Abstract
Exposure to addictive drugs causes changes in synaptic function within the striatal complex, which can either mimic or interfere with the induction of synaptic plasticity. These synaptic adaptations include changes in the nucleus accumbens (NAc), a ventral striatal subregion important for drug reward and reinforcement, as well as the dorsal striatum, which may promote habitual drug use. As the behavioral effects of drugs of abuse are long-lasting, identifying persistent changes in striatal circuits induced by in vivo drug experience is of considerable importance. Within the striatum, drugs of abuse have been shown to induce modifications in dendritic morphology, ionotropic glutamate receptors (iGluR) and the induction of synaptic plasticity. Understanding the detailed molecular mechanisms underlying these changes in striatal circuit function will provide insight into how drugs of abuse usurp normal learning mechanisms to produce pathological behavior.
Introduction
The development, progression, and persistence of drug addiction are thought to involve dynamic alterations in synaptic transmission within the striatum and related basal ganglia circuits. Synapses in these regions exhibit various forms of long-term synaptic plasticity, which appear to be aberrantly engaged by exposure to addictive drugs [1,2]. These forms of plasticity include strengthening of synaptic connectivity, or long-term potentiation (LTP), as well as its weakening, or long-term depression (LTD). These synaptic changes often manifest themselves as changes in the number and function of iGluRs, including AMPA receptors (AMPARs) and NMDA receptors (NMDARs). Thus, it is of great interest to elucidate the mechanisms of synaptic plasticity in the striatal circuitry that underlie important aspects of addiction related behaviors.
While our understanding of drug-evoked synaptic plasticity has expanded greatly over the past decade, the intrinsic complexity of the striatal circuitry has hampered our ability to place these synaptic adaptations in the context of the neural circuits that mediate behavioral responses relevant to addiction. Recent work has begun to focus on defining the specific neuronal populations that are modified by drug experience, as well as delineating the temporal dynamics and mechanisms of the circuit adaptations that occur during repeated drug exposure, withdrawal or extinction, and events associated with relapse.
Striatal circuitry
The striatal circuitry functions to integrate a complex mix of excitatory, inhibitory and modulatory inputs to optimize adaptive motivated behaviors. At a macroscopic level, subregions of the striatum can be differentiated on the basis of their anatomical connections and behavioral functions. Ventral portions of the striatum, which include the nucleus accumbens (NAc) core and shell, are commonly differentiated from dorsal striatal regions, which include both dorsomedial and dorsolateral sectors. In non-human primates, these subregions are organized in a series of parallel circuits linked in an ascending spiral [3]. Aside from a small number of interneurons, the large majority of neurons in the striatum are medium spiny projection neurons (MSNs). Two distinct populations of MSNs can be distinguished by their different molecular and anatomical properties [4,5]. MSNs expressing D1 dopamine receptors project directly to the midbrain (direct pathway), while those expressing D2 dopamine receptors primarily project to pallidal nuclei (indirect pathway). MSNs are quiescent cells and as such their activity depends heavily on excitatory inputs from cortical and limbic regions [6]. Plastic changes at excitatory synapses thus profoundly influence the output of striatal circuits.
Synaptic properties of MSNs
Recent studies have compared the synaptic properties of direct and indirect pathway MSNs by using fluorescent protein expression in BAC transgenic mice [7,8] to guide targeted whole-cell recordings from acute brain slices. These studies have revealed that MSN populations exhibit different basal electrophysiological membrane and synaptic properties in both NAc core [9] and dorsal striatum [4,5,10,11]. On average, indirect pathway MSNs have a higher synaptic release probability and greater intrinsic excitability relative to direct pathway MSNs. Differences in NMDAR/AMPAR ratios and similarity in mEPSC amplitudes between these cell types suggest indirect MSNs express a greater amount of NMDARs relative to direct pathway MSNs. While NMDAR-dependent LTD occurs in both NAc MSN cell types of naïve animals [9], metabotropic glutamate receptor 5 (mGluR5)-dependent LTD is largely restricted to synapses on to indirect pathway MSNs in both dorsal striatum and NAc core [5,9,11]. In dorsal striatum, the mGluR-dependent LTD is due to the postsynaptic release of endocannabinoids, which activate presynaptic cannabinoid receptor 1 (CB1), to cause a long-lasting depression of transmitter release [5,11]. Synaptic activation of mGluR5 in the indirect pathway of the NAc core results in LTD via two bifurcating pathways; one that appears identical to that observed in dorsal striatal MSNs and a second novel pathway that requires activation of postsynaptic transient receptor potential vanilloid 1 (TRPV1) [9]. The presynaptic component of this LTD requires the active zone protein RIM1α while the postsynaptic component is dependent upon dynamin activity and therefore is likely due to endocytosis of AMPARs [9].
In vivo drug-evoked synaptic plasticity in the NAc
Single cocaine exposure
A single cocaine exposure alters the induction of LTD by mGluR5 in indirect pathway MSNs of the NAc core [9]. Possible mechanisms for this cocaine induced ablation of mGluR5 LTD will be discussed below. In contrast, a single exposure to cocaine does not detectably alter the function or number of iGluRs in the NAc [12–17]. However, acute drug administration often occurs in a novel environment, and environmental novelty can directly affect NAc synapses [16], so care must be taken when designing and interpreting studies of acute drug administration. A single dose of cocaine does alter basal synaptic function in ventral tegmental area dopamine neurons [18,19], a change that appears to be required for subsequent synaptic adaptations in NAc [14].
Chronic cocaine exposure
One well-established consequence of repeated exposure to cocaine and other psychostimulants is an increased density of dendritic spines in NAc MSNs [20], which develops following repeated drug exposure and is exaggerated in females [21]. This morphological change is mediated by epigenetic regulation involving the transcription factor ΔFosB [22] and likely involves histone 3 lysine 9 (H3K9) dimethylation and the lysine dimethyltransferase G9a, as chronic cocaine exposure downregulates G9a in a ΔFosB-dependent manner [22,23]. Several recent studies suggest that increases in spine density may be more robust in direct pathway MSNs in the NAc following exposure to cocaine [13,24–26] or methylphenidate [27]. Furthermore, that a cocaine-induced increase in mESPC frequency in direct pathway MSNs was observed in the NAc shell [13] but not in the NAc core [24] suggests that the NAc shell may be the site of initial synaptic adaptations caused by repeated cocaine exposure.
The formation of new spines may proceed through the generation of “silent” synapses that contain NMDARs but no detectable functional AMPARs [28]. Indeed, electrophysiological and biochemical analysis suggest an increased proportion of silent synapses in the NAc shell shortly following repeated cocaine exposure [15]. This likely contributes to the decreased ratio of AMPAR- to NMDAR-mediated currents in NAc shell MSNs at this same time point [12]. These silent synapses are comprised of newly generated GluN2B subunits of the NMDAR [15] driven by activation of the transcription factor CREB [29]. Both cocaine exposure and viral overexpression of consititutively active CREB lead to slower NMDAR current decay kinetics, consistent with synaptic incorporation of GluN2B. Furthermore, cocaine induces increased binding of pCREB with GluN2B but not GluN2A promoters and the cocaine-induced increase in spines and the synaptic incorporation of GluN2B were absent in MSNs from cocaine treated animals overexpressing dominant negative CREB [29]. These results are consistent with the hypothesis that silent synapses may provide a substrate for future circuit plasticity [30,31]. Changes in NMDAR subunit composition may also influence the subsequent induction of synaptic plasticity, a process known as “metaplasticity” that has been reported in NAc following chronic cocaine self-adminstration [32] and in rats classified as “addicted” based on several behavioral criteria [33].
Protracted withdrawal from chronic drug exposure
The presence of silent synapses in NAc peaks towards the end of chronic cocaine exposure and dissipates during 1–2 weeks of withdrawal [15]. However, the increase in spine density caused by cocaine exposure persists throughout this period [20], and may be specific to direct pathway MSNs ([25]; but see [24]). At the same time, electrophysiological and biochemical evidence suggests that synaptic AMPAR content increases during withdrawal from chronic cocaine administration [12,34,35], a process that may also be specific to direct pathway MSNs in NAc [13,24]. This suggests that newly generated silent synapses are not lost during cocaine withdrawal; instead, they may be “unsilenced” by insertion of functional AMPARs. This process may not require cocaine withdrawal per se, but instead simply develop over time following repeated cocaine administration [24].
After weeks of withdrawal from cocaine self-administration, an important additional adaptation at NAc MSN synapses is a change in AMPAR stoichiometry that involves increased incorporation of receptors lacking the GluA2 subunit, leading to enhanced rectification of AMPAR synaptic currents [34]. This same change was also reported weeks after non-contingent cocaine administration in mice [14], although a separate report failed to replicate this result in rats [36]. This apparent discrepancy may reflect differences in the species used or the age of the animals at the time of cocaine administration. The subcellular redistribution of AMPAR subunits during protracted withdrawal from cocaine may involve transmembrane AMPAR regulatory proteins (TARPs), auxiliary subunits of AMPARs that are known to regulate trafficking and other biophysical properties of AMPARs [37]. Consistent with increases in GluA1following prolonged withdrawal, levels of γ-4 but not γ-2 TARPs are increased [38].
Extinction and reinstatement
Although repeated exposure to and withdrawal from cocaine is associated with enhanced AMPAR content at NAc synapses, this increase is reversed following re-exposure to cocaine [12,17, 39]. A similar decrease in excitatory synaptic strength in NAc shell MSNs was recently reported when mice that received repeated cocaine injections were subsequently exposed to a stressful experience, or following reinstatement of cocaine-conditioned place preference [40]. Both stress and drug re-exposure are potent triggers of relapse in human drug addicts, indicating that this specific synaptic adaptation is a common consequence of multiple experiences linked to relapse.
In addition to altering iGluR function, abstinence from cocaine can alter mGluR5-triggered LTD in the NAc in a subregion- and context-dependent manner [41,42]. For instance, mGluR5-LTD is absent in the NAc shell, but not core, following extended but not early withdrawal from chronic cocaine [42]. However, extinction training prevents mGluR5-induced LTD in the NAc core [41]. These findings in the NAc core are consistent with the critical dependence of group I mGluR function on the Homer family of scaffolding proteins and their expression patterns during withdrawal and extinction training. Withdrawal from chronic cocaine decreases the levels of the long isoforms of Homers [43], whereas, extinction training enhances expression of the long forms Homer1b/c. Increased levels of the long isoforms of Homer are thought to sequester mGluR5 away from the cell surface. Consistent with this action of Homer, overexpression of Homer1c prevented mGluR5-LTD and cue-induced reinstatement of cocaine seeking behavior [41].
Behavioral ramifications of NAc synaptic plasticity
A major challenge in this field is to provide experimental evidence that strongly supports causal links between plastic changes at NAc synapses and behavioral responses to abused drugs. For instance, a decade ago, the decrease in NAc synaptic strength following cocaine re-exposure was shown to occlude induction of NMDAR-dependent LTD at these synapses [17], suggesting these two phenomena share a common mechanistic basis. Based on these findings, subsequent experiments demonstrated that loading NAc MSNs with a peptide sequence that prevents endocytosis of AMPARs can block this form of LTD and also prevent the expression of behavioral sensitization following re-exposure to amphetamine [44]. Several other lines of evidence support the hypothesis that decreased synaptic activation of NAc MSNs enhance drug sensitivity and promotes drug-seeking behavior. Viral-mediated expression of a dominant negative glutamate receptor subunit in the NAc exacerbates reinstatement of cocaine-seeking [45] whereas overexpression of wild-type GluA1 enhances extinction and attenuates reinstatement [45,46]. However, the synaptic impact of these molecular manipulations needs to be directly verified. Furthermore, the process of extinction training (which decreases drug-seeking behavior) is associated with increased expression of iGluRs and other glutamatergic signaling molecules in the NAc shell [46,47].
However, such a simple conclusion is not supported by other findings that suggest the opposite; that synaptic activation of NAc MSNs promotes reinstatement of drug-seeking behavior. For example, classic behavioral pharmacology experiments showed that infusion of a glutamate receptor antagonist into NAc core prevented drug-primed reinstatement while intra-NAc infusion of AMPA itself caused reinstatement [48]. One caveat to this approach, however, is that high concentrations of AMPA may actually reduce MSN activation by causing depolarization block [49]. Another important finding suggesting that increased synaptic drive onto NAc MSNs promotes drug seeking behaviors is that intra-NAc infusion of drugs that selectively block GluA2-lacking AMPARs attenuates the drug-seeking in response to cocaine-associated cures following extended withdrawal from cocaine self-administration [34]. While this finding suggests activation of these receptors promotes drug-seeking in this model, it should be noted that GluA2-lacking AMPARs may internalize more readily following activation [50]. Finally, pharmacological manipulations of glutamate homeostasis in NAc that prevent reinstatement also decrease excitatory synaptic transmission in NAc core [51].
There are two obvious ways to reconcile these apparently contradictory sets of results, both related to distinctions between the functions of different striatal circuits. First, an emerging consensus is that activation of the projection from prelimbic cortex to NAc core promotes drug-seeking, whereas activation of the projection from infralimbic cortex to NAc shell inhibits this process [52]. Second, within a given striatal subregion, activation of direct and indirect pathway MSNs appear to produce divergent behavioral responses to drugs of abuse. Specifically, molecular manipulations that independently influence the activity of indirect and direct pathway MSNs suggest that activation of direct pathway MSNs in NAc enhances sensitivity to drugs of abuse, whereas activation of indirect pathway MSNs decreases drug sensitivity [53–56]. A similar pattern is emerging in dorsal striatum, with direct and indirect pathway MSNs facilitating and inhibiting movement, respectively [57,58]. Further application of cell type-specific molecular manipulations to different striatal subregions in the context of addiction models should provide a clearer picture of the impact of synaptic plasticity on addiction-related behavior.
Dorsal striatum: a home for bad habits?
The various subregions of the striatum are organized in an ascending spiral, with ventral areas projecting to midbrain dopamine neurons that subsequently project to more dorsal striatal regions [3]. Based on this anatomical organization, it has been proposed that initial adaptations in ventral striatal regions come to control more dorsal striatal regions over the course of chronic drug exposure, transforming drug-seeking into a compulsive habit [59]. Recent evidence to support this theory has come from elegant “disconnection” experiments, in which drug-seeking was reduced by lesioning NAc core in one hemisphere while infusing a dopamine receptor antagonist into the dorsolateral striatum of the opposite hemisphere [60].
MSNs in dorsal striatum also exhibit increased dendritic spine density following chronic cocaine exposure [26,27]. Months after repeated exposure to methamphetamine, spine density is increased in MSNs of the dorsolateral striatum but decreased in dorsomedial striatum [61]. As these striatal subregions respectively support habitual and flexible behavior [62], this pattern of structural plasticity is consistent with a shift towards more habitual control of behavior. Another recent study of primates chronically exposed to ethanol also reported increased spine density in the putamen [63], the primate (and human) analog to dorsolateral striatum in rodents. In this study, MSNs of the putamen also exhibited enhanced glutamatergic transmission and increased intrinsic excitability. Thus, limited evidence is consistent with the hypothesis that chronic drug exposure may engage synaptic plasticity in dorsolateral striatal subregions responsible for habit formation.
Conclusion and future directions
By outlining recent developments in the area of striatal synaptic plasticity and addiction, we have highlighted several emerging trends. A number of studies using a variety of approaches have provided convergent evidence regarding the time course of synaptic adaptations in the NAc, following both acute and repeated cocaine exposure, withdrawal/extinction, and re-exposure/reinstatement (Figure 1). However, we are just beginning to understand synaptic adaptations in dorsal striatal subregions, which may be particularly important for late stages of addiction involving habitual drug-seeking. The past several years of research have also greatly advanced our understanding of synaptic plasticity in specific neuronal populations of the striatum, particularly MSNs of the direct and indirect pathway, and the role of these particular cell types in behavioral responses to drugs of abuse. An important next step will be to expand these approaches to models of reinstatement and relapse – one of the most challenging problems in clinical treatment of addiction. Finally, optogenetic techniques have made it possible to dissect the function of different inputs to the striatum [64,65], an approach that should illuminate how synapses formed by these various inputs may be differentially modulated by drugs of abuse. In conclusion, the striatal complex should be examined in terms of its functionally distinct components, at the level of individual neuronal pathways in order to fully understand the cellular and behavioral mechanisms that underlie motivated behaviors, drug abuse and addiction.
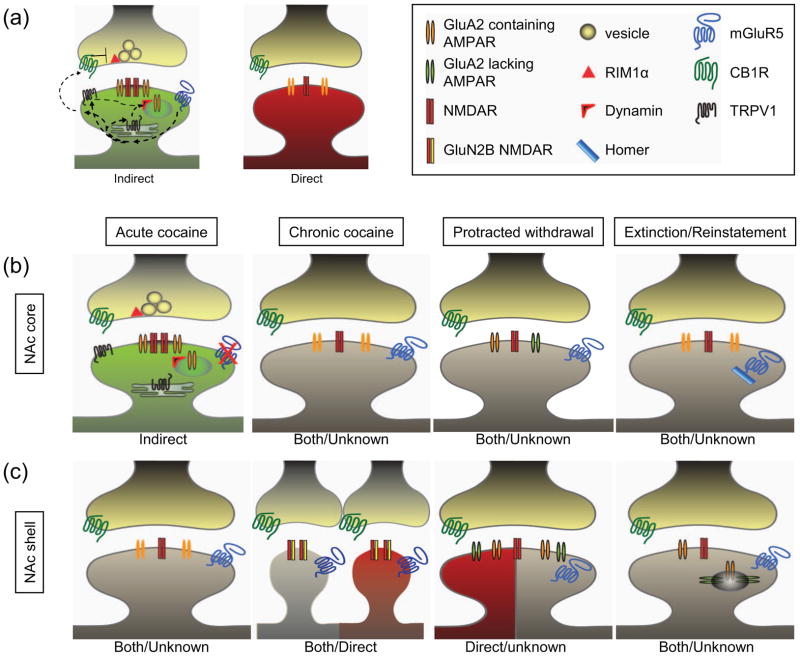
Figure 1. Pathway and experience-dependent differences in NAc MSN excitatory synapses.
(a) Simplified depiction of differences between excitatory synapses on indirect (green) and direct (red) pathway NAc MSNs. In naïve animals, synapses on indirect pathway MSNs express TRPV1 and CB1R-dependent LTD downstream of mGluR5 activation. Synapses on direct pathway MSNs have a lesser relative amount of NMDARs and do not normally express mGluR5-dependent LTD. (b) Simplified summary of the changes in excitatory synaptic properties in the NAc core. Acute cocaine exposure abolishes mGluR5-dependent LTD. Chronic cocaine administration does not change basal synaptic properties but abolishes NMDAR-dependent LTD (not shown). In contrast, protracted withdrawal results in synaptic incorporation of GluA2-lacking AMPARs. Extinction training also abolishes mGluR5-dependent LTD due to changes in Homer levels. (c) Simplified summary of the changes in excitatory synaptic properties in the NAc shell. Acute cocaine exposure has not been shown to alter synaptic function. Chronic cocaine administration causes a transient increase in GluN2B-containing “silent” synapses. Protracted withdrawal leads to an increase in AMPARs that includes incorporation of GluA2-lacking AMPARs. Following experiences known to cause reinstatement including drug re-exposure and stress, AMPARs are endocytosed. Dendritic spines in gray represent findings that either occur in both populations of MSNs or in an unknown population of MSNs. This summary diagram is not meant to depict all the reported synaptic adaptations in NAc MSNs associated with drug experiences.
Highlights.
Modifications in the properties and numbers of excitatory synapses in the striatum occur in response to administration of drugs of abuse.
Drug-induced synaptic modifications in the striatum are complex and depend on many factors including the duration of drug administration and the time point at which synapses are assayed relative to the drug experience.
D1 receptor-expressing direct pathway MSNs and D2 receptor expressing indirect pathway MSNs have different properties and are often modified differently by drugs of abuse.
The two different populations of MSNs are involved in different behavioral responses.
Cell type specific molecular manipulations will be essential for elucidating the detailed striatal circuit adaptations that contribute to addiction.
Acknowledgments
Work on our laboratory is supported by the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. This study provided the first evidence for serial connectivity between the ventral and dorsal striatum in a manner that could account for the transition from goal-directed to habitual behaviors during the development of addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. This study provided one of the first examples of cell-type specific synaptic plasticity in the striatum. [DOI] [PubMed] [Google Scholar]
- 12.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Park BH, Lee JH, Park SK, Kim JH. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry. 2011;69:1026–1034. doi: 10.1016/j.biopsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 15•.Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. Experiments demonstrating that cocaine administration generates silent synapses in the NAc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothwell PE, Kourrich S, Thomas MJ. Environmental novelty causes stress-like adaptations at nucleus accumbens synapses: Implications for studying addiction-related plasticity. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 18.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 19.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 20.Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology. 2011;61:217–227. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. This study provides evidence for molecular mechanisms underlying the cocaine-induced increase in spine density on NAc medium spiny neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maze I, Feng J, Wilkinson MB, Sun H, Shen L, Nestler EJ. Cocaine dynamically regulates heterochromatin and repetitive element unsilencing in nucleus accumbens. Proc Natl Acad Sci U S A. 2011;108:3035–3040. doi: 10.1073/pnas.1015483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31:1895–1904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren Z, Sun WL, Jiao H, Zhang D, Kong H, Wang X, Xu M. Dopamine D1 and N-methyl-D-aspartate receptors and extracellular signal-regulated kinase mediate neuronal morphological changes induced by repeated cocaine administration. Neuroscience. 2010;168:48–60. doi: 10.1016/j.neuroscience.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P. Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens. Proc Natl Acad Sci U S A. 2009;106:2915–2920. doi: 10.1073/pnas.0813179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malenka RC, Nicoll RA. Silent synapses speak up. Neuron. 1997;19:473–476. doi: 10.1016/s0896-6273(00)80362-1. [DOI] [PubMed] [Google Scholar]
- 29.Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: Silent synapse and beyond. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marie H, Morishita W, Yu X, Calakos N, Malenka RC. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron. 2005;45:741–752. doi: 10.1016/j.neuron.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 34•.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. (2) Study demonstrating that during prolonged withdrawal from cocaine self-administartion, NAc MSN synapses express GluA2-lacking AMPARs. Inhibition of these receptors within the NAc decreases subsequent behavioral response to drug associated cues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Rothwell PE, Kourrich S, Thomas MJ. Synaptic adaptations in the nucleus accumbens caused by experiences linked to relapse. Biol Psychiatry. 2011;69:1124–1126. doi: 10.1016/j.biopsych.2010.12.028. This study demonstrates that either a stressful experience or readministration of cocaine following prolonged withdrawal from cocaine caused a decrease in synaptic strength in NAc shell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH, Hsu KS. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 2011;31:4194–4203. doi: 10.1523/JNEUROSCI.5239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, Phelps L, Phillips AG, Wang YT. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310:1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- 45.Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur J Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- 46.Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 47.Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Region specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res. 2009 doi: 10.1016/j.brainres.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 48.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huppe-Gourgues F, O’Donnell P. Periadolescent changes of D(2) -AMPA interactions in the rat nucleus accumbens. Synapse. 2011 doi: 10.1002/syn.20976. [DOI] [PubMed] [Google Scholar]
- 50.Biou V, Bhattacharyya S, Malenka RC. Endocytosis and recycling of AMPA receptors lacking GluR2/3. Proc Natl Acad Sci U S A. 2008;105:1038–1043. doi: 10.1073/pnas.0711412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 53•.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. (2) This study demonstrates that optogenetic activation of direct pathway D1+ NAc MSNs promotes cocaine-elicited conditioned place preference (CPP) whereas actviation of indirect pathway D2+ NAc MSNs inhibits CPP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, Schiffmann SN, de Kerchove d’Exaerde A. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- 55.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci U S A. 2010;107:14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 60•.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. This study provides direct evidence that serial connections between NAc and dorsal striatum are critical for compulsive cocaine seeking behavior. [DOI] [PubMed] [Google Scholar]
- 61.Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci. 2007;25:847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- 62.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 63.Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-yerm, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]