Abstract
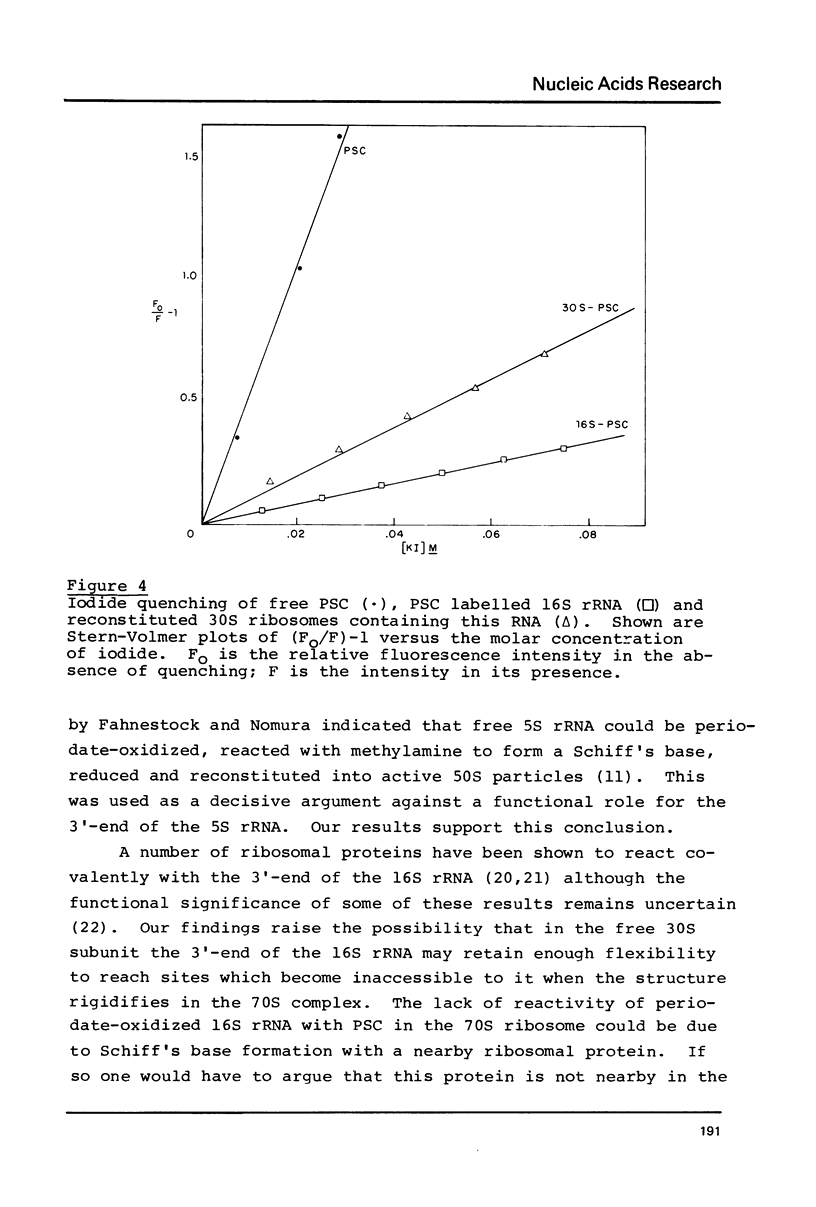
The accessibility of the 3'-ends of E. coli in various states has been probed by reaction, after periodate oxidation, with the fluorescent dye proflavine semicarbazide. Free oxidized 16S and 23S rRNAs each react with 2 equivalents of dye. The 23S rRNA is equally reactive in the 50S subunit and the 70S ribosome. The 16S RRNA 3'-end is accessible in the 30S subunit. In the intact 70S particle, periodate can reach the 3'-end of the 16S rRNA but the dye cannot. The 5S rRNA is relatively inaccessible to periodate oxidation or dye reaction in the 70S particle. Dye-labelled 16S rRNA will reconstitute into 30S particles but they are inactive in polypeptide synthesis. This is apparently due to the inability of the 30S particles to form tight complexes with 50S subunits. Iodide quenching studies indicate that the environment of the 3'-end of 16S rRNA in the 30S particle is different from that of the free rRNA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman C. M., Sidikaro J., Nomura M. Specific inactivation of ribosomes by colicin E3 in vitro and mechanism of immunity in colicinogenic cells. Nat New Biol. 1971 Dec 1;234(48):133–137. doi: 10.1038/newbio234133a0. [DOI] [PubMed] [Google Scholar]
- Chapman N. M., Noller H. F. Protection of specific sites in 16 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1977 Jan 5;109(1):131–149. doi: 10.1016/s0022-2836(77)80049-1. [DOI] [PubMed] [Google Scholar]
- Chung S. I., Folk J. E. Transglutaminase from hair follicle of guinea pig (crosslinking-fibrin-glutamyllysine-isoenzymes-purified enzyme). Proc Natl Acad Sci U S A. 1972 Feb;69(2):303–307. doi: 10.1073/pnas.69.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernilofsky A. P., Kurland C. G., Stöffler G. 30S ribosomal proteins associated with the 3'-terminus of 16S RNA. FEBS Lett. 1975 Oct 15;58(1):281–284. doi: 10.1016/0014-5793(75)80279-1. [DOI] [PubMed] [Google Scholar]
- Erdmann V. A., Sprinzl M., Pongs O. The involvement of 5S RNA in the binding of tRNA to ribosomes. Biochem Biophys Res Commun. 1973 Oct 1;54(3):942–948. doi: 10.1016/0006-291x(73)90785-7. [DOI] [PubMed] [Google Scholar]
- Fields R., Dixon H. B. A spectrophotometric method for the microdetermination of periodate. Biochem J. 1968 Aug;108(5):883–887. doi: 10.1042/bj1080883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Kenner R. A. A protein-nucleic acid crosslink in 30S ribosomes. Biochem Biophys Res Commun. 1973 Apr 16;51(4):932–938. doi: 10.1016/0006-291x(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Laughrea M., Moore P. B. On the relationship between the binding of ribosomal protein S1 to the 30 S subunit of Escherichia coli and 3' terminus of 16 S RNA. J Mol Biol. 1978 Jun 5;121(4):411–430. doi: 10.1016/0022-2836(78)90391-1. [DOI] [PubMed] [Google Scholar]
- Reines S. A., Cantor C. R. New fluorescent hydrazide reagents for the oxidized 3'-terminus of RNA. Nucleic Acids Res. 1974 Jun;1(6):767–786. doi: 10.1093/nar/1.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer M., Shane S. Area of 16S ribonucleic acid at or near the interface between 30S and 50S ribosomes of Escherichia coli. J Bacteriol. 1977 May;130(2):900–910. doi: 10.1128/jb.130.2.900-910.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thammana P., Cantor C. R. Studies on ribosome structure and interactions near the m62Am62A sequence. Nucleic Acids Res. 1978 Mar;5(3):805–823. doi: 10.1093/nar/5.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thammana P., Held W. A. Methylation of 16S RNA during ribosome assembly in vitro. Nature. 1974 Oct 25;251(5477):682–686. doi: 10.1038/251682a0. [DOI] [PubMed] [Google Scholar]
- Van Duin J., Kurland C. G., Dondon J., Grunberg-Mangago M., Branlant C., Ebel J. P. New aspects of the IF3-ribosome interaction. FEBS Lett. 1976 Feb 15;62(2):111–114. doi: 10.1016/0014-5793(76)80030-0. [DOI] [PubMed] [Google Scholar]
- WOOD W. B., BERG P. The effect of enzymatically synthesized ribonucleic acid on amino acid incorporation by a soluble protein-ribosome system from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:94–104. doi: 10.1073/pnas.48.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukioka M., Hatayama T., Omori K. Nucleotide sequence of a region in 23-S RNA adjacent to peptidyl transferase catalytic center of Escherichia coli ribosomes. Eur J Biochem. 1977 Mar 1;73(2):449–459. doi: 10.1111/j.1432-1033.1977.tb11337.x. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L., Scott J. F. PARTIAL PURIFICATION OF SOLUBLE RNA. Proc Natl Acad Sci U S A. 1960 Jun;46(6):811–822. doi: 10.1073/pnas.46.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]



