Abstract
Knowledge about the functional status of the frontal cortex in infancy is limited. This study investigated the effects of polymorphisms in four dopamine system genes on performance in a task developed to assess such functioning, the Freeze-Frame task, at 9 months of age. Polymorphisms in the catechol-O-methyltransferase (COMT) and the dopamine D4 receptor (DRD4) genes are likely to impact directly on the functioning of the frontal cortex, whereas polymorphisms in the dopamine D2 receptor (DRD2) and dopamine transporter (DAT1) genes might influence frontal cortex functioning indirectly via strong frontostriatal connections. A significant effect of the COMT valine158methionine (Val158Met) polymorphism was found. Infants with the Met/Met genotype were significantly less distractible than infants with the Val/Val genotype in Freeze-Frame trials presenting an engaging central stimulus. In addition, there was an interaction with the DAT1 3′ variable number of tandem repeats polymorphism; the COMT effect was present only in infants who did not have two copies of the DAT1 10-repeat allele. These findings indicate that dopaminergic polymorphisms affect selective aspects of attention as early as infancy and further validate the Freeze-Frame task as a frontal cortex task.
Keywords: frontal cortex, infancy, dopamine genes, attention, frontal-subcortical circuits
The frontal cortex is associated with important cognitive functions such as working memory and various aspects of cognitive control (for a review, see Fuster, 1997; Gazzaley & D’Esposito, 2007). Despite years of intensive study of this area in adults and nonhuman primates, relatively little is known about the functional status of the frontal cortex in infancy.
The frontal cortex has a more protracted development than other areas of the brain, with synaptogenesis continuing well into middle childhood (Glantz, Gilmore, Hamer, Lieberman, & Jarskog, 2007; Huttenlocher, 1990). Glucose metabolism and regional cerebral blood flow also peak later in the frontal cortex (Chugani & Phelps, 1986; Chugani, Phelps, & Mazziotta, 1987; Franceschini et al., 2007). Despite this protracted developmental course, infant neuroimaging studies have shown activation in the frontal cortex during language processing, processing of novel stimuli, and working memory (Baird et al., 2002; Bell, 2001; Bell & Fox, 1992, 1997; Dehaene-Lambertz, Dehaene, & Hertz-Pannier, 2002; Homae, Watanabe, Nakano, & Taga, 2007; Nakano, Watanabe, Homae, & Taga, 2009). Furthermore, Diamond and colleagues have shown that performance on a task that has been directly associated with the frontal cortex, the A-not-B task (Piaget, 1954), improves drastically during the second half of the first year of life (Diamond, 1985; Diamond & Goldman-Rakic, 1989; Diamond, Zola-Morgan, & Squire, 1989).
A previous report sought to validate a new infant frontal cortex task, the Freeze-Frame task, by investigating the relationship between this task and other infant and toddler frontal cortex tasks (Holmboe, Fearon, Csibra, Tucker, & Johnson, 2008). The Freeze-Frame task was developed to assess various aspects of inhibitory control in infancy using eye movements as the dependent measure. In this task, infants are encouraged to stay fixated on an animated cartoon in the center of a computer screen. On every trial, a peripheral distractor (a white square) is presented. If the infant looks to this distractor, the animation is frozen for a brief period of time. Furthermore, the task involves two alternating trial types. In the “interesting” trials, a dynamic and changeable animation is presented, whereas the “boring” trials present the same simple animation (a rotating orange star) every time.
In the study by Holmboe et al. (2008), 9-month-old infants stopped looking to the distractors during the course of the test session. Infants also looked less to the distractors in the interesting trials right from the beginning of the session. No evidence of an interaction between trial type and phase of the test session was found. Individual performance indices suggested that infants who looked less to the distractors in the interesting trials than the boring trials early in the Freeze-Frame session performed better on the A-not-B task at 9 months of age. Another Freeze-Frame index, which assessed infants’ ability to selectively learn to inhibit looks to the distractors, was associated with significantly better performance on a frontal cortex task (the Spatial Conflict task; Gerardi-Caulton, 2000; Rothbart, Ellis, Rueda, & Posner, 2003) at 24 months of age, suggesting that Freeze-Frame performance at 9 months is predictive of later frontal cortex functioning (Holmboe et al., 2008).
Even though these results indicate that performance on the Freeze-Frame task shares a significant proportion of its variance with performance on other infant and toddler frontal cortex tasks, this is nonetheless relatively indirect evidence that the task depends on the frontal cortex. More definitive evidence that the task is indeed associated with the functioning of the frontal cortex would involve establishing a direct relationship between performance on the task and biological markers of frontal cortex functioning. One way to address this issue is to investigate the potential effect of genetic variation. In the present study, we therefore investigated the relationship between performance on the Freeze-Frame task and well-characterized candidate polymorphisms in dopamine system genes.
The neurotransmitter dopamine plays a major role in the frontal cortex. For example, depletion of dopamine, but not noradrenaline or serotonin, in the dorsolateral prefrontal cortex causes delayedresponse deficits similar to those seen after ablation of that area (Brozoski, Brown, Rosvold, & Goldman, 1979; Collins, Roberts, Dias, Everitt, & Robbins, 1998; Roberts et al., 1994). Furthermore, recordings from prefrontal dopamine-sensitive neurons in primates have shown these neurons to be active during the delay period in working memory tasks (Goldman-Rakic, Muly, & Williams, 2000; Sawaguchi & Goldman-Rakic, 1991; Vijayraghavan, Wang, Birnbaum, Williams, & Arnsten, 2007). Finally, Diamond and colleagues investigated children treated early and continuously for phenylketonuria (PKU) and found that estimated dopamine levels in the frontal cortex affected children’s performance on frontal cortex tasks throughout infancy and early childhood (Diamond, Prevor, Callender, & Druin, 1997).
We investigated two dopamine system genes that have been demonstrated to impact on frontal cortex function in several studies: the catechol-O-methyltransferase gene (COMT) and the dopamine D4 receptor gene (DRD4). However, the dopamine system is not restricted to the frontal cortex. It also plays an important role in subcortical areas such as the striatum. We therefore included two dopaminergic polymorphisms believed to affect neurotransmission primarily in the striatum: the dopamine D2 receptor gene (DRD2) TaqIA polymorphism and the 40-bp 3′ variable number of tandem repeats (VNTR) polymorphism in the dopamine transporter gene (DAT1, SLC6A3). These polymorphisms could potentially affect performance in the Freeze-Frame task via frontal subcortical circuits linking the frontal cortex to distinct areas of the striatum (Alexander, DeLong, & Strick, 1986; Cummings, 1993; Cummings & Miller, 2007; Di Martino et al., 2008; Nieoullon, 2002).
The striatum used to be regarded as a subcortical relay of information from diverse cortical areas, especially in relation to movement control (reviewed in Alexander et al., 1986). However, Alexander et al. proposed a model whereby distinct basal ganglia-thalamocortical circuits process information relevant to different functional domains. Two of these circuits involve parts of the prefrontal cortex (the dorsolateral prefrontal and the lateral orbitofrontal circuits), and one involves the anterior cingulate. In support of this model, work on experimental animals as well as neuropsychological studies of human patients have shown deficits in the functions associated with specific frontal areas (e.g., working memory function associated with the dorsolateral prefrontal cortex) after lesion of other nodes in the relevant frontal-subcortical circuit (Cummings, 1993; Divac, Rosvold, & Szwarcbart, 1967; Stuss et al., 1998; Yehene, Meiran, & Soroker, 2008). Furthermore, the existence of strong functional connections between the striatum and different parts of the frontal cortex has been confirmed in an analysis of human functional magnetic resonance imaging (fMRI) data (Di Martino et al., 2008). Given this extensive evidence for frontal-subcortical networks, it seemed important to investigate not just dopamine genes likely to affect processing in the frontal cortex but also dopamine genes acting primarily at the subcortical level.
Looking at the individual genes in more detail, the COMT gene codes for the COMT enzyme, which metabolizes catecholamines, such as dopamine and noradrenaline (Chen et al., 2004; Männistö & Kaakkola, 1999; Tunbridge, Harrison, & Weinberger, 2006). The role of COMT in catabolizing dopamine in the frontal cortex is particularly important because of the relative lack of dopamine transporters and the positioning of these transporters at a distance from synaptic release sites (Sesack, Hawrylak, Matus, Guido, & Levey, 1998). Thus, COMT accounts for approximately 50 – 60% of the metabolic degradation of dopamine in the frontal cortex (Karoum, Chrapusta, & Egan, 1994; Yavich, Forsberg, Karayiorgou, Gogos, & Männistö, 2007). In contrast, COMT catabolism plays only a minor role in the striatum where the dopamine transporter is abundant and better situated for dopamine reuptake (Karoum et al., 1994; Yavich et al., 2007; for a review, see Tunbridge et al., 2006). Consistent with this, studies of COMT-deficient mice have demonstrated increased dopamine availability in the frontal cortex but not the striatum (Gogos et al., 1998; Yavich et al., 2007). The important role of COMT in the cortex compared with the striatum has also recently been shown in vivo in the human brain using positron emission tomography (PET; Slifstein et al., 2008).
The Val158Met polymorphism (rs4680) in the COMT gene affects the activity level of the COMT enzyme. The polymorphism is an evolutionarily recent G (guanine) to A (adenine) missense mutation at codon 158, resulting in a substitution of methionine (Met) for valine (Val) in the COMT enzyme (Chen et al., 2004; Lachman et al., 1996; Tunbridge et al., 2006, 2007). The Val and Met alleles are almost equally frequent in populations of European descent (Met-allele frequency = .47; heterozygosity = .48), whereas the Val allele is more common in other parts of the world (Met-allele frequency = .16 –.34; heterozygosity = .27–.45; Palmatier, Kang, & Kidd, 1999).
The Met variant of the enzyme is less stable at body temperature (Chen et al., 2004; Lotta et al., 1995), resulting in three to four times less COMT enzyme activity in the human liver and red blood cells (Männistö & Kaakkola, 1999). In the human brain this difference is smaller, but still considerable, with Met/Met homozygotes having approximately 40% less COMT activity than Val/Val homozygotes in the prefrontal cortex (Chen et al., 2004). The alleles are codominant, resulting in Val/Met heterozygotes having an intermediate level of COMT activity (Egan et al., 2001; Männistö & Kaakkola, 1999; Tunbridge et al., 2006). This evidence strongly suggests that Met/Met homozygotes have the highest baseline level of dopamine available in the prefrontal cortex (because less dopamine is catabolized) with Val/Met heterozygotes having an intermediate level and Val/Val homozygotes having the lowest level of prefrontal dopamine (Tunbridge et al., 2006, 2007).
Several studies have demonstrated a relationship between the COMT Val158Met polymorphism and performance on tasks associated with the frontal cortex. For example, in an initial study, Egan et al. (2001) found that the COMT Val158Met polymorphism affected performance on the Wisconsin Card Sorting Test. Val/Val homozygotes performed significantly worse than Met/Met homozygotes and heterozygotes. Furthermore, the number of Met alleles (0 –2) that an individual had significantly predicted neural efficiency in the frontal cortex during an N-back task, as measured by fMRI. In this task, all genotype groups performed at the same level, but Val/Val homozygotes showed significantly greater activation (indicating lower neural efficiency) in the frontal cortex than heterozygotes, and heterozygotes showed significantly greater activation than Met/Met homozygotes (Egan et al., 2001). Even though there are also negative findings in the literature (Barnett, Scoriels, & Munafò, 2008; Dennis et al., in press), the evidence for an effect of the COMT Val158Met polymorphism on neural efficiency as well as on a range of frontal cortex tasks has been replicated in several studies (Barnett, Jones, Robbins, & Müller, 2007; Bertolino et al., 2006; Blasi et al., 2005; Caldú et al., 2007; Diaz-Asper et al., 2008; Krämer et al., 2007; Mattay et al., 2003; Meyer-Lindenberg et al., 2006; Sheldrick et al., 2008; Stefanis et al., 2005). This evidence has been extended to a mouse model by Papaleo et al. (2008). The effect of the COMT Val158Met polymorphism on prefrontal efficiency has also recently been confirmed by meta-analysis (Mier, Kirsch, & Meyer-Lindenberg, in press).
Finally, a study by Diamond, Briand, Fossella, and Gehlbach (2004) demonstrated an effect of the COMT Val158Met polymorphism on school-age children’s performance on a task hypothesized to depend on dopamine in the prefrontal cortex. This finding demonstrates the potential effect of variation in COMT activity at younger ages and opens up the possibility that the COMT Val158Met polymorphism might already have an effect on frontal cortex functioning in infancy.
The second candidate gene in our study was the DRD4 gene. Knowledge about the distribution of the D4 receptor in the human brain is limited because of the lack of appropriate radioligands (Hurd & Hall, 2005; Oak, Oldenhof, & Van Tol, 2000). However, existing evidence suggests that D4 receptors are most abundant in the retina, followed by the prefrontal cortex (Oak et al., 2000). Hurd and Hall (2005) suggested that transmission via D4 receptors is predominantly inhibitory in nature, resulting in disinhibition of excitatory transmission when these receptors are blocked. Thus, a lack of, or less efficient, D4 receptors may lead to deficits in frontal cortex functioning.
The most widely studied polymorphism of the DRD4 gene is located in the third exon and contains a 48 – base pair variable number of tandem repeats (48-bp VNTR). Nine alleles of the DRD4 48-bp VNTR have been identified worldwide, with the number of repeats ranging between 2 and 10. The 4- and 7-repeat alleles are the most common globally, though the 2-repeat allele is prevalent in South and East Asian populations. In a population of mixed European ancestry, allele frequencies are .57, .21, and .12 for the 4-, 7-, and 2-repeat alleles, respectively (Chang, Kidd, Livak, Pakstis, & Kidd, 1996).
The number of 48-bp repeats has been hypothesized to affect the transmitted signal in the postsynaptic neuron. However, findings from in vitro studies have shown that the DRD4 48-bp VNTR does not significantly alter D4 receptor activity (Oak et al., 2000). A more recent study suggests that the different repeat sequences may affect gene expression differentially, that is, the density of D4 receptors in the brain. This study found that the 7-repeat allele had reduced expression compared with the 2-repeat and 4-repeat alleles (Schoots & Van Tol, 2003).
The DRD4 48-bp VNTR has been extensively studied in relation to attention deficit hyperactivity disorder (ADHD; Li, Sham, Owen, & He, 2006). ADHD has been linked to performance deficits on tasks assessing frontal cortex functions, such as response inhibition, selective attention, and set shifting (for a review, see Cornish et al., 2005). The 7-repeat allele has been consistently associated with ADHD in meta-analyses (Faraone et al., 2005; Li et al., 2006). Furthermore, the DRD4 48-bp VNTR has been shown to affect prefrontal gray matter volume in a sample of boys diagnosed with ADHD, their siblings, and controls (Durston et al., 2005). Recently, the 7-repeat allele has also been found to be associated with impulsivity and lower levels of response inhibition in healthy adults, both on its own (Congdon, Lesch, & Canli, 2008) and in combination with other polymorphisms in dopamine system genes (Congdon et al., 2008; Eisenberg et al., 2007). Finally, the 7-repeat allele has been linked to faster habituation in infancy and increased novelty seeking in adolescence (Laucht, Becker, & Schmidt, 2006) and to sensation seeking in toddlers when combined with poor parenting (Sheese, Voelker, Rothbart, & Posner, 2007). Therefore, the DRD4 48-bp VNTR can be considered a candidate polymorphism for frontal cortex functioning in infancy.
Turning to the genes most likely to act at the subcortical level, we note that the D2 receptor is considerably less prevalent in the cerebral cortex than in the striatum (Ito, Okubo, Halldin, & Farde, 1999; Lidow, Goldman-Rakic, Rakic, & Innis, 1989). The DRD2 TaqIA polymorphism was identified during the chromosomal localization of the gene. However, this polymorphism is located in the 3′ untranslated region, 10 kb downstream from the DRD2 gene, actually in the adjacent gene ANKK1 (Neville, Johnstone, & Walton, 2004); therefore, newer nomenclature refers to this polymorphism as the DRD2/ANKK1 TaqIA polymorphism. The A1 allele is the minor allele, and the A1-present (A1+) genotype group (A1/A1 and A1/A2 genotypes) has a prevalence of approximately 31% in Caucasian individuals (Noble, 2000). The presence of this allele has been associated with lower D2 receptor density in the human brain, as measured with PET, especially in the striatum (Jönsson et al., 1999; Pohjalainen et al., 1998; Ritchie & Noble, 2003; Thompson et al., 1997). Therefore, this polymorphism might serve as a good genetic marker for D2 receptor density in the brain.
In contrast to the DRD4 48-bp VNTR, the DRD2 TaqIA polymorphism is not associated with ADHD (Faraone et al., 2005). However, the A1 allele has been associated with various addictions (Munafò, Matheson, & Flint, 2007; Young, Lawford, Nutting, & Noble, 2004) and a more impulsive response style in a monetary reward task in healthy adults (Eisenberg et al., 2007). Little evidence exists for a role of the DRD2 TaqIA polymorphism in frontal cortex functioning. However, Reuter et al. (2005) showed a significant interaction between the DRD2 TaqIA polymorphism and the COMT Val158Met polymorphism on a Stroop-like task where participants had to respond as quickly as possible to the written form of color words written in incongruent colors. The interaction effect accounted for 13% of the variance in performance on this task. This result opens up the possibility that the DRD2 gene (and perhaps other subcortical dopaminergic genes) impacts indirectly on frontal cortex functioning via interactions with genes affecting dopaminergic neurotransmission directly in the frontal cortex (e.g., COMT and DRD4).
Finally, we investigated the potential effect of a well-known polymorphism of the dopamine transporter gene (DAT1). The dopamine transporter is primarily expressed in the mesencephalon (a subcortical area with strong dopaminergic projections to the striatum and frontal cortex), with the highest density in the basal ganglia (Hurd & Hall, 2005). The DAT1 gene contains a 40-bp VNTR in the 3′ untranslated region. Alleles range from 3 to 13 repeats, but the most common are the 9-repeat and 10-repeat alleles (Cornish et al., 2005). In populations of European ancestry, the frequencies of the 9- and 10-repeat alleles vary, but most studies report frequencies of approximately .30 for the 9-repeat allele and .70 for the 10-repeat allele (Kang, Palmatier, & Kidd, 1999). Although analyses of mRNA levels in brain regions resulted in contradictory findings (Mill, Asherson, Browes, D’Souza, & Craig, 2002; Wonodi et al., 2009), two independent large-scale in vivo single photon emission computed tomography (SPECT) studies have shown that healthy individuals with at least one copy of the 9-repeat allele (9/9 and 9/10 genotypes) have higher transporter density, and therefore presumably more effective dopamine removal at the synapse, than individuals with the 10/10 genotype (van de Giessen et al., 2008; van Dyck et al., 2005).
In terms of phenotypes, the DAT1 gene has been studied extensively in relation to ADHD because stimulant medication used in its treatment acts by blocking the dopamine transporter. Evidence suggests that 10/10 homozygosity is associated with a slightly increased risk of ADHD (Faraone et al., 2005; Yang et al., 2007). Furthermore, Cornish et al. (2005) reported an association between the 10/10 genotype and ADHD symptoms in a general population sample. This group also found an independent association between the 10/10 genotype and poorer performance on measures of selective attention and response inhibition in their selected high- and low-risk sample. A similar trend was found by Congdon et al. (2008) in a sample of healthy adults. Despite these findings, recent neuroimaging studies in adults have indicated a more efficient neural response in the prefrontal cortex of 10/10 homozygotes during a working memory task (Bertolino et al., 2006; Caldú et al., 2007), a pattern similar to that seen in participants with the COMT Met/Met genotype. One recent study also found higher levels of impulsivity in healthy adults with at least one 9-repeat allele (Forbes et al., 2009), contradicting other behavioral results. The behavioral effects of DAT1 3′ VNTR polymorphism may depend on the population studied.
In summary, the present study investigated whether performance on the Freeze-Frame task at 9 months of age was associated with genetic polymorphisms affecting important aspects of dopamine function in the brain. Because dopamine plays an important role in both the frontal cortex and the striatum, direct effects of the COMT Val158Met and DRD4 48-bp VNTR were hypothesized, with potential interacting or indirect effects of the DRD2 TaqIA and DAT1 3′ VNTR polymorphisms.
Method
Sample
Infants were recruited from the greater London area. Data from two independent cohorts of infants were combined in the present study. Cohort 1 consisted of a small group of infants (N = 24). Behavioral results from this cohort have been reported previously (Holmboe et al., 2008). Cohort 2 consisted of a considerably larger group of infants (N = 104) who took part in a longitudinal study of frontal cortex functioning during the first year of life. Ninety-four infants from the original cohort of 104 infants (recruited at 4 months) participated in the study at 9 months. Data from this cohort have not been reported previously.
Data on parental education and household income were only collected in Cohort 2 but generally represent families recruited for studies at our laboratory. Parents were in their mid-thirties (mothers: M = 34.43, SD = 4.90; fathers: M = 36.45, SD = 6.61) and were primarily, but not exclusively, of middle or upper-middle-class socioeconomic status (maternal years of education: M = 17.80, SD = 3.55; household income in U.K. pounds: M = 65,076, SD = 61,854).1 Seventy-nine percent of the infants tested (Cohorts 1 and 2 combined) had a White/Caucasian ethnic background (approximately three fourths of these infants were of British or Irish descent), and 21% had other or mixed ethnic background. Of the infants with other than Caucasian ethnic background (N = 26), .8% had an Asian ethnic background, 15% had a Black ethnic background, and 77% had a mixed ethnic background (e.g., mother Asian and father Caucasian). Ethical permission for the study was obtained from the School of Psychology ethics board at Birkbeck, University of London.
The Freeze-Frame Task
A detailed description of the Freeze-Frame task can be found in Holmboe et al. (2008). In short, infants were presented with animations in the center of a 19-in (48.3-cm) color monitor. Infants were seated in their parent’s lap at a 60-cm distance from the monitor. On every trial, a white square was flashed on the right or the left side of the screen (the distractor). If the infant looked to the distractor, the animation was stopped for 3,000 ms. If the infant did not look to the distractor, the animation continued after distractor presentation for the duration of the trial. Distractor duration was calibrated individually for each infant by increasing it by 40 ms on every trial where the infant did not look to the distractor. When the infant had looked to the distractor on two consecutive trials, distractor duration was fixed at the current duration for the rest of the test session. The even-numbered trials presented dynamic and colorful animations changing every 2 s (interesting trials), whereas the odd-numbered trials always presented the same uninteresting rotating orange star (boring trials). Infants were encouraged to complete 60 trials.
A few minor adjustments were made to the task used in Cohort 2. Most importantly, the animations were slightly smaller and a different set of animations was used for the interesting trials. The procedure used in Cohort 2 was the same as the procedure used in Cohort 1; however, in the new version, distractor duration did not increase beyond 1,200 ms. Infants were encouraged to complete 80 trials. The data were analyzed as described in Holmboe et al. (2008). That is, the session was divided into phases (from two trials before the calibration trial), invalid trials were excluded, and the proportion of looks to the distractors was calculated separately for boring and interesting trials in each phase. However, the additional data collection allowed an extra phase in the analyses. Thus, there were four phases of the experiment, each containing 16 trials (8 boring and 8 interesting).
Video recordings of each infant’s behavior were coded offline. The coding procedure in Cohort 2 was similar to the procedure reported in Holmboe et al. (2008). The trial was considered invalid if the infant was not looking at the central stimulus at distractor onset or looked away during the distractor. The trial was also considered invalid if the infant blinked, that is, if the pupils were fully covered, during distractor presentation (unless the infant made a clear saccade to the distractor at the same time). In addition, the trial was considered invalid if these behaviors occurred during the 1,000 ms following distractor presentation. This criterion was added because in trials where the infant looks away immediately following distractor presentation, it is impossible to know whether the infant would have looked to the distractor had he or she not looked away from the screen. On rare occasions, a trial was excluded because the infant’s eyes were out of view (e.g., if the infant’s hand was in front of his or her eyes); such trials were considered invalid if the eyes were out of view for more than two frames (80 ms) during distractor presentation or within the 1,000 ms following distractor presentation. Finally, trials in which a saccade toward the distractor was initiated earlier than three frames (120 ms) after distractor onset were also considered invalid; such saccades were most likely anticipatory or random. Intercoder reliability in Cohort 2 was satisfactory for both looking behavior (κ = .94) and trial validity (κ = .86) based on data from 10 participants. (Intercoder reliability in Cohort 1 was similar; see Holmboe et al., 2008.)
Collection of Buccal Swabs and DNA Extraction
Buccal (cheek) swabs were collected when infants were 3.5 years of age in Cohort 1 as part of a follow-up study and when infants were 4 months old in Cohort 2. The buccal swab was collected by the parent in the lab (by rubbing a cotton bud on the inside of the child’s cheeks for approximately 5–10 s) and then put in a sample tube by the experimenter. Two swabs per DNA sample tube were collected, and two independent samples per infant were shipped and isolated separately with a DNA-purification kit obtained from Gentra (Minneapolis, US), yielding a total of 2–10 μ g DNA per sample.
Genotyping
Genotyping procedures were carried out using published protocols (DRD2 TaqIA: Grandy, Zhang, & Civelli, 1993; DRD4 48-bp VNTR: Ronai et al., 2000; COMT Val158Met: Tarnok et al., 2007; DAT1 3′ VNTR: Vandenbergh et al., 1992). The two DNA samples from each infant were genotyped separately for all of the investigated polymorphisms. In order to ensure successful geno-typing, we took the following precautions: In case of unsuccessful amplification of the DRD4 48-bp and DAT1 3′ VNTR polymorphisms (~10%) the polymerase chain reaction (PCR) was repeated. In addition, we carried out independent amplification reactions for 50% of the samples at the DRD4 48-bp VNTR because of the problematic amplification of the longer alleles (Ronai et al., 2000); this quality control step yielded the same genotypes as the ones originally obtained. For the DRD2 TaqIA polymorphism, genotyping was repeated in case of unsuccessful amplification (~5%) or nonidentical results for the two samples at the restriction enzyme digestion (~8%). The COMT Val158Met polymorphism (rs4680) was also genotyped by an alternative method with a predesigned TaqMan kit (C_25746809_50, Applied Biosystems, Foster City, CA) on a 7300 Real-Time PCR System; the genotypes were in accordance with the original ones. Unsuccessful genotyp-ing of the COMT Val158Met polymorphism occurred in 1–3% of samples (1% with the real-time PCR method, 3% with the allele-specific amplification method by Tarnok et al., 2007). Genotyping of these samples was repeated. After regenotyping, the genotyping success rate was 100% for all four polymorphisms.
Data Analyses
Data were analyzed with SPSS for Windows, Version 15. We analyzed behavioral data using repeated measures analysis of variance (ANOVA) and genotype data using the Linear Mixed Model (LMM) procedure in SPSS, assuming a diagonal covariance structure, and with a random intercept term. Participant ID was entered as the subject variable (treated as a random effect). Phase and trial type were entered as repeated measures, and proportion of looks to the distractors was entered as the dependent variable. Genotype was entered as a between-subjects factor. All main effects and interaction terms were entered into the model as fixed effects (full factorial design).
The advantage of LMM is that data from participants with missing data points, in this case missing data from one or more phases of the experiment, can be included in the analysis (Garson, 2008). Missing data points are inevitable in infant studies and, given the fact that the genotype effects in which we were interested were likely to be modest in magnitude, we wished to include as much of the data in the analyses as possible.
Because of the risk of population stratification in ethnically mixed samples (Hutchison, Stallings, McGeary, & Bryan, 2004), genotype analyses were carried out on both the entire sample and on the subsample of infants of Caucasian ethnic origin. Significant main effects and interactions were followed up by post hoc tests and checked against a false discovery rate (FDR) adjusted p value based on the total number of post hoc tests carried out across all genotype analyses in both the total sample and the Caucasian subsample (33 post hoc tests in total). The FDR was controlled at p < .05 with the method described by Benjamini, Drai, Elmer, Kafkafi, and Golani, (2001). Only post hoc comparisons that remained significant after controlling the FDR are reported.
The Hardy–Weinberg equilibrium test was calculated with Knud Christensen’s program (Christensen, 1999); for the DAT1 3′ VNTR, the three common genotypes from two frequent alleles (9- and 10-repeat) were included in the analysis, and for the DRD4 48-bp VNTR, genotypes from four common alleles (2-, 3-, 4-, and 7-repeat) were analyzed. For the COMT Val158Met and DRD2 TaqIA polymorphisms, there were only three genotypes and therefore, all infants could be included in the Hardy–Weinberg test.
In the analyses investigating potential genotype effects on Freeze-Frame performance, we compared the most frequent 10/10 genotype of the DAT1 3′ VNTR with all other genotypes (9/9, 9/10, and other types of heterozygotes, i.e., 3/10, 7/10, 10/11). The latter group is referred to as the non-10/10 group. Genotype grouping for the DRD4 48-bp VNTR polymorphism was based on the presence or absence of the 7-repeat allele (the 7+ group and the 7− group, respectively). One infant with the genotype 4/8 was included in the 7+ group.
Results
Genotype and Allele Distribution
Genotype data were available for 19 of the 24 infants in Cohort 1. Seventeen of these infants were of Caucasian ethnic origin. In Cohort 2, genotype data were available for all 94 infants (71 Caucasian) tested at 9 months of age. When the two cohorts were pooled, genotype data were available for 113 infants (88 Caucasian). One hundred and two of these infants (79 Caucasian) calibrated in the task (see below) and could be included in the analyses. Genotype frequencies for each of the four polymorphisms are presented in Table 1, and allele frequencies are presented in a supplemental table online. Alleles and genotypes were in Hardy–Weinberg equilibrium for all polymorphisms, with the exception of the DRD4 48-bp VNTR polymorphism in the total sample (see note to Table 1). When the Hardy–Weinberg analysis of the DRD4 48-bp VNTR was restricted to the Caucasian sub-sample, the p value increased to .45. In order to ensure a genetically homogenous population, we carried out every genotype analysis in the Caucasian subsample as well. Allele and genotype frequencies were generally in agreement with the frequencies reported for a mixed European population (see introduction) and were similar in the total sample and the Caucasian subsample.
Table 1.
Genotype Frequencies in the Total Sample and the Caucasian Subsample for the Four Dopamine System Gene Polymorphisms
| Genotype | Total | Caucasian | Grouping |
|---|---|---|---|
| DRD4 48-bp VNTR | |||
| 2/3 | 2 (2.0) | 2 (2.5) | 7− |
| 2/4 | 10 (9.8) | 9 (11.4) | 7− |
| 2/7 | 8 (7.8) | 4 (5.1) | 7+ |
| 3/4 | 8 (7.8) | 6 (7.6) | 7− |
| 3/7 | 2 (2.0) | 2 (2.5) | 7+ |
| 4/4 | 48 (47.1) | 36 (45.6) | 7− |
| 4/5 | 2 (2.0) | 0 (0.0) | 7− |
| 4/7 | 21 (20.6) | 19 (24.1) | 7+ |
| 4/8 | 1 (1.0) | 1 (1.3) | 7+ |
| 7+ | 32 (31.4) | 26 (32.9) | |
|
| |||
| COMT Val158Met | |||
| Met/Met | 28 (27.5) | 19 (24.1) | Met/Met |
| Val/Met | 47 (46.1) | 37 (46.8) | Val/Met |
| Val/Val | 27 (26.5) | 23 (29.1) | Val/Val |
|
| |||
| DRD2 TaqIA | |||
| A1/A1 | 4 (3.9) | 4 (5.1) | A1+ |
| A1/A2 | 29 (28.4) | 20 (25.3) | A1+ |
| A2/A2 | 69 (67.6) | 55 (69.6) | A1− |
| A1+ | 33 (32.4) | 24 (30.4) | |
|
| |||
| DAT1 3′ VNTR | |||
| 3/10 | 1 (1.0) | 0 (0.0) | Non-10/10 |
| 7/10 | 1 (1.0) | 0 (0.0) | Non-10/10 |
| 9/9 | 4 (3.9) | 3 (3.8) | Non-10/10 |
| 9/10 | 35 (34.3) | 30 (38.0) | Non-10/10 |
| 10/10 | 60 (58.8) | 45 (57.0) | 10/10 |
| 10/11 | 1 (1.0) | 1 (1.3) | Non-10/10 |
| Non-10/10 | 42 (41.2) | 34 (43.0) | |
|
| |||
| 102a | 79a | ||
Note. Percentages are presented in brackets. Only data from infants who calibrated in the Freeze-Frame task are included in the table (data from infants who did not calibrate could not be used in the analyses). All polymorphisms except the dopamine D4 receptor (DRD4) 48 – base pair (48-bp) variable number of tandem repeats (VNTR) polymorphism conformed to Hardy–Weinberg equilibrium: DRD4 48-bp VNTR: χ2(6) = 12.95, p = .044 (all participants); χ2(6) = 5.80, p = .45 (Caucasian participants only). Catechol-O-methyltransferase (COMT) valine158methionine (Val158Met): χ2(1) = 0.63, p = .43 (all participants); χ2(1) = 0.29, p = .59 (Caucasian participants only). Dopamine D2 receptor (DRD2) Taq1A: χ2(1) = 0.18, p = .67 (all participants); χ2(1) = 1.37, p = .24 (Caucasian participants only). DAT1 3′ VNTR: χ2(1) = 0.16, p = .69 (all participants); χ2(1) = 0.54, p = .46 (Caucasian participants only).
Total N.
Freeze-Frame Behavioral Results
One hundred and two infants of the 113 infants with genotype data available calibrated in the Freeze-Frame task (79 in the Caucasian subsample), that is, they looked to the distractor on two consecutive trials (6 infants did not calibrate, and 5 infants were incorrectly calibrated by the experimenter; these infants could not be included in the analyses). Distractor duration, on average, was calibrated in 5.53 trials (SD = 8.13; range = 2 to 64), and the mean calibrated distractor duration was 324 ms (SD = 181; range = 200 to 1,200). The average proportion of valid trials was 0.82 (SD = 0.10). Infants in Cohort 2 had a slightly lower proportion of valid trials than did infants in Cohort 1 (0.81 vs. 0.90), probably because of the session being a few minutes longer in Cohort 2, but the groups did not differ significantly in terms of calibration data (data not shown).
The proportion of looks to the distractors in each phase and trial type is presented in Table 2. Freeze-Frame results from Cohort 1 have been reported previously (Holmboe et al., 2008). In the previous study, a repeated measures ANOVA indicated that there were significant main effects of phase and trial type but no interaction. Results were unchanged in the sample of infants from Cohort 1 for whom genotype data were available (data not shown). These results were also replicated in Cohort 2—for trial type, F(1, 68) = 79.29, p < .001, ; for phase, F(2, 136) = 99.63, p < .001, ; for phase × trial type, F(2, 136) = 0.63, p = .53—and in the total sample: for trial type, F(1, 81) = 105.99, p < .001, ; for phase, F(2, 162) = 117.42, p < .001, ; for phase × trial type, F(2, 162) = 0.59, p = .55. The same significant effects were found when four phases were included in an ANOVA using data from Cohort 2 (data not shown). These results indicate that there is a clear main effect of trial type on looks to the distractors such that infants looked less to the distractors in the interesting trials than in the boring trials. Infants also showed a decrease in looks to the distractors during the test session, and this decrease was similar in the two trial types, that is, there was no interaction (Table 2).
Table 2.
Descriptive Statistics for the Proportion of Looks to the Distractors Across Phases and Trial Types in the Freeze-Frame Task
| Trial type and phase | M | SD |
|---|---|---|
| Boring trials | ||
| Phase 1 | .69 | .22 |
| Phase 2 | .42 | .28 |
| Phase 3 | .40 | .24 |
| Phase 4a | .39 | .25 |
| Total | .48 | .17 |
| Interesting trials | ||
| Phase 1 | .45 | .25 |
| Phase 2 | .19 | .19 |
| Phase 3 | .13 | .16 |
| Phase 4a | .11 | .15 |
| Total | .22 | .14 |
Only infants in Cohort 2 completed four phases.
For the genotype analyses, we wished to combine the data from the two cohorts to increase power. In order to combine all of the available data, it was important to establish that infants in the two cohorts performed the task in the same way. A few minor parameters of the Freeze-Frame task differed between the two cohorts (see Method). Therefore, the repeated measures ANOVA was repeated with cohort as a between-subjects variable. This analysis clearly replicated the main effects and lack of interaction (data not shown). It is important to note that there was no significant main effect of, or interactions involving, cohort (all ps > .30). Given this lack of significant differences between the two cohorts, we deemed it appropriate to pool the data for the genotype analyses.
In all of the genotype analyses reported below, the main effects of phase and trial type remained significant, with no interaction between phase and trial type (data not shown). Furthermore, none of the polymorphisms was associated with basic task parameters such as the calibrated distractor duration or proportion of valid trials after controlling the FDR.
The COMT Val158Met Polymorphism and Freeze-Frame Performance
All four phases of the Freeze-Frame task were included in the LMM, as this analysis incorporates all available data. The LMM analysis indicated that there was a significant main effect of COMT Val158Met genotype on the proportion of looks to the distractors, F(2,564.08) = 3.01, p < .050. No interactions involving COMT Val158Met genotype reached significance in the total sample (all ps > .15). When the analysis was restricted to Caucasian infants, this picture changed. The main effect of COMT Val158Met genotype was no longer significant, F(2,418.32) = 2.20, p = .112, but the COMT Val158Met genotype × trial type interaction was significant, F(2,418.32) = 4.38, p = .013, indicating that COMT Val158Met genotype affected performance in the two trial types differentially. No other interactions approached significance (all ps > .70).
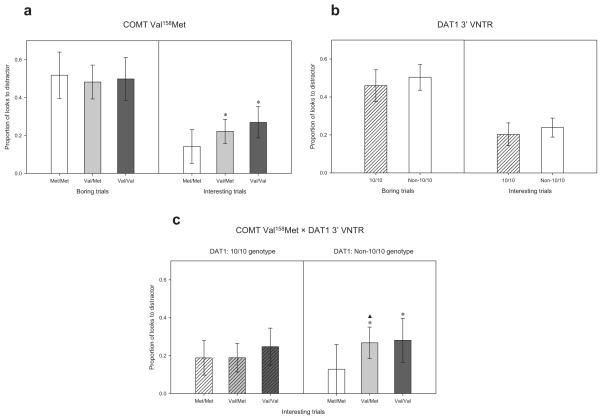
Post hoc analyses of the main effect of COMT Val158Met genotype in the total sample indicated that none of the differences between genotype groups survived the FDR correction. Post hoc analyses of the COMT Val158Met genotype × trial type interaction observed in the Caucasian subsample indicated a significant difference in looks to the distractors in interesting trials both between the Met/Met and Val/Val groups ( p < .0001) and between the Met/Met and Val/Met groups ( p < .01). No other post hoc comparisons reached significance after controlling the FDR. The COMT Val158Met genotype differences in the Caucasian sub-sample are illustrated in Figure 1a.
Figure 1.
The effect of the catechol-O-methyltransferase (COMT) valine158methionine (Val158Met) and dopamine transporter (DAT1)3′ variable number of tandem repeats (VNTR) polymorphisms on Freeze-Frame performance. Error bars indicate the 95% confidence interval of the mean. (a) The mean proportion of looks to the distractors in the boring and interesting Freeze-Frame trials in the three COMT Val158Met genotype groups in the Caucasian subsample (Met/Met, n = 19; Val/Met, n = 37; Val/Val, n = 23). An asterisk indicates a significant difference from the Met/Met group at p < .01. (b) The mean proportion of looks to the distractors in the boring and interesting Freeze-Frame trials in the DAT1 3′ VNTR 10/10 and non-10/10 genotype groups (10/10, n = 60; non-10/10, n = 42); the overall difference between the two genotype groups (across trial types) was significant at p < .05. (c) The effect of the COMT Val158Met polymorphism on the mean proportion of looks to the distractors in the interesting Freeze-Frame trials in the two DAT1 genotype groups (10/10 + Met/Met, n = 19; 10/10 + Val/Met, n = 26; 10/10 + Val/Val, n = 15; non-10/10 + Met/Met, n = 9; non-10/10 + Val/Met, n = 21; non-10/10 + Val/Val, n = 12). An asterisk indicates a significant difference from the Met/Met group at p < .01 within the non-10/10 group, and a triangle indicates a significant difference at p < .01 between the Val/Met group in the non-10/10 group compared with the Val/Met group in the 10/10 group.
The DRD4 48-bp VNTR Polymorphism and Freeze-Frame Performance
The LMM analysis of the effect of the DRD4 48-bp VNTR on performance in the Freeze-Frame task showed no significant effects involving genotype in either the total or the Caucasian subsample (all ps > .15). This indicates that, in the current sample, the 7+ group did not differ from the 7− group in terms of Freeze-Frame performance at 9 months of age.
The DRD2 TaqIA Polymorphism and Freeze-Frame Performance
The LMM showed no significant effects involving DRD2 TaqIA genotype (all ps > .70). This result was unchanged when the analysis was restricted to Caucasian infants (all ps > .20). The DRD2 TaqIA polymorphism did not therefore have any significant effect on Freeze-Frame performance in the present sample.
The DAT1 3′ VNTR Polymorphism and Freeze-Frame Performance
The LMM analysis of the DAT1 3′ VNTR showed a significant main effect of genotype in the total sample, F(1,569.52) = 3.98, p = .047. No interactions reached significance (all ps > .15). When the analysis was restricted to Caucasian infants, the main effect of DAT1 3′ VNTR genotype was only significant at trend level, F(1,427.74) = 2.92, p = .088. There was also a marginally significant DAT1 3′ VNTR genotype × phase interaction, F(3,191.66) = 2.34, p = .075. The main effect of DAT1 3′ VNTR genotype in the total sample was due to the 10/10 group looking less to the distractors overall than the non-10/10 group. This difference is illustrated in Figure 1b. No post hoc analyses were carried out, as only the main effect of DAT1 3′ VNTR genotype was significant.
Analysis of the Combined Effect of the COMT Val158Met and DAT1 3′ VNTR Polymorphisms on Freeze-Frame Performance
The genotype distribution of the COMT Val158Met and DAT1 3′ VNTR, which had genotype × genotype group sizes between 9 and 26 participants (see legend to Figure 1), allowed us to investigate the potential interaction between these two polymorphisms.(Genotype frequencies for the other polymorphisms in the study resulted in group sizes that were too small to allow investigation of interactions, with the size of minor genotype × genotype groups being less than 5.) An LMM where both DAT1 3′ VNTR genotype and COMT Val158Met genotype were entered as independent variables showed a significant main effect of COMT Val158Met genotype, F(2,528.98) = 3.41, p = .034, and a trend significant effect of DAT1 3′ VNTR genotype, F(1,529.47) = 3.30, p = .070. In addition to these main effects, there was a significant COMT Val158Met genotype × trial type interaction, F(2,528.98) = 3.19, p = .042, and a significant DAT1 3′ VNTR genotype × COMT Val158Met genotype × trial type interaction, F(2,528.98) = 4.09, p = .017. The DAT1 3′ VNTR genotype × phase interaction approached significance, F(3,240.18) = 2.24, p = .084, as did the DAT1 3′ VNTR genotype × COMT Val158Met genotype × phase interaction, F(6,241.10) = 1.91, p = .079. No other interactions approached significance in the total sample (all ps > .35).
In the Caucasian subsample alone, the results were slightly different. The main effect of COMT Val158Met genotype approached significance, F(2,373.00) = 2.82, p = .061. The same was the case for the DAT1 3′ VNTR genotype, F(1,374.07) = 3.82, p = .051. Again, the COMT Val158Met genotype × trial type interaction was significant, F(2,373.00) = 4.13, p = .017. Finally, the DAT1 3′ VNTR genotype × phase interaction was significant in the Caucasian subsample, F(3,170.63) = 2.98, p = .033. No other interactions reached significance in the Caucasian subsample (all ps > .20).
Post hoc analyses were restricted to the novel interaction effects involving COMT Val158Met and DAT1 3′ VNTR because all significant and near-significant main effects were qualified by a significant interaction and because other interactions, such as the COMT Val158Met genotype × trial type interaction, essentially indicated the same genotype effects as the analyses of the two polymorphisms separately. Post hoc analyses of the DAT1 3′ VNTR genotype × COMT Val158Met genotype × trial type interaction in the total sample indicated that within the DAT1 non-10/10 group there was a significant difference in looks to the distractors in the interesting trials between the Met/Met and Val/ Val groups ( p < .001) and between the Met/Met and Val/Met groups ( p = .001). In contrast, no COMT genotype differences reached significance in the DAT1 10/10 group after controlling the FDR. This pattern of results is illustrated in Figure 1c. With regard to performance in each COMT genotype group across DAT1 genotypes, infants with the Val/Met genotype who also had the DAT1 10/10 genotype looked significantly less to the distractors in the interesting trials than did infants with the Val/Met genotype in the DAT1 non-10/10 group ( p < .01). The other COMT genotype groups did not differ significantly across DAT1 genotype groups in the interesting trials (Figure 1c). None of the post hoc tests of the DAT1 3′ VNTR genotype × COMT Val158Met genotype × trial type interaction showed significant effects in the boring trials after controlling the FDR.
Post hoc analyses indicated that the DAT1 3′ VNTR genotype × phase interaction found in the Caucasian subsample was due to a highly significant difference in proportion of looks to the distractors between the 10/10 group and the non-10/10 group in phase 3 of the Freeze-Frame session ( p < .001).
Discussion
In the present study, we investigated whether performance in a novel task developed to assess frontal cortex functioning in infancy, the Freeze-Frame task (Holmboe et al., 2008), was associated with common polymorphisms in four dopamine system genes. Previous research has clearly shown that dopamine plays an important role in the frontal cortex (Brozoski et al., 1979; Collins et al., 1998; Diamond et al., 1997; Goldman-Rakic et al., 2000; Roberts et al., 1994; Sawaguchi & Goldman-Rakic, 1991; Vijayraghavan et al., 2007).
Behaviorally, we replicated previous findings on the Freeze-Frame task (Holmboe et al., 2008). In relation to the polymorphisms likely to impact directly on frontal cortex function, we found a significant association between Freeze-Frame performance and the COMT Val158Met polymorphism. Given the extensive evidence for an association between the COMT Val158Met polymorphism and performance on a range of frontal cortex tasks (Diamond et al., 2004; Diaz-Asper et al., 2008; Egan et al., 2001; Mattay et al., 2003; Sheldrick et al., 2008; Stefanis et al., 2005; see also Papaleo et al., 2008), as well as effects on neural efficiency in the frontal cortex during performance of these tasks (Bertolino et al., 2006; Blasi et al., 2005; Caldú et al., 2007; Egan et al., 2001; Krämer et al., 2007; Mattay et al., 2003; Meyer-Lindenberg et al., 2006; Mier et al., in press), it seems likely that COMT Val158Met genotype affects dopamine levels in the frontal cortex and thereby Freeze-Frame performance in our infant sample.
Furthermore, it is worth noting that this effect was specific to the interesting trials, at least in the Caucasian subsample (Figure 1a). This suggests that the COMT Val158Met effect is not a general effect impacting on infants’ distractibility level in any given situation. Rather, it seems to be the case that infants with the low-enzyme activity Met/Met genotype became particularly focused on the central stimulus compared with the high-enzyme activity Val/ Val genotype when this stimulus was engaging. However, it should be noted that the interaction with trial type was significant in the Caucasian subsample only and therefore might not generalize to other populations.
We found little evidence that the DRD4 48-bp VNTR polymorphism affects performance on the Freeze-Frame task at 9 months of age, though the sample was too small to detect subtle effects. In terms of the polymorphisms that are likely to act in the striatum, we did not observe any effect of the DRD2 TaqIA either. We did, however, observe an effect of the DAT1 3′ VNTR polymorphism. In contrast to the effect of the COMT Val158Met polymorphism, this effect did not appear to be specific to a particular trial type. Instead, we found evidence of an overall difference in the proportion of looks to the distractors, with the 10/10 group looking less to the distractors than did the non-10/10 group (Figure 1b). The results therefore suggest that the DAT1 3′ VNTR polymorphism modulates overall distractibility in the Freeze-Frame task, though there was a tendency for this genotype effect to be stronger at the end of the test session. Given the fact that the dopamine transporter plays an important role in the striatum (Hurd & Hall, 2005; Karoum et al., 1994), this effect could be due to modulation of general attentional mechanisms mediated by the subcortical dopamine system or frontal-subcortical connections (Alexander et al., 1986; Cummings, 1993).
Finally, we investigated the potential interaction between the COMT Val158Met and DAT1 3′ VNTR polymorphisms on Freeze-Frame performance. The results of these analyses broadly replicated the main effects and interactions found in the analysis of each polymorphism separately. However, the analysis also revealed a significant DAT1 3′ VNTR genotype × COMT Val158Met genotype × trial type interaction, suggesting that the DAT1 3′ VNTR polymorphism modulated the effect of the COMT Val158Met polymorphism on Freeze-Frame performance. Basically, the effect of the COMT Val158Met polymorphism on the proportion of looks to the distractors in the interesting trials was strong in the DAT1 non-10/10 group, with particularly large differences between the Met/Met group and the two other genotype groups (Figure 1c, right panel). In contrast, the equivalent effect in the DAT1 10/10 group virtually disappeared (Figure 1c, left panel).
Presuming that a lower level of distractibility in the interesting trials is an expression of a higher degree of selective inhibition, these results suggest that infants with higher COMT enzyme activity (Val/Val and Val/Met) actually benefit from having the DAT1 10/10 genotype, whereas this is not the case for infants with low COMT enzyme activity (Met/Met). This was confirmed at least for the Val/Met genotype; this genotype showed a significant reduction in looks to the distractors in the interesting trials when combined with the 10/10 genotype rather than with the non-10/10 genotype (Figure 1c). Though preliminary given the sample size, these findings are particularly interesting because they suggest that the interaction between a predominantly frontal dopaminergic polymorphism (COMT Val158Met) and a predominantly striatal dopaminergic polymorphism (DAT1 3′ VNTR) results in large performance differences on the Freeze-Frame task as early as 9 months of age.
Despite the likely effect of both frontal and subcortical mechanisms in the reported results, it is not possible to establish the exact neural substrate of this interaction from the current data. Previous studies have found additive genetic effects of the DAT1 3′ VNTR and COMT Val158Met polymorphisms on neural efficiency in the frontal cortex but no epistasis (Bertolino et al., 2006; Caldú et al., 2007). Further research involving neuroimaging data will help elucidate the potential role of the frontal cortex and the striatum in the genotype effects found in the present study.
It should be mentioned that it would have been ideal to investigate all possible interactions among the four polymorphisms in the study. However, only the COMT Val158Met and the DAT1 3′ VNTR polymorphisms had genotype frequencies providing enough power to investigate interaction effects (see Results). For the DRD4 48-bp VNTR and the DRD2 TaqIA polymorphisms, the genotype frequencies involving the minor allele were too low to test meaningful interactions. A clear limitation of the study is therefore the relatively small sample size (though large for an infant study). Future studies should address the question of interactions between all four (and additional) polymorphisms in dopamine system genes in a larger infant cohort. Furthermore, assessment of additional genetic ancestry-informative markers would prevent the sample reduction caused by analyzing the Caucasian only sample.
The current study constitutes a snapshot in time at 9 months of age. Future studies over a wider age range may help elucidate which patterns of Freeze-Frame performance are adaptive throughout infancy and early childhood and how these patterns relate to polymorphisms in dopamine system genes. Some progress has already been made toward this at the behavioral level in the work by Holmboe et al. (2008), where performance indices on early frontal cortex tasks showed both positive and negative associations with later performance. Nevertheless, an important conclusion to be drawn from the results of the present study is that polymorphisms in dopamine system genes play an important role as early as infancy. Previous studies have found effects of the DRD4 48-bp VNTR on temperament and relatively broad aspects of attention in infancy (Auerbach et al., 1999; Auerbach, Benjamin, Faroy, Geller, & Ebstein, 2001; Auerbach, Faroy, Ebstein, Kahana, & Levine, 2001; Ebstein et al., 1998; Laucht et al., 2006; Sheese et al., 2007). The current study adds to this evidence by showing that the COMT Val158Met polymorphism, which is thought to play an important role specifically in the frontal cortex, affects performance on a simple saccadic inhibition task in infancy.
In conclusion, the results of the present study further validate the Freeze-Frame task and demonstrate that variation in dopaminergic neurotransmission in the frontal cortex and associated subcortical structures can have an impact on infant attention as early as 9 months of age. The exact neural substrate and developmental course of these genotypic differences is a fruitful area for future research. This research holds the promise of deepening our understanding of the genetic underpinnings of individual differences in the important functions mediated by the frontal cortex from an early age.
Supplementary Material
Acknowledgments
Some of the work reported in this article formed part of Karla Holmboe’s doctoral dissertation submitted to the University of London in 2008. The work was supported by the United Kingdom Medical Research Council (Programme Grant G9715587 and Postdoctoral Fellowship G0800054) and the Hungarian Scientific Research Fund (OTKA Grant T048576). We would particularly like to thank the parents and children who participated in this study. We would also like to thank Sarah Lloyd-Fox, Tamsin Osborne, Cara Grayling, Agnes Volein, and Leslie Tucker for invaluable help with data collection and coding.
Footnotes
Supplemental materials:http://dx.doi.org/10.1037/a0018180.supp
Approximate equivalent in U.S. dollars: M = 120,511, SD = 114,544, based on the average U.K. pound per U.S. dollar exchange rate of 0.54 in 2006 (NationMaster.com, 2009), when the majority of the data were collected
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Reviews in Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Benjamin J, Faroy M, Geller V, Ebstein R. DRD4 related to infant attention and information processing: A developmental link to ADHD? Psychiatric Genetics. 2001;11:31–35. doi: 10.1097/00041444-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Faroy M, Ebstein R, Kahana M, Levine J. The association of the dopamine D4 receptor gene (DRD4) and the serotonin transporter promoter gene (5-HTTLPR) with temperament in 12-month-old infants. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:777–783. doi: 10.1111/1469-7610.00774. [DOI] [PubMed] [Google Scholar]
- Auerbach J, Geller V, Lezer S, Shinwell E, Belmaker RH, Levine J, et al. Dopamine D4 receptor (D4DR) and serotonin transporter promoter (5-HTTLPR) polymorphisms in the determination of temperament in 2-month-old infants. Molecular Psychiatry. 1999;4:369–373. doi: 10.1038/sj.mp.4000531. [DOI] [PubMed] [Google Scholar]
- Baird AA, Kagan J, Gaudette T, Walz KA, Hershlag N, Boas DA. Frontal lobe activation during object permanence: Data from near-infrared spectroscopy. NeuroImage. 2002;16:120–126. doi: 10.1006/nimg.2002.1170. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Müller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: A meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Molecular Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafò MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/ 108Met polymorphism. Biological Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bell MA. Brain electrical activity associated with cognitive processing during a looking version of the A-not-B task. Infancy. 2001;2:311–330. doi: 10.1207/S15327078IN0203_2. [DOI] [PubMed] [Google Scholar]
- Bell MA, Fox NA. The relations between frontal brain electrical activity and cognitive development during infancy. Child Development. 1992;63:1142–1163. [PubMed] [Google Scholar]
- Bell MA, Fox NA. Individual differences in object perma-nence at 8 months: Locomotor experience and brain electrical activity. Developmental Psychobiology. 1997;31:287–297. [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. Journal of Neuroscience. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevåg B, Callicott JH, Das S, et al. Effect of catechol-O-methyltransferase Val158Met geno-type on attentional control. The Journal of Neuroscience. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Caldú X, Vendrell P, Bartrés-Faz D, Clemente I, Bargalló N, Jurado M, et al. Impact of the COMT Val108/158 Met and DAT geno-types on prefrontal function in healthy subjects. NeuroImage. 2007;37:1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Human Genetics. 1996;98:91–101. doi: 10.1007/s004390050166. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in Catechol-O-Methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. American Journal of Human Genetics. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K. Calculation of Chi-square test for deviation from Hardy-Weinberg equilibrium. 1999 Retrieved from http://www.husdyr.kvl.dk/htm/kc/popgen/genetik/applets/kitest.htm.
- Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science. 1986;231:840–843. doi: 10.1126/science.3945811. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of Neurology. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. Perseveration and strategy in a novel spatial self-ordered sequencing task for nonhuman primates: Effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. Journal of Cognitive Neuroscience. 1998;10:332–354. doi: 10.1162/089892998562771. [DOI] [PubMed] [Google Scholar]
- Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: Implications for impulsivity. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, et al. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Molecular Psychiatry. 2005;10:686–698. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Miller BL. Conceptual and clinical aspects of the frontal lobes. In: Miller BL, Cummings JL, editors. The human frontal lobes: Functions and disorders. 2nd ed Guilford Press; New York: 2007. pp. 12–21. [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Need AC, LaBar KS, Waters-Metenier S, Cirulli ET, Kragel J, et al. COMT Val108/158Met genotype affects neural but not cognitive processing in healthy individuals. Cerebral Cortex. doi: 10.1093/cercor/bhp132. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: A resting state fMRI study. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Diamond A. Development of the ability to use recall to guide action, as indicated by infants’ performance on AB. Child Development. 1985;56:868–883. [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. American Journal of Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Diamond A, Goldman-Rakic PS. Comparison of human infants and rhesus monkeys on Piaget’s A-not-B task: Evidence for dependence on dorsolateral prefrontal cortex. Experimental Brain Research. 1989;74:24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- Diamond A, Prevor MB, Callender G, Druin DP. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monographs of the Society for Research in Child Development. 1997;62(4) [PubMed] [Google Scholar]
- Diamond A, Zola-Morgan S, Squire L. Successful performance by monkeys with lesions of the hippocampal formation on AB and object retrieval, two tasks that mark developmental changes in human infants. Behavioural Neuroscience. 1989;103:526–537. doi: 10.1037//0735-7044.103.3.526. [DOI] [PubMed] [Google Scholar]
- Diaz-Asper CM, Goldberg TE, Kolachana BS, Straub RE, Egan MF, Weinberger DR. Genetic variation in catechol-O-methyltransferase: Effects on working memory in schizophrenic patients, their siblings, and healthy controls. Biological Psychiatry. 2008;63:72–79. doi: 10.1016/j.biopsych.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. Journal of Comparative and Physiological Psychology. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Casey BJ, Pol HEH, Galvan A, Schnack HG, et al. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Molecular Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Levine J, Geller V, Auerbach J, Gritsenko I, Belmaker RH. Dopamine D4 receptor and serotonin transporter promoter in the determination of neonatal temperament. Molecular Psychiatry. 1998;3:238–246. doi: 10.1038/sj.mp.4000363. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DTA, MacKillop J, Modi M, Beauchemin J, Dang D, Lisman SA, et al. Examining impulsivity as an endophenotype using a behavioral approach: A DRD2 TaqI A and DRD4 48-bp VNTR association study [Electronic version] Behavioral and Brain Functions. 2007;3 doi: 10.1186/1744-9081-3-2. Retrieved from http://www.behavioralandbrainfunctions.com/content/3/1/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/ hyperactivity disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini MA, Thaker S, Themelis G, Krishnamoorthy KK, Bortfeld H, Diamond SG, et al. Assessment of infant brain development with frequency-domain near-infrared spectroscopy. Pediatric Research. 2007;61:546–551. doi: 10.1203/pdr.0b013e318045be99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: Anatomy, physiology, and neuropsychology of the frontal lobe. 3rd ed Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- Garson GD. Linear mixed models: Random effects, hierarchical linear, multilevel, random coefficients, and repeated measures models. Statnotes: Topics in multivariate analysis. 2008 Retrieved from http://www2.chass.ncsu.edu/garson/pa765/statnote.htm.
- Gazzaley A, D’Esposito M. Unifying prefrontal cortex function: Executive control, neural networks, and top-down modulation. In: Miller BL, Cummings JL, editors. The human frontal lobes: Functions and disorders. 2nd ed Guilford Press; New York: 2007. pp. 187–206. [Google Scholar]
- Gerardi-Caulton G. Sensitivity to spatial conflict and development of self-regulation in children 24 –36 months of age. Developmental Science. 2000;3:397–404. [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proceedings of the National Academy of Sciences of the USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, III, Williams GV. D1 receptors in prefrontal cells and circuits. Brain Research Reviews. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Grandy DK, Zhang Y, Civelli O. PCR detection of the TaqA RFLP at the DRD2 locus. Human Molecular Genetics. 1993;2:2197. doi: 10.1093/hmg/2.12.2197-a. [DOI] [PubMed] [Google Scholar]
- Holmboe K, Fearon RMP, Csibra G, Tucker LA, Johnson MH. Freeze-Frame: A new infant inhibition task and its relation to frontal cortex tasks during infancy and early childhood. Journal of Experimental Child Psychology. 2008;100:89–114. doi: 10.1016/j.jecp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Taga G. Prosodic processing in the developing brain. Neuroscience Research. 2007;59:29–39. doi: 10.1016/j.neures.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Hall H. Human forebrain dopamine systems: Characterization of the normal brain and in relation to psychiatric disorders. In: Dunnett SB, Bentivoglio M, Björklund A, Hökfelt T, editors. Handbook of chemical neuroanatomy: Dopamine. Elsevier; Amsterdam: 2005. pp. 525–571. [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: Fatal threat or red herring? Psychological Bulletin. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Ito H, Okubo Y, Halldin C, Farde L. Mapping of central D2 dopamine receptors in man using [11C]raclopride: PET with anatomic standardization technique. NeuroImage. 1999;9:235–242. doi: 10.1006/nimg.1998.0401. [DOI] [PubMed] [Google Scholar]
- Jönsson EG, Nöthen MM, Grünhage F, Farde L, Nakashima Y, Propping P, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Kang AM, Palmatier MA, Kidd KK. Global variation of a 40-bp VNTR in the 3′-untranslated region of the dopamine transporter gene (SLC6A3) Biological Psychiatry. 1999;46:151–160. doi: 10.1016/s0006-3223(99)00101-8. [DOI] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: Reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. Journal of Neurochemistry. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Krämer UM, Cunillera T, Càmara E, Marco-Pallarés J, Cucurell D, Nager W, et al. The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. The Journal of Neuroscience. 2007;27:14190–14198. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Laucht M, Becker K, Schmidt MH. Visual exploratory behaviour in infancy and novelty seeking in adolescence: Two developmentally specific phenotypes of DRD4? Journal of Child Psychology and Psychiatry. 2006;47:1143–1151. doi: 10.1111/j.1469-7610.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Human Molecular Genetics. 2006;15:2276–2284. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P, Innis RB. Dopamine D2 receptors in the cerebral cortex: Distribution and pharmacological characterization with [3H]raclopride. Proceedings of the National Academy of Sciences of the USA. 1989;86:6412–6416. doi: 10.1073/pnas.86.16.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen I, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): Biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacological Reviews. 1999;51:593–628. [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proceedings of the National Academy of Sciences of the USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, et al. Impact of complex genetic variation in COMT on human brain function. Molecular Psychiatry. 2006;11:867–877. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: A meta-analysis. Molecular Psychiatry. doi: 10.1038/mp.2009.36. (in press) [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. American Journal of Medical Genetics. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Matheson IJ, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: A meta-analysis of case– control studies and evidence of publication bias. Molecular Psychiatry. 2007;12:454–461. doi: 10.1038/sj.mp.4001938. [DOI] [PubMed] [Google Scholar]
- Nakano T, Watanabe H, Homae F, Taga G. Prefrontal cortical involvement in young infants’ analysis of novelty. Cerebral Cortex. 2009;19:455–463. doi: 10.1093/cercor/bhn096. [DOI] [PubMed] [Google Scholar]
- NationMaster.com Currency statistics > official exchange rate > LCU per US$, period average (2006) by country. 2009 Retrieved from http://www.nationmaster.com/graph/cur_off_exc_rat_lcu_per_us_per_avelcu-per-us-period-average&date=2006.
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Human Mutation. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Progress in Neurobiology. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Noble EP. Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: A review. European Psychiatry. 2000;15:79–89. doi: 10.1016/s0924-9338(00)00208-x. [DOI] [PubMed] [Google Scholar]
- Oak JN, Oldenhof J, Van Tol HHM. The dopamine D4 receptor: One decade of research. European Journal of Pharmacology. 2000;405:303–327. doi: 10.1016/s0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biological Psychiatry. 1999;46:557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. The Journal of Neuroscience. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. The construction of reality in the child. Routledge & Kegan Paul; London: 1954. [Google Scholar]
- Pohjalainen T, Rinne JO, Någren K, Lehikoinen P, Anttila K, Syvälahti EKG, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Molecular Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Reuter M, Peters K, Schroeter K, Koebke W, Lenardon D, Bloch B, et al. The influence of the dopaminergic system on cognitive functioning: A molecular genetic approach. Behavioural Brain Research. 2005;164:93–99. doi: 10.1016/j.bbr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochemical Research. 2003;28:73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, et al. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: Possible interactions with subcortical dopamine. Journal of Neuroscience. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronai Z, Guttman A, Nemoda Z, Staub M, Kalasz H, Sasvari-Szekely M. Rapid and sensitive genotyping of dopamine D4 receptor tandem repeats by automated ultrathin-layer gel electrophoresis. Electrophoresis. 2000;21:2058–2061. doi: 10.1002/1522-2683(20000601)21:10<2058::AID-ELPS2058>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of temperamental effortful control. Journal of Personality. 2003;71:1113–1143. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Schoots O, Van Tol HHM. The human dopamine D4 receptor repeat sequences modulate expression. The Pharmacogenomics Journal. 2003;3:343–348. doi: 10.1038/sj.tpj.6500208. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. Journal of Neuroscience. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Development and Psychopathology. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- Sheldrick AJ, Krug A, Markov V, Leube D, Michel TM, Zerres K, et al. Effect of COMT val158met genotype on cognition and personality. European Psychiatry. 2008;23:385–389. doi: 10.1016/j.eurpsy.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, et al. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Molecular Psychiatry. 2008;13:821–827. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Stefanis CN. Effect of COMT Val158Met polymorphism on the Continuous Performance Test, Identical Pairs Version: Tuning rather than improving performance. American Journal of Psychiatry. 2005;162:1752–1754. doi: 10.1176/appi.ajp.162.9.1752. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, et al. The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of the International Neuropsychological Society. 1998;4:265–278. [PubMed] [Google Scholar]
- Tarnok Z, Ronai Z, Gervai J, Kereszturi E, Gadoros J, Sasvari-Szekely M, et al. Dopaminergic candidate genes in Tourette syndrome: Association between tic severity and 3′UTR polymorphism of the dopamine transporter gene. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2007;144B:900–905. doi: 10.1002/ajmg.b.30517. [DOI] [PubMed] [Google Scholar]
- Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry EK, et al. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: Reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7:479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-O-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biological Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS, et al. Catechol-O-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cerebral Cortex. 2007;17:1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- van de Giessen EM, de Win MML, Tanck MWT, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. The Journal of Nuclear Medicine. 2008;50:45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. The Journal of Nuclear Medicine. 2005;46:745–751. [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wonodi I, Hong LE, Stine OC, Mitchell BD, Elliott A, Roberts RC, et al. Dopamine transporter polymorphism modulates oculomotor function and DAT1 mRNA expression in schizophrenia. American Journal of Medical Genetics Part B. 2009;150B:282–289. doi: 10.1002/ajmg.b.30811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Chan RCK, Jing J, Li T, Sham P, Chen RYL. A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3′-UTR of dopamine transporter gene and attention deficit hyperactivity disorder. American Journal of Medical Genetics Part B. 2007;144B:541–550. doi: 10.1002/ajmg.b.30453. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Männistö PT. Site-specific role of Catechol-O-Methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. Journal of Neuroscience. 2007;27:10196–10202. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehene E, Meiran N, Soroker N. Basal ganglia play a unique role in task switching within the frontal-subcortical circuits: Evidence from patients with focal lesions. Journal of Cognitive Neuroscience. 2008;20:1079–1093. doi: 10.1162/jocn.2008.20077. [DOI] [PubMed] [Google Scholar]
- Young RM, Lawford BR, Nutting A, Noble EP. Advances in molecular genetics and the prevention and treatment of substance misuse: Implications of association studies of the A1 allele of the D2 dopamine receptor gene. Addictive Behaviors. 2004;29:1275–1294. doi: 10.1016/j.addbeh.2004.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.