brain endothelium
Endothelial cell adhesion molecules (eCAMs)
Using a well-established in vitro model of the human BBB, it has been previously demonstrated that unstimulated human brain endothelial cells constitutively express low levels of ICAM-1 and barely detectable VCAM-1 (Wong and Dorovini-Zis 1992, 1995). However, activation with bacterial LPS or cytokines maximally upregulates VCAM-1 and ICAM-1. Evidence for the colocalization of VCAM-1 at sites of ICAM-1 clustering has been seen before on endothelial cells treated with TNF-α, suggesting upregulation during inflammation. Biochemical pull-down assays showed that ICAM-1 clustering induced its association to VCAM-1, suggesting a physical link between these two adhesion molecules. CAMs on endothelia play an important role in T-cell adhesion and migration across HMVEC monolayers (Wong et al. 1999). It was shown that T-cell crawling to sites permissive for diapedesis across BBB endothelium is mediated by endothelial ICAM-1 and ICAM-2. An increase in DC trafficking has been shown to require integrin-dependent adhesion to ICAM-1 and VCAM-1 (Steiner et al. 2010), expressed on inflamed lymphatic endothelium. Increasing evidence shows, however, that DC trafficking via cell adhesion molecules is integrin independent, suggesting another ligand is responsible for ICAM-1/VCAM-1 interaction. Integrin interaction and DC interaction with these CAMs on brain endothelial cells remain to be explored.
Transmigration
Transmigration is the final step in the cascade, in which dendritic cells squeeze through the basement membrane to enter the perivascular space of the CNS.
Receptors and ligands on DCs
Integrin-mediated podosomes
Podosomes are assembled actin-rich structures on mature DCs that are thought to be necessary, through the integrin β2 (CD18) subunit, for tight adhesion to ICAM-1 (Burns et al. 2004). As maturation progresses, DCs once again become rounded and devoid of podosomes. In immature DCs, failure to form podosomes or selective inhibition of the CD18 component of podosomes resulted in a similarly reduced ability to adhere to ICAM-1, indicating that podosomes, through CD18, are necessary for tight adhesion to this ligand. Thus, directional cell protrusion due to podosome assembly during DC maturation also suggests that it may be a critical step toward early transmigration through endothelium.
Receptors and ligands on brain endothelium
PECAM, JAM-A, and other junctional proteins
Platelet Endothelial Cell Adhesion Molecule (PECAM/CD31) is expressed on the surfaces of transmigrating leukocytes and concentrated at the borders of endothelial cells. PECAM is capable of homophilic interaction and subsequent transmigration. Blockade of PECAM function selectively blocked transendothelial migration but had no effect on adhesion of leukocytes to the apical surface of endothelium, nor did it interfere with the ability of leukocytes to move. Its only effect is on migration across the endothelial junction (Muller and Randolph 1999). Monocyte transmigration has been shown to be a result of accumulation of endothelial PECAM-1 (Hashimoto et al. 2011). There can be little doubt that transmigration of DCs involves engagement of PECAM-1 on the brain endothelium.
Similarly, Junctional Adhesion Molecule-A (JAM-A), a 32-kDa transmembrane glycoprotein belonging to the immunoglobulin superfamily of proteins, is expressed at the intercellular junctions of epithelial and endothelial cells, may have a role in binding leukocytes and in directing their transmigration through endothelial junctions, both by homophilic binding and by linking integrin LFA-1 (Cera et al. 2004; Ostermann et al. 2002). JAM-A was first identified in 1998 as a novel protein that is selectively concentrated at intercellular junctions of endothelial and epithelial cells of different origins (Martin-Padura et al. 1998). A monoclonal antibody directed to JAM-A was found to inhibit spontaneous and chemokine-induced monocyte transmigration through an endothelial cell monolayer in vitro. JAM-A consists of an intracellular PDZ-domain binding motif, a transmembrane segment, and two extracellular immunoglobulin (Ig) domains. The PDZ-domain binding motif has been shown to associate with the TJ components in occludin, zonula occludens 1 (ZO-1), and cingulin and is involved in cell signaling. During leukocyte migration, the homophilic transendothelial interactions between these receptors must be disrupted to enable a migrating leukocyte to pass through the junction. Recently JAM-A expression has been found to be localized not only at tight junctions of endothelial and epithelial cells but also on circulating leukocytes and DCs (Ogasawara et al. 2009), implying that homophilic binding could also occur between DCs and the junctional molecule on endothelium. However, the idea that the presence of LFA-1 on DCs can compete with JAM-A homophilic interactions across cell junctions to weaken these junctions and allow transmigration (Wojcikiewicz et al. 2009) seems plausible. L1CAM found on DCs is also found on endothelial cells under inflammatory conditions and could be an example of another homophilic interaction to enable transmigration across the BBB (Maddaluno et al. 2009). In addition, TJ proteins ZO-1, ZO-2, and claudin-4, -7, -8, and −9 were all detected on DCs (Ogasawara et al. 2009). We speculate on whether expression of TJ proteins on mature DCs may be needed to help them transmigrate easily across the BBB.
Potential in vivo imaging tools to study DC trafficking into the CNS
From the clinical standpoint as well as for capture of inflammatory disease timecourse measurements, noninvasive methods are desirable for imaging migration of leukocytes into areas of infection and inflammation. A host of imaging tools has been developed over the past decade for these types of measurements. For example, near-infrared fluorescence (NIRF) and bioluminescence imaging (BI) can now be routinely performed using standard optical imaging systems for rodents such as the Caliper Lifesciences IVIS (detailed below), Kodak Image Station-XF and LI-COR Bioscience Pearl Impulse imager (NIRF only). These systems acquire largely 2D data on optically engineered cells or fluorescent probe distribution. BI is exquisitely sensitive in small animals and is routinely used to detect gene transcription within engineered cells or micrometastasis within any tissue long before those cells are detectable using other modalities. One of the biggest drawbacks of the optical systems, however, is lack of depth penetration for photons of decreasing wavelength (a few millimeters tissue depth) leading to non-tomographic and semi-quantitative data, especially true for fluorescene-based imaging. Magnetic resonance imaging (MRI) can generate very high-resolution images of contrast-enhanced cellular migration in the context of spatial localization, providing good contrast between the soft tissues of the body for detailed visualization of internal structures. With MRI, however, sensitivity can be a limiting factor as is the mode of contrast. With T1-type relaxation probes, Gd3+ is the most frequently used contrast material to influence the spin of water protons surrounding the probe while Fe2O3, magnetite, directly and more potently contributes to T2* relaxivity and is employed most commonly as in vitro phagocytosed nanoparticle beads for cell tracking (Balagopalan et al. 2011; Kraitchman et al. 2005; Lange et al. 2005). A balance between sensitivity, non-invasiveness, generation of tomo-graphic data and resolution exists in the form of nuclear imaging in which leukocytes may be directly labeled on the cell surface in vitro or repeatedly labeled in vivo with specific radiolabeled antibodies or small molecule ligands or internally labeled with radiolabeled particles for phagocytosis prior to administration to the animal model. These imaging techniques are widely used in nuclear medicine and are generally referred to as positron emission tomography (PET) and single-photon emission computed tomography (SPECT). PET is capable of generating three-dimensional images of the migration of leukocytes in both humans and animal models. In the context of leukocyte recruitment at the BBB during neuroinflammation, direct observation of subcellular microcirculation by imaging methods is impaired because brain and spinal cord are obscured by the skull and spinal column, respectively. For these purposes, videomicroscopy of the surgical cranial window has been developed for animals that allows observation of the CNS microcirculation in real time. Use of this technique, despite its invasive nature, is important for studying leukocyte interaction with the CNS microvasculature. Further, advancement in intravital imaging, namely invention of two-photon videomicroscopy, has enabled better visualization of immune cell recruitment. Here we will discuss the utility of these tools in studying DC trafficking into the CNS.
Molecular imaging for DC based therapeutic intervention
Leukocyte interaction with target sites has traditionally been visualized using histologic staining of animal tissues. However, noninvasive imaging tools are increasingly used for improved clinical translation. Dynamic migration of DCs can also be visualized using these noninvasive methods. DC-based therapies have shown promise in recent years for diseases such as cancer, autoimmune conditions, neurodegeneration, and stroke. The first therapeutic cancer vaccine Sipuleucel-T (Provenge, Dendron Corp.) was approved for treatment of prostate cancer. Besides ex vivo generation of autologous DCs and subsequent pulsing with antigen, a novel approach to create vaccines via DCs is based on DC targeting (Caminschi et al. 2009; Flacher et al. 2009; Palucka et al. 2011). In this method, antigens are delivered directly to DCs in vivo using chimeric proteins made of anti-DC receptor Ab fused to the selected antigen (Ueno et al. 2011). Such DC immunotherapies (i.e. derived from patient) that act to activate T cells in the immune system are being successfully tested in clinical trials. Different DC subsets elicit different T cells responses and this phenomenon can be harnessed to develop therapeutic vaccines to treat either autoimmunity or cancer (Palucka et al. 2011; Ueno et al. 2011).
Efficacy of these therapies can best perceived concurrently with imaging tools to detect in vivo migration of these DCs generated ex vivo, so as to determine cellular fate and mechanistic efficacy post injection. The need to use therapeutic intervention in conjunction with in vivo imaging to view real-time physiological events within the host has led to rapid advances in molecular imaging, in particular noninvasive imaging tools such as optical imaging, MRI, and PET/SPECT.
Optical imaging of DC migration
A relatively new high-resolution technique, confocal laser scanning microscopy, incorporates spectroscopy and anisotropy imaging capabilities to permit visualization of specific cell types at high spatial resolution in vivo via optical imaging (Bigelow et al. 2003). Usually, fluorescent antibodies are injected intradermally into an animal model to label selected dermally localized targets in situ. The availability of a wide variety of fluorescently conjugated antibodies renders this technique very attractive for many applications in immunology and tumor biology. This technique has been used successfully in labeling endogenous DCs at the site of mammary tumor grown intradermally in the ears of BALB/c mice (Cummings et al. 2008). In another instance, optical imaging was performed using an IVIS (Caliper Life Sciences, Hopkinton, MS) fluorescence reflectance whole-mouse imaging system with a charge-coupled camera, which illuminates in vivo fluorescent sources (in this case, a fluorescein-myristic acid conjugate linked with an 11-mer polyarginine peptide). This conjugate was shown to be taken up by both mature and immature DCs upon short incubation. When these exogenously labeled DCs were injected subcutaneously into mice, green fluorescence was detected in a proximal lymph node within 24 h (Pham et al. 2007) and confirmed by histological analysis. In addition to directly labeled antibodies, targeted quantum dots (QD) are currently being investigated for use as luminescent biologic probes. These nanoparticles have attractive optical characteristics, including high-absorption cross-section, relatively narrow and symmetric luminescence bands, and high resistance to photobleaching. In one example of NIR QD-assisted tracking of DCs, in vitro DC-phagocytosed QDs have been injected into the hindleg footpad of a C57Bl/6 mouse and allowed to migrate (Noh et al. 2008). Preconditioning of the injection site with proinflammatory cytokines such as TNF-α led to 10-fold enhanced DC migration to the draining lymph nodes. Within 12 to 48 h after injection, the QD NIR fluorescence signals in the popliteal lymph node (pLN) were further increased. NIR scanning has also been used to detect dyes coated onto perfluorocarbons (PFCs). Because of the phagocytic properties of the DCs, synthesized IRDye800-coated perfluorooctyl bromide (PFOB) nanoemulsions could be easily incorporated into DCs in culture, even in the absence of cell penetrating peptides. To test in vivo migration of DCs, our laboratory has successfully used NIR imaging to trace peripherally injected exogenous BMDCs with transient adenovirus expressing PSMA to the site of an inflammatory tumor (unpublished data). Also, recent evidence of both leukocyte and lymphocyte (macrophages, B cells) recruitment to the vicinity of inflammatory brain tumors was shown in orthotopic tumor models in mice upon injecting IRDye800-labeled specific antibodies (anti-CD68 Ab (macrophages), anti-CD20 (B cells) and isotype control) and imaging the mice at four days post-antibody injection (Fig. 4, unpublished data).
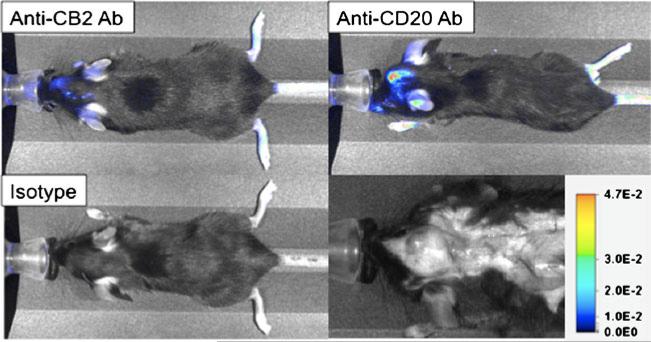
Fig. 4.
Lymphocytes and activated leukocytes can be tracked and imaged non-invasively using NIRF imaging in mouse models of brain tumors. Three antibodies (anti-cannabinoid receptor 2, anti-CD20 and IgG2a isotype control) were labeled with LiCOR IRDye800-NHS and purified using Sephadex G-25 desalting resin. Female C57bl6 bearing intracranial 3LL Lewis Lung carcinoma cells (day 6 post-implantation) were then injected intra-peritoneally with 10 mg of optically-labeled antibody as indicated in PBS. At 72 h post-antibody injection, each mouse was then imaged using a LiCOR Pearl Impulse imager exciting at 745 nm and detecting at 800 nm. Images show accumulation of anti-CB2 Ab at the site of the 3LL brain tumor (tumor in red arrow in bottom left white-light photo) showing the presence of activated macrophages and microglia in the vicinity of the tumor. Similarly, strong accumulation of anti-CD20 Ab indicates the presence of numerous B lymphocytes present in or around the tumor. Negative isotype control antibody presence within the tumor (but present in spleen, data not shown) indicates specific binding by anti-CB2 and anti-CD20 antibodies
To explore the in vivo survival and trafficking of DCs, another important optical imaging technique employs the use of bioluminescence imaging (BLI). This method has proved to be a sensitive technique for visualizing the trafficking and survival of cellular populations in living animals, due to the high tissue penetrating capability of the photons produced. BLI is based on the introduction of a reporter gene encoding for the bioluminescent protein luciferase (Luc). Dual-function reporter genes that express Luc in addition to a fluorescent reporter (such as green fluorescent protein [GFP]) bear the potential of linking high-resolution ex vivo histology and in vivo imaging. This offers an opportunity to refine and accelerate studies of cellular fate and function after intravenous transfer to unlabeled recipient animals. In a study demonstrating that DCs can be monitored in vivo with BLI, bone marrow-derived DCs (BMDCs) were transduced with a retroviral vector encoding Luc and GFP. The GFP positivity of DCs enabled fluorescence microscopy on excised tissues that were carefully sampled based on the findings of in vivo BLI. On day one following transfer, DCs were detected in the lungs and spleen of mice. Later, DCs were found in mesenteric lymph nodes, Peyer's patches, spleen, and thymus for up to 6 weeks after transfer (Schimmelpfennig et al. 2005).
While most of this work has imaged the peripheral lymph nodes, enhanced DC migration into areas of the CNS can be studied using similar techniques during neuroinflammation. Our preliminary studies focused on the bioluminescence detection of Luc- and GFP-transduced DCs by BLI imaging. Highly inflammatory murine Lewis Lung carcinoma 3LL cells were stereotactically introduced into the right mesocortical lobe of C57BL/6 mice and tail-vein injected transduced DCs were subsequently tracked ex vivo towards the site of the tumor. Histochemical analysis detected ex vivo DC-GFP fluorescence in the cervical lymph nodes (cLN), supporting DC migration into the CNS and drainage into cLN (unpublished data). These initial observations must be validated in further comprehensive in-depth investigations.
MRI in DC migration
An established modality in imaging peripheral and CNS soft tissue, MRI has been used pre-clinically to noninvasively track cellular migration. Para-magnetic contrast agents such as superparamagnetic iron oxide (SPIO) and monocrystalline iron oxide nanocolloid (MION) nanoparticles are frequently used to label many migratory cells of interest including various stem (Kraitchman et al. 2005; Lange et al. 2005) and myeloid lineage (Balagopalan et al. 2011; Filippi and Grossman 2002) cells. These nanoparticles contain an iron core coated with dextran. For example, DCs externally labeled using SPIOs and injected into a mouse footpad accumulated in peripheral lymph nodes (pLNs) within 24 h as indicated by the increase in signal intensity of SPIO labeling (Kobukai et al. 2010). SPIO nanoparticles have been repeatedly used for tracking the migratory capability of T cells in the context of EAE. In mice with magnetic nanoparticle-labeled Tcells, EAE lesions were observed in the lower thoracic and lumbar cord as discrete hypointense regions on in vivo MRI, indicating the presence of the SPIO nanoparticle-labeled cells. Mice receiving unlabeled cells did not exhibit similar hypointense regions in the thoracolumbar cords (Anderson et al. 2004). Labeled myelin-reactive Tcells were systemically transferred to naive rats in order to examine the cells’ infiltration in the spinal cord and the brain. Ex vivo imaging of the brain and spinal cord showed hypointense areas in the sacral part of the spinal cord, rostral to the cauda equina, indicating the presence of the transferred SPIO-labeled cells (Baeten et al. 2010). MRI studies of macrophage infiltration in carotid atherosclerotic plaque, stroke, brain tumor, and MS are reviewed elsewhere (Corot et al. 2004). Recently, magnetosome-like nanoparticles prepared by coating polyethylene glycol-phospholipid (PEG-phospholipid) onto ferromagnetic iron oxide nanocubes (FIONs), were used to demonstrate efficient uptake in breast cancer cells (Lee et al. 2011). The magnetization of FIONs being higher than that of SPIO, the r2 reflexivity of FIONs become two- to three-fold greater making them highly sensitive MR contrast agents. These nanocubes were further used to label pancreatic islets, which were infused into diabetic rats. In vivo MR imaging performed by a clinical 1.5 T scanner showed that the the islets were clearly observed as dark spots representing single cells within the liver in the T2* MR images. Transplantation of cells brought down glucose levels in rats given the pancreatic islets.
Another class of nanoparticles includes the PFCs, including perfluoropolyethers (PFPEs). Perfluorocarbons are highly chemically stable and are attractive compounds for formulating MRI reagents owing to their biological and chemical inertness. They are not degraded by any known enzyme found in the body and maintain their structure at typical lysosomal pH values, thereby providing long-lasting intracellular labeling. Intriguingly, PFPE is both hydrophobic and lipophobic and does not become associated with cell membranes. The nanoemulsion clearance is ultimately via the reticuloendothelial system and exhalation through the lungs (Castro et al. 1984). PFC-labeled cells have been described recently with the use of a novel imaging platform relying on 19F MRI. As with SPIO nanoparticle labeling, DCs and other cells are labeled ex vivo with PFPE and reintroduced into mice to monitor cellular trafficking. Compared with conventional 1H-based MRI, the fluorine-based MRI data have no background signal, which is a key advantage of the technique (Zhang 2004). This difference in MRI technique was demonstrated when PFOB, a PFC, was used as an 19F and 1H MR imaging nanoprobe in in vitro labeled DCs. MRI contrast was observed from DCs co-labeled with IRDye800-coated PFOB nanoemulsions. When the DCs were injected subcutaneously into the mice, 1H-based MRI provided a whole-body image whereas the 19F-based MR technique showed only signals generated from the injected DCs labeled with optically co-detected IRDye800-coated PFOB nanoemulsions (Lim et al. 2009). Human DCs have also been effectively labeled with commercially available PFPE without significant impact on cell viability, phenotype, or function (Helfer et al. 2010). PFPE-labeled injected human DCs were clearly detected in immunodeficient mice by 19F MRI, with mature DCs being shown to migrate selectively towards draining lymph node regions within 18 h. Interestingly, immature DCs were not observed to leave the injection site; however, by 18 h a distinct population of the PFPE-labeled mature DCs was observed located away from the injection site near the draining inguinal lymph nodes.
In light of the promise of MRI for in vivo imaging, detecting migration of DCs into the CNS during infection or inflammation should be feasible. Once this technique is established, mechanistic properties of the cells including functionality of cell surface markers in the recruitment and transmigration process across the BBB can be explored. In example, the function of RAGE (Receptor for Advanced Glycation End Products) has been studied in DC homing to lymph nodes using SPIO MRI techniques. Mice were studied after intravenous and subcutaneous injection of SPIO-labeled DCs. MRI showed that as opposed to wild-type DCs, RAGE−/− DCs injected into the footpads of wild-type or RAGE−/− mice failed to migrate to the draining pLNs, supporting the notion that expression of RAGE by maturing DCs, but not on lymphatic endothelium, is required for DC migration (Manfredi et al. 2008). It would be enlightening to study the in vivo interaction of surface receptors and ligands found on DCs with the BBB endothelium in this manner.
Radionucleotide techniques in DC migration
One of the most sensitive techniques among the current tomographic in vivo imaging tools is nuclear imaging using radioisotope cell tagging, such as PET and SPECT. In an attempt to label DCs for use in this type of imaging, a novel method of labeling with the positron-emitting radioisotope 18F using N-succinimidyl-4-[F-18] fluorobenzoate was developed, which covalently binds to the lysine residues of cell surface proteins. A PiPET (projection imager/positron emission tomograph) scanner configured for planar projection imaging was used, which enable comparable resolution to PET using a standard circular detector array. Planar projection imaging is less susceptible to statistical uncertainties because the depth dimension is collapsed onto a two-dimensional plane. Four hours after injection of 18F-labeled BMDCs into the hind footpad, the majority of the injected dose remained at the site of injection in the footpad but significant activity was detected in the draining lymph node as well as the liver, kidneys, and bladder (Olasz et al. 2002). PETand SPECT images have also been acquired on 7th day post-DC inoculation showing a significant accumulation of 18F-FIAU in lung and liver of a “DC vaccine” against coccidioidomycosis, a fungal infection, as compared with a control group. The molecular imaging technique was based on the use of 18F-labeled 2′-fluoro-2′-deoxy-1β-d-arabinofuranosyl-5-iodouracil (FIAU), a specific substrate for herpes simplex virus (HSV1)-thymidine kinase (TK). The DCs were cotransfected with Coccidioides-Ag2/PRA-cDNA and a plasmid DNA encoding HSV1-TK. HSV1-TK phosphorylates 18F-FIAU to phospho-18F-FIAU, which is then metabolically trapped and detected by the PET system (Vilekar et al. 2010). As noted by this study, 111In-oxine SPECT imaging for DC trafficking does not ensure the integrity of radiolabel and DC association in vivo, has poor resolution, and lastly allows imaging only up to 3 or 4 days. Although the resolution of PET is higher than that of SPECT, the latter meets the criteria of reasonable cost affordability by most researchers. SPECT imaging agents generally have longer physical half-lives than those used with PET, thereby permitting longer and more detailed neurochemical studies, when needed, are possible with PET. Hence, both of these tools are highly permissive in their abilities to track DC trafficking into the CNS. Our laboratory is presently using nuclear imaging to trace the path of radiolabeled DCs into the CNS in the classic neuroinflammatory model of EAE in mice. The first indications have shown that radiolabeled endogenous DCs do have the capacity to migrate to mediastinal nodes, while we are still pursuing migration of exogenously injected DCs using this method (unpublished data).
Intravital fluorescence videomicroscopy in DC migration
Traditionally, intravital microscopy has been employed in tissues that are thin enough to allow white light transmittance through the sample. The successful use of brightfield videomicroscopy for macrophage and neutrophil migration is reviewed elsewhere (Megens et al. 2011). Brightfield intravital microscopy is cost-effective and reliable; however, the major shortcoming is the lack of discrimination between cell subsets involved in the leukocyte recruitment cascade. This limitation has been overcome by the introduction of epifluorescence intravital microcopy, which involves labeling of cells with fluorescent antibodies before injection. This improvised epifluorescence videomicroscopy has been widely used to visualize T-cell interactions with CNS white matter microvasculature during EAE via VCAM-1 binding (Vajkoczy et al. 2001). We and others have initiated efforts to employ this technique in investigating functional roles of cell surface markers in DC migration through the BBB and established α4-integrins as crucial players in the multistep cascade of DC trafficking into the CNS, in addition to their proven role in T cell trafficking (Jain et al. 2010). Pretreatment of cells with the α4-integrin blocking antibody (PS/2) did not have a significant effect on initial contact and rolling or capture of both nonactivated and activated DCs with the endothelial cells of the BBB. However, a dramatic difference was observed at the level of firm adhesion calculated as the number of permanently adhering DCs within a given field of view. Because blocking of α4-integrin did not affect the initial contact (rolling/capture) of DCs, it is possible that two separate mechanisms are involved in the initial recruitment of DCs to the BBB and their subsequent diapedesis across the endothelial wall.
Intravital two-photon microscopy in DC migration
A fast, resonant-scanning based two-photon platform has been established by modifying traditional videomicroscopy. It allows for imaging deeper within tissues and can acquire images at close to video rate acquisition speeds. A further advantage of this setup is the stability achieved by use of a vacuum chamber placed on a thoracic window, whereby the organ can be gently immobilized (Looney et al. 2011; Megens et al. 2011). Also, intravital two-photon fluorescence microscopy is different from confocal microscopy in that the fluoropore molecule absorbs two photons instead of a single photon. Thus, the excitation area can be confined to the focal point on the objective lens, concentrating photons into a small area. This gives rise to bright, high-resolution images and (unlike with optical imaging) can penetrate up to 1 mm inside tissues and organs. Thus cellular imaging can be undertaken under the preservation of vascular and lymphatic flow, innervation, oxygen metabolism, and possibly soluble gradients. A procedure to visualize in real time the behavior of 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester–labeled innate immune cells, such as DC and natural killer (NK) cells, in the lymph nodes of live, anesthetized mice has been established (Celli et al. 2008). Moreover, intrinsic biologic structures, including collagen fibers, muscle, brain, cornea, and bone can be visualized without labeling them with exogenous probes (Ishii and Ishii 2011). Antigen-loaded DCs were labeled with fluorescent dye and then injected into the footpad of hindlimbs of a wild-type mouse (Stoll et al. 2002). After 18 h, these antigen-loaded DCs were found in the pLN by intravital two-photon imaging. For further insight on the use of two-photon microscopy in viewing DC behavior in secondary lymphoid organs and peripheral tissue under homeostatic and inflammatory conditions, refer to Cavanagh and Weninger (Cavanagh and Weninger 2008).
Intravital two-photon microscopy has been used successfully in visualizing brain autoantigen-specific T cell behavior in EAE (Kawakami and Flugel 2010). These T cells have been seen to crawl along the intraluminal surface of CNS blood vessels before they extravasate into the perivascular environment where they meet DCs and macrophages. Here, T cells can find antigen, be further activated, and produce cytokines, resulting in massive immune cell recruitment and clinical disease. For a further comprehensive review on migration of effector T cells in the course of EAE using two photon microscopy (refer to (Flugel et al. 2007)). The use of intravital two-photon microscopy in DC recruitment into the CNS remains to be explored.
Conclusions
In conclusion, the evidence supporting the fact that that dendritic cells, like other leukocytes can migrate into the immunoprevileged regions of the CNS are increasingly being provided. Despite being the most potent antigen presenting cells in the body contributing towards both primary as well as secondary immune response, their phenotype and function as they are recruited across the BBB has been least explored amongst all leukocytes. Therefore, there are significant gaps in knowedge about the characteristic receptors and ligands they utilize in order to accomplish this transmigratory process. However, it is being revealed that these cells are equipped with the right machinery to realize their functional goal as defense against disease of the brain and spinal cord. A look at the future applications of DCs in immunotherapy against inflammation or immunosuppression of the CNS can certainly benefit from understanding how these cells respond in vivo. Therapies such as receptor inhibitors can impair migration thereby reducing the contribution of these cells to the inflammatory process. On the other hand, understanding how chemoattraction and protein synthesis in dendritic cells facilitate transmigration can help one develop strategies to create a sufficiently suitable environment for their migration towards areas of immunosupression. To meet this goal, taking advantage of both established as well as novel molecular imaging techniques is essential. The ultimate aim is to be able to manipulate these cells in the context of the body's natural ability to defend itself against CNS disease and use them to launch a controlled yet potent immune response.
Acknowledgements
Authors wish to acknowledge United States Public Health Service/National Institutes of Health grants R01 AI077414 to PJ and R21 AI 093172–01 to ZKK.
Abbreviations
- APC
antigen presenting cell
- DC
dendritic cell
- CNS
central nervous system
- CLN
cervical lymph node
- CSF
cerebrospinal fluid
- BBB
blood-brain barrier
- ECM
extracellular matrix
- EAE
experimental autoimmune encephalomyelitis
- FION
ferrimagnetic iron oxide nanocubes
- HEV
high endothelial venule
- HIVE
HIVencephalopathy
- HSV-1
herpes simplex virus-1
- ICAM
intracellular cell adhesion molecule
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- JAM
junctional adhesion molecule
- LCMV
lymphocytic choriomeningitis virus
- LFA
lymphocyte function-associated antigen
- MCP
monocyte chemotactic protein
- MIP
macrophage inflammatory protein
- MVEC
microvascular endothelial cell
- MS
multiple sclerosis
- MRI
magnetic resonance imaging
- NIR
near infrared
- PECAM
platelet endothelial cell adhesion molecule
- PET
positron emission tomography
- PFC
perfluorocarbons
- PSGL
P-selectin glycoprotein ligand
- RANTES
regulated upon activation, normal T-cell expressed and secreted
- SCI
spinal cord injury
- SDF-1
stromal-derived factor-1
- SGPG
sulfoglucuronosyl paragloboside
- SPECT
single photon emission computed tomography
- TJ
tight junction
- TNF-α
tumor necrosis factor-α
- VCAM
vascular cell adhesion molecule
- VLA
very late antigen
- ZO
zona occludens
Footnotes
Authors declare no conflict of interest
Contributor Information
Divya Sagar, Drexel Institute for Biotechnology and Virology Research, and Department of Microbiology and Immunology, Drexel University College of Medicine, Philadelphia, PA 19129, USA.
Catherine Foss, Department of Radiology and Radiological Sciences, Johns Hopkins Medical Institutions, Baltimore, MD 21231, USA.
Rasha El Baz, Drexel Institute for Biotechnology and Virology Research, and Department of Microbiology and Immunology, Drexel University College of Medicine, Philadelphia, PA 19129, USA.
Martin G. Pomper, Department of Radiology and Radiological Sciences, Johns Hopkins Medical Institutions, Baltimore, MD 21231, USA
Zafar K. Khan, Drexel Institute for Biotechnology and Virology Research, and Department of Microbiology and Immunology, Drexel University College of Medicine, Philadelphia, PA 19129, USA
Pooja Jain, Drexel Institute for Biotechnology and Virology Research, and Department of Microbiology and Immunology, Drexel University College of Medicine, Philadelphia, PA 19129, USA; Department of Microbiology & Immunology, Drexel Institute for Biotechnology & Virology Research, Drexel University College of Medicine, 3805 Old Easton Road, Doylestown, PA 18902, USA pjain@drexelmed.edu.
References
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- Alt C, Laschinger M, Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur J Immunol. 2002;32:2133–2144. doi: 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Columba-Cabezas S, Serafini B, Muscella A, Aloisi F. Astrocytes are the major intracerebral source of macrophage inflammatory protein-3alpha/CCL20 in relapsing experimental autoimmune encephalomyelitis and in vitro. Glia. 2003;41:290–300. doi: 10.1002/glia.10193. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2005;64:706–715. doi: 10.1097/01.jnen.0000173893.01929.fc. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Shukaliak-Quandt J, Jordan EK, Arbab AS, Martin R, McFarland H, Frank JA. Magnetic resonance imaging of labeled T-cells in a mouse model of multiple sclerosis. Ann Neurol. 2004;55:654–659. doi: 10.1002/ana.20066. [DOI] [PubMed] [Google Scholar]
- Antonelli-Orlidge A, Saunders KB, Smith SR, D'Amore PA. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci USA. 1989;86:4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjmandi A, Liu K, Dorovini-Zis K. Dendritic cell adhesion to cerebral endothelium: role of endothelial cell adhesion molecules and their ligands. J Neuropathol Exp Neurol. 2009;68:300–313. doi: 10.1097/NEN.0b013e31819a8dd1. [DOI] [PubMed] [Google Scholar]
- Austyn JM, Kupiec-Weglinski JW, Hankins DF, Morris PJ. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J Exp Med. 1988;167:646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten K, Adriaensens P, Hendriks J, Theunissen E, Gelan J, Hellings N, Stinissen P. Tracking of myelin-reactive T cells in experimental autoimmune encephalomyelitis (EAE) animals using small particles of iron oxide and MRI. NMR Biomed. 2010;23:601–609. doi: 10.1002/nbm.1501. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53:637–644. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Balagopalan L, Sherman E, Barr VA, Samelson LE. Imaging techniques for assaying lymphocyte activation in action. Nat Rev Immunol. 2011;11:21–33. doi: 10.1038/nri2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De Silva S, Allt G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J Neurocytol. 2001;30:35–44. doi: 10.1023/a:1011965307612. [DOI] [PubMed] [Google Scholar]
- Barkalow FJ, Goodman MJ, Mayadas TN. Cultured murine cerebral microvascular endothelial cells contain von Willebrand factor-positive Weibel-Palade bodies and support rapid cytokine-induced neutrophil adhesion. Microcirculation. 1996;3:19–28. doi: 10.3109/10739689609146779. [DOI] [PubMed] [Google Scholar]
- Barratt-Boyes SM, Zimmer MI, Harshyne LA, Meyer EM, Watkins SC, Capuano S, 3rd, Murphey-Corb M, Falo LD, Jr, Donnenberg AD. Maturation and trafficking of monocyte-derived dendritic cells in monkeys: implications for dendritic cell-based vaccines. J Immunol. 2000;164:2487–2495. doi: 10.4049/jimmunol.164.5.2487. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, Nelson RM. Selectins. J Clin Invest. 1993;91:379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi G, D'Amico G, Varone L, Sozzani S, Mantovani A, Allavena P. In vitro studies on the trafficking of dendritic cells through endothelial cells and extra-cellular matrix. Dev Immunol. 2000;7:143–153. doi: 10.1155/2000/39893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Sauter A, Brouwer N, Copray SC, Boddeke HW. Ischemia-induced neuronal expression of the microglia attracting chemokine Secondary Lymphoid-tissue Chemokine (SLC). Glia. 2001;34:121–133. doi: 10.1002/glia.1047. [DOI] [PubMed] [Google Scholar]
- Bigelow CE, Conover DL, Foster TH. Confocal fluorescence spectroscopy and anisotropy imaging system. Opt Lett. 2003;28:695–697. doi: 10.1364/ol.28.000695. [DOI] [PubMed] [Google Scholar]
- Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- Boven LA, Montagne L, Nottet HS, De Groot CJ. Macrophage inflammatory protein-1alpha (MIP-1alpha), MIP-1beta, and RANTES mRNA semiquantification and protein expression in active demyelinating multiple sclerosis (MS) lesions. Clin Exp Immunol. 2000;122:257–263. doi: 10.1046/j.1365-2249.2000.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britschgi MR, Favre S, Luther SA. CCL21 is sufficient to mediate DC migration, maturation and function in the absence of CCL19. Eur J Immunol. 2010;40:1266–1271. doi: 10.1002/eji.200939921. [DOI] [PubMed] [Google Scholar]
- Brown KA, Bedford P, Macey M, McCarthy DA, Leroy F, Vora AJ, Stagg AJ, Dumonde DC, Knight SC. Human blood dendritic cells: binding to vascular endothelium and expression of adhesion molecules. Clin Exp Immunol. 1997;107:601–607. doi: 10.1046/j.1365-2249.1997.d01-951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–970. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW. Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell Immunol. 2011;267:109–123. doi: 10.1016/j.cellimm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns S, Hardy SJ, Buddle J, Yong KL, Jones GE, Thrasher AJ. Maturation of DC is associated with changes in motile characteristics and adherence. Cell Motil Cytoskeleton. 2004;57:118–132. doi: 10.1002/cm.10163. [DOI] [PubMed] [Google Scholar]
- Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol. 2009;39:931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- Cannella B, Cross AH, Raine CS. Relapsing autoimmune demyelination: a role for vascular addressins. J Neuroimmunol. 1991;35:295–300. doi: 10.1016/0165-5728(91)90183-8. [DOI] [PubMed] [Google Scholar]
- Carrithers MD, Visintin I, Kang SJ, Janeway CA., Jr Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain. 2000;123(Pt 6):1092–1101. doi: 10.1093/brain/123.6.1092. [DOI] [PubMed] [Google Scholar]
- Castro O, Nesbitt AE, Lyles D. Effect of a perfluorocarbon emulsion (Fluosol-DA) on reticuloendothelial system clearance function. Am J Hematol. 1984;16:15–21. doi: 10.1002/ajh.2830160103. [DOI] [PubMed] [Google Scholar]
- Cavanagh LL, Weninger W. Dendritic cell behaviour in vivo: lessons learned from intravital two-photon microscopy. Immunol Cell Biol. 2008;86:428–438. doi: 10.1038/icb.2008.25. [DOI] [PubMed] [Google Scholar]
- Celli S, Breart B, Bousso P. Intravital two-photon imaging of natural killer cells and dendritic cells in lymph nodes. Methods Mol Biol. 2008;415:119–126. doi: 10.1007/978-1-59745-570-1_7. [DOI] [PubMed] [Google Scholar]
- Cera MR, Del Prete A, Vecchi A, Corada M, Martin-Padura I, Motoike T, Tonetti P, Bazzoni G, Vermi W, Gentili F, Bernasconi S, Sato TN, Mantovani A, Dejana E. Increased DC trafficking to lymph nodes and contact hypersensitivity in junctional adhesion molecule-A-deficient mice. J Clin Invest. 2004;114:729–738. doi: 10.1172/JCI21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Ou YC, Chang CY, Pan HC, Liao SL, Raung SL, Chen SY. TNF-alpha and IL-1beta mediate Japanese encephalitis virus-induced RANTES gene expression in astrocytes. Neurochem Int. 2011;58:234–242. doi: 10.1016/j.neuint.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Chui R, Dorovini-Zis K. Regulation of CCL2 and CCL3 expression in human brain endothelial cells by cytokines and lipopolysaccharide. J Neuroinflammation. 2010;7:1. doi: 10.1186/1742-2094-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavarra RP, Stephens A, Nagy S, Sekellick M, Steel C. Evaluation of immunological paradigms in a virus model: are dendritic cells critical for antiviral immunity and viral clearance? J Immunol. 2006;177:492–500. doi: 10.4049/jimmunol.177.1.492. [DOI] [PubMed] [Google Scholar]
- Columba-Cabezas S, Serafini B, Ambrosini E, Aloisi F. Lymphoid chemokines CCL19 and CCL21 are expressed in the central nervous system during experimental autoimmune encephalomyelitis: implications for the maintenance of chronic neuroinflammation. Brain Pathol. 2003;13:38–51. doi: 10.1111/j.1750-3639.2003.tb00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corot C, Petry KG, Trivedi R, Saleh A, Jonkmanns C, Le Bas JF, Blezer E, Rausch M, Brochet B, Foster-Gareau P, Baleriaux D, Gaillard S, Dousset V. Macrophage imaging in central nervous system and in carotid atherosclerotic plaque using ultrasmall superparamagnetic iron oxide in magnetic resonance imaging. Invest Radiol. 2004;39:619–625. doi: 10.1097/01.rli.0000135980.08491.33. [DOI] [PubMed] [Google Scholar]
- Crone C, Christensen O. Electrical resistance of a capillary endothelium. J Gen Physiol. 1981;77:349–371. doi: 10.1085/jgp.77.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M, McCandless EE, Patel JR, Luker GD, Littman DR, Russell JH, Klein RS. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J Exp Med. 2011;208:327–339. doi: 10.1084/jem.20102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings RJ, Mitra S, Lord EM, Foster TH. Antibody-labeled fluorescence imaging of dendritic cell populations in vivo. J Biomed Opt. 2008;13:044041. doi: 10.1117/1.2966122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico G, Bianchi G, Bernasconi S, Bersani L, Piemonti L, Sozzani S, Mantovani A, Allavena P. Adhesion, transendothelial migration, and reverse transmigration of in vitro cultured dendritic cells. Blood. 1998;92:207–214. [PubMed] [Google Scholar]
- Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet. 1997;17:346–349. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diab A, Abdalla H, Li HL, Shi FD, Zhu J, Hojberg B, Lindquist L, Wretlind B, Bakhiet M, Link H. Neutralization of macrophage inflammatory protein 2 (MIP-2) and MIP-1alpha attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect Immun. 1999;67:2590–2601. doi: 10.1128/iai.67.5.2590-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore-Duffy P, Balabanov R, Washington R, Swanborg RH. Transforming growth factor beta 1 inhibits cytokine-induced CNS endothelial cell activation. Mol Chem Neuropathol. 1994;22:161–175. doi: 10.1007/BF03160103. [DOI] [PubMed] [Google Scholar]
- Doring A, Wild M, Vestweber D, Deutsch U, Engelhardt B. E- and P-selectin are not required for the development of experimental autoimmune encephalomyelitis in C57BL/6 and SJL mice. J Immunol. 2007;179:8470–8479. doi: 10.4049/jimmunol.179.12.8470. [DOI] [PubMed] [Google Scholar]
- dos Santos AC, Barsante MM, Arantes RM, Bernard CC, Teixeira MM, Carvalho-Tavares J. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis–an intravital microscopy study. J Neuroimmunol. 2005;162:122–129. doi: 10.1016/j.jneuroim.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Dos Santos AC, Roffe E, Arantes RM, Juliano L, Pesquero JL, Pesquero JB, Bader M, Teixeira MM, Carvalho-Tavares J. Kinin B2 receptor regulates chemokines CCL2 and CCL5 expression and modulates leukocyte recruitment and pathology in experimental autoimmune encephalomyelitis (EAE) in mice. J Neuroinflammation. 2008;5:49. doi: 10.1186/1742-2094-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn AM, Horst E, Pals ST, Rouse BN, Steere AC, Picker LJ, Meijer CJ, Butcher EC. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988;130:147–155. [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Vestweber D, Hallmann R, Schulz M. E- and P-selectin are not involved in the recruitment of inflammatory cells across the blood-brain barrier in experimental autoimmune encephalomyelitis. Blood. 1997;90:4459–4472. [PubMed] [Google Scholar]
- Engelhardt B, Kempe B, Merfeld-Clauss S, Laschinger M, Furie B, Wild MK, Vestweber D. P-selectin glycoprotein ligand 1 is not required for the development of experimental autoimmune encephalomyelitis in SJL and C57BL/6 mice. J Immunol. 2005;175:1267–1275. doi: 10.4049/jimmunol.175.2.1267. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol. 1993a;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- Fabry Z, Sandor M, Gajewski TF, Herlein JA, Waldschmidt MM, Lynch RG, Hart MN. Differential activation of Th1 and Th2 CD4+ cells by murine brain microvessel endothelial cells and smooth muscle/pericytes. J Immunol. 1993b;151:38–47. [PubMed] [Google Scholar]
- Figdor CG. Molecular characterization of dendritic cells operating at the interface of innate or acquired immunity. Pathol Biol (Paris) 2003;51:61–63. doi: 10.1016/s0369-8114(03)00097-x. [DOI] [PubMed] [Google Scholar]
- Filippi M, Grossman RI. MRI techniques to monitor MS evolution: the present and the future. Neurology. 2002;58:1147–1153. doi: 10.1212/wnl.58.8.1147. [DOI] [PubMed] [Google Scholar]
- Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P. Targeting of epidermal Langerhans cells with antigenic proteins: attempts to harness their properties for immunotherapy. Cancer Immunol Immunother. 2009;58:1137–1147. doi: 10.1007/s00262-008-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugel A, Odoardi F, Nosov M, Kawakami N. Autoaggressive effector T cells in the course of experimental autoimmune encephalomyelitis visualized in the light of two-photon microscopy. J Neuroimmunol. 2007;191:86–97. doi: 10.1016/j.jneuroim.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances transinfection of Tcells. Cell. 2000a;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000b;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G, Thorpe JW, Kidd D, Kendall BE, Moseley IF, Thompson AJ, Keir G, Miller DH, Feldmann M, Thompson EJ. Soluble E-selectin in multiple sclerosis: raised concentrations in patients with primary progressive disease. J Neurol Neurosurg Psychiatry. 1996;60:20–26. doi: 10.1136/jnnp.60.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW, Ransohoff RM. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol. 1996;156:4363–4368. [PubMed] [Google Scholar]
- Gourmala NG, Limonta S, Bochelen D, Sauter A, Boddeke HW. Localization of macrophage inflammatory protein: macrophage inflammatory protein-1 expression in rat brain after peripheral administration of lipopolysaccharide and focal cerebral ischemia. Neuroscience. 1999;88:1255–1266. doi: 10.1016/s0306-4522(98)00295-4. [DOI] [PubMed] [Google Scholar]
- Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kataoka N, Nakamura E, Hagihara K, Hatano M, Okamoto T, Kanouchi H, Minatogawa Y, Mohri S, Tsujioka K, Kajiya F. Monocyte trans-endothelial migration augments subsequent transmigratory activity with increased PECAM-1 and decreased VE-cadherin at endothelial junctions. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Hatterer E, Touret M, Belin MF, Honnorat J, Nataf S. Cerebrospinal fluid dendritic cells infiltrate the brain parenchyma and target the cervical lymph nodes under neuroinflammatory conditions. PLoS One. 2008;3:e3321. doi: 10.1371/journal.pone.0003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer BM, Balducci A, Nelson AD, Janjic JM, Gil RR, Kalinski P, de Vries IJ, Ahrens ET, Mailliard RB. Functional assessment of human dendritic cells labeled for in vivo (19)F magnetic resonance imaging cell tracking. Cytotherapy. 2010;12:238–250. doi: 10.3109/14653240903446902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Thompson Y, Sprinkle A, Carroll J, Smith J. E-selectin expression on human brain microvascular endothelial cells. Neurosci Lett. 1996;213:37–40. doi: 10.1016/0304-3940(96)12837-8. [DOI] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CR. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24:1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- Ifergan I, Kebir H, Bernard M, Wosik K, Dodelet-Devillers A, Cayrol R, Arbour N, Prat A. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain. 2008;131:785–799. doi: 10.1093/brain/awm295. [DOI] [PubMed] [Google Scholar]
- Ireland DD, Reiss CS. Gene expression contributing to recruitment of circulating cells in response to vesicular stomatitis virus infection of the CNS. Viral Immunol. 2006;19:536–545. doi: 10.1089/vim.2006.19.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Ishii M. Intravital two-photon imaging: a versatile tool for dissecting the immune system. Ann Rheum Dis. 2011;70(Suppl 1):i113–115. doi: 10.1136/ard.2010.138156. [DOI] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA, Butterfiled DA, Banks WA. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer's disease. Brain Behav Immun. 2009;23:507–517. doi: 10.1016/j.bbi.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Coisne C, Enzmann G, Rottapel R, Engelhardt B. Alpha4beta1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. J Immunol. 2010;184:7196–7206. doi: 10.4049/jimmunol.0901404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Johnston GI, Bliss GA, Newman PJ, McEver RP. Structure of the human gene encoding granule membrane protein-140, a member of the selectin family of adhesion receptors for leukocytes. J Biol Chem. 1990;265:21381–21385. [PubMed] [Google Scholar]
- Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57:123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T, Yamawaki M, Ariga T, Yu RK. Interleukin 1 beta up-regulates the expression of sulfoglucuronosyl paragloboside, a ligand for L-selectin, in brain microvascular endothelial cells. Proc Natl Acad Sci USA. 1995;92:7897–7901. doi: 10.1073/pnas.92.17.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami N, Flugel A. Knocking at the brain's door: intravital two-photon imaging of autoreactive T cell interactions with CNS structures. Semin Immunopathol. 2010;32:275–287. doi: 10.1007/s00281-010-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KJ, Strieter RM, Kunkel SL, Lukacs NW, Karpus WJ. Acute and relapsing experimental autoimmune encephalomyelitis are regulated by differential expression of the CC chemokines macrophage inflammatory protein-1alpha and monocyte chemotactic protein-1. J Neuroimmunol. 1998;92:98–108. doi: 10.1016/s0165-5728(98)00187-8. [DOI] [PubMed] [Google Scholar]
- Kenwrick S, Watkins A, De Angelis E. Neural cell recognition molecule L1: relating biological complexity to human disease mutations. Hum Mol Genet. 2000;9:879–886. doi: 10.1093/hmg/9.6.879. [DOI] [PubMed] [Google Scholar]
- Kieffer JD, Fuhlbrigge RC, Armerding D, Robert C, Ferenczi K, Camphausen RT, Kupper TS. Neutrophils, monocytes, and dendritic cells express the same specialized form of PSGL-1 as do skin-homing memory T cells: cutaneous lymphocyte antigen. Biochem Biophys Res Commun. 2001;285:577–587. doi: 10.1006/bbrc.2001.5230. [DOI] [PubMed] [Google Scholar]
- Kielian T, van Rooijen N, Hickey WF. MCP-1 expression in CNS-1 astrocytoma cells: implications for macrophage infiltration into tumors in vivo. J Neurooncol. 2002;56:1–12. doi: 10.1023/a:1014495613455. [DOI] [PubMed] [Google Scholar]
- Kim JS, Gautam SC, Chopp M, Zaloga C, Jones ML, Ward PA, Welch KM. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. J Neuroimmunol. 1995;56:127–134. doi: 10.1016/0165-5728(94)00138-e. [DOI] [PubMed] [Google Scholar]
- Kobukai S, Baheza R, Cobb JG, Virostko J, Xie J, Gillman A, Koktysh D, Kerns D, Does M, Gore JC, Pham W. Magnetic nanoparticles for imaging dendritic cells. Magn Reson Med. 2010;63:1383–1390. doi: 10.1002/mrm.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, Young RG, Marcelino M, Pittenger MF, Solaiyappan M, Boston RC, Tsui BM, Wahl RL, Bulte JW. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M, Theil D, Steinmeyer F, Cepok S, Hemmer B, Hofbauer M, Farina C, Derfuss T, Junker A, Arzberger T, Sinicina I, Hartle C, Newcombe J, Hohlfeld R, Meinl E. CCL19 is constitutively expressed in the CNS, up-regulated in neuroinflammation, active and also inactive multiple sclerosis lesions. J Neuroimmunol. 2007;190:72–79. doi: 10.1016/j.jneuroim.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Ishikawa F, Yasuda T, Aritomi K, Nakano H, Tanaka Y, Okada Y, Lipp M, Kakiuchi T. CCR7 ligands are required for development of experimental autoimmune encephalomyelitis through generating IL-23-dependent Th17 cells. J Immunol. 2009;183:2513–2521. doi: 10.4049/jimmunol.0800729. [DOI] [PubMed] [Google Scholar]
- Lange C, Togel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- Lee N, Kim H, Choi SH, Park M, Kim D, Kim HC, Choi Y, Lin S, Kim BH, Jung HS, Park KS, Moon WK, Hyeon T. Magnetosome-like ferrimagnetic iron oxide nanocubes for highly sensitive MRI of single cells and transplanted pancreatic islets. Proc Natl Acad Sci USA. 2011;108:2662–2667. doi: 10.1073/pnas.1016409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ransohoff RM. The roles of chemokine CXCL12 in embryonic and brain tumor angiogenesis. Semin Cancer Biol. 2009;19:111–115. doi: 10.1016/j.semcancer.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Lim YT, Noh YW, Kwon JN, Chung BH. Multifunctional perfluorocarbon nanoemulsions for (19)F-based magnetic resonance and near-infrared optical imaging of dendritic cells. Chem Commun (Camb) 2009:6952–6954. doi: 10.1039/b914006a. [DOI] [PubMed] [Google Scholar]
- Lin MS, Sun YY, Chiu WT, Chang CY, Hung CC, Shie FS, Tsai SH, Lin JW, Hung KS, Lee YH. Curcumin attenuates the expression and secretion of RANTES following spinal cord injury in vivo and lipopolysaccharide-induced astrocyte reactivation in vitro. J Neurotrauma. 2011 doi: 10.1089/neu.2011.1768. [DOI] [PubMed] [Google Scholar]
- Liu KK, Dorovini-Zis K. Regulation of CXCL12 and CXCR4 expression by human brain endothelial cells and their role in CD4 + and CD8+ T cell adhesion and transendothelial migration. J Neuroimmunol. 2009;215:49–64. doi: 10.1016/j.jneuroim.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW, Krummel MF. Stabilized imaging of immune surveillance in the mouse lung. Nat Methods. 2011;8:91–96. doi: 10.1038/nmeth.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaluno L, Verbrugge SE, Martinoli C, Matteoli G, Chiavelli A, Zeng Y, Williams ED, Rescigno M, Cavallaro U. The adhesion molecule L1 regulates transendothelial migration and trafficking of dendritic cells. J Exp Med. 2009;206:623–635. doi: 10.1084/jem.20081211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi AA, Capobianco A, Esposito A, De Cobelli F, Canu T, Monno A, Raucci A, Sanvito F, Doglioni C, Nawroth PP, Bierhaus A, Bianchi ME, Rovere-Querini P, Del Maschio A. Maturing dendritic cells depend on RAGE for in vivo homing to lymph nodes. J Immunol. 2008;180:2270–2275. doi: 10.4049/jimmunol.180.4.2270. [DOI] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Ueta H, Shu Z, Xue-Dong X, Sawanobori Y, Kitazawa Y, Bin Y, Yamashita M, Shi C. The microstructure of secondary lymphoid organs that support immune cell trafficking. Arch Histol Cytol. 2010;73:1–21. doi: 10.1679/aohc.73.1. [DOI] [PubMed] [Google Scholar]
- McDonnell GV, McMillan SA, Douglas JP, Droogan AG, Hawkins SA. Serum soluble adhesion molecules in multiple sclerosis: raised sVCAM-1, sICAM-1 and sE-selectin in primary progressive disease. J Neurol. 1999;246:87–92. doi: 10.1007/s004150050313. [DOI] [PubMed] [Google Scholar]
- McEver RP. Properties of GMP-140, an inducible granule membrane protein of platelets and endothelium. Blood Cells. 1990;16:73–80. discussion 80–73. [PubMed] [Google Scholar]
- McKimmie CS, Graham GJ. Astrocytes modulate the chemokine network in a pathogen-specific manner. Biochem Biophys Res Commun. 2010;394:1006–1011. doi: 10.1016/j.bbrc.2010.03.111. [DOI] [PubMed] [Google Scholar]
- Megens RT, Kemmerich K, Pyta J, Weber C, Soehnlein O. Intravital imaging of phagocyte recruitment. Thromb Haemost. 2011;105 doi: 10.1160/TH10-11-0735. [DOI] [PubMed] [Google Scholar]
- Mossner R, Fassbender K, Kuhnen J, Schwartz A, Hennerici M. Circulating L-selectin in multiple sclerosis patients with active, gadolinium-enhancing brain plaques. J Neuroimmunol. 1996;65:61–65. doi: 10.1016/0165-5728(96)00003-3. [DOI] [PubMed] [Google Scholar]
- Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- Muratori C, Mangino G, Affabris E, Federico M. Astrocytes contacting HIV-1-infected macrophages increase the release of CCL2 in response to the HIV-1-dependent enhancement of membrane-associated TNFalpha in macrophages. Glia. 2010;58:1893–1904. doi: 10.1002/glia.21059. [DOI] [PubMed] [Google Scholar]
- Noh YW, Lim YT, Chung BH. Noninvasive imaging of dendritic cell migration into lymph nodes using near-infrared fluorescent semiconductor nanocrystals. FASEB J. 2008;22:3908–3918. doi: 10.1096/fj.08-112896. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, Ihara F. The microvasculature of the human cerebellar meninges. Acta Neuropathol. 2002;104:608–614. doi: 10.1007/s00401-002-0592-y. [DOI] [PubMed] [Google Scholar]
- Nottet HS, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, Sharer LR, McComb RD, Swindells S, Soderland C, Gendelman HE. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–1295. [PubMed] [Google Scholar]
- Ogasawara N, Kojima T, Go M, Fuchimoto J, Kamekura R, Koizumi J, Ohkuni T, Masaki T, Murata M, Tanaka S, Ichimiya S, Himi T, Sawada N. Induction of JAM-A during differentiation of human THP-1 dendritic cells. Biochem Biophys Res Commun. 2009;389:543–549. doi: 10.1016/j.bbrc.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Mori E, Tung MM, Thomas WS, del Zoppo GJ. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- Olasz EB, Lang L, Seidel J, Green MV, Eckelman WC, Katz SI. Fluorine-18 labeled mouse bone marrow-derived dendritic cells can be detected in vivo by high resolution projection imaging. J Immunol Methods. 2002;260:137–148. doi: 10.1016/s0022-1759(01)00528-2. [DOI] [PubMed] [Google Scholar]
- Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- Ovanesov MV, Ayhan Y, Wolbert C, Moldovan K, Sauder C, Pletnikov MV. Astrocytes play a key role in activation of microglia by persistent Borna disease virus infection. J Neuroinflammation. 2008;5:50. doi: 10.1186/1742-2094-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paemeleire K. The cellular basis of neurovascular metabolic coupling. Acta Neurol Belg. 2002;102:153–157. [PubMed] [Google Scholar]
- Palucka K, Ueno H, Banchereau J. Recent developments in cancer vaccines. J Immunol. 2011;186:1325–1331. doi: 10.4049/jimmunol.0902539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancook JD, Reisfeld RA, Varki N, Vitiello A, Fox RI, Montgomery AM. Expression and regulation of the neural cell adhesion molecule L1 on human cells of myelomonocytic and lymphoid origin. J Immunol. 1997;158:4413–4421. [PubMed] [Google Scholar]
- Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124:480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- Pashenkov M, Teleshova N, Kouwenhoven M, Smirnova T, Jin YP, Kostulas V, Huang YM, Pinegin B, Boiko A, Link H. Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. J Neuroimmunol. 2002;122:106–116. doi: 10.1016/s0165-5728(01)00451-9. [DOI] [PubMed] [Google Scholar]
- Patel N, Kirmi O. Anatomy and imaging of the normal meninges. Semin Ultrasound CT MR. 2009;30:559–564. doi: 10.1053/j.sult.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Pendl GG, Robert C, Steinert M, Thanos R, Eytner R, Borges E, Wild MK, Lowe JB, Fuhlbrigge RC, Kupper TS, Vestweber D, Grabbe S. Immature mouse dendritic cells enter inflamed tissue, a process that requires E- and P-selectin, but not P-selectin glycoprotein ligand 1. Blood. 2002;99:946–956. doi: 10.1182/blood.v99.3.946. [DOI] [PubMed] [Google Scholar]
- Pham W, Xie J, Gore JC. Tracking the migration of dendritic cells by in vivo optical imaging. Neoplasia. 2007;9:1130–1137. doi: 10.1593/neo.07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- Prodinger C, Bunse J, Kruger M, Schiefenhovel F, Brandt C, Laman JD, Greter M, Immig K, Heppner F, Becher B, Bechmann I. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011 doi: 10.1007/s00401-010-0774-y. [DOI] [PubMed] [Google Scholar]
- Raine CS, Lee SC, Scheinberg LC, Duijvestin AM, Cross AH. Adhesion molecules on endothelial cells in the central nervous system: an emerging area in the neuroimmunology of multiple sclerosis. Clin Immunol Immunopathol. 1990;57:173–187. doi: 10.1016/0090-1229(90)90032-l. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M. Peripheral cytokines profile in Parkinson's disease. Brain Behav Immun. 2009;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Ricart BG, John B, Lee D, Hunter CA, Hammer DA. Dendritic cells distinguish individual chemokine signals through CCR7 and CXCR4. J Immunol. 2011;186:53–61. doi: 10.4049/jimmunol.1002358. [DOI] [PubMed] [Google Scholar]
- Robert C, Fuhlbrigge RC, Kieffer JD, Ayehunie S, Hynes RO, Cheng G, Grabbe S, von Andrian UH, Kupper TS. Interaction of dendritic cells with skin endothelium: A new perspective on immunosurveillance. J Exp Med. 1999;189:627–636. doi: 10.1084/jem.189.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TK, Buckner CM, Berman JW. Leukocyte transmigration across the blood-brain barrier: perspectives on neuroAIDS. Front Biosci. 2010;15:478–536. doi: 10.2741/3631. [DOI] [PubMed] [Google Scholar]
- Rollins BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198–204. doi: 10.1016/1357-4310(96)88772-7. [DOI] [PubMed] [Google Scholar]
- Rosen SD. Cell surface lectins in the immune system. Semin Immunol. 1993;5:237–247. doi: 10.1006/smim.1993.1028. [DOI] [PubMed] [Google Scholar]
- Schakel K, Kannagi R, Kniep B, Goto Y, Mitsuoka C, Zwirner J, Soruri A, von Kietzell M, Rieber E. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity. 2002;17:289–301. doi: 10.1016/s1074-7613(02)00393-x. [DOI] [PubMed] [Google Scholar]
- Schimmelpfennig CH, Schulz S, Arber C, Baker J, Tarner I, McBride J, Contag CH, Negrin RS. Ex vivo expanded dendritic cells home to T-cell zones of lymphoid organs and survive in vivo after allogeneic bone marrow transplantation. Am J Pathol. 2005;167:1321–1331. doi: 10.1016/S0002-9440(10)61219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosshauer B. The blood-brain barrier: morphology, molecules, and neurothelin. Bioessays. 1993;15:341–346. doi: 10.1002/bies.950150508. [DOI] [PubMed] [Google Scholar]
- Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21:3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- Schulz M, Engelhardt B. The circumventricular organs participate in the immunopathogenesis of experimental autoimmune encephalomyelitis. Cerebrospinal Fluid Res. 2005;2:8. doi: 10.1186/1743-8454-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Kim YO, Jo I. Differential expression of stromal cell-derived factor 1 in human brain microvascular endothelial cells and pericytes involves histone modifications. Biochem Biophys Res Commun. 2009;382:519–524. doi: 10.1016/j.bbrc.2009.03.049. [DOI] [PubMed] [Google Scholar]
- Shukaliak JA, Dorovini-Zis K. Expression of the beta-chemokines RANTES and MIP-1 beta by human brain micro-vessel endothelial cells in primary culture. J Neuropathol Exp Neurol. 2000;59:339–352. doi: 10.1093/jnen/59.5.339. [DOI] [PubMed] [Google Scholar]
- Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol. 1998;84:238–249. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- Smits HA, Rijsmus A, van Loon JH, Wat JW, Verhoef J, Boven LA, Nottet HS. Amyloid-beta-induced chemokine production in primary human macrophages and astrocytes. J Neuroimmunol. 2002;127:160–168. doi: 10.1016/s0165-5728(02)00112-1. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Steel CD, Hahto SM, Ciavarra RP. Peripheral dendritic cells are essential for both the innate and adaptive antiviral immune responses in the central nervous system. Virology. 2009;387:117–126. doi: 10.1016/j.virol.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner O, Coisne C, Cecchelli R, Boscacci R, Deutsch U, Engelhardt B, Lyck R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J Immunol. 2010;185:4846–4855. doi: 10.4049/jimmunol.0903732. [DOI] [PubMed] [Google Scholar]
- Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ohta H, Oda A, Nakagaito Y, Kiyota Y, Shintani Y. Macrophage inflammatory protein-3alpha plays a key role in the inflammatory cascade in rat focal cerebral ischemia. Neurosci Res. 2009;64:75–82. doi: 10.1016/j.neures.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Thanabalasundaram G, El-Gindi J, Lischper M, Galla HJ. Methods to assess pericyte-endothelial cell interactions in a coculture model. Methods Mol Biol. 2011;686:379–399. doi: 10.1007/978-1-60761-938-3_19. [DOI] [PubMed] [Google Scholar]
- Tripathy D, Thirumangalakudi L, Grammas P. RANTES upregulation in the Alzheimer's disease brain: a possible neuroprotective role. Neurobiol Aging. 2010;31:8–16. doi: 10.1016/j.neurobiolaging.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada N, Miyagi K, Matsuda M, Yanagisawa N. Soluble E-selectin in the serum and cerebrospinal fluid of patients with multiple sclerosis and human T-lymphotropic virus type 1-associated myelopathy. Neurology. 1995;45:1914–1918. doi: 10.1212/wnl.45.10.1914. [DOI] [PubMed] [Google Scholar]
- Ueno H, Klechevsky E, Schmitt N, Ni L, Flamar AL, Zurawski S, Zurawski G, Palucka K, Banchereau J, Oh S. Targeting human dendritic cell subsets for improved vaccines. Semin Immunol. 2011;23:21–27. doi: 10.1016/j.smim.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago L, Nebuloni M, Bonetto S, Pellegrinelli A, Zerbi P, Ferri A, Lavri E, Capra M, Grassi MP, Costanzi G. Rantes distribution and cellular localization in the brain of HIV-infected patients. Clin Neuropathol. 2001;20:139–145. [PubMed] [Google Scholar]
- Vajkoczy P, Laschinger M, Engelhardt B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108:557–565. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y, Geijtenbeek TB. A novel adhesion pathway that regulates dendritic cell trafficking and T cell interactions. Immunol Rev. 2002;186:47–56. doi: 10.1034/j.1600-065x.2002.18605.x. [DOI] [PubMed] [Google Scholar]
- Venetz D, Ponzoni M, Schiraldi M, Ferreri AJ, Bertoni F, Doglioni C, Uguccioni M. Perivascular expression of CXCL9 and CXCL12 in primary central nervous system lymphoma: T-cell infiltration and positioning of malignant B cells. Int J Cancer. 2010;127:2300–2312. doi: 10.1002/ijc.25236. [DOI] [PubMed] [Google Scholar]
- Vilekar P, Awasthi V, Lagisetty P, King C, Shankar N, Awasthi S. In vivo trafficking and immunostimulatory potential of an intranasally-administered primary dendritic cell-based vaccine. BMC Immunol. 2010;11:60. doi: 10.1186/1471-2172-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela MC, Mansur DS, Lacerda-Queiroz N, Rodrigues DH, Lima GK, Arantes RM, Kroon EG, da Silva Campos MA, Teixeira MM, Teixeira AL. The chemokine CCL5 is essential for leukocyte recruitment in a model of severe Herpes simplex encephalitis. Ann N YAcad Sci. 2009;1153:256–263. doi: 10.1111/j.1749-6632.2008.03959.x. [DOI] [PubMed] [Google Scholar]
- Villablanca EJ, Russo V, Mora JR. Dendritic cell migration and lymphocyte homing imprinting. Histol Histopathol. 2008;23:897–910. doi: 10.14670/HH-23.897. [DOI] [PubMed] [Google Scholar]
- Wang X, Feuerstein GZ. Induced expression of adhesion molecules following focal brain ischemia. J Neurotrauma. 1995;12:825–832. doi: 10.1089/neu.1995.12.825. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Downie SA, Lyman WD, Berman JW. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol. 1998;161:6896–6903. [PubMed] [Google Scholar]
- Wekerle H. Experimental autoimmune encephalomyelitis as a model of immune-mediated CNS disease. Curr Opin Neurobiol. 1993;3:779–784. doi: 10.1016/0959-4388(93)90153-p. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz EP, Koenen RR, Fraemohs L, Minkiewicz J, Azad H, Weber C, Moy VT. LFA-1 binding destabilizes the JAM-A homophilic interaction during leukocyte transmigration. Biophys J. 2009;96:285–293. doi: 10.1529/biophysj.108.135491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote EH, Risau W, Engelhardt B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- Wong D, Dorovini-Zis K. Upregulation of intercellular adhesion molecule-1 (ICAM-1) expression in primary cultures of human brain microvessel endothelial cells by cytokines and lipopolysaccharide. J Neuroimmunol. 1992;39:11–21. doi: 10.1016/0165-5728(92)90170-p. [DOI] [PubMed] [Google Scholar]
- Wong D, Dorovini-Zis K. Expression of vascular cell adhesion molecule-1 (VCAM-1) by human brain microvessel endothelial cells in primary culture. Microvasc Res. 1995;49:325–339. doi: 10.1006/mvre.1995.1028. [DOI] [PubMed] [Google Scholar]
- Wong D, Dorovini-Zis K. Regualtion by cytokines and lipopolysaccharide of E-selectin expression by human brain microvessel endothelial cells in primary culture. J Neuropathol Exp Neurol. 1996;55:225–235. doi: 10.1097/00005072-199602000-00011. [DOI] [PubMed] [Google Scholar]
- Wong D, Prameya R, Dorovini-Zis K. In vitro adhesion and migration of T lymphocytes across monolayers of human brain microvessel endothelial cells: regulation by ICAM-1, VCAM-1, E-selectin and PECAM-1. J Neuropathol Exp Neurol. 1999;58:138–152. doi: 10.1097/00005072-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Wu GF, Shindler KS, Allenspach EJ, Stephen TL, Thomas HL, Mikesell RJ, Cross AH, Laufer TM. Limited sufficiency of antigen presentation by dendritic cells in models of central nervous system autoimmunity. J Autoimmun. 2011;36:56–64. doi: 10.1016/j.jaut.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia MQ, Qin SX, Wu LJ, Mackay CR, Hyman BT. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer's disease brains. Am J Pathol. 1998;153:31–37. doi: 10.1016/s0002-9440(10)65542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K, Wakita H, Takeda K, Takahashi K. Conditional expression of microRNA against E-selectin inhibits leukocyte-endothelial adhesive interaction under inflammatory condition. Biochem Biophys Res Commun. 2008;371:747–751. doi: 10.1016/j.bbrc.2008.04.160. [DOI] [PubMed] [Google Scholar]
- Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002;22:146–167. doi: 10.1002/med.10001. [DOI] [PubMed] [Google Scholar]
- Zhang H. Molecular Imaging and Contrast Agent Database (MICAD) [database online] National Library of Medicine (US); Bethesda (MD): 2004. Perfluoro-15-crown-5 ether-labeled dendriic cells. NCBI; 2004-2009. Available from: http://micad.nih.gov. [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Phillips ML, Rosenbloom CL, Cruz R, Manning A. E-selectin in focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab. 1996;16:1126–1136. doi: 10.1097/00004647-199611000-00006. [DOI] [PubMed] [Google Scholar]
- Zhou S, Halle A, Kurt-Jones EA, Cerny AM, Porpiglia E, Rogers M, Golenbock DT, Finberg RW. Lymphocytic choriomeningitis virus (LCMV) infection of CNS glial cells results in TLR2-MyD88/Mal-dependent inflammatory responses. J Neuroimmunol. 2008;194:70–82. doi: 10.1016/j.jneuroim.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozulya AL, Reinke E, Baiu DC, Karman J, Sandor M, Fabry Z. Dendritic cell transmigration through brain microvessel endothelium is regulated by MIP-1alpha chemokine and matrix metalloproteinases. J Immunol. 2007;178:520–529. doi: 10.4049/jimmunol.178.1.520. [DOI] [PMC free article] [PubMed] [Google Scholar]