Abstract
Using morphological, morphometrical, and molecular methods, we describe Leptopharynx bromelicola n. sp. from tank bromeliads of Jamaica. We add significant data to Leptopharynx costatus and briefly characterize and review the genus Leptopharynx Mermod, 1914, including four new combinations. Nine species can be distinguished when applying the following main features and assuming that most or all have the ability to produce macrostomes (MAs): distinct ridges along the right side ciliary rows; special features like spines or wings on the body and of the oral basket; dikinetids present vs. absent from somatic kinety 3; number of kinetids in kinety 6 as two for the costatus pattern and ≥ five for the bromelicola pattern; beginning and structure of kinety 9 as either underneath or far underneath the adoral membranelles and with or without dikinetids; postoral complex present vs. absent; and preoral kinety 4 continuous vs. discontinuous. The 18S rDNA sequences of L. bromelicola and L. costatus differ by 1.7% and show that Leptopharynx forms a distinct clade within the Nassophorea Small & Lynn, 1981. Leptopharynx bromelicola is possibly closely related to Leptopharynx euglenivora Kahl, 1926, which, however, lacks the basket nose so typical of the former. Leptopharynx forms thin-walled, non-kinetosome-resorbing resting cysts maintaining most of the trophic organelles.
Keywords: Bromeliaceae, Leptopharynx costatus, macrostomy, Nassophorea, new combinations, review
The genus Leptopharynx was established by Mermod (1914). Type by monotypy is Leptopharynx costatus Mermod, 1914. Trichopelma Levander, 1900 is possibly a senior synonym of Leptopharynx, but preoccupied by an arachnid (Aescht 2001). Furthermore, Trichopelma sphagnetorum Levander, 1900 might be not identical with L. costatus because it is rather large (~ 60 μm) and usually contains symbiotic green algae (i.e. zoochlorellae). See Prelle (1961) and Aescht (2001) for taxonomic and nomenclatural details. Today, this nassulid genus is either classified into the Microthoracidae Wrześniowski, 1870 (Foissner 1985, 1997) or into the Leptopharyngidae Kahl, 1926, containing also the genus Pseudomicrothorax Mermod, 1914 (Corliss 1979; Lynn 2008).
Of the nine taxa recognized as possibly distinct species (see “Discussion”), only L. costatus has been investigated with modern methods by Prelle (1961), Thompson (1972), Njine (1979), Njine and Didier (1980) and, especially, by Foissner (1979, 1989) and Foissner, Berger, and Kohmann (1994), who provided detailed line drawings and morphometrics as well as light and scanning electron micrographs (SEMs). The poor knowledge of this genus is very likely caused by the small body size (<50 μm), which makes the species difficult to investigate, especially the oral structures. Thus, it is not surprising that some descriptions contain serious mistakes, for instance, that the nasse kinetosomes are ciliated (Njine 1979).
The discovery of a new Leptopharynx species in tank bromeliads prompted us to reinvestigate the genus with both morphological and molecular technics. This showed that the oral structures are highly similar to those described by Peck (1974) in Pseudomicrothorax dubius. Likewise, ontogenesis is quite similar in these taxa (Njine 1979; Peck 1974).
MATERIAL AND METHODS
Material and morphological methods
Leptopharynx bromelicola n. sp. was discovered in a tankwater sample from epiphytic bromeliads on the northern slope of the Blue Mountains in Jamaica, surroundings of the village of Silver Hill, 18°12′N, 76°40′W. The sample was collected in March 2006 and sent to the Salzburg laboratory, where it was screened for the species present and then treated in the following way: the tankwater was sieved through a 500-μm net to remove crustaceans and insect larvae. A portion of the cleaned sample and some squashed wheat grains were used to establish a raw culture. Leptopharynx bromelicola n. sp. and some other ciliates grew well in this culture, where they fed on bacteria and heterotrophic flagellates. Then, some millilitres of this culture were transferred to Eau de Volvic, a French mineral water, enriched with some squashed wheat grains. Here, the ciliates developed in masses, providing sufficient specimens for live observations and various preparations.
Basically the same method was applied for two populations of L. costatus. One is from a tank bromeliad, Tillandsia heterophylla, of a cloud mountain forest near the town of Jalapa, Veracruz province, Mexico. The other population occurred in a European Paramecium culture used for feeding fish larvae. Here, L. costatus developed considerable abundances in old cultures. The source of this culture, which was used for the molecular investigations, is unknown, but is likely from the Max-Plank Institute, Freiburg, Germany. Furthermore, we reinvestigated the slides of the alpine population studied by Foissner (1989). Preliminary investigations were made on three new species from Australia, Finland, and Brazil, respectively; they were cultivated with the non-flooded Petri dish method, described in Foissner, Agatha, and Berger (2002).
Cells were studied in vivo using a high-power oil immersion objective (N. A. 1.32) and differential interference contrast optics. The infraciliature and various cytological structures were revealed by SEM and silver impregnation, as described by Foissner (1991). For protargol impregnation, the specimens were fixed in 70% (w/v) ethanol, which resulted in excellent impregnations, mainly because food vacuoles and other cytoplasmic inclusions did not impregnate. However, the cells became rather strongly inflated, especially the oral basket. Thus, body and basket size were measured also in Chatton–Lwoff silver nitrate preparations, which preserve these features almost perfectly because the fixative contains osmium tetroxide.
Counts and measurements on prepared specimens were performed at a magnification of 1,000X. In vivo measurements were conducted at magnifications of 100–1,000X. Although these are only rough estimates, it is worth giving such data as specimens may change in preparations. Illustrations of live specimens were based on free-hand sketches and micrographs, while those of prepared cells were made with a drawing device.
Molecular methods
To extract genomic DNA for 18S rDNA phylogenies, about 50 specimens each of L. bromelicola n. sp. and the German L. costatus were picked with a micropipette and transferred into 180 μl ATL buffer (Qiagen, Hildesheim, Germany) and 20 μl Proteinase K (20 mg/ml). Subsequently, the genomic DNA was extracted using the protocol for cultured animal cells of the DNEasy Tissue Kit (Qiagen). The 18S rDNA was amplified using the universal eukaryotic primers EukA and EukB (Medlin et al. 1988). The amplification reaction contained 10–20 ng of DNA template, 2.5 U HotStar Taq DNA polymerase (Qiagen) in the manufacturer-provided reaction buffer, 1.5 mM MgCl2, 200 μM of each dNTP, and 0.5 μM of each oligonucleotide primer. The final volume was adjusted to 50 μl with sterile distilled water. The PCR protocol for 18S rDNA gene amplification consisted of an initial hot start incubation of 15 min at 95 °C followed by 30 identical amplification cycles (i.e. denaturing at 95 °C for 45 s, annealing at 55 °C for 1 min, and extension at 72 °C for 2.5 min), and a final extension at 72 °C for 7 min. Negative control reactions included Escherichia coli DNA as a template. The resulting PCR products were cleaned with the PCR MinElute Kit (Qiagen) and cloned into a vector using the TA-Cloning kit (Invitrogen, Carlsbad, CA). Plasmids were isolated with Qiaprep Spin Miniprep Kit (Qiagen) from overnight cultures and PCR re-amplified using M13F and M13R primers to screen for inserts of the expected size (i.e. ~ 1.8 kb in case of the 18S rDNA fragment). Three clones were sequenced bidirectionally using M13 primers with the Big Dye terminator kit on an ABI 3730 automated sequencer (Applied Biosystems, Foster City, CA).
For assessment of the phylogenetic placement of L. bromelicola n. sp. and L. costatus 18S rDNA sequences were aligned to 18S rDNA sequences of other nassophorean ciliates available in GenBank. Furthermore, we included representative sequences of the classes Prostomatea, Oligohymenophorea, Colpodea, Phyllopharyngea, and Plagiopylea in order to account for the polyphyly of the class Nassophorea (Gong et al. 2009). Additionally, we included four environmental sequences (Accession numbers EF024334, EF0242001, FJ810605, and FJ810609), which had the highest sequence similarity to Leptopharynx in a BLASTN search against the NCBI nucleotide data base release 179. As out-groups we chose two spirotrich species Strombidium purpureum and Halteria grandinella. Alignments were constructed using MUSCLE (Edgar 2004), and were refined using Gblocks (Castresana 2000), followed by inspection by eye and manual refinement. The resulting alignment included 1,646 characters and 28 taxa and is available by the authors upon request. Distance, maximum-likelihood and maximum-parsimony analyses were conducted for phylogenies. Neighbour-joining evolutionary distance (BioNJ) and parsimony analyses were carried out in the SEAVIEW program package (vers. 4.2, Galtier, Gouy, and Gautier 1996). Maximum-likelihood bootstrapping analyses were carried out with 100 replicates using RAxML with the setting as described in Stamatakis, Hoover, and Rougement (2008). Maximum likelihood analyses were conducted online on the CIPRES Portal V 2.0 (http://www.phylo.org). Pairwise sequence similarities were calculated with the module pairalign as implemented in the JAguc software package (http://wwwagak.informatik.unikl.de/JAguc). The GenBank accession numbers of L. bromelicola n. sp. and L. costatus are HQ 668466 and HQ 668467.
RESULTS
Description of Leptopharynx bromelicola n. sp. (Tables 1–3 and Fig. 1–17, 22–67)
Table 1.
Morphometric data on macrostome (upper line) and microstome (lower line) Leptopharynx bromelicola n. sp.
| Characteristicsa | Mean | M | SD | SE | CV | Min | Max | n | % Increaseb |
|---|---|---|---|---|---|---|---|---|---|
| Body, length in protargol preparations (μm) | 49.7 | 50.0 | 4.3 | 1.0 | 8.6 | 41.0 | 58.0 | 20 | 46.0 |
| 34.0 | 34.0 | 3.4 | 0.7 | 10.0 | 29.0 | 39.0 | 21 | ||
| Body, width in protargol preparations (μm) | 39.0 | 39.0 | 4.2 | 0.9 | 10.8 | 30.0 | 46.0 | 20 | 57.3 |
| 24.8 | 25.0 | 2.6 | 0.6 | 10.4 | 20.0 | 30.0 | 21 | ||
| Body, length in Chatton–Lwoff silver nitrate preparations (μm) | 54.5 | 53.0 | 5.2 | 1.2 | 9.6 | 47.0 | 65.0 | 20 | 50.6 |
| 36.2 | 37.0 | 4.1 | 0.9 | 11.3 | 30.0 | 45.0 | 21 | ||
| Body, width in Chatton–Lwoff silver nitrate preparations (μm) | 32.4 | 32.5 | 4.0 | 0.9 | 12.3 | 26.0 | 40.0 | 20 | 45.9 |
| 22.2 | 22.0 | 3.5 | 0.8 | 15.9 | 18.0 | 32.0 | 21 | ||
| Body, length in SEM macrostomes (μm) | 43.1 | 44.0 | 4.6 | 1.1 | 10.6 | 31.0 | 50.0 | 17 | — |
| Body, width in SEM macrostomes (μm) | 28.9 | 30.0 | 2.7 | 0.7 | 9.4 | 24.0 | 34.0 | 17 | — |
| Body length: width, ratio in the SEM | 1.5 | 1.5 | 0.1 | 0.1 | 8.8 | 1.3 | 1.7 | 17 | — |
| Body length: width, ratio in protargol preparations | 1.3 | 1.2 | 0.1 | 0.1 | 11.3 | 1.0 | 1.5 | 20 | −7 |
| 1.4 | 1.3 | 0.2 | 0.1 | 16.9 | 1.1 | 1.9 | 21 | ||
| Body length: width, ratio in Chatton–Lwoff silver nitrate preparations | 2.1 | 2.0 | 0.3 | 0.1 | 15.0 | 1.5 | 3.0 | 20 | 23.5 |
| 1.7 | 1.7 | 0.1 | 0.1 | 8.2 | 1.3 | 1.9 | 21 | ||
| Anterior body end to adoral membranelles, distance (μm) | 8.0 | 8.0 | 1.2 | 0.3 | 15.0 | 6.0 | 10.0 | 20 | 14.3 |
| 7.0 | 7.0 | 1.0 | 0.2 | 14.3 | 5.0 | 9.0 | 21 | ||
| Anterior body end to macronucleus, distance (μm) | 18.2 | 18.5 | 2.6 | 0.6 | 14.5 | 13.0 | 22.0 | 20 | 32.8 |
| 13.7 | 14.0 | 2.7 | 0.6 | 19.5 | 9.0 | 19.0 | 21 | ||
| Anterior body end to excretory pore of contractile vacuole (μm) | 28.5 | 29.0 | 2.6 | 0.6 | 9.3 | 23.0 | 33.0 | 20 | 62.9 |
| 17.5 | 18.0 | 2.0 | 0.4 | 11.4 | 14.0 | 21.0 | 21 | ||
| Macronucleus, length (μm) | 9.0 | 9.0 | 0.7 | 0.2 | 7.7 | 8.0 | 10.0 | 20 | 26.8 |
| 7.1 | 7.0 | 0.9 | 0.2 | 11.9 | 6.0 | 10.0 | 21 | ||
| Macronucleus, width (μm) | 8.4 | 9.0 | 0.8 | 0.2 | 9.0 | 7.0 | 9.0 | 20 | 20.0 |
| 7.0 | 7.0 | 0.6 | 0.1 | 9.0 | 6.0 | 8.0 | 21 | ||
| Micronucleus, diameter (μm) | 2.7 | 2.6 | — | — | — | 2.0 | 3.0 | 20 | 12.5 |
| 2.4 | 2.3 | — | — | — | 2.0 | 2.8 | 21 | ||
| Oral basket at distal end, long axis (μm), protargol impregnation | 13.1 | 13.0 | 1.1 | 0.2 | 8.0 | 11.0 | 15.0 | 20 | 77.0 |
| 7.4 | 7.0 | 0.9 | 0.2 | 12.5 | 5.0 | 9.0 | 21 | ||
| Oral basket at distal end, short axis (μm), protargol impregnation | 8.3 | 8.0 | 1.0 | 0.2 | 12.4 | 6.0 | 10.0 | 20 | 69.4 |
| 4.9 | 5.0 | 0.7 | 0.2 | 14.3 | 3.0 | 6.0 | 21 | ||
| Oral basket at distal end, short axis (μm), Chatton–Lwoff preparation | 2.1 About 1 μm |
2.0 | 0.3 | 0.1 | 15.0 | 1.5 | 3.0 | 20 | — |
| Oral basket of macrostomes at distal end, long axis in SEM micrographs (μm) | 10.6 | 11.0 | 1.2 | 0.3 | 11.7 | 8.0 | 13.0 | 23 | — |
| Oral basket of macrostomes at distal end, short axis in SEM micrographs (μm) | 2.2 | 2.2 | 0.5 | 0.1 | 23.5 | 1.4 | 3.0 | 17 | — |
| Oral basket, number of rods (nasse kinetosomes) | 47.9 | 48.5 | 3.1 | 0.7 | 6.5 | 42.0 | 52.0 | 20 | 49.2 |
| 32.1 | 32.0 | 2.8 | 0.6 | 8.5 | 25.0 | 36.0 | 21 | ||
| Somatic kineties, number | 9.0 | 9.0 | 0.0 | 0.0 | 0.0 | 9.0 | 9.0 | 20 | 0.0 |
| 9.0 | 9.0 | 0.0 | 0.0 | 0.0 | 9.0 | 9.0 | 21 | ||
| Somatic kinety 1, number of dikinetids | 17.4 | 17.5 | 1.4 | 0.3 | 7.8 | 14.0 | 20.0 | 20 | 55.4 |
| 11.2 | 11.0 | 1.5 | 0.3 | 13.1 | 8.0 | 13.0 | 21 | ||
| Somatic kinety 1, number of monokinetids | 2.3 | 2.0 | 1.2 | 0.3 | 53.7 | 1.0 | 6.0 | 20 | 27.8 |
| 1.8 | 2.0 | 0.7 | 0.2 | 41.4 | 0.0 | 3.0 | 21 | ||
| Somatic kinety 2, number of dikinetids | 14.1 | 14.0 | 1.8 | 0.4 | 12.5 | 11.0 | 17.0 | 20 | 131.1 |
| 6.1 | 6.0 | 1.0 | 0.2 | 16.3 | 4.0 | 8.0 | 21 | ||
| Somatic kinety 2, number of monokinetids | 25.6 | 26.5 | 2.9 | 0.6 | 11.3 | 21.0 | 31.0 | 20 | 37.6 |
| 18.6 | 18.0 | 3.4 | 0.7 | 18.4 | 14.0 | 26.0 | 21 | ||
| Somatic kinety 3, number of monokinetids (does not have dikinetids) | 45.5 | 45.0 | 4.1 | 0.9 | 8.9 | 34.0 | 51.0 | 20 | 91.2 |
| 23.8 | 22.0 | 4.0 | 0.9 | 16.8 | 18.0 | 30.0 | 21 | ||
| Somatic kinety 4, number of monokinetids (does not have dikinetids) | 47.2 | 47.0 | 4.3 | 1.0 | 9.1 | 36.0 | 55.0 | 20 | 67.4 |
| 28.2 | 28.0 | 3.2 | 0.7 | 11.4 | 23.0 | 35.0 | 21 | ||
| Somatic kinety 5, number of monokinetids (does not have dikinetids) | 34.5 | 34.0 | 5.2 | 1.2 | 15.1 | 18.0 | 42.0 | 20 | 128.5 |
| 15.1 | 15.0 | 3.1 | 0.7 | 20.6 | 12.0 | 21.0 | 21 | ||
| Somatic kinety 6, number of monokinetids (does not have dikinetids) | 18.6 | 19.0 | 2.7 | 0.6 | 14.7 | 12.0 | 24.0 | 20 | 70.6 |
| 10.9 | 10.0 | 1.7 | 0.4 | 15.5 | 9.0 | 15.0 | 21 | ||
| Somatic kinety 7, number of monokinetids (does not have dikinetids) | 9.1 | 9.0 | 1.0 | 0.2 | 11.0 | 8.0 | 11.0 | 20 | 13.8 |
| 8.0 | 8.0 | 1.0 | 0.2 | 13.1 | 6.0 | 10.0 | 21 | ||
| Somatic kinety 8, number of monokinetids (does not have dikinetids) | 4.0 | 4.0 | 0.5 | 0.1 | 11.5 | 3.0 | 5.0 | 20 | 5.3 |
| 3.8 | 4.0 | 0.5 | 0.1 | 14.3 | 3.0 | 5.0 | 21 | ||
| Somatic kinety 9, number of dikinetids | 9.2 | 9.0 | 2.4 | 0.5 | 26.4 | 4.0 | 15.0 | 20 | 283.3 |
| 2.4 | 2.0 | 0.5 | 0.1 | 20.9 | 2.0 | 3.0 | 21 | ||
| Somatic kinety 9, number of monokinetids | 14.0 | 14.0 | 3.5 | 0.8 | 25.4 | 7.0 | 23.0 | 20 | 11.0 |
| 12.7 | 13.0 | 2.5 | 0.6 | 20.0 | 8.0 | 18.0 | 21 | ||
| Preoral ciliary rows, number | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 20 | 0 |
| 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 | ||
| Preoral kinety 1, number of dikinetids | 2.1 | 2.0 | — | — | — | 2.0 | 3.0 | 20 | 5.0 |
| 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 | ||
| Preoral kinety 1, number of monokinetids | 0.2 | — | — | — | — | 0.0 | 1.0 | 20 | — |
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21 | ||
| Preoral kinety 2, number of dikinetids | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 20 | 29.0 |
| 3.1 | 3.0 | — | — | — | 3.0 | 4.0 | 21 | ||
| Preoral kinety 2, number of monokinetids | 1.9 | 2.0 | 0.6 | 0.1 | 31.7 | 1.0 | 3.0 | 20 | 58.3 |
| 1.2 | 1.0 | — | — | — | 1.0 | 2.0 | 21 | ||
| Preoral kinety 3, number of dikinetids | 6.1 | 6.0 | 0.4 | 0.1 | 7.3 | 5.0 | 7.0 | 20 | 45.2 |
| 4.2 | 4.0 | — | — | — | 4.0 | 5.0 | 21 | ||
| Preoral kinety 3, number of monokinetids | 1.8 | 2.0 | 0.6 | 0.1 | 34.2 | 1.0 | 3.0 | 20 | 5.9 |
| 1.7 | 2.0 | 0.8 | 0.2 | 47.7 | 1.0 | 3.0 | 21 | ||
| Preoral kinety 4, number of dikinetids | 9.1 | 9.0 | 1.0 | 0.2 | 10.6 | 8.0 | 11.0 | 20 | 59.6 |
| 5.7 | 6.0 | 0.6 | 0.1 | 10.2 | 5.0 | 7.0 | 21 | ||
| Preoral kinety 4, number of monokinetids | 5.1 | 5.0 | 1.2 | 0.3 | 23.7 | 3.0 | 8.0 | 20 | 27.5 |
| 4.0 | 4.0 | 1.0 | 0.2 | 25.0 | 2.0 | 5.0 | 21 | ||
| Oral anlage, number of dikinetids | 5.5 | 6.0 | 0.7 | 0.2 | 12.6 | 4.0 | 6.0 | 20 | 175 |
| 2.0 | 2.0 | — | — | — | 2.0 | 3.0 | 21 | ||
| Oral anlage, number of monokinetids | 0.7 | 1.0 | — | — | — | 0.0 | 2.0 | 20 | −30 |
| 1.0 | 1.0 | — | — | — | 0.0 | 1.0 | 21 | ||
| Adoral membranelle 1, number of basal bodies | 3.6 | 4.0 | 0.8 | 0.2 | 21.4 | 2.0 | 4.0 | 20 | −5 |
| 3.8 | 4.0 | 0.5 | 0.1 | 13.4 | 2.0 | 4.0 | 21 | ||
| Adoral membranelle 2, number of basal bodies | 17.1 | 18.0 | 2.0 | 0.4 | 11.5 | 15.0 | 21.0 | 20 | 28.6 |
| 13.3 | 12.0 | 2.2 | 0.5 | 16.5 | 9.0 | 18.0 | 21 | ||
| Adoral membranelle 3, number of basal bodies | 20.4 | 21.0 | 2.1 | 0.5 | 10.2 | 18.0 | 24.0 | 20 | 29.9 |
| 15.7 | 15.0 | 1.9 | 0.4 | 11.9 | 12.0 | 21.0 | 21 | ||
| Resting cysts from macrostomes, length (μm) | 36.3 | 36.0 | 2.4 | 0.8 | 6.6 | 32.0 | 40.0 | 9 | 26.5 |
| Resting cysts from macrostomes, width (μm) | 25.2 | 26.0 | 2.0 | 0.7 | 7.9 | 21.0 | 28.0 | 9 | 43.2 |
| Resting cysts from microstomes, length (μm) | 28.7 | 28.0 | 2.2 | 0.7 | 7.8 | 27.0 | 31.0 | 11 | |
| Resting cysts from microstomes, width (μm) | 17.6 | 17.0 | 1.3 | 0.4 | 7.3 | 16.0 | 20.0 | 11 |
Data based, if not mentioned otherwise, on protargol-impregnated, randomly selected specimens from a single culture. See species description, for distinguishing microstomes from macrostomes.
% Increase, the increase in the mean value for macrostomes relative to microstomes.
CV, coefficient of variation in %; mean, arithmetic mean; M, median; Max, maximum; Min, minimum; n, number of specimens investigated; SD, standard deviation; SE, standard error of mean; SEM, scanning electron microscopy.
Table 2.
Distribution of main characteristics in various species and populations of Leptopharynx.
| Characteristics | bromelicola a | costatus 1b | costatus 2c | costatus 3d | n. sp. 1e | n. sp .2f | n. sp. 3g |
|---|---|---|---|---|---|---|---|
| Microstomes | Present | Present | Present | Present | Present | Present | Absent |
| Macrostomes | Present | Present | Present | ? | Present | Absent? | Present |
| Nose | Present | Absent | Absent | Absent | Absent | Absent | Absent |
| Distinct ridges along ciliary rows of macrostomes | Present | Absent | Absent | Absent | Present | Absent | Absent |
| Spines and wings on left side | Absent | Absent | Absent | Absent | Absent | Present | Absent |
| Adoral membranelle 1 in microstomes | Present | Absent | Variable | Absent | Absent | Absent | — |
| Adoral membranelle 1 in macrostomes | Present | Present | Variable | ? | Present | — | Present |
| Number of ciliary rows in microstomes | 9 | 9 | 9 | 9 | 9 | 9 | — |
| Number of ciliary rows in macrostomes | 9 | 10 | 10 | — | 9 | — | 9 |
| Dikinetids in kinety 3 | Absent | Present | Present | Present | Present | Present | Present |
| Number of kinetids comprising kinety 6 in (MA) | >5 | 2 | 2 | 2 | >5 | 2 | 2 |
| Begin of kinety 9 relative to the adoral membranelles | Underneath | Far underneath | Far underneath | Far underneath | Far underneath | Far underneath | Far underneath |
| Preoral kinety 4 | Continuous | Discontinuous | Discontinuous | Discontinuous | Discontinuous | Discontinuous | Discontinuous |
| Postoral complex | Lacking | Present | Present | Present | Present | Present | Present |
For details, see text and Table 1.
A German population. Numerical values based on five specimens.
A population from a tank bromeliad of Mexico. Numerical values based on five specimens.
A population from alpine soil of Austria. For details, see Foissner (1989).
Australian population from jungle soil in the Botanical Gardens of Cairns. Will be described later.
From floodplain soil in Brazil. Will be described later.
From coniferous litter in Finland. Will be described later.
Table 3.
Distinguishing features of Leptopharynx bromelicola and Leptopharynx costatus.
| Characteristics | L. bromelicola a |
L. costatus
|
||||
|---|---|---|---|---|---|---|
| Foissner (1989) b | Prelle (1961) c | Njine (1979) d | Mexico (unpubl.)e | Germany (unpubl.)e | ||
| Distinct triangular area between kineties 1 and 2 | Present | Absent | Absent | Absent | Absent | Absent |
| Distinct cortical ridges in macrostomes | Present | Absent | Absent | Absent | Absent | Absent |
| Dikinetids in kinety 3 | 0 (MI+MA) | 4 (MI) | 4 (MI) | 13 (MI) 16 (MA) | 9 (MA) | 4 (MI) |
| Dikinetids in kinety 4 | 0 (MI+MA) | 0 (MI) | 0 (MI) | 1? (MI) 11(MA) | 0 (MI+MA) | Several (MA) |
| Number of basal bodies in kinety 6 | 11 (MI) 19 (MA) | 2 (MI) | 2 (MI) | 1 (MI) 2 (MA) | 2 (MA) | 2 (MA) |
| Kinety 9 commencing underneath or far underneath of adoral membranelles |
Underneath | Far underneath | Far underneath | Far underneath | Far underneath | Far underneath |
| Dikinetids present/absent in kinety 9 | Present | Absent | Absent | Absent | Absent | Absent |
| Total number of somatic basal bodies | 212 (MI) 343 (MA) | 185 (MI) | ? | ? | 186 (MI) 247 (MA) | 182 (MI) 264 (MA) |
| Preoral kineties ventral or ventrolateral | Ventrolateral | Ventral | Ventral, ventrolateral | Ventral | Ventral (MI+MA) | Ventral (MI+MA) |
| Preoral kinety 4 continuous or discontinuous | Continuous | Discontinuous | Discontinuous | Discontinuous | Discontinuous | Discontinuous |
| Macrostomes present/absent | Present | ? | Absent | Present | Present | Present |
| Percentage (%) of macrostomes (n 100) | 57 | — | — | — | 6 | 11 |
| Number of adoral membranelles in microstomes | 3 | 2 | 2 | 3 | 2 | 2 |
| Number of adoral membranelles in macrostomes | 3 | — | ? | 3 | 2 | 3 |
| Number of adoral membranelles in dividers | 3 | ? | ? | 3 | 3 | ? |
| Basket nose in macrostomes present/absent | Present | — | ? | Absent | Absent | Absent |
| Postoral complex present/absent | Absent | Present | Present | Present | Present | Present |
For details, see Table 1.
Re-investigated; average values from detailed Table in Foissner (1989).
From the description and figures provided (Fig. 2a, b).
Assuming that his L. macrostoma is the macrostome of his L. costatus (see chapter on “Comparison with macrostome congeners”). However, we would not exclude the possibility that it is a distinct species (see chapter on “Comparison with L. costatus”).
Numbers based on averages from five macrostome specimens each.
MA, macrostomes; MI, microstomes.
Fig. 1–10.
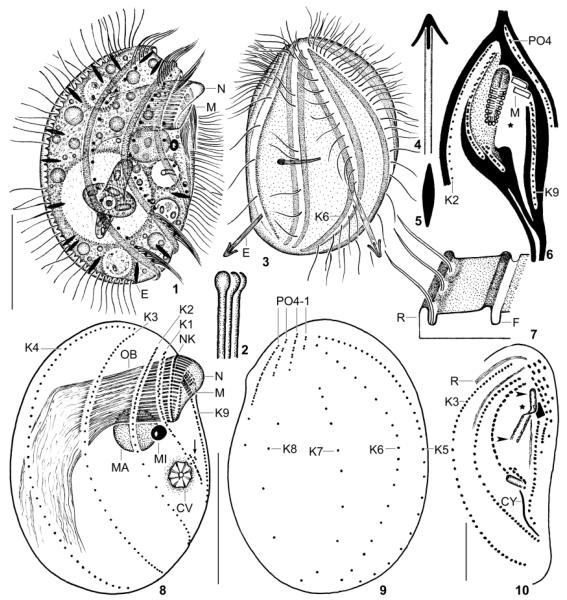
Leptopharynx bromelicola n. sp., macrostome specimens from life (1, 3–5), redrawn from scanning electron microscopy micrographs (2, 6, 7), after protargol impregnation (8, 9), and after Chatton–Lwoff silver nitrate impregnation (10). 1, 3. Right and left side view of representative specimens, the left one has just captured an euglenid flagellate. Note the conspicuous ridges and furrows as well as the comparatively densely ciliated kinety 6, a main difference to L. costatus, which has only two cilia in this kinety. 2. Distal end of oral basket rods. 4, 5. Exploded and resting extrusome (~ 4 × 0.8 μm). 6. Ridge pattern (black) of ventral side. The asterisk marks a rather deep concavity left and underneath the oral basket. The stippled area is also deepened but less than that of the deep concavity. The oral basket is covered by membranous material in the anterior half and oblique to the body’s main axis. 7. Ridge and furrow pattern of the cortex. 8, 9. Right and left side view of macrostome hapantotype specimen, length 52 μm. The arrow marks the oral primordium. 10. Ventrolateral view showing the narrow opening of the oral basket (arrowheads) and the anterior elongation of the basket (nose, asterisk). AM, adoral membranelles; CV, contractile vacuole; CY, cytopyge; E, resting and exploded trichocysts; F, cortex furrow; K1–9, somatic kineties; MA, macrostome; MI, microstome; N, nose; NK, nasse kinetosomes; OB, oral basket; PO1–4, preoral kineties; R, cortical ridge; Scale bars 15 μm (Fig. 10) and 25 μm (Fig. 1, 2, 8, 9).
Fig. 11–29.
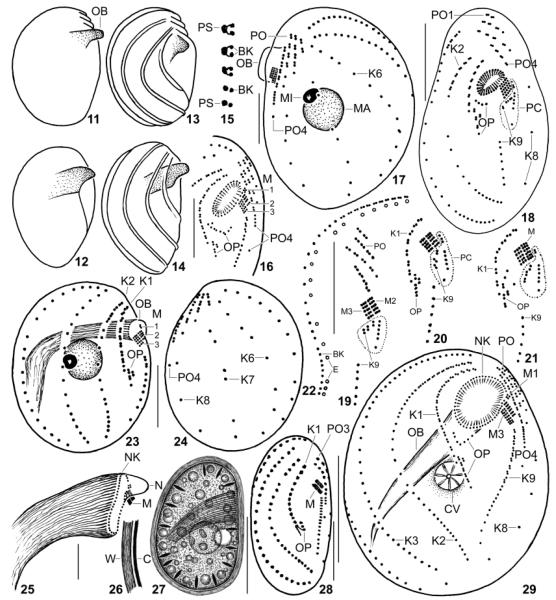
Leptopharynx bromelicola n.sp. (11–17, 22–29) and Leptopharynx costatus (18–21) from life (11, 12, 26, 27), redrawn from scanning electron microscopy micrographs (13, 14), after silver nitrate impregnation (15), and after protargol impregnation (16–25, 28, 29). 11–14. Variability of macrostome body shape; cortical ridges not shown in Fig. 11, 12. 15. Structure of kinety 2. 16, 29. Ventral views of a microstome (16) and of a transition stage (29) to a macrostome, where the oral basket opening is broadly elliptical due to insufficient fixation. 17. Left side view of a transition stage showing the preoral kineties on the left side. 18–21. L. costatus, ventral overview (18, from Foissner 1989) and details (19–21, originals from same population), showing the location and variability of the postoral complex (PC, encircled by dotted line), which is absent from L. bromelicola. 22. Arrangement of basal bodies and extrusomes in kinety 2. 23, 24. Right and left side view of microstome hapantotype, length 28 μm; drawn to scale relative to the macrostome shown in Fig. 8, 9. 25. Oral basket of a macrostome. 26, 27. Right side view of a macrostome resting cyst. 28. Ventral view of a microstome resting cyst. BK, basal body; C, cortex; CV, contractile vacuole; E, extrusomes; K1–9, somatic kineties; M(1–3), adoral membranelles; MA, macrostome; MI, microstome; N, nose; NK, nasse kinetosomes; OB, oral basket; OP, oral primordium; PC, postoral complex; PO(1–4), preoral kineties; PS, parasomal sac; W, cyst wall. Scale bars = 10 μm (Fig. 16–18, 23–25, 28) and 20 μm (Fig. 22, 27, 29).
Fig. 30–42.
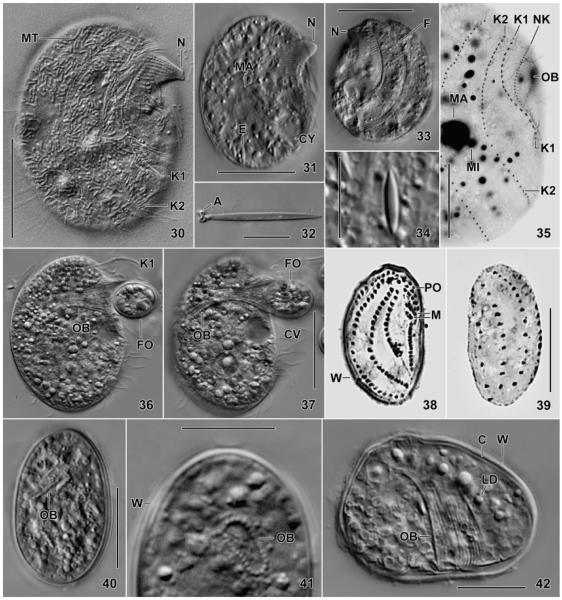
Leptopharynx bromelicola n. sp., trophic (30–37) and cystic (38–42) macrostome (30–37, 41, 42) and microstome (38–40) specimens from life (30–34, 36, 37, 40–42), after protargol impregnation (35), and Chatton–Lwoff silver nitrate impregnation (38, 39). 30, 31. Right side views of slightly squashed specimens, showing the typical nose produced by elongated oral basket rods. Note the numerous, sausage-shaped mitochondria (30, MT). 32, 34. Exploded and resting extrusome. 33. Left side view showing two deep furrows accompanied by ridges. 35. Right side view showing kineties 1 and 2 strongly diverging posteriorly. 36, 37. A specimen feeding on a resting cyst of a flagellate (Polytomella?). The cytoplasm is studded with lipid droplets. The cilia of kinety 1 (K1) form a membranous structure during feeding. 38–42. The resting cysts are covered by an about 1 μm thick wall, well recognizable in the squashed specimen shown in Fig. 42. The infraciliature, the oral basket, and some extrusomes are maintained, while the cilia are possibly resorbed. A, anchors; C, cortex; CV, contractile vacuole; CY, cytopyge; E, extrusomes; F, furrow; FO, food; K1, 2, somatic kineties; LD, lipid droplets; M, adoral membranelles; MA, macrostome; MI, microstome; MT, mitochondria; N, nose; NK, nasse kinetosomes; OB, oral basket; PO, preoral kineties; W, cyst wall. Scale bars = 5 μm (Fig. 32, 34), 15 μm (Fig. 35, 38–42), 25 μm (Fig. 36, 37), and 30 μm (Fig. 30, 31, 33).
Fig. 43–49.
Leptopharynx bromelicola n. sp., somatic and oral ciliary pattern of macrostome (43–47) and microstome (48, 49) specimens after protargol (43–47) and Chatton–Lwoff silver nitrate (48, 49) impregnation. The width of the oral basket opening has strongly increased due to insufficient fixation (cf. Fig. 48, 57, 59, 63). 43, 45. Ventrolateral view of a specimen with very large oral basket recognizable by the nasse kinetosomes. The asterisk marks the triangular area between kineties 1 and 2. Figure 45 shows the adoral ciliature at higher magnification, especially the minute membranelle 1 consisting of only four basal bodies. 44, 47. Ventral views, where the preoral kineties are on the left side. 48, 49. The microstomes have the same ciliary pattern as the macrostomes, but are much smaller and have much less basal bodies. The arrow (48) marks part of the oral primordium. Scale bars = 5 μm (Fig. 46) and 20 μm (Fig. 43, 44, 47–49).
Fig. 50–60.
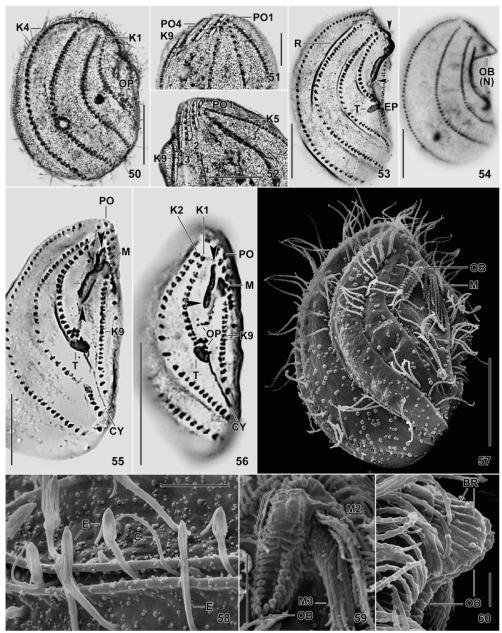
Leptopharynx bromelicola n. sp., macrostome (50–55, 58–60) and microstome (56, 57) specimens after Klein–Foissner (50–52) and Chatton–Lwoff (53–56) silver nitrate impregnation, and in the scanning electron microscopy (57–60). 50, 53, 54. Right side views, showing the dense ciliation of kineties 1–4, the dense cortical granulation (50), and the large oral basket (53, ends marked by arrowheads) with a distinct nose (54). The asterisk marks kineties 1 and 2 greatly widening posteriorly. 51, 52. Ventrolateral view showing a narrowly meshed silverline pattern associated with the preoral kineties and the anterior portion of kinety 9. 55, 56. Ventral view showing macrostomes and microstomes differing mainly in the length of the oral basket opening (arrowheads) and the number of basal bodies. The lines (55) indicate the width of the basket opening, while the asterisk denotes the transition site of long and short basket rods. 57. Ventrolateral view of a microstome postdivider with oral basket marked by arrowhead. 58. Part of left side, showing exploding extrusomes within and between the cortical furrows. 59, 60. Frontal and lateral view of oral basket, which is covered by a membranous structure in the upper, nose-forming half (bracket). Scale bars = 2 μm (Fig. 60), 5 μm (Fig. 58, 59), 10 μm (Fig. 51, 52), 15 μm (Fig. 57), and 20 μm (Fig. 50, 53–56). Explanation of abbreviations for Fig. 43–60: BR, basket rods; C, cilium; CY, cytopyge; E, extrusomes; EP, excretory pore; K1–9, somatic kineties; MA, macrostome; MI, microstome; M(1–3), adoral membranelles; NK, nasse kinetosomes; OB, oral basket; OP, oral primordium; PO(1–4), preoral kineties; R, cortical ridge; T, excretory tube.
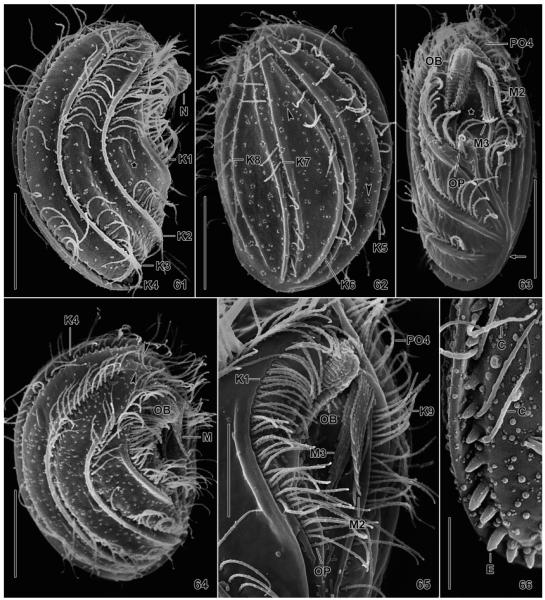
Fig. 61–66.
Leptopharynx bromelicola n. sp., macrostome specimens in the scanning electron microscopy. 61. Right side view, showing the typical nose, the ridges accompanying kineties 1–4, and the lack of cilia in mid-body. Kineties 1 and 2 diverge posteriorly, producing an elongate triangular area (asterisk). 62. Left side view, showing the kineties in deep furrows and accompanied by a ridge each right and left. The arrowheads mark circular accumulations of blebs possibly related to the extrusomes. 63, 65. Ventral and ventrolateral view, showing the oral ciliary pattern and the oral basket. The asterisk (63) marks the oral concavity. The arrow indicates the merging ridges of kineties 4 and 9. 64. Right side view of a broadly elliptical specimen showing the preoral kineties (arrowheads) occupying the ventral side, as in Leptopharynx costatus. 66. Right posterior corner, showing exploding extrusomes. C, cilia; E, extrusomes; K1–9, somatic kineties; M2, 3, adoral membranelles; N, nose; OB, oral basket; OP, oral primordium; PO4, preoral kinety 4. Scale bars = 5 μm (Fig. 66), 10 μm (Fig. 65), and 15 μm (Fig. 61–64).
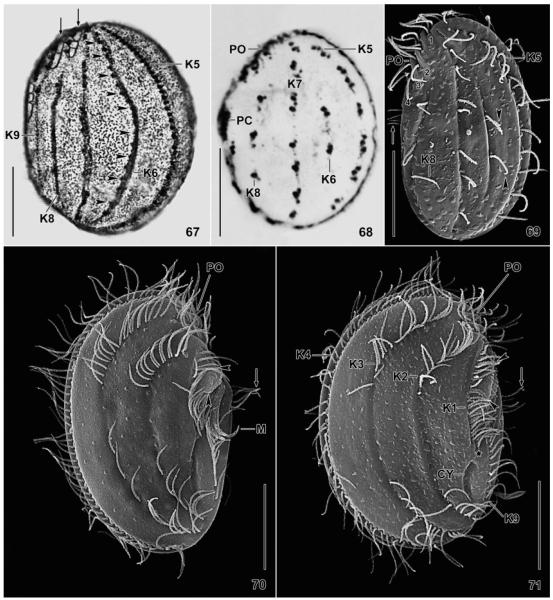
Fig. 67–71.
Leptopharynx bromelicola n. sp. (67) and Leptopharynx costatus (68–71) after Klein–Foissner silver nitrate impregnation (67, 68) and in the scanning electron microscopy (69–71). 67. Left side view, showing the dense cortical granulation and the comparatively dense ciliation of kinety 6 (arrowheads), a main feature of this species (bromelicola pattern). The arrows denote a narrow-meshed silverline pattern left of the preoral kineties and in the anterior portion of kinety 9. 68. Left side view of an Austrian population (from Foissner 1979), showing kinety 6 consisting of only two kinetids (costatus pattern). 69. Left side view of a German population, showing kinety 6 composed of two monokinetids. The arrow marks the cilia of the postoral complex. Numerals denote the preoral kineties. 70, 71. Right side views of a microstome and a macrostome specimen from a tank bromeliad of Mexico. The long axes of the oral baskets are marked by arrowheads. The arrows mark the ciliated portion of the postoral complex. CY, cytopyge; K1–9, somatic kineties; M, adoral membranelles; PC, postoral complex; PO, preoral kineties. Scale bars = 15 μm.
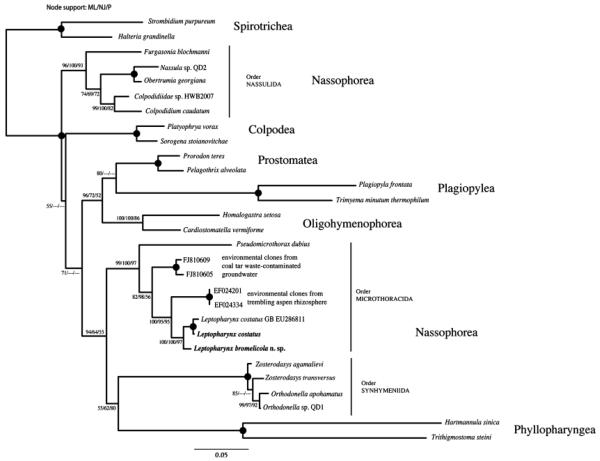
Fig. 72.
The 18S rDNA neighbour joining (NJ) tree showing the phylogenetic placement of Leptopharynx bromelicola n. sp. and the German population of Leptopharynx costatus sequenced in this study (in bold). Bootstrap values above 50 for the NJ evolutionary distance (BioNJ, 1,000 replicates), maximum-likelihood (ML, 100 replicates) analyses, and parsimony (P, 1,000 replicates) are given at the individual nodes. Filled circles at nodes indicate full support from all tree methods. For details about tree construction, see “Materials and Methods.”
In the environmental samples, this species attracted us by the huge oral basket and the rather large size, when compared with the common L. costatus. In laboratory cultures, both microstome (MI) and MA individuals develop with a ratio of about 1.3 (Table 3). Microstomes and MA are described together because they are very similar morphologically. Briefly, they differ in two main features useable for identification in vivo, in silver preparations, and the SEM: the MI lack the very distinct cortical ridges present in the MA; and most morphometrics are higher/larger in the MA than the MI, differing by 0% (e.g. number of ciliary rows) to 283% (number of dikinetids in somatic kinety 9). These and other differences are shown in Table 1, both as real values and as percentages.
As there are many transition stages, MI and MA cannot be separated unequivocally. Preliminary measurements suggested to classify protargol-impregnated specimens <40 μm as MI and those >40 μm as MA. This limit was increased to 45 μm for the Chatton–Lwoff silver nitrate-impregnated specimens where shrinkage is usually only about 5% (data not shown).
Foissner (1989) showed that L. costatus has an ordinary intrapopulation variability (i.e. most variability coefficients are <20%). This applies also to L. bromelicola n. sp. when MI and MA are analysed separately (Table 1). Thus, these values are usually not repeated in the description.
In vivo, the MI of L. bromelicola n. sp. have a size of 30–50 × 20–35 μm, usually it is 40 × 25 μm, while the MA are 45–70 × 25–45 μm, frequently about 60 × 35 μm, as calculated from some in vivo measurements and the Chatton–Lwoff silver nitrate-impregnated specimens, where size and shape are well preserved due to the osmic acid contained in the fixative. In the protargol preparations, some continuous features are highly distorted due to the poor alcohol fixation, for instance, the width of the oral basket. Likewise, the specimens shrink considerably in SEM preparations (Table 1).
Basically, the body shape is as in L. costatus, that is, slenderly to broadly ellipsoidal with distinctly convex dorsal side and flat to slightly convex ventral side more or less receding preorally. The MI are very broadly to ordinarily elliptical with a range of 1.3–1.9:1 and an average of 1.7:1. The MA are more slender with a length:width ratio of 1.5–3:1, on average 2.1:1. Ovate, obovate, or almost circular specimens rarely occur. The cells are laterally flattened up to 2:1 and are slenderly elliptical to slenderly obovate in ventral and dorsal view (Table 1 and Fig. 1, 3, 11–14, 30, 31, 33, 36, 57, 61–64). Compared with the congeners, L. bromelicola n. sp. has two conspicuous features best recognizable in the MA: distinct ridges and furrows along the ciliary rows and a curious, nose-like process subapically, produced by elongated rods of the oral basket.
The nuclear apparatus is in the anterior body half underneath the oral basket and in or left of body midline; rarely, it is a very near to the ventral surface. The macronucleus, which is rather small (i.e. ~ 18% of body length, as in many nassulids; Foissner et al. 2002), is globular to broadly ellipsoidal and contains globular, argyrophilic masses (i.e. probably nucleoli) up to 2 μm across. The globular micronucleus is usually attached to the ventral half of the macronucleus and becomes conspicuously fusiform in very early dividers (Table 1 and Fig. 1, 8, 17, 23, 31, 35, 47). The contractile vacuole and the cytopyge are located as in the congeners, i.e. in the third quarter of the ventral side. The contractile vacuole, which is near mid-body, has a distinct canal recognizable in vivo and in silver nitrate preparations; it contains fibre bundles forming a star-like pattern (Fig. 1, 8, 29, 31, 37, 53). The cytopyge is underneath the contractile vacuole, usually forming a blister containing food remnants (e.g. bacterial spores); in silver nitrate preparations, the cytopyge is represented by a thick silverline extending between the posterior portion of somatic kineties 2 and 9 (Table 1 and Fig. 10, 48, 55, 56). The extrusomes are also as in the congeners, that is, bluntly fusiform and very compact, producing bright dots left of the ciliary rows on the right body side and within the furrows of the left side; furthermore, the extrusomes are scattered between the ciliary rows. In vivo and when resting, the extrusomes are about 4 × 0.8 μm in size, while up to 20 μm long and with four rod-like anchors when exploded; the anchor rods are 3–4 μm long in scanning micrographs (Table 1 and Fig. 3–5, 22, 31, 44, 58, 66).
Basically, the cortex is as in the congeners, i.e. comparatively thick (~ 1 μm), glossy, and usually studded with up to 1 μm blebs occasionally forming a circular pattern, indicating that some blebs could result from exocytotic processes related to extrusome release (Fig. 62); likely, the blebs produce the dense cortical granulation recognizable in dry silver nitrate preparations (Fig. 50, 62, 67). Underneath the cortex, there are countless sausage-shaped mitochondria up to 3 μm long (Fig. 30). The cortex of the MI individuals is only slightly furrowed, thus resembling that of L. costatus (Fig. 57, 69, 70). The MA, in contrast, have a complex ridge and furrow pattern (Fig.1, 2, 13, 14, 30, 33, 61–65). The ridges, which are 1–2 μm width, are sometimes rather high and almost wing-like, especially those of kineties 6 and 7. The ridges right of kineties 1, 2, and 3 are conspicuous, while those of kineties 4 and 5 are less distinct when the specimen is viewed laterally, because they are on the dorsal margin of the cell; the ridge accompanying kinety 1 commences subapically (Fig. 6, 63). Kineties 6, 7, and 8 each extend in a furrow produced by a ridge right and left of the kineties. Kinety 9 is separated from the concave oral field by a ridge merging into the ridge of kinety 4 posteriorly. The ridges separating the preoral kineties are of ordinary appearance (Fig. 63, 64, 71). A rather complex ridge pattern shown in the semischematic Fig. 6 extends on the ventral side. Briefly, the oral primordium extends in a furrow formed by an Y-shaped ridge; kinety 9 is separated from the concave oral field by a ridge; and the adoral membranelles are anchored in the angle formed by the ridge separating kinety 9 and preoral kinety 4 (Fig. 58, 59, 62, 65, 71).
The microthoracids do not have a classical silverline pattern, but the whole cortex is studded with minute, argyrophilic granules, except of the preoral area, where distinct small meshes occur, first described by Klein (1928) in Microthorax pusillus. In L. bromelicola n. sp., the meshes are very distinct and extend left of the preoral kineties and along the anterior, dikinetidal portion of kinety 9 (Fig. 50–52, 67). The silverline meshes of the individual preoral kineties are not connected with each other. The pattern is not known in L. costatus.
The cytoplasm is colourless but may be spotted by slightly orange-coloured food vacuoles, when cells feed on chrysomonads. Well-fed specimens are studded with lipid droplets up to 5 μm across, usually 2–3 μm (Fig. 1, 31, 36).
Leptopharynx bromelicola n. sp. is a planktonic species, swimming continuously and moderately rapidly in the culture dishes. In contrast, L. costatus is a periphytic species gliding on various substrates, ranging from soil particles to the bottom of the culture dishes; however, in blooming pure cultures L. costatus behaves like L. bromelicola n. sp.
All somatic cilia of L. bromelicola n. sp. are about 10 μm long in vivo and 6–9 μm in SEM micrographs. Invariably, there are nine kineties, each showing a specific kinetid number and pattern, the latter being almost identical in MI and MA, while the kinetid numbers are highly different (Table 1 and Fig. 8, 9, 23, 24). Kineties 1 and 9 limit the ventral side, kineties 2 and 3 occupy the right side, kineties 4 and 5 limit the dorsal side, and kineties 6–8 extend on the left side. About half of the kineties are bipolar, while rows 1, 7, 8, and 9 are shortened anteriorly and/or posteriorly. A special pattern is formed by kineties 1 and 2: they are close anteriorly and then strongly diverge, especially in the MA, forming a conspicuous, elongate-triangular area, which is not observed in L. costatus (comp. Fig. 43, 53, 61 with 70, 71).
The detailed description of the kineties is based on the data compiled in Table 1 and the Fig. 8–10, 17, 23, 24, 29, 43, 44, 47–49, 53–57, 61–64. Cortex sculpturing has been described above. Kinety 1 extends at the right margin of the oral field and ends in mid-body. It is composed of ciliated dikinetids spaced so narrowly that the cilia form a membrane-like structure (Fig. 36). The kinetids in the anterior region are usually obliquely arranged, and an average of two barren monokinetids is at the posterior end. Kinety 2, which is on the right side, is bipolar, slightly sigmoidal, and contains the highest number of kinetids (i.e. 54 on average). In the anterior third, it is composed of narrowly spaced, slightly oblique dikinetids, while the posterior portion consists of monokinetids, many of which are barren in mid-body. Kineties 3 and 4 are bipolar and composed of monokinetids throughout. Kinety 3 is widely separate from kinety 2, while rows 3 and 4 are close anteriorly. Kinety 5 is on the dorsal margin and consists of ciliated monokinetids throughout. The distances between the kinetids gradually and strongly increase from anterior to posterior. Kinety 6 is on the left side and bipolar, consisting of rather evenly spaced, ciliated monokinetids. Kinety 7 is slightly shortened anteriorly and consists of widely spaced, ciliated monokinetids, forming more or less distinct pairs. Kinety 8 is distinctly shortened anteriorly and slightly posteriorly. It consists of an average of four widely spaced, ciliated monokinetids. Kinety 9, which limits the left margin of the oral field, is distinctly shortened anteriorly and slightly so posteriorly; it is bipartite, commencing underneath the adoral membranelles with some dikinetids, followed by a long tail of monokinetids.
As usual, there are four preoral kineties composed of ciliated dikinetids and a few monokinetids posteriorly (Table 1 and Fig. 3, 9, 10, 24, 29, 44, 47, 55, 56, 62, 71). In most specimens, the kineties occupy the left preoral half of the ventral side and extend slightly obliquely, appearing straight when seen from the left side (Fig. 9, 17, 24). In the more circular specimens, the preoral kineties can be seen from both sides of the cell because they extend obliquely across the entire ventral side, as in L. costatus (Fig. 18, 57, 64).
Of the four preoral kineties, kinety 4 is of special interest because it is not interrupted ( = continuous) and thus conspicuously long, posteriorly ending with four to seven densely spaced and one to three widely spaced monokinetids (Fig. 9, 10, 17, 24, 29, 47). This “continuous” pattern differs markedly from the discontinuous pattern of L. costatus (Fig. 18–21). Here, a “postoral complex” is formed by the posterior portion of the interrupted preoral kinety 4 and the anterior portion of the interrupted somatic kinety 9. The posterior portion of preoral kinety 4 forms a highly characteristic bundle of four to six cilia, while the dikinetids of the anterior portion of somatic kinety 9 are possibly barren. This interpretation assumes that species of the costatus group have a break each in preoral kinety 4 and somatic kinety 9, whose anterior dikinetidal portion appears as a more or less disordered short row opposite to the narrowly spaced kinetids of the interrupted preoral kinety 4. Unfortunately, this special pattern is not mentioned in the ontogenetic study of Njine (1979).
The oral apparatus of L. bromelicola n. sp. is located subapically within a broadly fusiform oral field having a rather deep concavity underneath the oral basket (Fig. 6, 16, 29, 43, 44, 47, 63, 65). The structure of the oral apparatus is as typical for the Microthoracida: there is a distinct oral basket made of nematodesmata, three adoral membranelles, and a highly specific paroral reduced to a single row of basal bodies (i.e. “nasse kinetosomes”) subapically connected with the basket rods (Peck 1974).
The up to 5 μm long bases of the adoral membranelles insert obliquely at the left anterior corner of the oral basket. The membranellar cilia have different lengths, so that two obconical, posteriorly directed bundles are formed about 12 μm long in vivo and in SEM preparations. The membranellar cilia are almost motionless, as evident from in vivo and SEM observations, where the bundles invariably have the same shape and location, i.e. are directed posteriorly between the oral basket and the ridge accompanying kinety 9 (Fig. 59, 63, 65).
Membranelle 1 (M1) is at the right anterior margin of the membranellar assemblage and composed of an average of only four basal bodies, with extremes of two to eight basal bodies in the about 70 specimens observed. This membranelle, which is quite distinct in the protargol preparations (Fig. 16, 29, 43–45, 47), is not recognizable in silver nitrate and SEM preparations where, however, one specimen showed two cilia possibly belonging to M1. Membranelle 2 (M2) is underneath and distinctly longer than M1, but slightly shorter than M3. It is composed of three rows of basal bodies, each having an average of six and four basal bodies in the MA and MI, respectively; the posterior row is possibly unciliated. Membranelle 3 (M3) is very close to M2 and composed of three basal body rows, each having an average of seven and five basal bodies in the MA and MI, respectively; possibly, only the anterior row is ciliated (Table 1 and Fig. 1, 8, 16, 23, 29, 43–48, 56, 59, 63–65).
The opening of the oral basket is slightly to distinctly oblique with respect to the main body axis (Fig. 47, 48, 55, 57, 63, 71). When seen frontally, the basket opening is very slenderly elliptical (~ 4:1) because it is about 11 μm long and only 2–3 μm wide in the MA (Table 1 and Fig. 6, 10, 59, 63, 71). However, in protargol preparations, the basket opening is broadly elliptical due to the poor alcohol fixation (Table 1 and Fig. 29, 43, 47). Thus, the basket rods form a flattened tube extending to body midline, where it abruptly curves to the dorsal posterior body end and the nematodesmata become rather disordered. The length of the oral basket opening is highly different in MI and MA: about 5 and 11 μm on average (Table 1), and the basket becomes very conspicuous in MA with an about 15 μm long opening and a distinct nose described in the following paragraph.
The oral basket rods are gradually elongated in the anterior half of the basket, forming a highly characteristic, up to 7 μm long, nose-like projection in the MA (Fig. 1, 8, 11–14, 25, 30, 61). The elongated rods are also present but indistinct in the MI, suggesting that they are a feature of this species, not depending on macrostomy. The distinctness and shape of the nose vary considerably, depending on the life cycle, its size, and the angle a specimen is viewed; usually, it is a rounded or acute process appearing triangular when seen laterally. The frontal part of the nose is covered by some material appearing membranous in the SEM (Fig. 1, 8, 10, 11–14, 25, 30, 31, 33, 53–56, 59, 60, 61, 63–65, 71).
As mentioned above, the microthoracids have a highly specific paroral ciliature composed of single, barren basal bodies (i.e. “nasse kinetosomes”) producing the basket rods (Peck 1974). The basal bodies are not at the distal end of the rods but subapically, and the distal portion of the rods is slightly angled (Fig. 8, 16, 23, 25, 29, 35, 43, 44, 47).
The last kinetosomal structures to be described are the oral anlagen, which, in the microthoracids, are present throughout the life cycle, similar to the scutica of the scuticociliates. They consist of two kinetosomal fields (Table 1 and Fig. 6, 8, 16, 23, 29, 43, 47, 56, 63, 65). The upper field is underneath the oral basket, forming a more or less disordered, concave row of basal bodies, which are barren and usually too faintly impregnated to be counted. The lower field consists of two rows of ciliated dikinetids left of the posterior end of somatic kinety 1. These rows produce the opisthe adoral membranelles (Njine 1979; Peck 1974). Usually, there is a ciliated monokinetid at the posterior end of the longer row. Occasionally, the second, short row is lacking, especially in the MI.
Resting cyst (Table 1 and Fig. 26–28, 38–42)
Resting cysts were obtained with two methods. Macrostomes were picked with a fine pipette into a few drops of fresh culture medium on a microscope slide deposited in a wet chamber. The cysts were studied after 1 wk. Microstome cysts were obtained by putting a coverslip on the culture surface, where encysting specimens attached. Microstome cysts occurred also in the culture mud and the bottom of the culture dish and were used for silver impregnations. In the cultures, only MI cysts were found, suggesting that the MAs develop to MIs before encysting.
The cysts from MI and MA have the same shape and structure, but differ considerably in size (Table 1): the former have an average of 29 × 17 μm, the latter of 36 × 25 μm. The cysts are ellipsoidal to slightly reniform or ovate and circular in transverse view. The compact cyst wall is about 1 μm thick, smooth, and structureless. Both, the wall and the cytoplasm are colourless; the latter is studded with granules and lipid droplets up to 3 μm across (Fig. 27, 42). Live observation (Fig. 27, 40–42) as well as silver nitrate (Fig. 38, 39) and protargol impregnation showed that the somatic and oral infraciliature as well as the oral basket, some extrusomes, and the contractile vacuole are maintained, while the cilia are possibly resorbed because the encysted cell did not start to move on prolonged observation under slight coverslip pressure. The resting cysts of the German population of L. costatus are highly similar to those of L. bromelicola n. sp.
Ecology and distribution
In the environmental samples and raw laboratory cultures, MI and MA of L. bromelicola n. sp. occur concomitantly in a similar ratio (Table 3). We do not know the factor(s) that induce macrostomy; possibly, it is a low abundance of small food items, as in Bromeliothrix metopoides, which stays microstomous as long as there are sufficient bacteria, but becomes macrostomous when bacterial abundance decreases and flagellates become numerous (Foissner 2010).
The MI of L. bromelicola possibly feed mainly on bacteria collected in food vacuoles up to 6 μm across. The MA possibly feed also on bacteria but, additionally, ingest resting cysts of flagellates, such as Polytomella sp. and Ochromonas sp. (Fig. 36, 37), as well as middle-sized euglenids (Peranema sp.) and ciliates (Glaucomides sp.), where the oral basket opens widely; possibly cannibalism occurs also. Intact prey organisms occur rarely in the predator’s cytoplasm, suggesting that they are digested rapidly.
Leptopharynx bromelicola n. sp. is very common in various bromeliads of Mexico, the Dominician Republic, Jamaica, Costa Rica, Ecuador, and Peru. Thus, it is possibly distributed throughout the bromeliad region.
Molecular phylogeny (Fig. 72)
The amplified 18S rDNA sequences of L. bromelicola n. sp. and L. costatus are 1,713 and 1,757 bp long and are available under GenBank accession numbers 1414065 and 1414068, respectively. The two sequences share a primary structure similarity of 98.34%. As in previous analyses (Gong et al. 2009), the class Nassophorea is polyphyletic in phylogenetic analyses based on the SSU rDNA. The orders Synhymeniida, Microthoracida, and Nassulida are each held together with significant support from all tree construction methods, with both Leptopharynx sequences branching within the order Microthoracida. The closest described relative to L. bromelicola n. sp. and L. costatus is P. dubius sharing 91.1% sequence similarity with both Leptopharynx species. Unfortunately, no further described members of the order Microthoracida are available. However, both sequences are relatively closely related to environmental clones, two of which (FJ810605 and FJ810609) were retrieved from coal tar waste-contaminated groundwater, and the other two sequences (EF024201 and EF024334) from trembling aspen rhizosphere under elevated CO2 concentrations. Our analyses identified these environmental clones as microthoracid sequences.
DISCUSSION
A brief review on the Leptopharynx species described
Eleven species have been described within or transferred to Leptopharynx (masculine gender fixed by the type species costatus) and Trichopelma (neuter): Leptopharynx sphagnetorum (Levander 1900) n. comb. (basionym: T. sphagnetorum Levander 1900) is possibly a distinct species because it is rather large (~ 60 μm) and usually contains symbiotic green algae; L. costatus Mermod, 1914; Trichopelma (Trochiliopsis) opacum (Penard, 1922) Kahl, 1931 has been redescribed and transferred back to Trochiliopsis by Augustin, Foissner, and Adam (1987); Leptopharynx euglenivorus (nom. corr.) Kahl, 1926 (a distinct species); Leptopharynx eurystoma (Kahl, 1931) n. comb. (basionym: Trichopelma eurystoma Kahl, 1931) is possibly a distinct species; Trichopelma torpens Kahl, 1931 is now type of the genus Lopezoterenia Foissner, 1997; Leptopharynx stenostomatus (Gellért, 1942) n. comb. (basionym: Trichopelma stenostomatum Gellért, 1942; nom. corr.) is possibly a distinct species because it has trichocysts and six kinetids in kinety 6; Leptopharynx agilis (Savoie, 1957) n. comb. (basionym: Trichopelma agile Savoie, 1957; nom. corr.) may be a distinct species because it lacks trichocysts and has four kinetids in kinety 6; Leptopharynx ambiguus (nom. corr.) Dragesco and Dragesco-Kernéis, 1986 is based on the L. costatus described by Njine (1979) and is possibly a distinct species; Leptopharynx minimus Alekperov, 1993 has been poorly described but may be a distinct species because it is <30 μm in size and very sparsely ciliated; Leptopharynx margaritatus (nom. corr.) Alekperov, 2005 is too poorly described to be identifiable and very likely a synonym of L. costatus; and L. bromelicola n. sp. described in the present paper.
Features for distinguishing Leptopharynx species
Kahl (1931) and Gellért (1942) used the following features: body size and shape, cortical ridges distinct vs. indistinct, oral basket opening narrow vs. wide, and food preferences (i.e. with or without green algae). Njine (1979), the first who used protargol impregnation, did not mention the features on which he based the new species Leptopharynx macrostoma. Alekperov (1993, 2005) used details of the ciliary pattern recognizable after silver nitrate impregnation, but did not substantiate their taxonomic value by detailed morphometric analyses; further, he did not consider the left body side.
Based on previous studies of L. costatus (Foissner 1989; Njine 1979; Prelle 1961), the present investigation, including two undescribed populations of L. costatus, and three new, not yet described species, we can discuss the taxonomic significance of various features, some summarized in Table 2, more precisely. First, we note those characteristics not informative at the present state of knowledge, either because they are the same in all species described or because their value is not known: body size and shape because of the frequent occurrence of MIs and MAs, nuclear apparatus, location of the contractile vacuole and the cytopyge, extrusome size and shape, and the silverline pattern. Furthermore, ciliation is a difficult feature because some or even many of the basal bodies recognizable in silver preparations are barren. The useful features include:
Detailed morphometrics, as provided by Foissner (1989) and in the present study, are indispensable and very informative.
The ability to produce MAs usually needs cultivation. However, if the species is numerous in the environment or in environmental cultures, the presence or absence of MIs or MAs should be also useable. According to the data available (Table 2), there are species that produce only MIs (i.e. a new species from Finland and possibly some populations of L. costatus), only MAs (i.e. a new species from Australia), or both (i.e. L. bromelicola n. sp., some L. costatus populations). However, we would be not surprised when future research shows that all Leptopharynx species can produce both MIs and MAs.
Cortical differentiations, such as ridges, furrows, and wings. Although such features have a considerable phenotypical variability in general, they can be useful. We performed several experiments, including the addition of various predators of L. costatus, to elicit distinct ridges or wings, but without any success. This suggests a low phenotypic variability in this genus. Thus, we rate the spine on the left side of a population from Finland, as a reliable species character (Table 2).
Ecology, biogeography, and zoochlorellae. Little is known about these features, except for L. costatus, which seems to be a cosmopolitan with wide environmental ranges and occurs in limnetic and terrestrial habitats (for a brief review, see Foissner et al. 1994). However, L. costatus possibly consists of several cryptic species (see Table 2 and below “Comparison with L. costatus”), including Sphagnum pond populations with symbiotic green algae, one of which was investigated by Prelle (1961). Such populations and non-European L. costatus should be investigated with modern methods to confirm or refute a cosmopolitan distribution. Leptopharynx bromelicola n. sp. is possibly restricted to tank bromeliads, i.e. to the southern hemisphere, as are many other protists (Foissner 2006, 2010; Foissner et al. 2003).
The somatic basal body (not ciliary!) pattern is a main feature, which, however, should be used with care and must be supplemented by a detailed morphometry. All populations investigated have 9 (MIs) or 10 (MAs) somatic kineties: three (kineties 1–3) or four (in MAs) on the right side, two (kineties 4 and 5) on the dorsal side, three or four (kineties 6–8/9) on the left side, and one (kinety 9 or 10) on the ventral side (Fig. 8, 9). As mentioned above, the basal bodies are frequently barren in the middle third of the body, a feature highly variable within and between populations (Foissner 1989; this study; and unpubl. data). Most important are the numbers of dikinetids in the anterior half of kineties 1–4, the number and location of the basal bodies in kinety 6, and the location and composition of kinety 9. Concerning kinety 6, two patterns can be distinguished (Fig. 9, 62, 68, 69): the costatus pattern, where the kinety consists of only one to three, usually two ciliated or barren kinetids in the middle third of the left side; and the bromelicola pattern, where kinety 6 is bipolar and composed of many basal bodies. Kinety 9 can commence underneath or far underneath the adoral membranelles and may begin with mono- or dikinetids. The total number of basal bodies is possibly also a valuable feature, but needs detailed investigations in further species and populations (Table 2).
As concerns the oral structures, four features should be evaluated: the preoral kineties and the postoral complex as well as the number of adoral membranelles and the shape of the oral basket. The four preoral kineties are either on the ventral side in L. costatus or ventro-laterally located, as in L. bromelicola n. sp. However, the distinction is vague and the character thus of limited value. Only kinety 4 is of interest: it may be continuous as in L. bromelicola n. sp. or discontinuous as in L. costatus, with the postoral complex, which may be present (e.g. L. costatus) or absent (e.g. L. bromelicola n. sp.). When continuous, kinety 4 is much longer and composed of many more basal bodies than kineties 1–3.
The basic adoral ciliature of Leptophrynx very likely includes three membranelles, as indicated by ontogenetic data (Njine 1979; present study, data not shown) and morphostatic specimens. Membranelle 1 is very small, consisting of only two to eight kinetids, both in morphostatic and/or microstomous specimens. However, it may be reduced in morphostatic and/or microstomous specimens. For instance, M1 is absent from the MIs of L. costatus population 1, while present in the MAs (Table 2). Certainly, the presence/absence and the structure of M1 can be used as a taxonomic feature, provided one has excellent preparations because M1 can be very small and thus difficult to observe. The structure of M2 and M3 is possibly also important, but the data are too incomplete for a definite conclusion. The oral basket of the MAs of L. bromelicola is unique in having elongated rods, forming a nose-like projection anteriorly (Fig. 11–14, 30, 61).
Comparison with Leptopharynx costatus
Detailed data for comparing L. bromelicola n. sp. are available only for L. costatus (Table 3). This shows (i) distinct differences between these species and (ii) a remarkable homogeneity of the five costatus populations (Table 3). Notable differences in L. costatus are the presence vs. absence of adoral M1 and the ability to produce MAs. Only Njine (1979) describes three adoral membranelles in L. costatus. As it is a population from tropical Africa, this observation might be correct and indicative of a different species. Indeed, Dragesco and Dragesco-Kernéis (1986) classified it as a new species, L. ambiguus. On the other hand, we observed in one of our populations that M1 is present only in dividers, and in another population M1 occurred only in the MAs (Tables 2 and 3). The two new populations investigated produce MAs with basket openings up to 15 μm wide. Prelle (1961), who worked with cultures, as we did, does not mention the formation of MAs, indicating a restricted ability of L. costatus-like populations to produce MAs.
At first glance, the MIs of L. bromelicola n. sp. and ordinary specimens of L. costatus look very similar. However, they differ unequivocally in the following features, ordered according to taxonomic significance: number of basal bodies in kinety 6, beginning of kinety 9 underneath vs. far underneath of adoral membranelles, presence vs. absence of dikinetids in anterior portion of kinety 9, and postoral complex absent vs. present. The MAs of L. bromelicola n. sp., additionally, have a highly characteristic nose (Fig. 60, 61), hardly recognizable in L. costatus (Fig. 71). The number of basal bodies in kinety 6 (i.e. ≥ 10 or only 1–3) produces very different patterns in L. bromelicola n. sp. and L. costatus. The distinctness of L. bromelicola n. sp. from L. costatus is emphasized by a 1.7% difference in the ribosomal genes.
Comparison with MA congeners
Three MA Leptopharynx species have been described: L. euglenivorus Kahl, 1926; L. eurystoma Kahl, 1931; and L. macrostoma Njine, 1979. We add L. bromelicola n. sp. and two MA-forming populations of L. costatus (Tables 2 and 3). The new MA species described by Njine (1979) is possibly the MA of the L. costatus described by Njine (1979) in the same paper. This interpretation is supported in that both were found in ephemeral pools on the campus of the University of Yaoundé and the highly similar oral and somatic ciliary pattern. Taking this and other data into account, the MAs of L. bromelicola n. sp. and L. costatus differ in the following features (Tables 2 and 3): postoral complex absent vs. present; by kineties 3 and 4, which have monokinetids vs. dikinetids anteriorly; by kinety 6, which consists of about 10 vs. 2 kinetids (bromelicola vs. costatus pattern); by kinety 9 commencing underneath vs. far underneath the adoral membranelles and having vs. lacking dikinetids in the anterior region; and by the oral basket having vs. lacking a nose.
No recent data are available for Kahl’s (1926, 1931) species. Leptopharynx eurystoma is possibly the MA of L. costatus because the ciliary pattern is highly similar. However, we cannot exclude the possibility that it is a distinct species lacking MIs (cf. Table 2). Leptopharynx euglenivorus resembles L. bromelicola n. sp. in body size (i.e. 40–50 μm) and shape, in its distinct cortical ridges, and the habitat of decomposing Phragmites leaves. The most conspicuous difference is the lack of a nose. We can be certain of this because Kahl (1926, 1931) observed abundant populations of this species 2 times. Whether L. euglenivorus feeds only on green euglenids is not known.
TAXONOMIC SUMMARY
Class Nassophorea Small & Lynn, 1981
Order Microthoracida Jankowski, 1967
Family Leptopharyngidae Kahl, 1926
Genus Leptopharynx Mermod, 1914
Diagnosis
Small (15 to about 50 μm, MIs) to medium-sized (up to 100 μm, MAs), discoidal Microthoracida with 9 (MIs) or 10 (MAs) somatic kineties having partially reduced the cilia, especially on left body side and in middle third of right side. Cortex rigid, with or without distinct ridges and/or furrows containing or accompanying ciliary rows. Oral apparatus in anterior half of body with homomorphic oral basket and four preoral kineties; two to three adoral membranelles, M1, if present, consisting of <10 barren basal bodies; frequently produces MIs and MAs. Limnetic and terrestrial habitats.
Type species (by monotypy)
Leptopharynx costatus Mermod, 1914.
Remarks
The diagnosis is based on the present investigations, Foissner (1985, 1989, 1997), Foissner and O’Donoghue (1990), Kahl (1931), and Leitner and Foissner (1997). As concerns the definition of the family Microthoracidae and of the genera included, see Corliss (1979), Foissner (1985, 1997), and Lynn (2008). Foissner (1985, 1997), who included Leptopharynx into the Microthoracidae, provided diagnoses for the (sub)order Microthoracina and the families Microthoracidae, Pseudomicrothoracidae, and Discotrichidae.
Leptopharynx bromelicola n. sp.
Diagnosis
Size of MI in vivo 40 × 25 μm on average, that of MAs 60 × 35 μm. Leptopharynx costatus shaped. Nine ciliary rows accompanied by distinct ridges in (MA); kinety 3 composed of monokinetids; kinety 6 composed of 11 and 19 monokinetids on average in (MI) and (MA), respectively; kinety 9 commences with some dikinetids underneath three adoral membranelles. Preoral kineties usually on left half of ventral side; kinety 4 continuous and thus extending beyond adoral membranelles. Postoral complex lacking. Oral basket with elongated rods in anterior half, forming a distinct nose, especially in (MA). For minor features, see Tables 2 and 3.
Type locality
Bromeliad tanks on the northern slope of the Blue Mountains, Jamaica, Silver Hill village, 18°12′N, 76°40′W.
Type material
Four slides with protargol-impregnated specimens and nine slides with silver nitrate-impregnated cells (Klein–Foissner and Chatton–Lwoff methods) have been deposited in the Biologiezentrum of the Oberösterreichische Landesmuseum in Linz (LI), Austria. Hapantotypes, paratypes, and other relevant specimens have been marked by black ink circles on the coverslip. The sequence of the SSU rRNA genes has been deposited in GenBank, Accession Number: HQ 668466.
Etymology
Composite of Bromeliaceae, the plants in whose leaf-tanks it occurs, and the Latin verb colere (to dwell), referring to its typical habitat.
ACKNOWLEDGMENTS
This study was supported by the Austrian Science Foundation (FWF, project 20360-B17) and the German Science Foundation (DFG, project STO 414/3-1). The technical assistance of Mag. Barbara Harl, Robert Schörghofer, Andreas Zankl, and Hans-Werner Breiner are greatly acknowledged. Special thanks to Carlos Durán Ramirez for providing the Mexican sample, to Dr. Walter Stoiber and Sabine Götter for the German population of L. costatus, and to Dr. Remigius Geiser for help with Latin and Greek names.
LITERATURE CITED
- Aescht E. Catalogue of the generic names of ciliates (Protozoa, Ciliophora) Denisia. 2001;1:1–350. [Google Scholar]
- Alekperov IK. Free-living ciliates in the soils of St. Petersburg parks (Protozoa) Zoosyst. Rossica. 1993;2:13–28. [Google Scholar]
- Alekperov IK. Atlas of free-living infusoria (Classes Kinetofragminophora, Colpodea, Oligohymenophora, Polyhymenophora) Nat. Akad. Azerbaijan, Inst. Zool. NAN Azerbaijan, Baku. 2005 (in Russian) [Google Scholar]
- Augustin H, Foissner W, Adam H. Revision of the genera Acineria, Trimyema and Trochiliopsis (Protozoa, Ciliophora) Bull. Br. Mus. nat. Hist. (Zool.) 1987;52:197–224. [Google Scholar]
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Corliss JO. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature. 2nd ed Pergamon Press; Oxford: 1979. [Google Scholar]
- Dragesco J, Dragesco-Kernéis A. Ciliés libres de l’Afrique intertropicale. Faune tropicale. 1986;26:1–559. [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner W. Ökologische und systematische Studien über das Neuston alpiner Kleingewässer, mit besonderer Berücksichtigung der Ciliaten. Int. Revue ges. Hydrobiol. 1979;64:99–140. [Google Scholar]
- Foissner W. Morphologie und Infraciliatur der Genera Microthorax und Stammeridium und Klassifikation der Microthoracina Jankowski, 1967 (Protozoa:Ciliophora) Zool. Anz. 1985;214:33–53. [Google Scholar]
- Foissner W. Morphologie und Infraciliatur einiger neuer und wenig bekannter terrestrischer und limnischer Ciliaten (Protozoa, Ciliophora) Sber. Akad. Wiss. Wien. 1989;196:173–247. [Google Scholar]
- Foissner W. Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. Eur. J. Protistol. 1991;27:313–330. doi: 10.1016/S0932-4739(11)80248-8. [DOI] [PubMed] [Google Scholar]
- Foissner W. Infraciliature and systematic position of the marine interstitial ciliates (Protozoa, Ciliophora) Lopezoterenia torpens (Kahl, 1931) nov. gen., nov. comb., Discotricha papillifera Tuffrau, 1954, and Paraspathidium fuscum (Kahl, 1928) Fjeld, 1955. Revta Soc. mex. Hist. nat. 1997;47:41–63. [Google Scholar]
- Foissner W. Biogeography and dispersal of micro-organisms: a review emphasizing protists. Acta Protozool. 2006;45:111–136. [Google Scholar]
- Foissner W. Life cycle, morphology, ontogenesis, and phylogeny of Bromeliothrix metopoides nov. gen., nov. spec., a peculiar ciliate from tank bromeliads. Acta Protozool. 2010;49:159–193. [PMC free article] [PubMed] [Google Scholar]
- Foissner W, O’Donoghue PJ. Morphology and infraciliature of some freshwater ciliates (Protozoa:Ciliophora) from western and south Australia. Invertebr. Taxon. 1990;3:661–696. [Google Scholar]
- Foissner W, Agatha S, Berger H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha region and the Namib Desert. Denisia. 2002;5:1–1459. [Google Scholar]
- Foissner W, Berger H, Kohmann F. Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems – Band III: Hymenostomata, Prostomatida, Nassulida. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1994;1/94:1–548. [Google Scholar]
- Foissner W, Strüder-Kypke M, van der Staay GWM, Moon-van der Staay S-Y, Hackstein JHP. Endemic ciliates (Protozoa, Ciliophora) from tank bromeliads: a combined morphological, molecular, and ecological study. Eur. J. Protistol. 2003;39:365–372. [Google Scholar]
- Galtier N, Gouy M, Gautier C. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- Gellért J. Életegyüttes a fakéreg zöldporos bevonatában. Acta Sci. math.-nat. Univ. Kolozsvár. 1942;8:1–36. [Google Scholar]
- Gong J, Stoeck T, Yi Z, Miao M, Zhang Q, Roberts D. McL., Warren A, Song W. Small subunit rRNA show that the class Nassophorea is not monophyletic (Phylum Ciliophora) J. Eukaryot. Microbiol. 2009;56:339–347. doi: 10.1111/j.1550-7408.2009.00413.x. [DOI] [PubMed] [Google Scholar]
- Jankowski AV. A new system of ciliate protozoa (Ciliophora) Trudy Zool. Inst., Leningr. 1967;43:3–52. (in Russian) [Google Scholar]
- Kahl A. Neue und wenig bekannte Formen der holotrichen und heterotrichen Ciliaten. Arch. Protistenk. 1926;55:197–438. [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 2. Holotricha außer den im 1. Teil behandelten Prostomata. Tierwelt Dtl. 1931;21:181–398. [Google Scholar]
- Klein BM. Die Silberliniensysteme der Ciliaten. Arch. Protistenk. 1928;62:177–260. [Google Scholar]
- Leitner AR, Foissner W. Morphology and infraciliature of Microthorax pusillus Engelmann 1862 and Spathidium deforme Kahl 1928, two ciliates (Protozoa, Ciliophora) from activated sludge. Linzer Biol. Beitr. 1997;29:349–368. [Google Scholar]
- Levander KM. Zur Kenntniss des Lebens in den stehenden Kleingewässern auf den Skäreninseln. Acta Soc. Fauna Flora Fenn. 1900;18:1–107. [Google Scholar]
- Lynn DH. The Ciliated Protozoa. Characterization, Classification, and Guide to the Literature. 3rd ed Springer; Dordrecht: 2008. [Google Scholar]
- Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Mermod G. Recherches sur la faune infusoriennes des tourbi res et des eaux voisines de Sainte-Croix (Jura vaudois) Revue suisse Zool. 1914;22:31–114. [Google Scholar]
- Njine T. Structure et morphogen se buccales du cilié Leptopharynx (Mermod, 1914) Protistologica. 1979;15:459–465. [Google Scholar]
- Njine T, Didier P. Étude ultrastructurale des ciliés du genre Leptopharynx Mermod, 1914. Protistologica. 1980;16:155–166. [Google Scholar]
- Peck RK. Morphology and morphogenesis of Pseudomicrothorax, Glaucoma and Dexiotricha, with emphasis on the types of stomatogenesis in holotrichous ciliates. Protistologica. 1974;10:333–369. [Google Scholar]
- Penard E. Études sur les Infusoires d’Eau Douce. Georg et Cie; Geneva: 1922. [Google Scholar]
- Prelle A. Contribution a l’étude de Leptopharynx costatus (Mermod) (infusoire cilié) Bull. Biol. Fr. Belg. 1961;95:731–752. [Google Scholar]
- Savoie A. Le cilié Trichopelma agilis n. sp. J. Protozool. 1957;4:276–280. [Google Scholar]
- Small EB, Lynn DH. A new macrosystem for the phylum Ciliophora Doflein, 1901. Biosystems. 1981;14:387–401. doi: 10.1016/0303-2647(81)90045-9. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougement J. A rapid bootstrap algorithm for the RAxML web-servers. Syst. Biol. 2008;75:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Thompson JC. Ciliated protozoa of the Antarctic Peninsula. Antarc. Res. Ser. 1972;20:261–288. [Google Scholar]
- Wrześniowski A. Beobachtungen über Infusorien aus der Umgebung von Warschau. Z. Wiss. Zool. 1870;20:467–511. [Google Scholar]